Abstract
Background
Epicardial adipose tissue (EAT) secretes proatherogenic and proinflammatory cytokines and affects cardiac morphology and functions. The aim of this study was to measure EAT thickness in patients without previous coronary artery disease (CAD) who were admitted with acute coronary syndrome (ACS), and determine its relationship to ACS clinical risk scores, and the severity and complexity of CAD.
Methods
This study enrolled 150 patients (mean age 59.7 ± 11.1 years, 83% men), including 75 with non-ST elevated myocardial infarction (NSTEMI group) and 75 with ST elevated myocardial infarction (STEMI group). Cardiovascular risk factors and laboratory analyses were recorded. The Global Registry of Acute Coronary Events (GRACE) risk score, TIMI clinical, SYNTAX and Gensini angiographic scores were calculated according to guidelines. EAT thickness was measured by echocardiography above the free wall of the right ventricle, perpendicular to the aortic annulus.
Results
There were no significant differences in CAD risk factors, clinical, demographic features, anthropometric measurements, or EAT thickness (mean 5.94 ± 1.17 mm) between the two groups. In the patients with ACS, there were no direct correlations between EAT thickness and TIMI, GRACE, SYNTAX and Gensini scores. There were positive and significant correlations between the thickness of EAT and SYNTAX (r = 0.243, p = 0.035) and Gensini (r = 0.394, p < 0.001) scores only in the NSTEMI group. Multivariate linear regression analysis showed that EAT predicted SYNTAX (β = 0.06, p < 0.001) and Gensini (β = 0.04, p = 0.006) scores, but not TIMI score (β = 0.1, p = 0.06) in the patients overall.
Conclusions
EAT thickness measured by 2D echocardiography was not correlated with the extent or complexity of CAD in the ACS patients. However, after adjusting for confounding factors, multivariate linear regression analysis showed that EAT predicted SYNTAX and Gensini scores in these patients.
Keywords: Acute coronary syndrome, Clinic and angiographic risk scores, Epicardial adipose tissue
INTRODUCTION
Acute coronary syndrome (ACS) represents a major clinical endpoint in coronary atherosclerosis, and includes non-ST elevated myocardial infarction (NSTEMI) and ST elevated myocardial infarction (STEMI).1 Clinical and angiographic risk scoring systems have been developed to identify high-risk ACS patients, develop appropriate management strategies, and evaluate the severity and complexity of coronary artery disease (CAD). The SYNTAX score and Gensini scoring systems have been developed for determining the extent and severity of CAD.1-4 The Global Registry of Acute Coronary Events (GRACE) risk score, TIMI risk score, and TIMI-STEMI risk score are also commonly used in clinical practice.5 Although these scoring systems are helpful, cost-effective, non-invasive, and widely available risk stratification systems are needed to identify the extent and severity of CAD in ACS patients.
Epicardial adipose tissue (EAT) is derived from the splanchnopleuric mesoderm and is found on the surface of the heart between the myocardium and visceral pericardium. It communicates locally with coronary vessels and myocardium through paracrine or vasocrine pathways,6 and also secretes pro-atherogenic and pro-inflammatory cytokines which affects cardiac morphology and local functions. Furthermore, it receives branches from coronary arteries, protects cardiomyocytes from toxic effects by storing excessive fatty acids, and provides free fatty acids for cardiomyocytes in case of urgent need.7
The aim of this study was to measure EAT thickness in patients admitted with a diagnosis of ACS and determine the relationships between ACS clinical risk scores and CAD severity and complexity.
METHODS
Study population
This prospective study enrolled 150 patients (75 patients with STEMI and 75 patients with NSTEMI). All of the patients visited Ondokuz Mayis University Medical Faculty, Department of Emergency, from January 2013 to September 2013, and were admitted to the coronary intensive care unit with a diagnosis of ACS based on clinical, electrocardiographic, laboratory, and imaging studies.
Patients who did not consent to participate, those with a known history of CAD, percutaneous coronary interventions (PCI), coronary artery bypass graft (CABG) surgery, heart failure, cardiomyopathy, pericardial effusion, significant valvular disease, surgery for cardiac valves, chronic renal failure (eGFR < 60 mL/dk/1.73 m2), pregnancy, and poor echocardiographic image quality were excluded from the study. Significant valvular disease was defined as critical aortic stenosis, aortic regurgitation, mitral stenosis and regurgitation, and poor echocardiographic image quality was defined as the inability to assess EAT. Age, gender, Killip and New York Heart Association (NYHA) classifications, heart rate and arterial blood pressures were recorded. The patient’s information with regards to smoking, hypertension (HT), diabetes mellitus (DM), hyperlipidemia, and a family history of CAD was recorded.
