Abstract
Background
The location of the accessory pathway (AP) can be precisely identified on surface electrocardiography (ECG) in adults with Wolff-Parkinson-White (WPW) syndrome. However, current algorithms to locate the AP in pediatric patients with WPW syndrome are limited.
Objective
To propose an optimal algorithm that localizes the AP in pediatric patients with WPW syndrome.
Methods
From 1992 to 2016, 180 consecutive patients aged below 18 years with symptomatic WPW syndrome were included. After the exclusion of patients with non-descriptive electrocardiography (ECG), multiple APs, congenital heart diseases, non-inducible tachycardia, and those who received a second ablation, 104 patients were analyzed retrospectively. Surface ECG was obtained before ablation and evaluated by using previously documented algorithms, from which a new pediatric algorithm was developed.
Results
Previous algorithms were not highly accurate when used in pediatric patients with WPW syndrome. In the new algorithm, the R/S ratio of V1 and the polarity of the delta wave in lead I could distinguish right from the left side AP with 100% accuracy. The polarity of the delta wave of lead V1 could distinguish free wall AP from septal AP with an accuracy of 100% in left-side AP, compared to 88.6% in leads III and V1 for right-side AP. The overall accuracy was 92.3%.
Conclusions
This simple, novel algorithm could differentiate left from right AP and septal from free wall AP in pediatric patients with WPW syndrome.
Keywords: Accessory pathway, Algorithm, Children, Localization, WPW syndrome
INTRODUCTION
Wolff-Parkinson-White (WPW) syndrome was discovered by Louis Wolff, Sir John Parkinson, and Paul Dudley White in 1930.1 It is the most common cause of tachycardia in children.2 WPW syndrome can be defined as short PR, a delta wave, and wide QRS interval on a 12-lead surface electrocardiogram (ECG).
An electrophysiological study (EPS) is a procedure carried out if abnormal heart rhythms and/or conduction disorders are suspected, and radiofrequency catheter ablation (RFCA) is one of the standard treatments for WPW syndrome. An accurate prediction of the accessory pathway (AP) location before ablation can save procedural time and decrease fluoroscopic exposure. When the AP is suspected of being close to normal conduction, a safer procedure can be used such as cryoablation.
ECG characteristics are used in identifying the AP location in individuals with WPW syndrome. Rosenbaum et al. first classified WPW syndrome as type A (AP on the left side) and type B (AP on the right side) using the V1 R/S ratio.3 Subsequently, more precise algorithms were developed for adults.4-10 The major criteria for identifying AP by 12-lead surface ECG for adults include the axis of delta waves, the polarity of QRS, or both in different leads. Adult algorithms have a high accuracy of above 90%. However, Wren et al. found that adult algorithms are less efficient in localizing AP in pediatric patients, especially right side AP.11 Boersma et al. developed a pediatric algorithm, however the location of right-side AP was still difficult to identify.12
In this study, we assessed several adult algorithms for their accuracy in a pediatric cohort. We then created a new pediatric algorithm after referencing these adult algorithms.
METHODS
Patients
This retrospective study was approved by the Institutional Committee on Human Research Board at Taipei-Veterans General Hospital (2018-02-010AC). From July 1992 to February 2016, a total of 180 consecutive patients aged below 18 years with clinically symptomatic WPW syndrome who underwent successful RFCA with no WPW pattern on ECG or tachycardia for more than 6 months after ablation were included retrospectively. Patients with congenital structural heart diseases, RFCA more than once, more than one AP, and those with faded thermal printing on 12-lead surface ECG that made analysis very difficult were excluded. Before ablation, informed consent for EPS and RFCA was obtained from the parents of the patients who were younger than 18 years. If the patient was taking antiarrhythmic agents, they were asked to stop treatment for at least 5 half-lives before catheterization.13
EPS and RFCA
The patients underwent EPS and RFCA as a routine procedure described in a previous study.14 The RFCA was carried out at the atrioventricular (AV) ring at the AV fusion region using an intracardiac electrogram (EGM).
