Abstract
Background
Zero fluoroscopy during ablation of arrhythmias has been introduced to reduce radiation exposure. However, the safety, feasibility and efficacy of the technique in pediatric populations have yet to be delineated.
Objectives
To investigate the safety, feasibility and effectiveness of zero fluoroscopic-guided transcatheter ablation of right-sided supraventricular tachycardia substrates in a pediatric population.
Methods
Patients < 20 years of age who received ablation of right-sided accessory pathway-mediated arrhythmia and atrioventricular nodal reentrant tachycardia at our hospital between January 2014 and July 2018 were enrolled, and their medical records were reviewed. Patients undergoing ablations with conventional fluoroscopic guidance were enrolled as the control group, and those undergoing ablations with zero fluoroscopic guidance were enrolled as the study group. Repaired or complex congenital heart disease patients were excluded.
Results
One hundred and two patients (55 male; 47 female) received a total of 109 ablation procedures: 68 procedures in the control group and 41 in the study group. The mean procedure duration was 104.7 ± 65.1 minutes in the control group and 98.6 ± 57.6 minutes in the study group (p = 0.62). The mean fluoroscopy time was 30.9 ± 23.9 minutes in the control group, while all procedures in the study group were performed without fluoroscopy (p < 0.001). In subgroup analysis, the results were similar. Acute procedural success rates were high in both groups (98.5% vs. 97.6%, p = 1.0). The recurrence rate was 11.5% (7/61) in the control group and 7.5% (3/40) in the study group (p = 0.78) at mid-term follow-up.
Conclusions
Using the zero fluoroscopy technique during pediatric ablation procedures for right-sided supraventricular tachycardia substrates is safe and significantly reduces radiation exposure.
Keywords: Arrhythmia ablation, Pediatric, Zero fluoroscopy
INTRODUCTION
Supraventricular tachycardia (SVT) is the most common tachyarrhythmia in children and adolescents. The mainstay of treatment for SVT is transcatheter ablation using radiofrequency energy or cryoenergy.1-3 In the past decades, fluoroscopy has been used to guide transcatheter ablation of arrhythmias. However, the stochastic risks of ionizing radiation, including malignancies and hereditary defects are of great concern.4-6 The estimated lifetime risk of cancer mortality can be markedly reduced by performing a fluoroless procedure.7 In pediatric populations, this issue has even greater importance because of a longer life expectancy in which to express the stochastic effects.8-11
Recent advances in technology have led to the development of non-fluoroscopic electroanatomic mapping systems to reduce radiation exposure in ablation procedures.12,13 A recent study showed a potential 96% reduction in the estimated risks of cancer incidence and mortality using a zero fluoroscopy (ZF) approach compared with a conventional approach.14 However, few studies have delineated the safety, feasibility and effectiveness of the ZF technique in guiding transcatheter ablation of arrhythmias in a pediatric population.15 In children and adolescents, atrioventricular reentrant tachycardia (AVRT) and atrioventricular nodal reentrant tachycardia (AVNRT) are the two most common types of arrhythmia.16 The aim of this study was to investigate the safety, feasibility and effectiveness of ZF-guided transcatheter ablation of right-sided SVT substrates, including accessory pathway (AP)-related AVRT and AVNRT in a pediatric population.
METHODS
In December 2016, the EnSite PrecisionTM Cardiac Mapping System (Abbot, St. Paul, MN, USA) was introduced to our electrophysiology (EP) laboratory and was used to perform ZF in pediatric ablation procedures for cardiac arrhythmias.
Study population
All patients undergoing EP studies and ablation procedures performed between January 2014 and April 2018 were included in the initial data collection. Those who received ablation procedures for right-sided AP-related AVRT and AVNRT and those aged < 20 years were enrolled in the study. Patients with repaired or complex congenital heart diseases were excluded from the study.
Study design
We conducted a retrospective case-control study to investigate the safety, feasibility, and efficacy of ZF in ablations of right-sided SVT substrates, including right-sided AP-related AVRT and AVNRT. Reviewing the medical records, those who received ablation procedures through conventional fluoroscopy guidance from January 2014 to November 2016 were enrolled as the control group, and those who received ablation procedures under ZF guidance from December 2016 to April 2018 were enrolled as the study group. The demographics of the patients and records of EP procedures were collected. This research project was approved by the National Taiwan University Hospital Research Ethics Committee, and the requirement for informed consent was waived.