This study was approved by the Ethics Committee of the Medical Faculty of Ondokuz Mayis University (Decision No: 2013/386).
Risk stratification
Initially, patients with ACS who met the inclusion criteria were divided into two groups: those with STEMI (n = 75) and those with NSTEMI (n = 75). The presence of STEMI was ascertained by the presence of new-onset left bundle branch block (LBBB) or > 1.0 mV ST segment elevation in at least two related leads, while the presence of new horizontal or down-sloping ST depression greater than 0.05 mV and/or T wave inversion greater than 0.1 mV or enzyme elevation without significant electrocardiography changes were considered to indicate NSTEMI. A single serum cardiac troponin I (cTnI) measurement of > 0.1 ng/ml was considered as the threshold for elevated cardiac markers.
Clinical risk stratification in the NSTEMI and STEMI groups was performed using GRACE and TIMI risk scoring systems, respectively.8,9 The parameters used for risk assessment in the GRACE scoring system include age, heart rate, systolic blood pressure, serum creatinine level, Killip class at presentation, ST segment depression on admission electrocardiography, elevated cardiac marker levels, and cardiac arrest.5 The TIMI STEMI score includes age, DM, history of HT or angina, systolic blood pressure, heart rate, Killip class, weight, anterior ST elevation or LBBB, and time to treatment.9 The NSTEMI patients were stratified into three risk groups as low-, moderate-, and high-risk based on a GRACE risk score of ≤ 108, 108 to 140, and ≥ 140, respectively. The STEMI patients were divided into low- and high-risk groups if they had a TIMI risk score of < 4 and ≥ 4, respectively.9
Assessment of the extent and severity of CAD
Coronary angiography
Angiographic assessment of stenosis was performed by two experienced cardiologists blinded to the study. The narrowing percentages in the left main coronary artery (LMCA), left anterior descending artery (LAD), circumflex artery (Cx), right coronary artery (RCA) and their branches were determined. Severe CAD was defined as angiographic ≥ 70% stenosis in coronary arteries.
SYNTAX score
The SYNTAX scoring system was used to assess the extent and severity of CAD. The SYNTAX score was calculated using the online data entry system at syntaxscore. com after examination of angiography records.10 Subjects with a SYNTAX score of < 22, 22 to 33, and > 33 were considered to be at low-, moderate-, and high-risk, respectively.3,10
Gensini score
The extent and severity of CAD were also evaluated using the Gensini score. Scores of 1, 2, 4, 8, 16, and 32 were assigned to narrowing percentages of 0-25%, 25-50%, 50-75%, 75-90%, 90-99%, and 100%, respectively.4
Subsequently, these scores were multiplied by factors defined in line with the functional importance of the myocardial area supplied by the corresponding vessel with stenosis to yield a summed score. These factors included five for the LMCA, 2.5 for the proximal LAD and proximal Cx, 1.5 for the LAD mid-segment, and 1 for the RCA, distal LAD, posterolateral artery, obtuse marginal artery, and 0.5 for the remaining vessels. A Gensini score < 20 was considered to indicate mild CAD, while a score > 20 was considered to indicate severe CAD.4
Anthropometric measurements
Weight and body fat mass were determined using a Body Composition Analyser (Model TBF-300 Tanita Corp., Tokyo, Japan), with the study subjects standing barefoot on the device at least 2 hours following a meal. Body mass index (BMI) [weight (kg)/height (m)2] was calculated. A BMI from 25 to 29.9 kg/m2 was considered to be overweight, and BMI ≥ 30 kg/m2 was considered to be obese. The waist circumference was measured at the end of expiration using an inelastic measurement tape placed between the lower costal margin and iliac crest at the level of the umbilicus. The threshold of the waist circumference was ≥ 102 cm in men and ≥ 88 cm in women. Hip circumference was measured in the standing position at the widest part of the gluteal region. Waist to hip ratio was calculated, with thresholds of ≥ 0.9 and ≥ 0.8 for males and females, respectively.11
Measurement of echocardiographic EAT
Echocardiographic studies were performed in the left lateral decubitus position in parasternal and apical views by the same cardiologist. Two-dimensional, pulsed and color flow Doppler echocardiographic assessments of all patients were performed by the same operator using a Vivid 7 device (GE Medical System, Horten, Norway; 3.5-MHz phased array transducer).