Locations of successful ablation
The nomenclature of AP location was adapted from that proposed by Chiang et al., and included eight locations in the tricuspid valve [right anterolateral (RAL), right anterior (RA), right anterior septal (RAS), right medial septal (RMS), right posteroseptal (RPS), right posterior (RP), right posterolateral (RPL), and right lateral (RL)], and five positions in the mitral valve [left anterolateral (LAL), left lateral (LL), left posterolateral (LPL), left posterior (LP), and left posteroseptal (LPS) region]. The success of ablation of these sites was evaluated by the patient’s attending electrophysiologist.
ECG analysis and validation of previous algorithms
ECG results were collected retrospectively. The 12-lead surface ECG tracings were obtained before catheter ablation. The recording speed of the ECG was set at 25 mm/s and the amplitude at 10 mm/mV. Senior and junior cardiac electrophysiologists analyzed the 12-lead surface ECG using previous algorithms without knowing the AP location. Table 1 shows the comparison and number of sites identified using the new algorithm and previous algorithms.
Table 1. Comparison of the previous WPW algorithms and our algorithm with the nomenclature.
| Our algorithm | LF | LF | LF | LF | LS | RS | RS | RS | RF | RF | RF | RF | RF |
| Arruda4 | LAL | LL | LPL | LP | PSMA | PSTA | MS | AS | RA | RAL | RL | RPL | RP |
| Boersma11 | LL | LL | LPS | LPS | LPS | PS | MS | MS | AS | RL | RL | RL | RPS |
| Chiang5 | LAL | LL | LPL | LP | LPS | RPS | MS | RAS | RA | RAL | RL | RPL | RP |
| d’Avila6 | LL | LL | LP | LPS | LPS | RPS | RMS | RAS | RAL | RAL | RAL | RPL | RPL |
| Iturralde8 | LPL/LAS | LPL/LAS | LIP/LI | LIP/LI | LIP/LI | RIP/RI | RASP | RASP | RA | RA | RA | RIP/RI | RIP/RI |
AS, anteroseptal; LAL, left anterolateral; LAS, left anterosuperior; LF, left free wall; LI, left inferior; LIP, left inferior paraseptal; LL, left lateral; LP, left posterior; LPL, left posterolateral; LPS, left posteroseptal; LS, left septal; MS, midseptal; PSMA, paraseptal mitral annulus; PSTA, paraseptal tricuspid annulus; RA, right anterior; RAL, right anterolateral; RAS, right anteroseptal; RASP, right anterosuperior paraseptal; RF, right free wall; RL, right lateral; RI, right inferior; RIP, right inferior paraseptal; RMS, right midseptal; RP, right posterior; RPL, right posterolateral; RS, right septal.
Development of the novel pediatric algorithm
The leads which were applied in the adult algorithms were referenced for the development of the new algorithm, considering that the frequency and usefulness of the previous algorithms have been validated. The strategies of the adult algorithms are summarized in Table 2. The new algorithm was generated theoretically and objectively, and was then revised until the main parts were distinguishable.
Table 2. Simple descriptions, total accuracy, right-side total accuracy and left-side total accuracy in pediatric patients with the previous algorithms and the newly developed algorithm.
| Simple descriptions | Total | Right side | Left side | |
| Arruda | 4 individual steps | 46.2% | 34.3% | 70.6% |
| 1. I (Δ < +) or V1 (RS > 1) → AVF (Δ) | ||||
| 2. II (Δ: -) | ||||
| 3. V1 (Δ < +) → AVF (Δ) | ||||
| 4. Remaining → AVF (Δ) | ||||
| Boersma | 3 sequential steps | 70.2% | 77.1% | 55.9% |
| V1 (RS), III (RS), II (RS) | ||||
| Chiang | 4 sequential steps | 48.1% | 32.9% | 79.4% |
| V2 (RS), III (Δ), AVF (Δ) / V1 (RS) | ||||
| d’Avila | 4 sequential steps | 30.8% | 11.4% | 70.6% |
| V1 (RS), III (RS), AVL (RS) / III (Q) → RSII(RS) | ||||
| Iturralde | 3 sequential steps | 46.2% | 41.4% | 55.9% |
| III (RS), V1 (RS), V2 (RS) | ||||
| Li | 3 sequential steps | 92.3% | 88.6% | 100% |
| V1 (RS), V1 (Δ) / I (Δ) → III (Δ) |
RS, R/S polarity; Δ, delta wave polarity; < +, ± or -; /, followed by the different result of previous step in the same level of algorithm; →, next step only following by the one of the previous step.
