Abstract
Background
YiqiHuoxue decoction (YHD) is frequently prescribed to prevent and treat cardiovascular diseases. YHD inhibits platelet aggregation, however the underlying mechanisms are unclear.
Methods
The in vitro and in vivo anti-platelet and antithrombotic effects of YHD and ethanol-precipitated YHD (EYHD) and underlying mechanisms were investigated. Forty-six Sprague-Dawley (SD) rats and 36 male Kunming mice were examined. Ten SD rats were used to assess the cytotoxicity of YHD and EYHD by releasing lactate dehydrogenase from treated platelets. The remaining 36 SD rats were divided into six groups (six per group), including control saline (5 mL/kg), aspirin (20 mg/kg), YHD low dosage (0.2 g/kg), YHD high dosage (2.0 g/kg), 75% EYHD low dosage (0.2 g/kg), and 75% EYHD high dosage (2.0 g/kg) groups to detect platelet aggregation; the 36 Kunming mice were divided into 6 groups to detect mesenteric arterial thrombosis induction. Thromboxane B2 (TXB2) levels were determined by enzyme immunoassay.
Results
YHD high dosage and 75% EYHD (low and high dosage) inhibited ADP-induced platelet aggregation. Moreover, collagen-induced platelet aggregation was significantly suppressed by YHD (high dosage), 75% EYHD (high dosage), and 75% EYHD (low dose). Rats given 75% EYHD (high dose) displayed a marked reduction in collagen-induced platelet aggregation at 2 h post-administration. YHD and EYHD markedly prolonged the onset of thrombosis causing loose attachment of the thrombus to the vascular endothelium, but bleeding and clotting times were not significantly changed. Finally, YHD and EYHD markedly reduced TXB2 levels.
Conclusions
YHD and EYHD effectively inhibit platelet activation and thrombosis, presumably by suppressing TXB2.
Keywords: EYHD, Platelet, Thrombosis, Thromboxane B2, YHD
INTRODUCTION
Thrombosis is a major pathological basis of many cardiovascular events. Adhesion, activation, and aggregation of platelets and thrombocytes are pivotal for thrombosis, and thus anti-platelet therapies are important for preventing and treating many cardiovascular diseases.1 Frequently used clinical antiplatelet agents, including aspirin and clopidogrel, effectively prevent both the occurrence of cardiovascular events in mid-to-high-risk patients and their presence in average-risk populations. However, inadvertent hemorrhaging is associated with anti-platelet therapies,2,3 causing concerns over their clinical application. Furthermore, a subset of patients are resistant to antiplatelet drugs.4-6 Thus, it is very important to identify alternative effective anti-platelet and anti-thrombotic agents from the vast library of traditional Chinese medicines.
Cardiovascular diseases associated with platelet activation and thrombus formation belong to the scopes of "chest obstruction and pain" in traditional Chinese medicine, wherein "Qi deficiency" and "blood stasis" are the major symptoms. Some researchers have suggested that platelet activation is the fundamental pathophysiology of blood stasis, and as such, platelet activation has been proposed to be an important microcosmic index for differentiating the syndromes of blood stasis.7 Drugs currently used clinically to improve blood circulation and eliminate blood stasis can also improve hemorheology, reduce platelet adhesion and aggregation, and inhibit thrombosis. YiqiHuoxue decoction (YHD) is frequently prescribed to treat Qi deficiency and blood stasis of coronary heart disease in clinical practice to ameliorate a patient’s symptoms and enhance their quality of life. YHD is derived from extracts of Panax ginseng, Astragalus mongholicus, Radix Paeoniae Rubra, and Carthamus tinctorius. Compounds containing Radix Paeoniae Rubra and C. tinctorius (Honghua in Chinese) and P. ginseng extract have been reported to possess antithrombotic activity.8-10 We previously demonstrated that YHD inhibits remodeling of left ventricular post-myocardial infarction in rats, while also promoting angiogenesis and reducing platelet aggregation.11,12 However, the mechanisms underlying these actions remain unclear.
In the present study, we extracted the constituents of YHD by ethanol precipitation (ethanol-precipitated YHD, EYHD). First, the cytotoxicity of YHD and EYHD toward platelets was assessed by measuring the release of lactate dehydrogenase (LDH). Next, the effects of YHD and EYHD on platelet aggregation were assayed in vitro via ADP- or collagen-induced platelet aggregation. Finally, rats were given YHD or EYHD by gavage and evaluated using various assays. The influence of treatment on collagen-induced platelet aggregation was observed 2 h later. We also measured the bleeding time and clotting time of blood drawn from the rat tails and the onset of FeCl3-induced mesenteric thrombosis in mice, and determined the level of thromboxane B2 (TXB2) in the washed platelets of rats treated with YHD or EYHD and untreated rats. Our results suggest the potential mechanisms underlying the effects of YHD and EYHD on platelet activation and thrombosis.
METHODS
Animals
Forty-six specific pathogen-free-grade Sprague-Dawley (SD) rats (250-300 g), 36 male Kunming mice (20-25 g), and stile feeds were purchased from the Animal Experiment Center. Animals were housed in individually ventilated cages at an ambient temperature of 22 ± 2 °C and 12-h light/12-h dark cycle at the Experimental Animal Center of the Second School of Medicine of Henan University of Chinese Medicine. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Second School of Medicine of Henan University of Chinese Medicine.