Epicardial fat tissue measurements were determined from the low echogenic density area perpendicular to lines drawn from the parasternal long and short axes to the free wall of the right ventricle, using two-dimensional and M-mode measurements. The aortic annulus was considered to be an anatomical landmark (Figure 1). Average values were measured in three separate cardiac cycles and recorded.12 The measurement of EAT thickness was performed by the same cardiologist who was unaware of the clinical and angiographic data.
Figure 1.
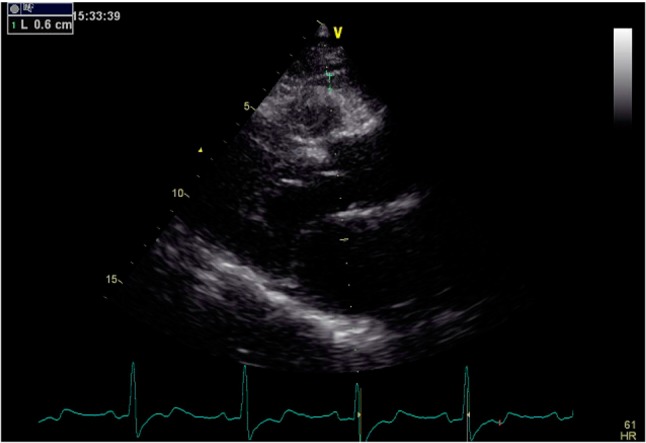
Two-dimensional echocardiographic epicardial fat tissue measurement, aortic annulus was considered as an anatomical landmark, and low echogenic density area perpendicular to the lines drawn from parasternal long axis to the free wall of right ventricle.
Biochemical marker assessments
Complete blood count, biochemistry tests, and cholesterol measurements were performed using an Abbott ARCHITECT c8000 (Abbott Laboratories, USA) auto-analyzer, and recorded.
Statistical analysis
All data were analyzed using SPSS v.15.0 software for Windows (SPSS Inc. Chicago, Illinois, USA). Normal distribution of the data was tested with the Kolmogorov-Smirnov test. For data without normal distribution, Kruskal Wallis analysis of variance and Mann-Whitney U tests were used. Data were presented as number, percentage, and arithmetic mean ± standard deviation. Groups were compared using the Student’s t-test and chi-square test, and correlations between variables were evaluated using Pearson’s correlation analysis. Multivariate linear regression analysis was used to predict the angiographic severity of ACS with regards to EAT. The parameters were determined using the enter method to explain causality in the regression model. SYNTAX, Gensini, TIMI and GRACE scores, BMI, age, and body fat mass parameters were entered into the model. GRACE score, BMI, age, and body fat mass parameters that were confounding factors were removed from the model. The level of significance was defined as a p-value < 0.05.
RESULTS
There were no significant differences in age, weight, height, BMI, waist and hip circumference, waist-to-hip ratio, fat mass, and epicardial fat thickness between the study groups. There were significantly more males than females in both groups. The STEMI patients had significantly lower systolic and diastolic blood pressures and ejection fraction (Table 1). Overall, the ACS patients (n = 150) were generally overweight, with a high BMI (mean 28.1 ± 3.7 kg/m2) and waist-to-hip ratio (mean 0.999 ± 0.4) (Table 1).
Table 1. Comparison of the clinical, demographic, anthropometric, risk factors, EAT, laboratory parameters in NSTEMI and STEMI groups.