In the new pediatric algorithm, we aimed to differentiate the AP as four main parts: the left septal region (LS), left free wall region (LF), right septal region (RS), and right free wall region (RF). Compared with Chiang’s algorithms, the LS was equal to the LPS, the LF was composed of the LAL, LL, LPL, and LP, and the RS consisted of the RPS, RMS, and RAS. In addition, the RF was composed of the RA, RAL, RL, RPL, and RP.
Statistical analysis
Continuous data were presented as mean ± SD. The overall accuracy was calculated by dividing the number of patients with correct predictions by the total number of patients. The right-side accuracy was calculated by dividing the number of patients with a correct prediction of right-side AP by the number of patients with the AP in the tricuspid region. The left-side accuracy was calculated by dividing the number of patients with a correct prediction of left-side AP by the number of patients with AP in the mitral region.
RESULTS
Patients’ characteristics
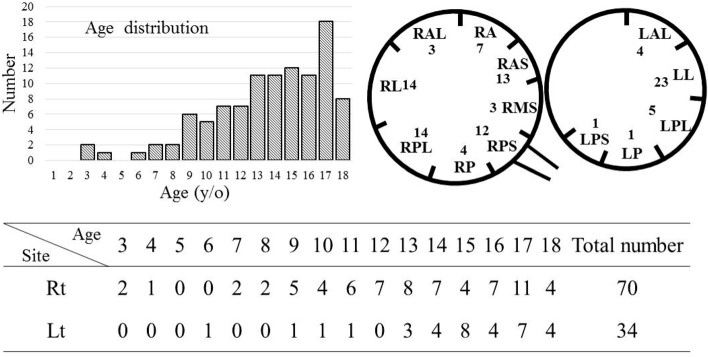
Of the 180 consecutive patients, 76 were excluded due to the following reasons: 45 had no ECG records or barely visible ECG waveform, 5 presented with Mahaim fibers, 7 also had congenital heart diseases, 17 had either two APs or had undergone second ablation, 1 also had Turner syndrome, and 1 was conjoined with no inducible arrhythmia. Finally, a total of 104 patients with symptomatic WPW syndrome and a single AP were enrolled. The mean age of the patients was 13.6 ± 3.4 years (range: 3-18 years), and the male-to-female ratio was 63:41. The age and location distribution and sex and location relationship are presented in Figure 1.
Figure 1.
Age (years) distribution, patient number in each location and gender distribution of pediatric patients with WPW syndrome.
Accuracy of the pediatric ECGs using the adult and pediatric algorithms
Table 2 summarizes the steps of each algorithm, and the total, right-side, and left-side accuracy of the adult and pediatric algorithms when used in our pediatric patients with WPW syndrome. In general, low total and right-side accuracy were observed when the adult algorithms were used, and relatively low total and right-side accuracy were noted when Boersma’s pediatric algorithm was used.
New pediatric algorithm
The new pediatric algorithm is shownin Figure 2. In summary, the first step involved the use of the V1RS ratio that was first reported by Rosenbaum in 1945.3 In our pediatric group, nine patients had left-side Kent conduction that was misinterpreted at the wrong location by Rosenbaum’s criteria. However, after the patients’ ECG tracings were subclassified based on the polarity of the delta wave in lead I, the new algorithm could differentiate left-side AP from right-side AP with 100% accuracy. In addition, right-side AP could partially differentiate free wall AP from septal AP by the negative polarity of the delta wave in lead III and the positive or isoelectric polarity of the delta wave in lead V1. The overall accuracy of the new algorithm in our patient group was 92.3% (96/104). The left-side accuracy was 100% (34/34), and the right-side accuracy was 88.6% (62/70).
Figure 2.