Preparation and EYHD
Briefly, 1.5 kg A. mongholicus, 750 g P. ginseng, 750 g Radix Paeoniae Rubra, and 500 g C. tinctorius were used to prepare YHD. P. ginseng was soaked at 4 °C in 1.5 L ethanol in a sealed container for 48 h. Subsequently, the ethanol solution was filtered through two layers of gauze and stored at 4 °C. The dregs of P. ginseng were soaked in 750 mL ethanol twice for 24 h, and the ethanol solutions were filtered through two layers of gauze and pooled together with the previous ethanol solution. The ethanol was then removed completely to obtain the extracts. The dregs of P. ginseng were decocted with the other three herbs first with 6 volumes of water and then 4 volumes of water. Each decoction was conducted for 1.5 h after boiling. The decoctions were pooled, filtered, and settled for 24 h. The supernatant was concentrated to an appropriate volume by heating and then mixing with the above ethanol extracts. The mixture was concentrated again to obtain YHD.
We then precipitated YHD with ethanol, as previously reported.13 In brief, prepared YHD was diluted with T buffer pH 7.4 (10 mM HEPES, 140 mM NaCl, 5 mM KCl, 3.2 mM MgCl2, and 2 mM D-glucose). Ethanol was slowly added to a final ratio of 50% v/v or 75% v/v. Following precipitation, the solution was concentrated to obtain three types of EYHD: 50% EYHD (precipitated in 50% ethanol), 50-75% EYHD (precipitated in 75% ethanol), and 75% EYHD (supernatant of 75% ethanol precipitation). The precipitates were dried and dissolved in T buffer, filtered through a 0.45-μm filter, and brought to a concentration of 200 mg/mL.
LDH release assay
The cytotoxicity of YHD and EYHD was assessed by measuring the release of LDH from treated platelets. Ten SD rats were anesthetized with 4% chloral hydrate by intraperitoneal injection at a dosage of 8 mL/kg. Blood was drawn from the abdominal aortas and mixed with 3.8% of the anti-coagulant trisodium citrate at a ratio of 9:1. The mixture was centrifuged at 120 × g for 15 min to obtain platelet-rich plasma. This platelet-rich plasma was centrifuged again at 600 × g for 5 min, and the pellets were washed with the T buffer to obtain washed platelets. The platelet concentration was adjusted to 3.0 × 108 cells/mL, and they were then treated with either YHD or EYHD for 10 min at 37 °C and centrifuged at 12,000 × g for 2 min to obtain the supernatant. The positive control was obtained by treating the same equal concentration of platelets with 0.2% Triton X-100. The samples were analyzed with an LDH assay kit, and light absorption was measured at 490 nm with a plate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Determination of platelet aggregation rates
To evaluate the platelet aggregation rate in vitro, 200 μL of the washed platelet suspension (3.0 × 108 cells/mL) was incubated with either YHD or EYHD for 5 min at 37 °C. The platelet aggregation rate was determined using a Platelet Aggregation Analyzer (AggRAM, Helena Laboratories, Beaumont, TX, USA) and in the presence of 5 μg/mL collagen or 20 μM ADP. The platelet aggregation rate was calculated according to the turbidimetric method.14
To determine the platelet aggregation rate ex vivo, 36 male SD rats were randomly assigned to six groups (six per group): control saline (5 mL/kg), aspirin (20 mg/kg), YHD low dosage (0.2 g/kg), YHD high dosage (2.0 g/kg), 75% EYHD low dosage (0.2 g/kg), and 75% EYHD high dosage (2.0 g/kg). The animal dosages were 6.25-fold of the human dosages. The rats were fasted for 12 h and treated by gavage. At 2 h post-gavage, blood was drawn from the abdominal aortas, and washed platelets were prepared as described above (Determination of platelet aggregation rates). The platelet aggregation rate was measured with a Platelet Aggregation Analyzer.
Determination of bleeding time
The rats were anesthetized with 4% chloral hydrate (8 mL/kg), and the tail tips were transected 3 mm from the end and immediately immersed in 0.9% saline at 37 °C. Bleeding time was measured from transection until the bleeding had stopped for 30 s. Bleeding times of longer than 30 min were recorded as 30 min.
Measurement of clotting time
Blood was initially drawn from 36 Kunming rats (randomly assigned to six groups equally: control saline, aspirin, YHD low dosage, YHD high dosage, 75% EYHD low dosage, and 75% EYHD high dosage) that had been treated by gavage. Blood samples were centrifuged at 3000 rpm for 10 min. The plasma was isolated from the supernatant, and prothrombin time (PT) and activated partial thromboplastin time (aPTT) were determined with a multifunctional blood coagulation analyzer (Shanghai General Detection Research Institute, Shanghai, China). To determine PT, 200 μL PT reagent pre-warmed to 37 °C was added to 100 μL platelet-poor plasma, and the clotting time was recorded. To determine aPTT, 100 μL aPTT reagent pre-warmed to 37 °C and 100 μL 25 mM CaCl2 solution also pre-warmed to 37 ° C were added to 100 μL platelet-poor plasma. The clotting time was recorded. All assays were completed within 2 h of plasma isolation.
Induction of mesenteric arterial thrombosis in mice15
Mesenteric arterial thrombosis was induced at 2 h post-gavage for 5 days. Mice were initially anesthetized with 4% chloral hydrate (8 mL/kg). Next, a 1-cm incision was made along the midline of the abdomen, and the intestine was gently squeezed out. With the mouse laid on its side in a 10-cm dish, the mesenterium was unfolded with a cotton bud under an inverted microscope (Ti-E, Nikon, Tokyo, Japan), after which 0.05% Rhodamine 6G (20 μL/10 g) was injected into the tail vein. A filter paper saturated with 4% FeCl3 solution was subsequently placed over the mesenteric artery for 2 min, and the mesenterium was observed to measure the time of thrombus formation by microscopy for 40 min in real-time.