| ACS (n = 150) | NSTEMI subgroup (n = 75) | STEMI subgroup (n = 75) | p value | |
| Age (year) | 59.7 ± 11.1 | 60.56 ± 10.9 | 58.91 ± 11.4 | NS |
| Gender | < 0.05 | |||
| Men | 125 | 58 (77.3%) | 67 (89.3%) | |
| Women | 25 | 17 (22.7%) | 8 (10.7%) | |
| Weight (kg) | 77.96 ± 11.9 | 77.9 ± 10.1 | 77.9 ± 13.5 | NS |
| Height (cm) | 166.4 ± 8.7 | 165.2 ± 9.3 | 167.6 ± 7.94 | NS |
| Body-mass Index (kg/m2) | 28.1 ± 3.7 | 28.6 ± 3.59 | 27.6 ± 3.8 | NS |
| Waist circumference (cm) | 99.6 ± 9.2 | 100.9 ± 8.5 | 98.4 ± 9.7 | NS |
| Hip circumference (cm) | 99.6 ± 7.3 | 101.1 ± 7.1 | 98 ± 7.2 | NS |
| Waist/hip ratio | 0.999 ± 0.04 | 0.99 ± 0.04 | 1 ± 0.04 | NS |
| Fat mass (kg) | 23.1 ± 8.1 | 24.3 ± 7.9 | 21.9 ± 8.1 | NS |
| EAT thickness (mm) | 5.94 ± 1.17 | 5.7 ± 1.1 | 6.09 ± 1.2 | NS |
| Systolic BP (mmHg) | 120.53 ± 15.9 | 109.83 ± 14.8 | < 0.001 | |
| Diastolic BP (mmHg) | 69.4 ± 10.1 | 65.7 ± 8.9 | 0.021 | |
| Heart rate (atım/dk) | 71.68 ± 9.5 | 74.6 ± 11.6 | NS | |
| Ejection fraction (%) | 54 ± 6.5 | 47.4 ± 6.8 | < 0.001 | |
| Total cholesterol (mg/dl) | 189.8 ± 35.3 | 181.4 ± 33.7 | NS | |
| LDL cholesterol (mg/dl) | 122.1 ± 31 | 119.7 ± 28.7 | NS | |
| HDL cholesterol (mg/dl) | 38.4 ± 8.8 | 40.6 ± 9.7 | NS | |
| Triglyceride (mg/dl) | 162.9 ± 76.1 | 133.5 ± 72.1 | 0.017 | |
| Glucose (mg/dl) | 128.6 ± 46.3 | 130 ± 56 | NS | |
| HbA1c (%) | 6.53 ± 1.21 | 6.3 ± 1.25 | NS | |
| Hemoglobin (gr/dl) | 13.9 ± 1.68 | 13.96 ± 1.79 | NS | |
| Creatinine (mg/dl) | 0.98 ± 0.3 | 0.94 ± 0.18 | NS | |
| Cigarette | 0.01 | |||
| (+) | 99 (66%) | 42 (56%) | 57 (76%) | |
| (-) | 51 (34%) | 33 (44%) | 18 (24%) |
p value < 0.01, highly significant; p < 0.05, significant; p > 0.05, non-significant (NS).
ACS, acute coronary syndrome; BP, blood pressure; EAT, epicardial adipose tissue; HDL, high density lipoprotein; HbA1c, hemoglobin A1c; LDL, low density lipoprotein; NSTEMI, non-ST elevated myocardial infarction; STEMI, ST elevated myocardial infarction.
With regards to CAD risk factors, there were no significant differences in HT, DM, dyslipidemia, family history, or sedentary lifestyle between the NSTEMI and STEMI groups, while there were significantly more smokers in the STEMI group (Table 1). There were no significant differences in laboratory results including total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, hemoglobin, creatinine, glucose, and hemoglobin A1c (HbA1c) between the two groups, while the NSTEMI patients had significantly higher triglyceride levels (Table 1). With regards to risk factors in the overall study group (n = 150), a small proportion of the patients had a known history of diabetes (31 and 119 patients with and without DM, respectively), while glucose (mean 129 ± 51.2 mg/dl) and HbA1c (mean 6.4 ± 1.23%) were significantly elevated in the group overall.
With regards to risk scores, there were no significant differences in SYNTAX and Gensini scores or in the number of diseased vessels between the two groups, while there were significantly more patients with Killip I and NYHA Class I status in the NSTEMI group (Table 2). In the whole study group (n = 150), there were significantly more patients with NYHA class I (66%) and II (30.7%), Killip Class I (89.3%), SYNTAX score < 22 (72.7%), Gensini score ≥ 20 (86%), and one-vessel disease (52.7%) (Table 2). The proportion of patients with a GRACE score ≥ 140 was significantly higher in the NSTEMI group (46.7%).