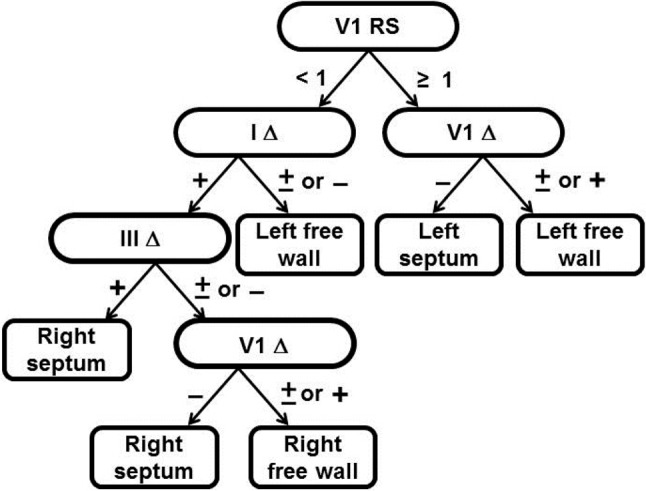
The validated pediatric algorithm. Step 1 analyzes R/S ratio in V1; if the R/S ≥ 1, left-side WPW is definite, and if the R/S < 1, the AP needs further evaluation by the polarity of the delta wave of Lead I. Step 2 analyzes the delta wave polarity in V1 ≥ 1 in V1 for differentiating free wall and septum in left-side AP and analyzing the delta wave polarity in Lead I to differentiate into left- and right-side AP. Step 3 uses the delta wave polarity in Lead III that can separate partial right septum AP form right free wall. Step 4 further separates the right septum and right free wall AP by the delta wave polarity of V1.
DISCUSSION
Primary finding
In this study, the accuracy of adult algorithms was low in predicting the AP in pediatric patients with symptomatic WPW syndrome. We developed a new and simple algorithm that could differentiate left-side AP from right-side AP, and septal AP from free wall AP in pediatric patients with WPW syndrome.
Prognosis of children with WPW syndrome
Compared adults, AP might disappear several months after birth and recur later in life. Perry et al. reported 140 children with symptomatic WPW syndrome who developed tachycardia as early as 0-2 months after birth, and 93% of their symptoms disappeared. However, 31% experienced recurrence at 12 years of age.15 The Taiwan National Health Insurance Program provided information on pediatric WPW syndrome and RFCA therapy to researchers. Between 2000 and 2014, the incidence of WPW syndrome was 0.17/1000 patient-years in those born between 2000 and 2008, of whom 28% were diagnosed by 1 year of age and 50% by 6 years of age. The cumulative percentage of RFCA in WPW syndrome was 1.7% in infancy, 5.8% before 5 years of age, and 31.8% before 10 years of age. Patients with WPW syndrome may undergo ablation at a younger age due to a high risk of sudden death if WPW syndrome is combined with other cardiac problems in children, not developing atrial fibrillation or cardiac death.16 In addition, unlike Brugada syndrome, patients with WPW syndrome are less likely to experience sudden death when AV reentry tachycardia occurs.17
Relationship of locations between symptomatic and non-symptomatic pediatric WPW patients
In this study, all of the patients had tachycardia, and more patient had right-side than left-side APs (R:L = 70:34). Poppone et al. studied 152 patients with asymptomatic WPW syndrome who were under 12 years of age, and reported that more patients had left-side than right-side APs (L:R = 87:65).18 The results suggest that right-side APs may trigger tachycardia more easily than left-side APs during childhood.
Risk of catheter-related complications and lethal ventricular arrhythmia in patients with WPW syndrome
In Taiwan, Wu et al. reported that WPW syndrome with major congenital heart diseases, cardiomyopathy, and/or onset during infancy or early childhood was associated with a significant risk of death.16 RFCA is effective in controlling arrhythmia in patients > 5 years, and it is also recommended for patients < 5 years when antiarrhythmic drugs fail to control the arrhythmia or when they cannot tolerate the side effects of drugs.16 In our study, three patients who were under 5 years of age underwent RFCA for frequent tachyarrhythmia and/or the inability to tolerate medications.