Determination of TXB2
Washed platelets (3.0 × 108 cells/mL) were pre-treated with YHD or 75% EYHD for 5 min and then incubated with 5 μg/mL collagen for 5 min. The reaction was terminated by adding 50 μM indometacin and 2 mM EDTA. The mixture was centrifuged at 12,000 × g for 10 min at 4 °C, after which the supernatant was collected and TXB2 levels were determined using an enzyme immunoassay kit according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI, USA).
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ± SEM. Differences among groups were analyzed by one-way analysis of variance followed by Fisher’s least significant difference test for two groups. A p-value of < 0.05 or < 0.01 was considered to be statistically significant.
RESULTS
Composition of EYHD
YHD contains a variety of substances. Through ethanol precipitation, polysaccharides, inorganic salts, water-soluble pigments, glycosides, resin, and impurities in YHD were separated. We used different concentrations of ethanol to extract the substances, and isolated 50% EYHD (starch and impurities), 50-75% EYHD (proteins, polysaccharides, and inorganic salts), and 75% EYHD (water-soluble pigments, glycosides, and resin). The yields of 100 g YHD following ethanol precipitation, purification, and drying are presented in Table 1. The yield of 75% EYHD (68.97%) was the highest among the three, while the yield of 50-75% EYHD (8.98%) was the lowest, suggesting that most water-soluble substances in YHD were isolated in 75% EYHD.
Table 1. Yield of ethanol-precipitated YHD.
| Constituent | Weight (g) | Yield (%) |
| EYHD 50% | 14.75 | 22.05% |
| EYHD 50-75% | 6.01 | 8.98% |
| EYHD 75% | 46.15 | 68.97% |
EYHD, ethanol-precipitated YiqiHuoxue decoction.
Effects of YHD and EYHD on platelet LDH release
Cytotoxic agents can rupture cell membranes and lead to the leakage of LDH from cells. Accordingly, we conducted an LDH-release assay to evaluate the cytotoxicity of YHD and EYHD on platelets. YHD and its various constituents, with dosages varying from 0.02 to 2.0 mg/mL, did not significantly affect LDH release by platelets (Figure 1). Moreover, LDH levels decreased inversely with herb substance concentrations, suggesting that YHD stabilizes the spontaneous activation of platelets.
Figure 1.
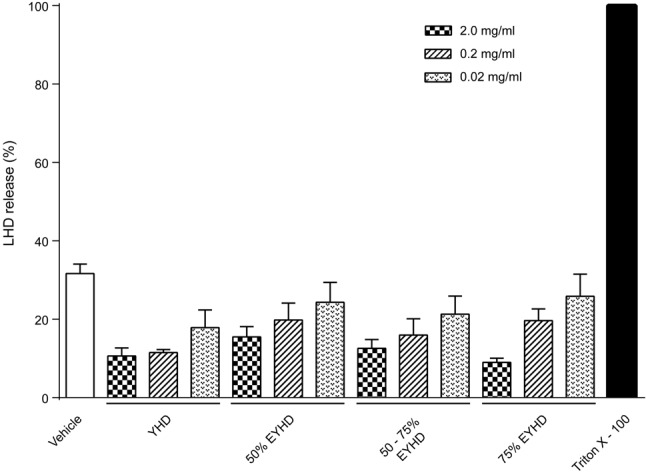
Effect of YHD and EYHD on platelet LDH release. Washed platelets (3.0 × 108 cells/ml) were incubated with 0.02, 0.2 and 2.0 mg/ml YHD/EYHD for 10 min prior to LDH determination (n = 3). EYHD, ethanol-precipitated YHD; LDH, lactate dehydrogenase; YHD, YiqiHuoxue decoction.
Effect of YHD and EYHD pre-treatment on platelet aggregation
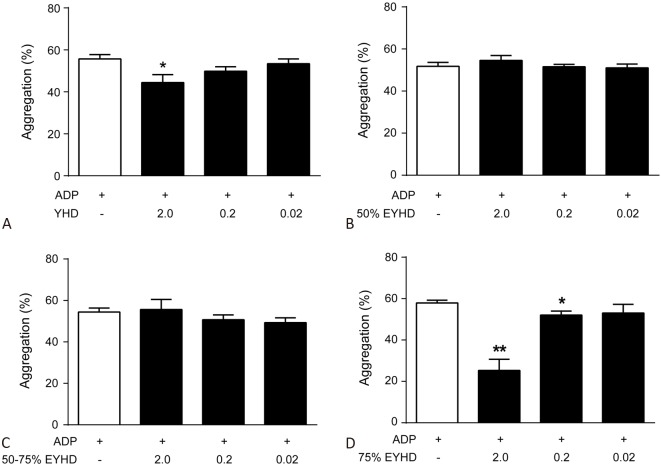
Platelets adhere to injured vascular endothelium and further promote platelet activation and aggregation by releasing endogenous stimulants such as collagen and ADP. We therefore investigated the interfering effect of YHD and EYHD on platelet aggregation using collagen and ADP as stimulants. Our data showed that induction with 20 μM ADP profoundly increased the in vitro rate of platelet aggregation (Figure 2), whereas 2.0 mg/mL YHD (Figure 2A) and 0.2 and 2.0 mg/mL of 75% EYHD (Figure 2D) attenuated this effect. Moreover, 75% EYHD inhibited platelet aggregation in a dose-dependent manner (Figure 2D).
Figure 2.