Table 2. Comparison of risk scores in NSTEMI and STEMI groups.
| ACS (n = 150) | NSTEMI subgroup (n = 75) | STEMI subgroup (n = 75) | p value | |
| Killip class | 0.006 | |||
| I | 134 (89.3%) | 73 (97.3%) | 61 (81.3%) | |
| II | 14 (9.3%) | 2 (2.7%) | 12 (16%) | |
| III | 2 (1.3%) | 0 | 2 (2.7%) | |
| NYHA class | 0.04 | |||
| I | 99 (66%) | 51 (68%) | 48 (64%) | |
| II | 46 (30.7%) | 22 (29.3%) | 24 (32%) | |
| III | 5 (3.3%) | 2 (2.7%) | 3 (4%) | - |
| TIMI score | ||||
| < 4 | - | 45 (60%) | ||
| ≥ 4 | - | 30 (40%) | ||
| GRACE score | - | |||
| < 108 | 13 (17.3%) | - | ||
| 108-140 | 27 (36%) | - | ||
| ≥ 140 | 35 (46.7%) | - | ||
| SYNTAX score | NS | |||
| < 22 | 109 (72.7%) | 55 (73.3%) | 54 (72%) | |
| 22-33 | 27 (18%) | 13 (17.3%) | 14 (18.7%) | |
| ≥ 33 | 14 (9.3%) | 7 (9.3%) | 7 (9.3%) | |
| Gensini score | NS | |||
| < 20 | 21 (14%) | 12 (16%) | 9 (12%) | |
| ≥ 20 | 129 (86%) | 63 (84%) | 66 (88%) | |
| Number of coronary arteries with > 70% stenosis | NS | |||
| 1 | 79 (52.7%) | 36 (48%) | 43 (57.3%) | |
| 2 | 43 (28.7%) | 24 (32%) | 19 (25.3%) | |
| 3 | 28 (18.7%) | 15 (20%) | 13 (17.3%) |
p value < 0.01, highly significant; p < 0.05, significant; p > 0.05, non-significant (NS).
Correlation analysis examining associations between EAT and TIMI, GRACE, SYNTAX, and Gensini scores showed significant and positive correlations with SYNTAX (r = 0.243, p = 0.035) and Gensini (r = 0.398, p < 0.001) scores (Table 3). In the STEMI group (n = 75) and the overall ACS group (n = 150), there were no correlations between EAT and risk scores (Table 4). In addition, there were no significant correlations between EAT, CAD risk factors, and anthropometric measurements in the overall ACS group (n = 150) (Table 4).
Table 3. Correlation analysis between the EAT thickness and risk scores.
| Risk scores | EAT thickness | |||||
| ACS (n = 150) | NSTEMI subgroup (n = 75) | STEMI subgroup (n = 75) | ||||
| r | p | r | p | r | p | |
| SYNTAX Score | 0.088 | 0.284 | 0.243* | 0.035 | 0.132 | 0.258 |
| Gensini Score | 0.081 | 0.327 | 0.394** | < 0.001 | 0.078 | 0.504 |
| TIMI Score | 0.090 | 0.273 | 0.052 | 0.659 | 0.122 | 0.297 |
| GRACE Score | 0.125 | 0.285 |
p value < 0.01, highly significant; p < 0.05, significant; p > 0.05, non-significant (NS).
Table 4. Correlation analysis between EAT to coronary artery disease risk factors and anthropometric measurements in patients with ACS (n = 150).
| EAT thickness | ||
| r | p | |
| Hypertension | -0.049 | 0.549 |
| Diabetes mellitus | -0.002 | 0.983 |
| Cigarette | 0.011 | 0.893 |
| History of family | -0.049 | 0.552 |
| Dyslipidemia | 0.121 | 0.139 |
| Body mass index | 0.089 | 0.279 |
| Body fat mass | 0.082 | 0.319 |
| Waist circumference | 0.134 | 0.101 |
| Waist hip ratio | 0.101 | 0.218 |
p value < 0.01, highly significant; p < 0.05, significant; p > 0.05, non-significant (NS).
Multivariate linear regression analysis showed that EAT predicted SYNTAX (β = 0.06, p < 0.001), and Gensini (β = 0.04, p = 0.006) scores, but not TIMI score (β = 0.1, p = 0.06) in the overall ACS group after adjustments for confounding factors (Table 5).
Table 5. Multivariate linear regression analysis of EAT predicting angiographic severity of acute coronary syndrome patients (n = 150).
| Beta | p value | |
| SYNTAX | 0.06 | < 0.001 |
| Gensini | 0.04 | 0.006 |
| TIMI | 0.1 | 0.06 |
| GRACE | 0.15 | 0.72 |
| Body mass index | 0.12 | 0.09 |
| Age | 0.16 | 0.81 |
| Body fat mass | 0.21 | 0.91 |
p value < 0.01, highly significant; p < 0.05, significant; p > 0.05, non-significant (NS).