Differences between the algorithms for adults and children
The ECG criteria used for the localization of AP included the polarity of the delta wave or QRS or both. D’Avila et al. used QRS polarity to predict the AP. The first step used the V1RS that divided the AP from left to right. However, in Rosenbaum’s research, using V1RS ratio as types A and B could not accurately differentiate right-side AP from left-side AP.3
When using the polarity of the delta wave to localize the AP, it is difficult to predict the delta wave when the AP is not fully pre-excited. Chiang et al. reported that using ECG criteria for AP localization was not effective due to the absence of the definition of the polarity of the delta wave in their algorithm.5 In addition, according to Gallagher’s and Chiang’s research, the best definition of the polarity of the delta wave was the initial 40 ms of the QRS wave. Moreover, the initial 40 ms of the polarity of the delta wave was in accordance with the morphological definition of the delta wave in adults.5,19 In this algorithm, the polarity of the delta wave in lead III could differentiate right anteroseptal AP from the other types. In addition, the V1 delta wave polarity could differentiate right free wall AP from septal wall AP. However, Chiang et al. did not use V1RS in the first step, causing the right-side AP to be misinterpreted as left-side AP, and some left APs with a V1RS polarity < 1 could not be differentiated from right-side APs when the delta wave in lead I or aVL was not used as the second step.
Iturralde et al. used the polarity of the QRS to localize the AP.8 This algorithm first separated the septal region and the free wall region and then divided the left side and right side using V1RS. Although the first step did not utilize the polarity of V1RS but lead III, the slight increase in right-side accuracy shows that lead IIIRS might be useful in differentiating septal and free wall regions.
Arruda’s algorithm, which is the most recent of these adult algorithms, provided the most accurate and important information, including: 1) the first step should be applied with the polarity of V1RS and lead I delta wave; 2) the algorithm separates the heart into four main parts (the left free wall, subepicardial, right free wall, and septal regions); and 3) the use of the polarity of V1 delta wave to separate the septal region and right free wall is another method that can be adapted.
Boersma et al. developed a pediatric algorithm which was validated based on d’Avila’s algorithm. As previously mentioned, d’Avila’s algorithm was not useful for the pediatric group. Although its accuracy was higher than that of the adult algorithms, the Boersma algorithm was still not effective because of the mixed left-side and right-side AP and mixed para-Hisian with a right free wall.
Because ablation of the AP in the septum carries a higher risk of AV block, precisely predicting the AP location near normal conduction is important. The risk must also be explained to the family before the procedure. Another critical point is right- or left-side AP ablation before vascular sheath insertion in pediatric patients due to their relatively small arteries.
In our new pediatric algorithm, we considered Rosenbaum’s ideas and also Arruda’s algorithm. First, the polarity of V1RS was used. However, its positive and biphasic/isoelectric polarity could only produce partial accuracy of left-side AP (73%). The negative polarity of V1RS and positive delta wave in lead I could subclassify left-side AP from right-side AP with 100% accuracy. Moreover, we slightly modified Chiang’s algorithm by applying the polarity of the V1 delta wave to differentiate LS and LF with 100% accuracy, and applying the polarity of lead III and V1 delta wave, which could differentiate RF and RS with 88.6% accuracy. We found the four RA APs were misinterpreted as RS, which is probably due to the location of the RA [located between the septal and free wall (11 o’clock-2 o’clock in LAO view)]. Two RL and 2 RPL APs were misinterpreted as RS due to AV nodal interactions. AV nodal function in children is more predominant and faster than in adults, and the AP location may be misinterpreted if the delta wave is not very clear during the evaluation of the AP location using adult algorithms. Boersma et al. showed that children have a less maximal pre-excited WPW ECG pattern due to a more robust AV nodal conduction.20 A new and simple pediatric AP location algorithm may be able to overcome these factors.
Resolution and accuracy
Some authors have reported that high resolution can help identify the ablation site more precisely. Josephson reported that an electrophysiologist cannot accurately identify the AP location using the initial 20-40 ms of the QRS (delta wave), the R/S ratio, or precordial lead transition in any leads in the absence of total pre-excitation. Moreover, a simple approach that regionalizes the AP is more reasonable. Teixeira et al. tested his adult patients with WPW syndrome using published adult and pediatric algorithms, and the overall absolute accuracy varied between 27% and 47% which increased to 40-76% when adjacent locations were included.21 Thus, high resolution may lead to more misinterpretations and may not shorten the procedure time in more than 50% of cases. However, how best to precisely regionalize the AP is a challenge. Josephson suggested using a multipolar catheter, such as a Halo catheter, to record the tricuspid annulus circumferentially, and that this may be useful in localizing the AP.