Effect of YHD and EYHD pre-treatment on ADP-induced platelet aggregation in vitro. PRP (3.0 × 108 cells/ml) was incubated with the indicated drugs for 5 min at 37 °C and subsequently with 20 μM ADP (n = 4). (A) YHD; (B) 50% EYHD; (C) 50-75% EYHD; (D) 75% EYHD. * p < 0.05 and ** p < 0.01 vs. control. EYHD, ethanol-precipitated YHD; YHD, YiqiHuoxue decoction.
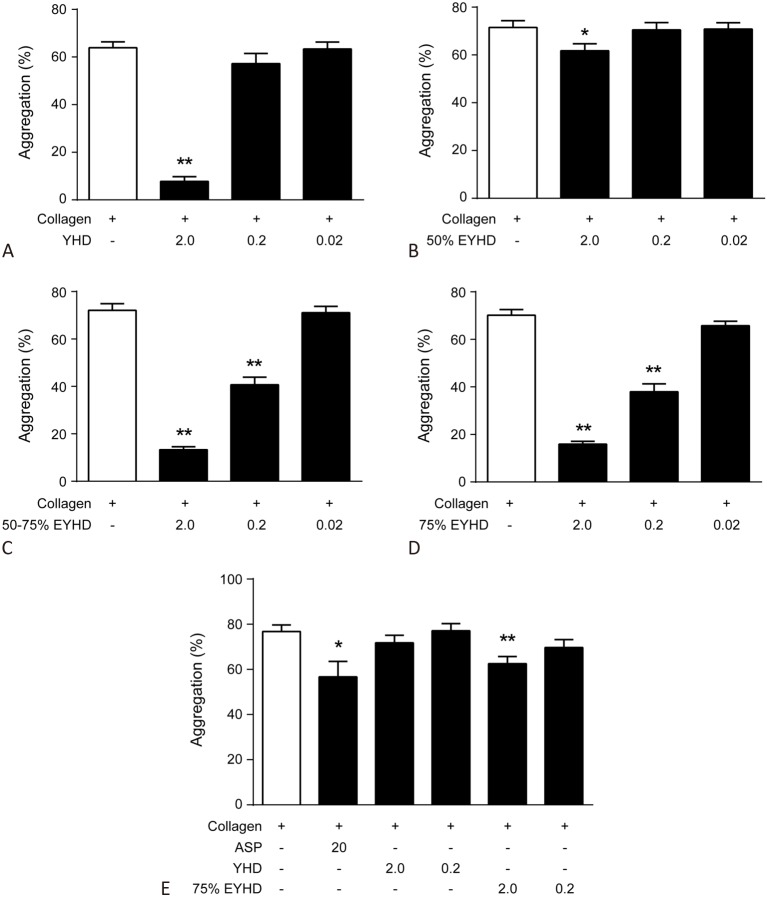
Similarly, pre-treatment with 5 μg/mL collagen enhanced the rate of platelet aggregation in vitro, which was markedly inhibited by 2.0 mg/mL YHD (Figure 3A), 2.0 mg/mL 75% EYHD, and 2.0 mg/mL 50-75% EYHD (Figure 3C & D). Moreover, the inhibitory effects of 50-75% and 75% EYHD on platelet aggregation were dose-dependent (Figure 3C & D). Taken together, these data demonstrated that YHD and EYHD had a greater inhibitory effect on collagen-induced platelet aggregation than on ADP-induced platelet aggregation, and that the 75% EYHD fraction contained the highest levels of components. Accordingly, we then investigated the prolonged effect of pre-treatment of rats with YHD and 75% EYHD on collagen-induced platelet aggregation ex vivo, as soluble YHD and EYHD were removed from aggregation testing. The platelet aggregation rate in un-pretreated rats was 76.77 ± 7.16% (Figure 3E). In contrast, rats pre-treated with varying dosages of YHD (low, 77.08 ± 7.89%; high, 71.75 ± 8.26%) or EYHD (low, 69.72 ± 8.5%; high, 62.58 ± 7.55%) showed reduced rates of platelet aggregation. Furthermore, there was a significant difference in the rates of platelet aggregation post-treatment with either 75% EYHD (1.0 g/kg) or control saline (p = 0.0075). We also assayed ADP-induced platelet aggregation ex vivo, and found no significant difference in the platelet aggregation rate following treatment with YHD, 75% EYHD, or saline (data not shown).
Figure 3.
Effect of YHD and EYHD pre-treatment on collagen-induced platelet aggregation in vitro and ex vivo. (A-D) Washed platelets (3.0 × 108 cells/ml) were incubated with the indicated drugs for 5 min at 37 °C and subsequently platelet aggregation was induced with 5 μg/ml collagen in vitro (n = 6). Blood was drawn 2 h following YHD or EYHD gavage. (E) Washed platelets (3.0 × 108 cells/ml) were induced aggregation with 5 μg/ml collagen ex vivo (n = 6). * p < 0.05 and ** p < 0.01 vs. control. ASP, aspirin; EYHD, ethanol-precipitated YHD; YHD, YiqiHuoxue decoction.