DISCUSSION
Despite significant advances in the treatment of ACS, mortality, re-hospitalization and re-infarction rates remain high. Although plaque rupture is the most common pathology in ACS, there are significant differences between unstable angina, NSTEMI, and STEMI in terms of clinical course, treatment modalities, morbidity and mortality rates.8,9 Risk assessment should be performed for each individual patient to choose the appropriate treatment. Clinical and angiographic risk scoring systems have been developed and are strongly recommended in guidelines for the management and follow-up of patients with ACS.8,9 Main areas of cardiology research aimed at preventing ACS and its catastrophic outcomes include the identification of triggering factors, emerging biomarkers, and novel therapeutic molecules.
EAT is a component of the visceral adipose tissue that is localized between the heart and pericardium, particularly in the atrioventricular and interventricular sulcus, lateral wall of the right ventricle, and around the coronary arteries.13,14 Iacobellis et al.15 were the first to report a significant association between increased epicardial fat and metabolic syndrome, insulin resistance, LDL cholesterol, adiponectin, and arterial blood pressure. Iacobellis et al.15 also found correlations between EAT measurements performed with echocardiography, cardiac computed tomography (CT), and cardiac magnetic resonance imaging (MRI). Of these imaging modalities, echocardiography offers the advantages of being simple, inexpensive, and radiation-free. Furthermore, close correlations have also been demonstrated between the epicardial fat thickness and abdominal fat, anthropometric measures, and metabolic parameters.14,16
EAT acts like an endocrine and inflammatory organ with impacts on cardiac morphology and functions via the local production of pro-atherogenic and pro-inflammatory cytokines.14 In the current study with patients with no known history of CAD who underwent coronary angiography after presenting with an index ACS, we examined associations between the epicardial fat tissue thickness and the extent and complexity of CAD, and also CAD risk factors and anthropometric measurements. The mean EAT was 5.94 ± 1.17 mm, which is higher than the average (5.3 ± 1.6 mm) value reported for healthy individuals with normal coronary arteries.17,18 Shemirani et al.19 stratified a group of subjects (n = 315) undergoing angiography into those with normal findings and those with CAD findings, and observed higher EAT in the latter group (4.4 ± 1.8 mm vs. 5.4 ± 1.9 mm). In a meta-analysis by Xu et al.20 involving 2872 patients, CAD patients had increased epicardial fat thickness and volume compared to subjects without CAD. Wang et al.21 showed that increased EAT volumes were related to coronary atherosclerosis and coronary calcium score and also CAD inflammatory biomarkers. In addition, EAT is associated with left ventricular hypertrophy, abnormal left ventricular geometry, microalbuminuria, carotid intima-media thickness and chronic inflammatory diseases such as rheumatoid arthritis.22-25
Our correlation analysis showed associations between EAT and TIMI, GRACE, SYNTAX and Gensini scores, including significant and positive correlations with SYNTAX (r = 0.243, p = 0.035) and Gensini (r = 0.398, p < 0.001) scores in the NSTEMI group. On the other hand, there were no correlations between EAT and TIMI, SYNTAX and Gensini scores in the STEMI patients. In addition, in the ACS patients overall without classification, correlation analysis showed no associations between EAT and risk scores. Multivariate linear regression analysis showed that EAT could predict SYNTAX (β = 0.06, p < 0.001) and Gensini (β = 0.04, p = 0.006) scores in the ACS patients overall. Eroğlu et al.26 compared CAD patients to individuals with normal coronary arteries, and found that the CAD patients had increased EAT, and that this was correlated with Gensini scores. Similarly, Bhuiyan27 observed a positive correlation between the presence and severity of CAD and EAT in patients undergoing angiography due to suspected CAD. Consistent with our findings, Erkan et al.28 found correlations between EAT and Gensini and SYNTAX scores in patients with unstable angina or myocardial infarctions, and suggested that EAT could be an independent predictor of these conditions. Gul et al.29 showed a correlation between EAT with GRACE risk score in NSTEMI patients, and Altun et al.30 observed a correlation between EAT and SYNTAX score in 65 patients with ACS, while no such correlation could be determined for the GRACE risk score. Wang et al.31 found a correlation between high SYNTAX score and EAT, while Ozcan et al.32 observed an independent association between EAT and TIMI risk score in USAP/NSTEMI patients. A systematic review by Benedek et al.33 showed correlations among EAT, the severity of coronary stenosis, and clinical and angiographic risk scores for ACS. Consistently, we also found varying associations between EAT and risk factors.