Benefit of the new pediatric algorithm
First, the adult algorithms usually predicted the AP location using 8-11 specific locations. However, high specificity and sensitivity were only found in their own groups, and some showed low sensitivity and specificity in other study groups. Therefore, too many locations may decrease the flexibility and application of AP location prediction. For example, some junior EP doctors may spend considerable time ablating the AP location where the algorithm states. This can also prolong the procedure time and increase patient risk. Moreover, most of algorithms are either complicated or difficult to apply clinically.
Second, in our algorithm, differentiating left from right and free wall to septal APs helped to narrow down the AP location and also expedite the interpretation time and treatment strategy making. It may therefore be useful for the initial interpretation, size and location of catheter insertion.
Third, the number of patients but not the patients’ heart size was the main problem limiting the number of AP locations. Nevertheless, it was sufficient for the initial interpretation and treatment decision making.
Study limitations
This was a retrospective study conducted over a long period of time, and nearly one-third of the data could not be analyzed because ECG lines fade with time. In addition there was no training group for the new algorithm in this study due to the limited patient number. By classifying the AP locations in our pediatric patients with symptomatic WPW syndrome into 13 areas as described by Chiang et al., the algorithm could not absolutely differentiate septal wall from free wall AP, particularly in the RA region. We accidentally discovered that the boys had a relatively higher incidence of symptomatic WPW syndrome before reaching the age of 18 years. However, further investigations should be conducted to clarify these findings.
CONCLUSIONS
The use of adult algorithms in predicting the AP location in pediatric patients with WPW syndrome is not ideal because of their substandard quality. We developed a simple and useful algorithm to differentiate left-side AP from right-side AP, allowing for a more precise vessel instrument assessment. Using this algorithm, high-risk regions such as the RMS and RAS regions can be identified with high accuracy. This novel algorithm, therefore, makes it easier to explain the risks.
Acknowledgments
This study was supported by Taipei Veterans General Hospital grant V107B-021. We also want to thank Dr. Ting-Yung Chang, Dr. Ting-Chun Huang, Dr. Chih-Min Liu, Dr. Cheng-I Wu, Dr. Chin-Yu Lin, Dr. Simon Salim, Dr. Minh Hoang Quang, Dr. Jennifer Jeanne B. Vicera, Dr. Abigail Louise D. Te, and Dr. Shinya Yamada to help us to complete this article with lots of good ideas.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare that they have no conflict of interest.
REFERENCES
- 1.Wolff L, Parkinson J, White P. Bundle-branch block with short P-R interval in healthy young people prone to paroxysmal tachycardia. 1930. Ann Noninvasive Electrocardiol. 2006;11:340–353. doi: 10.1111/j.1542-474X.2006.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanatani S, Potts JE, Reed JH, et al. The study of antiarrhythmic medications in infancy (SAMIS): a multicenter, randomized controlled trial comparing the efficacy and safety of digoxin versus propranolol for prophylaxis of supraventricular tachycardia in infants. Circ Arrhythm Electrophysiol. 2012;5:984–991. doi: 10.1161/CIRCEP.112.972620. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum FF, Hecht HH, Wilson FN, Johnston FD. The potential variations of the thorax and the esophagus in anomalous atrioventricular excitation (Wolff-Parkinson-White syndrome). Am Heart J. 1945;29:281–326. [Google Scholar]
- 4.Arruda MS, McClelland JH, Wang X, et al. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 1998;9:2–12. doi: 10.1111/j.1540-8167.1998.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 5.Chiang CE, Chen SA, Teo WS, et al. An accurate stepwise electrocardiographic algorithm for localization of accessory pathways in patients with Wolff-Parkinson-White syndrome from a comprehensive analysis of delta waves and R/S ratio during sinus rhythm. Am J Cardiol. 1995;76:40–46. doi: 10.1016/s0002-9149(99)80798-x. [DOI] [PubMed] [Google Scholar]