Effects of YHD and EYHD on bleeding and clotting
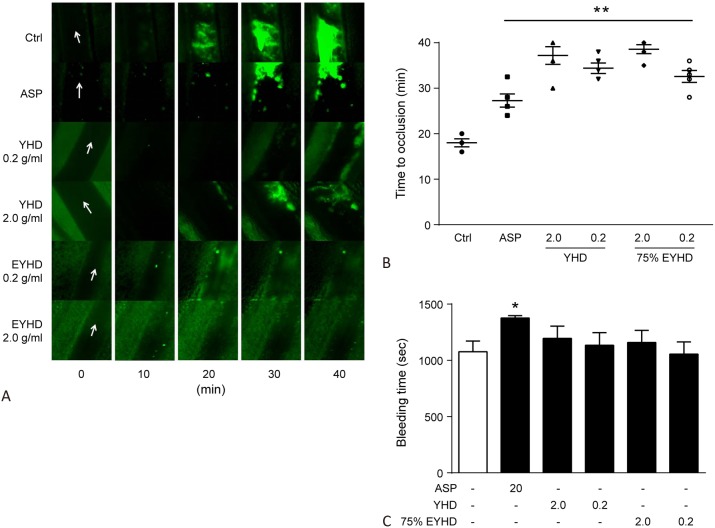
Blood coagulation is commonly measured as the time required for bleeding of a rat’s tail to cease. Therefore, if YHD or EYHD increase the risk of hemorrhage, the time until cessation would be prolonged. The bleeding times post-treatment with saline and aspirin were 1076 ± 223.25 and 1376.67 ± 52.52 s, respectively (Figure 4C). In comparison, the bleeding times were 1195.5 ± 265.37 and 1134 ± 277.68 s post-treatment with a high and low dosage of YHD, and 1159.7 ± 263.08 and 1056 ± 265.26 s post-treatment with a high and low dosage of EYHD, respectively. The bleeding times of YHD- or EYHD-pre-treated rats were not significantly different from those of control rats administered with saline. These results suggested that treatment with YHD or EYHD did not increase the risk of hemorrhage or significantly interfere with blood coagulation.
Figure 4.
Effect of YHD and EYHD on FeCl3-induced mesenteric arterial thrombosis in vivo and bleeding in rat tail. (A-B) Mouse mesenteric artery was exposed and in contact with a filter paper saturated with 4% FeCl3 solution for 2 min. The mesenterium was observed for 40 min under an inverted microscope. (A) Real-time recording of thrombosis in each group (white arrow indicates the direction of blood flow). (B) Onset of FeCl3-induced mesenteric arterial thrombosis in YHD- and 75% EYHD-treated mice (n=6). (C) After anesthesia, the rat’s tail tip was transected 3 mm from the end after which the time taken for bleeding to cease was recorded (n = 6). * p < 0.05 and ** p < 0.01 vs. control. ASP, aspirin; EYHD, ethanol-precipitated YHD; YHD, YiqiHuoxue decoction.
During blood clotting, the coagulation process is influenced by numerous anticoagulant factors such as plasmin and plasminogen activator as reflected in PT and aPTT. The PTs were: saline, 12.6 ± 1.46 s; aspirin, 13.15 ± 1.09 s; YHD high dosage, 12.22 ± 1.45 s; YHD low dosage, 11.67 ± 1.46 s; 75% EYHD high dose, 12.9 ± 1.35 s; and 75% EYHD low dose, 12.57 ± 0.67 s (Table 2). No significant difference was detected between these groups (p > 0.05). Similarly, there was no statistically significant difference in aPTT between these groups (p > 0.05).
Table 2. Effect of YHD and EYHD on PT and APTT in vitro.
| Groups | Dose (g/kg) | PT (sec) | APTT (sec) |
| Ctrl | 12.6 ± 1.46 | 25.55 ± 2.47 | |
| ASP | 0.02 | 13.15 ± 1.09 | 26.18 ± 2.66 |
| YHD | 2.0 | 12.22 ± 1.45 | 25.31 ± 2.68 |
| 0.2 | 11.67 ± 1.46 | 24.86 ± 2.47 | |
| 75% EYHD | 2.0 | 12.9 ± 1.35 | 26.53 ± 2.91 |
| 0.2 | 12.57 ± 0.67 | 25.2 ± 2.17 |
The data are expressed as means ± SEM. No significant difference was detected among each group (p > 0.05).
APTT, activated partial thromboplastin time; ASP, aspirin; EYHD, ethanol-precipitated YiqiHuoxue decoction; PT, prothrombin time; YHD, YiqiHuoxue decoction.
Effects of YHD and EYHD on the time to thrombus formation in vivo
FeCl3 can induce acute vascular endothelial injury, and exposure of the extracellular matrix causes platelet adhesion, activation, and aggregation, ultimately leading to thrombosis. Mice treated with saline developed a thrombus at 20 min, which enlarged over time. In comparison, thrombosis development was significantly delayed and thrombi were markedly smaller in volume in mice treated with aspirin. The onset of thrombosis was further delayed by YHD and EYHD, with the thrombus growing slowly over time. Moreover, the thrombi that developed post-treatment with YHD or EYHD were attached loosely to the injured endothelium and were mostly in motion. Thrombi in the YHD and the EYHD groups disappeared 10-15 min later compared to those in the saline or aspirin group (Figure 4A). The time for thrombi to form was significantly longer (p < 0.01) after aspirin treatment (27.3 ± 3.23 min) compared to saline (18.0 ± 2.0 min), indicating that treatment with aspirin prevented thrombosis (Figure 4B). The thrombus formation times were as follows: YHD high dosage, 37.2 ± 4.38 min; YHD low dosage, 34.4 ± 2.61 min; 75% EYHD high dose, 38.6 ± 2.19 min; and 75% EYHD low dose (32.6 ± 2.97 min). The thrombus formation times in YHD- and EYHD-treated mice were significantly longer than those in saline-treated mice (p < 0.01; Figure 4B).