ZF technique guidance procedures
Written informed consent was acquired from each patient or their parents before the procedures. Antiarrhythmic medications were discontinued for at least 3 days before the procedure, except for beta-blockers. Conscious sedation was employed via intravenous general anesthesia with propofol. The EnSite PrecisionTM Cardiac Mapping System was used to perform ZF. Detailed information about the PrecisionTM Cardiac Mapping system has been described previously.17,18. In brief, a steerable decapolar mapping catheter was used to depict the geometry of the venous route to the right atrium via femoral venous access. The catheter was then deployed into the coronary sinus. A 4 Fr. or 5 Fr. multipolar catheter was advanced through the geometry collected previously and placed into the right ventricle to record the His bundle and the right ventricle apex signals concomitantly. A quadripolar catheter was placed in the high right atrium in the same way. After placement of the three catheters, the cardiac geometry was sufficient for following mapping and ablation procedures.
Electrophysiological study and ablation procedure: general description
Standard EP protocols were performed, and the mechanism of arrhythmias at the target sites of ablation were confirmed by using standard burst pacing or extrastimulation pacing maneuver and standard atrioventricular (AV) ring mapping techniques. The diagnosis of AVNRT, parahisian, including antero-septal and mid-septal AP, and other non-parahisian APs was made using standard criteria. Cryoenergy was delivered with a 4-mm, 6-mm or 8-mm cryoablation catheter (CryoConsole, Freezor, Freezor Xtra, Freezor Max, Medtronic CryoCath LP, Quebec, Canada) for AVNRT and parahisian AP after 2015. Radiofrequency energy was delivered with a 5Fr. temperature-controlled catheter (5-mm tip, S/S curve, Fantasista, Japan Lifeline Co. Ltd, Tokyo, Japan or "IBI" TherapyTM Ablation Catheter, Irvine Biomedical, Inc., a St. Jude Medical Company, USA) in all other ablation procedures. Procedural success was defined as the loss of antegrade and retrograde AP conduction, and a lack of tachycardia induction in AVRT. For AVNRT, elimination of the slow pathway with no more than one echo beat was defined as a procedural success. Recurrence was defined as documentation of the reappearance of an arrhythmia targeted for ablation.
Statistics
Values are shown as mean ± standard deviation unless otherwise specified. The Statistical Package for Social Sciences statistical software (SPSS, Version 20 for Windows) was used for all statistical analyses. The independent t-test was used to compare the means among continuous variables, and associations among categorical variables were compared using the chi-squared test. Kaplan-Meier analysis and the log rank test were used for survival analysis of freedom from arrhythmia recurrence. A p value < 0.05 was considered to be statistically significant.
RESULTS
Patient demographics
During the study period, a total of 102 patients (55 male; 47 female) received 109 ablation procedures involving right-sided SVT substrates. The demographics of the patients are demonstrated in Table 1. The mean age at the procedure was younger in the study group (p = 0.004), and the body height and body weight were both lower in the study group (p = 0.016, and 0.017, respectively). However, after excluding the patients who received ablation procedures with cryoenergy, the mean age, body height and body weight were comparable between the control and study groups (age: 12.5 versus 12 years, p = 0.63; height: 155 versus 149.5 cm, p = 0.37; weight: 53.1 versus 46.1 kg, p = 0.264). Five patients in the control group received RF ablation of slow pathways. The overall schematic locations of the APs in the two groups are demonstrated in Figure 1. Five procedures (four in the control group and one in the study group) involved multiple arrhythmia substrates. The trend of procedure duration is shown in Figure 2, which shows that almost no learning curve was needed to use the ZF technique to guide ablation of right-sided SVT substrates.
Table 1. Demographics and results of control group and study group.
| Control group | Study group | p value | |
| Number of patients | 62 | 41 | - |
| Number of procedures | 68 | 41 | - |
| Follow-up duration (months), mean ± SD | 22.7 ± 15 | 10.7 ± 4.4 | - |
| Age (years), mean ± SD | 14 ± 3.5 | 12 ± 3.4 | 0.004 |
| Height (cm), mean ± SD | 157.3 ± 16.8 | 149.4 ± 16 | 0.016 |
| Weight (kg), mean ± SD | 52.7 ± 15.9 | 45.1 ± 15.5 | 0.017 |
| Sex (male: female) | 32:30 | 24:17 | 0.49 |
| Fluoroscopy time (minutes), mean ± SD | 30.9 ± 23.9 | 0 | < 0.001 |
| Procedure duration (minutes), mean ± SD | 104.7 ± 65.1 | 98.6 ± 57.6 | 0.62 |
| Arrhythmia mechanism, n (%) | |||
| AVNRT | 37 (59.7) | 10 (24.4) | - |
| Parahisian AP | 9 (14.5) | 9 (22). | - |
| Non-pararhisian AP | 12 (19.4) | 21 (51.2) | - |
| Multiple mechanisms | 4 (6.5) | 1 (2.4) | - |
| Types of ablation (n) | |||
| Radiofrequency ablation | 22 | 22 | - |
| Cryoablation | 46 | 19 | - |
AP, accessory pathway; AVNRT, atrioventricular nodal reentrant tachycardia.