- 6.d'Avila A, Brugada J, Skeberis V, et al. A fast and reliable algorithm to localize accessory pathways based on the polarity of the QRS complex on the surface ECG during sinus rhythm. Pacing Clin Electrophysiol. 1995;18:1615–1627. doi: 10.1111/j.1540-8159.1995.tb06983.x. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick AP, Gonzales RP, Lesh MD, et al. New algorithm for the localization of accessory atrioventricular connections using a baseline electrocardiogram. J Am Coll Cardiol. 1994;23:107–116. doi: 10.1016/0735-1097(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 8.Iturralde P, Araya-Gomez V, Colin L, et al. A new ECG algorithm for the localization of accessory pathways using only the polarity of the QRS complex. J Electrocardiol. 1996;29:289–299. doi: 10.1016/s0022-0736(96)80093-8. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi N, Yoshida N, Inden Y, et al. A simple algorithm for localizing accessory pathways in patients with Wolff-Parkinson-White syndrome using only the R/S ratio. J Arrhythm. 2014;30:439–443. [Google Scholar]
- 10.Xie B, Heald SC, Bashir Y, et al. Localization of accessory pathways from the 12-lead electrocardiogram using a new algorithm. Am J Cardiol. 1994;74:161–165. doi: 10.1016/0002-9149(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 11.Wren C, Vogel M, Lord S, et al. Accuracy of algorithms to predict accessory pathway location in children with Wolff-Parkinson-White syndrome. Heart. 2012;98:202–206. doi: 10.1136/heartjnl-2011-300269. [DOI] [PubMed] [Google Scholar]
- 12.Boersma L, Garcia-Moran E, Mont L, Brugada J. Accessory pathway localization by QRS polarity in children with Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 2002;13:1222–1226. doi: 10.1046/j.1540-8167.2002.01222.x. [DOI] [PubMed] [Google Scholar]
- 13.Lin CY, Chung FP, Lin YJ, et al. Safety and efficacy of epicardial ablation of ventricular tachyarrhythmias: experience from a tertiary referral center in Taiwan. Acta Cardiol Sin. 2018;34:49–58. doi: 10.6515/ACS.201801_34(1).20170724A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee PC, Hwang B, Chen SA, et al. The results of radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing Clin Electrophysiol. 2007;30:655–661. doi: 10.1111/j.1540-8159.2007.00727.x. [DOI] [PubMed] [Google Scholar]
- 15.Perry JC, Garson A., Jr. Supraventricular tachycardia due to Wolff-Parkinson-White syndrome in children: early disappearance and late recurrence. J Am Coll Cardiol. 1990;16:1215–1220. doi: 10.1016/0735-1097(90)90555-4. [DOI] [PubMed] [Google Scholar]
- 16.Wu MH, Chen HC, Kao FY, Huang SK. Postnatal cumulative incidence of supraventricular tachycardia in a general pediatric population: a national birth cohort database study. Heart Rhythm. 2016;13:2070–2075. doi: 10.1016/j.hrthm.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Chung FP, Lin CY, Lin YJ, Chen SA. Reappraisal of the worldwide prevalence of Brugada syndrome and Brugada phenotype: from the old to the new diagnostic criteria. Acta Cardiol Sin. 2018;34:278–279. doi: 10.6515/ACS.201805_34(3).20180305A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappone C, Manguso F, Santinelli R, et al. Radiofrequency ablation in children with asymptomatic Wolff-Parkinson-White syndrome. N Engl J Med. 2004;351:1197–1205. doi: 10.1056/NEJMoa040625. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher JJ, Pritchett EL, Sealy WC, et al. The preexcitation syndromes. Prog Cardiovasc Dis. 1978;20:285–327. doi: 10.1016/0033-0620(78)90015-4. [DOI] [PubMed] [Google Scholar]
- 20.Bar-Cohen Y, Khairy P, Morwood J, et al. Inaccuracy of Wolff-Parkinson-white accessory pathway localization algorithms in children and patients with congenital heart defects. J Cardiovasc Electrophysiol. 2006;17:712–716. doi: 10.1111/j.1540-8167.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira CM, Pereira TA, Lebreiro AM, Carvalho SA. Accuracy of the electrocardiogram in localizing the accessory pathway in patients with Wolff-Parkinson-White pattern. Arq Bras Cardiol. 2016;107:331–338. doi: 10.5935/abc.20160132. [DOI] [PMC free article] [PubMed] [Google Scholar]