Effects of YHD and EYHD on TXB2 formation
Thromboxane A2 (TXA2) is an unstable compound with a half-life of 30 s, after which it decomposes into TXB2. TXA2 activity can be estimated by measuring the levels of TXB2 secreted by platelets. To evaluate whether YHD and EYHD inhibit platelet activation and thrombosis by modulating TXA2 release, we stimulated platelet activation with collagen and then measured the level of TXB2. As shown in Figure 5, the level of TXB2 in negative controls (without collagen stimulation) was 4-fold higher (0.73 ± 0.10 ng/mL) than that in the positive control group treated with collagen (4.06 ± 0.51 ng/mL; p < 0.01). After treatment with 2.0 mg/mL YHD, the level of TXB2 was only 0.74 ± 0.23 ng/mL. After pre-treatment with various concentrations (0.02, 0.2, and 2.0 mg/mL) of 75% EYHD, the TXB2 levels were 3.53 ± 0.50, 1.8 ± 0.3, and 0.51 ± 0.03 ng/mL, respectively. The TXB2 levels after YHD- and EYHD-treatment were significantly different from those in the positive controls (YHD 2.0 mg/mL, p = 0.005; 75% EYHD 0.2 mg/mL, p = 0.003; 75% EYHD 2.0 mg/mL, p = 0.0027). However, 0.02 mg/mL 75% EYHD had no inhi-bitory effect on TXB2 formation (p > 0.05). The level of TXB2 decreased as the concentration of EYHD increased, indicating that 75% EYHD inhibited TXB2 formation in a dose-dependent manner. Taken together, these results suggested that YHD and EYHD inhibited TXB2 release, thereby suppressing platelet activation and thrombosis.
Figure 5.
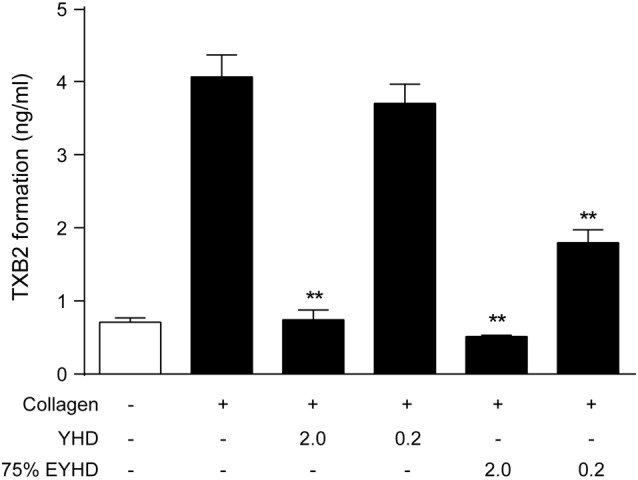
Effect of YHD and EYHD on collagen-induced TXB2 formation in platelets. Washed platelets (3.0 × 108 cells/ml) were incubated with YHD or 75% EYHD for 5 min, subsequently stimulated with 5 μg/ml collagen for 5 min, and TXB2 levels determined (n = 4). # p < 0.01 vs. negative control and * p < 0.05 and ** p < 0.01 vs. positive control.
DISCUSSION
In the present study, we first evaluated the effects of YHD and EYHD pre-treatment on platelet aggregation in vitro and ex vivo, and then investigated the relationships between YHD- and EYHD-mediated inhibition of TXB2 formation and their anti-thrombotic effects. Finally, we assessed the risk of hemorrhaging associated with YHD and EYHD treatment.
Over-activation of platelets plays an important role in the development of cardiovascular diseases.16 Collagen present in the extracellular matrix is exposed during endothelial injury, wherein it specifically binds to the glycoprotein VI receptor expressed on platelets17,18 to induce the release of platelet-derived granules containing TXA2 and ADP, further promoting platelet activation and thrombus volume growth.19,20 Our data suggest that pre-treatment with YHD and EYHD markedly inhibited collagen-induced platelet aggregation in a dose-dependent manner. These results are consistent with a report showing that the compound P. ginseng and extract inhibited collagen-induced platelet activation and thrombus formation by modulating early glycoprotein VI signaling events.10 Moreover, pre-treatment with YHD and EYHD inhibited ADP-induced platelet aggregation, although this inhibition was less prominent compared to the effect on collagen-induced platelet aggregation. These data indicate that YHD and EYHD could inhibit collagen activity to block further platelet activation, supporting that further studies are warranted to investigate the use of YHD and EYHD as anti-platelet drugs.
YHD can invigorate blood circulation while eliminating blood stasis, and thus has been used clinically to treat cardiovascular diseases for decades without any reports of an increased risk of hemorrhage. We investigated the potential effect of YHD on bleeding and coagulation in small animals. The rat-tail bleeding test measures the hemostatic time following transection, and is commonly used to assess the adverse effects of anticoagulants.21 Moreover, indicators of activation of an external coagulation system (PT) and internal coagulation system (aPTT) are commonly used to detect the risk of hemorrhage and hypercoagulability-induced thrombosis.22 Our results indicated that YHD and EYHD did not alter the bleeding time compared to the rats treated with saline, nor did it affect PT or aPTT, suggesting that YHD and EYHD would not pose a risk of hemorrhage or clinical concerns when used as an antiplatelet treatment.