Figure 1.
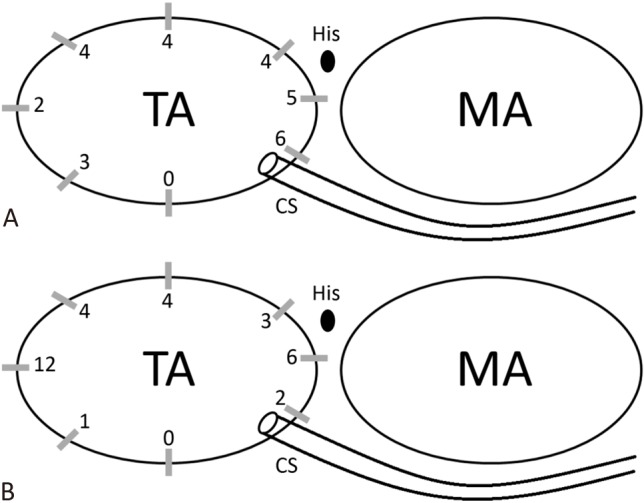
Schematic demonstration of accessory pathway locations in the two groups. Twenty-eight and 32 accessory pathway were treated in control group (A) and study group (B), respectively. MA, mitral annulus; TA, tricuspid annulus.
Figure 2.
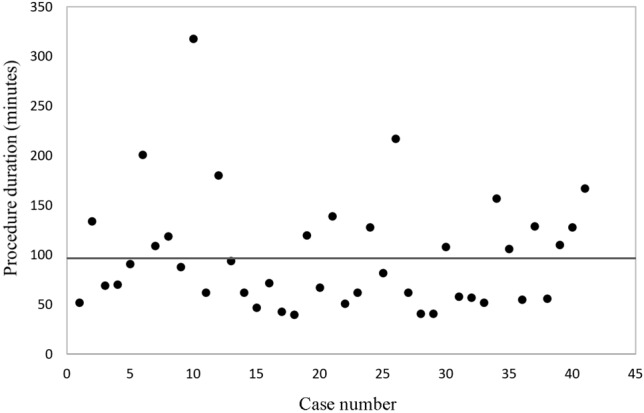
Dot-plot of procedure duration of each procedure demonstrating that almost no learning curve were needed in performing zero-fluoroscopy technique guidance ablation procedures. The grey line indicates the mean procedure duration of study group, which was 98.6 minutes.
Acute results
The procedure durations in the two groups were comparable [mean procedure duration 104.7 ± 65.1 minutes in the control group versus 98.6 ± 57.6 minutes in the study group (p = 0.62)]. A marked reduction in fluoroscopy time was achieved in the study group. The mean fluoroscopy time was 30.9 ± 23.9 minutes in the control group while none of the patients in the study group needed fluoroscopic guidance during the ablation procedures (p < 0.001) (Figure 3). Furthermore, in subgroup analysis, there were no significant differences in procedure duration between the two groups in the patients with AVNRT, parahisian AP or non-parahisian AP (AVNRT: 110.9 versus 93.7, p = 0.44; parahisian AP: 90.7 versus 118, p = 0.334; non-parahisian AP: 86.1 versus. 82.2, p = 0.83), and the reduction in fluoroscopy time was significant in all arrhythmia mechanisms (AVNRT: 26.5 versus 0, p < 0.001; parahisian AP: 31.2 versus 0, p < 0.001 ; non-parahisian AP: 41.6 versus 0, p = 0.002).
Figure 3.