In contrast to other studies, we did not have a control group of subjects with normal coronary arteries. The association between EAT and atherosclerosis has been clearly established in several studies, and our patients presented with ACS. EAT is linked with chronic inflammation through the secretion of pro-atherogenic and pro-inflammatory cytokines.6,7,12 In our NSTEMI group, the correlation with the angiographic extent and complexity of stenosis may be due to partial obstruction and opening of the lumen, and a prolonged course of inflammatory events compared to the STEMI group.
Although EAT was increased in all of our patients, the lack of correlations with the risk scores may be due to the different assessments of risk factors in the risk scoring systems as well as the effect of unknown factors in the pathophysiology of ACS. Our study patients generally had a low number of known risk factors for CAD. Apart from smoking, the presence of components of the metabolic syndrome which increase the risk of CAD and are known to be associated with EAT such as HT, DM, and dyslipidemia were relatively infrequent. The majority of our patients had NYHA and Killip class I status, and most of the patients (52%) had single vessel disease resulting in an ACS. SYNTAX and Gensini scores provide versatile assessments through the inclusion of many different parameters to evaluate the extent and complexity of CAD. Epicardial fat tissue represents a marker of visceral fat, and is independent of anthropometric measurements of obesity (such as BMI, waist circumference and fat percentage).11 Our findings also support the notion that EAT is a metabolically active inflammatory organ reflecting visceral fat that is independent of the anthropometric measurements of obesity.
In the current study, EAT was measured using two-dimensional transthoracic echocardiography, which is a simple and inexpensive method. However, EAT has a three-dimensional distribution, and its thickness and volume may be examined using more sophisticated and costly methods such as three-dimensional echocardiography and cardiac MRI in further studies to provide more objective assessments of its association with risk scores.
Limitations of the study
The small sample size represents a major limitation of our study. Operator dependency of the echocardiographic measurements, two-dimensional measurements and difficulty in differentiating pericardial fat, inclusion of low-risk patients in the study, and absence of other imaging modalities may also be considered as other limitations.
CONCLUSIONS
EAT thickness determined by two-dimensional echocardiographic measurements in ACS patients were not directly correlated SYNTAX, Gensini, TIMI and GRACE risk scores. EAT thickness was positively and significantly correlated with Gensini and SYNTAX scores in the NSTEMI group, but not with the STEMI group. After adjusting for confounding factors, multivariate linear regression analysis showed that EAT could predict SYNTAX and Gensini scores in the ACS patients. Although EAT assessments with two-dimensional echocardiography is a simple method, further studies are warranted.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
FUNDING STATEMENT
None.
FINANCIAL SUPPORT
None.
REFERENCES
- 1.Davies MJ. The pathophysiology of acute coronary syndromes. . Heart. 2000;83:361–366. doi: 10.1136/heart.83.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamm C, Heeschen C, Falk E, Fox KAA. Acute coronary syndromes: pathophysiology, diagnosis and risk stratification. In: Camm AJ, Luescher TF, Serruys PW, ed. The ESC Textbook of Cardiovascular Medicine. Oxford: UK: Blackwell Publishing; 2006. pp. 333–366. [Google Scholar]
- 3.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 4.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606–698. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 5.Fox KA, Goodman SG, Klein W, et al. Management of acute coronary syndromes. Variations in practice and outcome; findings from the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2002;23:1177–1189. doi: 10.1053/euhj.2001.3081. [DOI] [PubMed] [Google Scholar]
- 6.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–457. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 8.Hamm CW, Bassand JP, Agewall S, et al. ESC Committee for Practice Guidelines. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 9.Steg PG, James SK, Atar D, et al. ESC Committee for Practice Guidelines. ESC Guidelines for the management of acute myocardial infarction in patients with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 10.Http://www.syntaxscore.com/calculator/start.htm. [Google Scholar]