FeCl3 causes chemical damage and endothelial injury by oxidation, which induces platelet aggregation and results in thrombosis,20 and thus FeCl3-induced thrombosis is a widely used animal model of thrombosis. In the present study, we examined the influence of YHD and EYHD on the onset of FeCl3-induced thrombosis in mice. We found that YHD and EYHD had remarkable anti-thrombotic effects. YHD and EYHD not only delayed thrombosis but also reduced the adhesiveness of the thrombus. TXA2 plays a crucial role in platelet activation, and TXA2 activity is used as an indicator of thrombosis in cardiovascular diseases. However, TXA2 has a short half-life and is easily converted to TXB2. Thus, fluctuations in TXB2 levels are typically examined to reflect TXA2 activity. Our results showed that YHD and EYHD significantly reduced the level of platelet-derived TXB2 following collagen stimulation, suggesting that the antithrombotic actions of YHD and EYHD were associated with the inhibition of TXB2.
Acute coronary syndrome is considered to come under the category of "thoracic obstruction" in Chinese medicine, in which the main pathological basis is Qi deficiency and blood stasis. Herbs benefiting Qi and invigorating the blood are commonly used to treat cardiovascular thrombotic diseases in Chinese medicine. YHD is used to treat heart disease based on these principles, and our results showed that YHD had inhibitory effects on platelet activation. Previous studies have also shown that YiqiHuoxue herbs significantly alter blood viscosity (Musk Jiuxin Dropping Pills), inhibit platelet activation and aggregation, reduce thrombosis (Buyang-Huanwu Decoction), and improve GP IIb/IIIa, von Willebrand factor, and angiotensin II levels in patients with coronary heart disease associated with Qi deficiency and blood stasis syndrome (Shuxin Yin and Xuefu Zhuyu Decoction).23-25 Kou showed that a combination of YiqiHuoxue herbs and anti-thrombotic drugs further reduced the rate of platelet aggregation.26 Other reports have suggested that YiqiHuoxue herbs can effectively ameliorate the symptoms of blood stasis and Qi deficiency after acute coronary syndrome interventions, improve cardiac function, inhibit ventricular remodeling after infarction, shorten the duration of angina pectoris after percutaneous coronary interventions, and reduce the incidence of adverse time.27-32 These data demonstrate that the use of YiqiHuoxue herbs with or without Western medicine is reliable and safe for treating heart disease.
CONCLUSIONS
In summary, YHD and EYHD inhibited collagen-induced platelet activation and FeCl3-induced thrombosis, and these effects may have been associated with YHD- and EYHD-mediated inhibition of TXB2 formation. Furthermore, YHD and EYHD did not increase the risk of hemorrhage. Accordingly, we demonstrated that YHD and EYHD are safe and effective anti-platelet and antithrombotic medicines for preventing and treating cardiovascular diseases.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (Grant No. 81072968, 81473453), the Scientific Research Foundation for the Returned Oversea Chinese Scholars, State Ministry of Education of China, and Collaborative Innovation Center of Henan University of Chinese Medicine.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Sabouret P, Rushton-Smith SK, Kerneis M, et al. Dual antiplatelet therapy: optimal timing, management, and duration. Eur Heart J Cardiovasc Pharmacother. 2015;1:198–204. doi: 10.1093/ehjcvp/pvv015. [DOI] [PubMed] [Google Scholar]
- 2.Zullo A, Hassan C, Radaelli F. Gastrointestinal endoscopy in patients on anticoagulant therapy and antiplatelet agents. Ann Gastroenterol. 2017;30:7–14. doi: 10.20524/aog.2016.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schurtz G, Bauters C, Ducrocq G, et al. Effect of aspirin in addition to oral anticoagulants in stable coronary artery disease outpatients with an indication for anticoagulation. Panminerva Med. 2016;58:271–285. [PubMed] [Google Scholar]
- 4.Floyd CN, Ferro A. Antiplatelet drug resistance: molecular insights and clinical implications. Prostaglandins Other Lipid Mediat. 2015;120:21–27. doi: 10.1016/j.prostaglandins.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Dretzke J, Riley RD, Lordkipanidze M, et al. The prognostic utility of tests of platelet function for the detection of ‘aspirin resistance’ in patients with established cardiovascular or cerebrovascular disease: a systematic review and economic evaluation. Health Technol Assess. 2015;19:1–366. doi: 10.3310/hta19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Açar B, Maden O, Gülcihan Balci K, et al. Predictors of impaired reperfusion after percutaneous coronary intervention in patients with in-hospital acute stent thrombosis: a retrospective analyses of 5 years of data. Acta Cardiol Sin. 2017;33:384–392. doi: 10.6515/ACS20161026B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Yu G. Biomedical mechanisms of blood stasis syndrome of coronary heart disease by systems biology approaches. Chin J Integr Med. 2014;20:163–169. doi: 10.1007/s11655-013-1461-3. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Wang N. Antithrombotic effects of danggui, honghua and potential drug interaction with clopidogrel. J Ethnopharmacol. 2010;128:623–628. doi: 10.1016/j.jep.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Xie P, Cui L, Shan Y, Kang WY. Antithrombotic effect and mechanism of radix paeoniae rubra. Biomed Res Int. 2017;2017:9475074. doi: 10.1155/2017/9475074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endale M, Lee WM, Kamruzzaman SM, et al. Ginsenoside-Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and mapk activation. Br J Pharmacol. 2012;167:109–127. doi: 10.1111/j.1476-5381.2012.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han LH, Wang ZT, Wu H, Li J. Effects of yiqi huexue decoction on serum pciii, tnf-a and et-1 in rats after myocardial infarction with left ventricular remodeling. Shanghai Zhong Yi Yao Za Zhi. 2004;38:50–52. [Google Scholar]