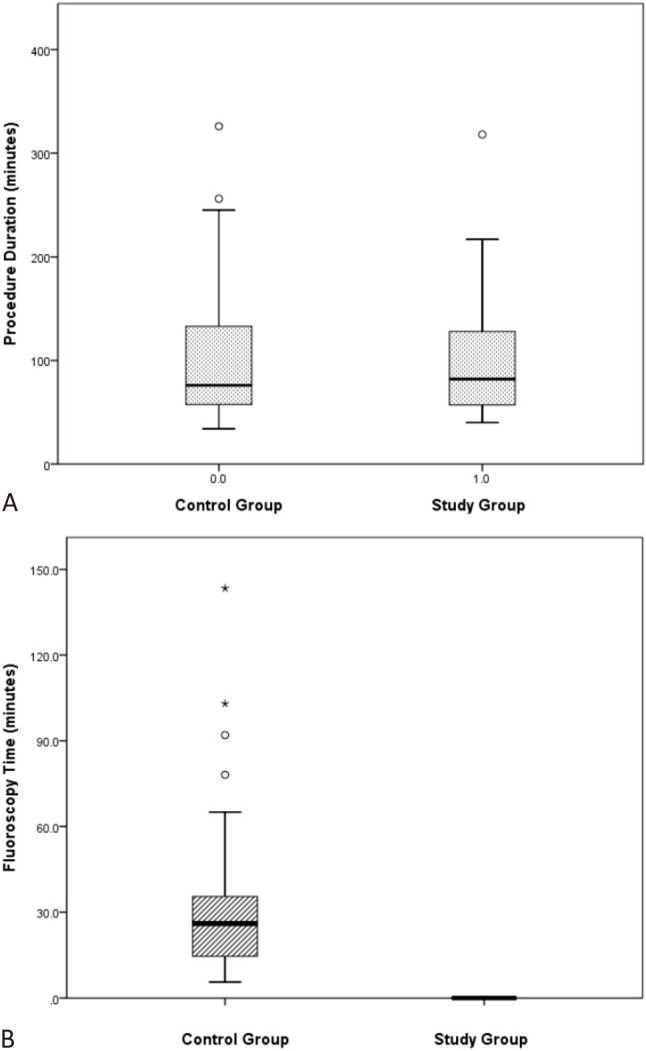
Comparisons of procedure duration (A) and fluoroscopy time (B) between study and control group. No significant difference of procedure duration between study and control group.
The overall acute procedural success rate was 98.5% (67/68 procedures) in the control group and 97.6% in the study group (40/41 procedures) (p = 1.0). One patient in the control group had AVNRT (slow-fast, fast-intermediate, fast-slow AVNRT) and received cryoablation of retrograde slow and intermediate pathways successfully. However, non-sustained SVT, suspected slow-fast AVNRT or ectopic atrial tachycardia could be induced at the end of the procedure. One patient in the study group was diagnosed with Wolff-Parkinson-White (WPW) syndrome with right antero-lateral and right lateral Kent pathways. Elimination of the delta wave could be achieved quickly during the procedure, however the delta wave recurred immediately in the EP laboratory. The procedure was aborted due to a prolonged total procedure time. During the study period, none of the patients experienced major procedural complications requiring emergency or ongoing treatment such as cardiac perforation, pericardial effusion with cardiac tamponade, high degree AV block (second or third degree AV block), thrombi, emboli or death. In addition, none of the patients had vascular complications such as pseudoaneurysm, or arteriovenous fistula during the procedure and follow-up care for the ablations.
Mid-term results
Recurrence of arrhythmia was monitored at a mean follow-up duration of 22.7 ± 15 months in the control group and 10.7 ± 4.4 months in the study group. The results are summarized in Table 2. The overall recurrence rates were 11.5% (7 out of 61 patients) in the control group and 7.5% (3 out of 40 patients) in the study group (p = 0.78), and the recurrence-free survival rate was similar between these two groups (p = 0.599) (Figure 4). In subgroup analysis, there were no significant differences in the recurrence rates between the study and control groups in AVNRT, parahisian AP and non-parahisian AP subgroups.
Table 2. Subgroup analysis of success rate and recurrence rate.
| Control group | Study group | p value | |
| All procedures (n) | 68 | 41 | - |
| Success rate (%) | 67/68 (98.5) | 40/41 (97.6) | 1.0 |
| Recurrence rate (%) | 7/61 (11.5) | 3/40 (7.5) | 0.78 |
| AVNRT (n) | 42 | 10 | - |
| Success rate (%) | 41/42 (97.6) | 10/10 (100) | 1.0 |
| Recurrence rate (%) | 4/41 (9.8) | 1/10 (10) | 0.64 |
| Parahisian AP (n) | 9 | 9 | - |
| Success rate (%) | 9/9 (100) | 9/9 (100) | - |
| Recurrence rate (%) | 1/9 (11.1) | 0/9 (0) | 0.26 |
| Non-parahisian AP (n) | 16 | 21 | - |
| Success rate (%) | 16/16 (100) | 21/21 (100) | - |
| Recurrence rate (%) | 2/16 (12.5) | 2/21 (9.5) | 0.83 |
AP, accessory pathway; AVNRT, atrioventricular nodal reentrant tachycardia.
Figure 4.