- 11.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–e841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 12.Eroğlu S. How do we measure epicardial adipose tissue thickness by transthoracic echocardiography? Anatol J Cardiol. 2015;15:416–419. doi: 10.5152/akd.2015.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachar GN, Dicker D, Kornowski R, Atar E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am J Cardiol. 2012;110:534–538. doi: 10.1016/j.amjcard.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Singh H, Khanijoun HK, Iacobellis G. Echocardiographic assessment of epicardial adipose tissue - a marker of visceral adiposity. McGill Journal of Medicine. 2007;10:26–30. [PMC free article] [PubMed] [Google Scholar]
- 15.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–1319. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Katlandur H, Ulucan S, Ozdil H, et al. Evaluation of echocardiographic epicardial fat thickness as a sign of cardiovascular risk in positive exercise test patients. Acta Cardiol Sin. 2016;32:684–689. doi: 10.6515/ACS20160110A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids on vitro. Int J Obes. 1990;14:1013–1022. [PubMed] [Google Scholar]
- 19.Shemirani H, Khoshavi M. Correlation of echocardiographic epicardial fat thickness with severity of coronary artery disease - an observational study. Anadolu Kardiyol Derg. 2012;12:200–205. doi: 10.5152/akd.2012.061. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Cheng X, Hong K, et al. How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta-analysis. Coron Artery Dis. 2012;23:227–233. doi: 10.1097/MCA.0b013e328351ab2c. [DOI] [PubMed] [Google Scholar]
- 21.Wang CP, Yeh LR, Lu LF, et al. Increased epicardial adipose tissue volume in coronary artery calcium and coronary atherosclerosis: possible role in inflammatory reaction. Acta Cardiol Sin. 2012;28:1–9. [Google Scholar]
- 22.Şeker T, Türkoğlu C, Harbalıoğlu H, et al. Epicardial fat thickness is associated with abnormal left ventricle geometry in newly diagnosed hypertension. Acta Cardiol Sin. 2018;34:280–287. doi: 10.6515/ACS.201805_34(3).20171209A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aydın E, Altın C, Sakallıoğlu O, et al. Epicardial adipose tissue thickness and carotid intima-media thickness in hemodialysis patients. Acta Cardiol Sin. 2017;33:266–272. doi: 10.6515/ACS20161023A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alpaydın S, Buyukterzi Z, Akkurt HE, et al. Impaired left ventricular diastolic functions and thickened epicardial adipose tissue in rheumatoid arthritis patients is correlated with DAS-28 Score. Acta Cardiol Sin. 2017;33:182–187. doi: 10.6515/ACS20160608B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozturk MT, Ebinç FA, Okyay GU, et al. Epicardial adiposity is associated with microalbuminuria in patients with essential hypertension. Acta Cardiol Sin. 2017;33:74–80. doi: 10.6515/ACS20160418A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eroglu S, Sade LE, Yildirir A, et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis. 2009;19:211–217. doi: 10.1016/j.numecd.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Bhuiyan GR, Roy GC, Siddique MA, et al. Relationship between echocardiographic epicardial adipose tissue (EAT) thickness and angiographically detected coronary artery disease. Mymensingh Med J. 2017;26:498–504. [PubMed] [Google Scholar]
- 28.Erkan AF, Tanindi A, Kocaman SA, et al. Epicardial adipose tissue thickness is an independent predictor of critical and complex coronary artery disease by Gensini and Syntax scores. Tex Heart Inst J. 2016;43:29–37. doi: 10.14503/THIJ-14-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gul I, Zungur M, Aykan AC, et al. The relationship between GRACE score and epicardial fat thickness in non-STEMI patients. Arq Bras Cardiol. 2016;106:194–200. doi: 10.5935/abc.20160024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altun B, Colkesen Y, Gazi E, et al. Could epicardial adipose tissue thickness by echocardiography be correlated with acute coronary syndrome risk scores. Echocardiography. 2013;30:1130–1134. doi: 10.1111/echo.12276. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Liu Q, Liu C, et al. Correlation of echocardiographic epicardial fat thickness with severity of coronary artery disease in patients with acute myocardial infarction. Echocardiography. 2014;31:1177–1181. doi: 10.1111/echo.12545. [DOI] [PubMed] [Google Scholar]
- 32.Ozcan F, Turak O, Canpolat U, et al. Association of epicardial fat thickness with TIMI risk score in NSTEMI/USAP patients. Herz. 2014;39:755–760. doi: 10.1007/s00059-013-3914-z. [DOI] [PubMed] [Google Scholar]
- 33.Benedek T, Opincariu D, Rat N, et al. The assessment of epicardial adipose tissue in acute coronary syndrome patients. A systematic review. J Cardiovasc Emerg. 2017;3:18–29. [Google Scholar]