- 12.Han LH, Wu H, Wang ZT, Li J. Effects of yiqi huexue decoction on platelet aggregation and aptt/pt/tt in rats after myocardial infarction with left ventricular remodeling. Jiangsu Zhong Yi Yao. 2005;26:55–56. [Google Scholar]
- 13.Fan Y, He Q, Luo A, et al. Characterization and antihyperglycemic activity of a polysaccharide from dioscorea opposita thunb roots. Int J Mol Sci. 2015;16:6391–6401. doi: 10.3390/ijms16036391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Yu G, Fan J. Alditols and monosaccharides from sorghum vinegar can attenuate platelet aggregation by inhibiting cyclooxygenase-1 and thromboxane-a2 synthase. J Ethnopharmacol. 2014;155:285–292. doi: 10.1016/j.jep.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Eckly A, Hechler B, Freund M, et al. Mechanisms underlying fecl3-induced arterial thrombosis. J Thromb Haemost. 2011;9:779–789. doi: 10.1111/j.1538-7836.2011.04218.x. [DOI] [PubMed] [Google Scholar]
- 16.Gabbasov Z, Sabo J, Petrovic D, et al. Impact of platelet phenotype on myocardial infarction. Biomarkers. 2015;20:17–25. doi: 10.3109/1354750X.2014.993707. [DOI] [PubMed] [Google Scholar]
- 17.Semeniak D, Kulawig R, Stegner D, et al. Proplatelet formation is selectively inhibited by collagen type I through syk-independent gpvi signaling. J Cell Sci. 2016;129:3473–3484. doi: 10.1242/jcs.187971. [DOI] [PubMed] [Google Scholar]
- 18.Carrim N, Walsh TG, Consonni A, et al. Role of focal adhesion tyrosine kinases in GPVI-dependent platelet activation and reactive oxygen species formation. PLoS One. 2014;9:e113679. doi: 10.1371/journal.pone.0113679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J, Li MP, Zhou HH, Chen XP. Platelet inhibition agents: current and future P2Y12 receptor antagonists. Curr Vasc Pharmacol. 2015;13:566–577. doi: 10.2174/1570161112666141127162209. [DOI] [PubMed] [Google Scholar]
- 20.Huang SW, Kuo HL, Hsu MT, et al. A novel thromboxane receptor antagonist, nstpbp5185, inhibits platelet aggregation and thrombus formation in animal models. Thromb Haemost. 2016;116:285–299. doi: 10.1160/TH15-12-0993. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Wang S, Diao X, et al. Design, synthesis and antithrombotic evaluation of novel dabigatran etexilate analogs, a new series of non-peptides thrombin inhibitors. Bioorg Med Chem. 2015;23:7405–7416. doi: 10.1016/j.bmc.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102:248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- 23.Shen X, Hou Q, Wang P, et al. Clinical efficacy and hemorheology of shexiang jiuxin dripping pills in treating coronary heart disease angina pectoris (qi deficiency and blood stasis syndrome). Chinese J Inform Tradit Chinese Med. 2007;4:65–66. [Google Scholar]
- 24.Tang N, Huang X, he Y, et al. Effect of shuxinyin and xuefu zhuyu decoction on platelet activation related factors in patients with angina pectoris of different syndromes. ACTA Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai. 2007;21:25–28. [Google Scholar]
- 25.Wang WR, Lin R, Zhang H, et al. The effects of buyang huanwu decoction on hemorheological disorders and energy metabolism in rats with coronary heart disease. J Ethnopharmacol. 2011;137:214–220. doi: 10.1016/j.jep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Kou N. The effect and bleeding risk of Yiqi-Huoxue herbs combined with dual antiplatelet therapy (Dissertation), Beijing: Beijing University of Traditional Chinese Medicine; 2016. (in Chinese). [Google Scholar]
- 27.Ge H, Li H, Li B. Clinic observation on yiqi huoxue decoction in the treatment of recurrent angina after percutaneous coronary intervention: a report of 38 cases. Chinese J Integr Med Cardio Cerebrovasc Dis. 2015;13:667–669. [Google Scholar]
- 28.Chen J, Han T, Wang X. Effects of chinese herbs on left ventricular remodeling and cardiac function in patients with acute coronary syndrome after percutaneous coronary intervention. Shaanxi J Tradit Chin Med. 2015;36:802–803. [Google Scholar]
- 29.Wang P, Wang S, Zhang D, et al. Effect of supplementing qi and activating blood circulation chinese herbs on the syndrome of qi deficiency and blood stasis syndrome in patients with acute coronary syndrome after intervention therapy. J Tradit Chin Med. 2015;56:2104–2107. [Google Scholar]
- 30.Wang P, Wang C, Wang S, et al. Quality of life of chinese herbal medicine for nourishing qi and removing blood stasis on acute coronary syndrome patients after percutaneous coronary intervention. Global Tradit Chinese Med. 2012;5:881–885. [Google Scholar]
- 31.Zhang D, Wang C, Wang P, et al. Evolutionof tcm syndrome elements in acute coronary syndrome treated with chinese herbs for nourishing qi and activating blood circulation fter percutaneous coronary intervention. Chinese J Integr Med Cardio Cerebrovasc Dis. 2013;11:385–388. [Google Scholar]
- 32.Zhou M, Li P, Kang Q, et al. Shen-Yuan-Dan capsule inhibiting inflammatory reaction by regulating insulin receptor substrate 1/PI3K/Akt/NF-κB signaling pathway in apoliprotein E knockout mice fed with a high-fat diet. Acta Cardiol Sin. 2017;33:285–291. doi: 10.6515/ACS20160901B. [DOI] [PMC free article] [PubMed] [Google Scholar]