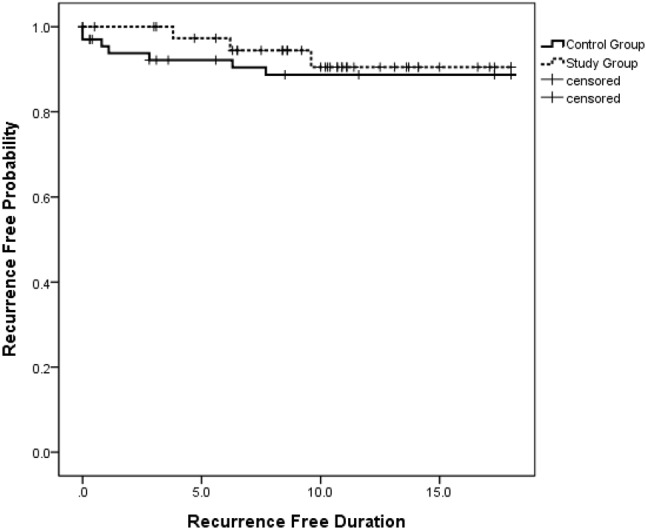
No significant difference of 18-month freedom from arrhythmia recurrence survival after ablation procedures between study and control group (p = .599 by log rank test).
DISCUSSION
There are three main findings to this study: 1) the use of ZF for right-sided SVT substrates in our pediatric population was safe and did not increase the risks of procedural complications; 2) the use of ZF in ablation procedures is feasible for AVNRT and right-sided AP-related AVRT regardless of the location of the APs in pediatric populations; 3) ablation procedures under ZF guidance are as effective as conventional fluoroscopy guidance regardless of the arrhythmia mechanisms and AP locations with regards to acute success and arrhythmia recurrence rates at mid-term follow-up.
In our EP laboratory, the as low as reasonably achievable (ALARA) principle has been universally followed for over 20 years during EP studies and ablation procedures.19 A previous study reported that the lifetime attributable risk of cancer mortality can be significantly reduced by performing a fluoroless procedure.7 The NO-PARTY trial further reported a 96% reduction in the estimated risks of cancer incidence and mortality with the use of a ZF approach compared to a conventional approach.14 Pediatric patients have a longer life expectancy and their organ systems are still developing, so they have a greater chance of expressing the stochastic effects of radiation. Thus, reducing the use of fluoroscopy following the ALARA principle during ablation procedures is even more important in pediatric populations. In our study, all procedures in the study group were performed uneventfully without fluoroscopy. That means that none of the patients in study group were exposed to a higher risk of cancer after the ablation procedure.
With improvements in technology and increased experience with ablation, the overall procedural success rate of ablation procedures has increased, and the number of procedural complications decreased. However, minor and major complications still occur. Minor vascular complications such as pseudoaneurysm, arteriovenous fistula and minor bleeding have been reported in 1%-3% of ablation procedures in pediatric populations.2 The most common serious complications are high degree AV block, catheter perforation, and thrombi or emboli, which have been reported to occur in approximately 1% of procedures. In pediatric populations, AV block has been reported to occur in 1.2% of cases.20 None of our patients experienced serious complications including high degree AV block, cardiac perforation, and thrombi or emboli regardless of the AP location or energy type. In addition, none of the patients in the study group developed minor vascular events. The results of our study confirm that using the ZF technique in ablation of right-sided SVT substrates, including AVNRT and AP-mediated AVRT, could avoid the harmful effects produced by radiation exposure without increasing the risks of developing minor or major vascular events and AV block in the ablation procedures.
Wan et al.21 reported factors associated with decreased fluoroscopy exposure with conventional catheter ablation versus electroanatomic navigation. They reviewed 234 substrates in which 107 (46%) procedures were performed under electroanatomical guidance. The fluoroscopy duration decreased significantly from a mean of 11.1 ± 10.1 to 3.5 ± 6.2 minutes in the conventional and electroanatomical guidance groups, respectively. For those with AVNRT, right free wall APs, and right septal APs, the mean fluoroscopy durations were 1.8, 2.8, and 2.8 minutes, respectively. Drago et al. recruited 21 WPW patients with right Kent pathways receiving ablations at a mean age of 11.3 ± 3.2 years, and the mean fluoroscopy time was 9.2 ± 7.7 minutes in the 12 patients in whom fluoroscopy was used.22 In their report, only 40% of the patients received mapping and ablation procedures without fluoroscopy. The reasons why the management of right-sided arrhythmia substrates requires less fluoroscopy exposure may be because right-sided procedures do not need high-risk maneuvers such as trans-septal puncture and retrograde trans-aortic maneuvers. However, few reports have thoroughly investigated ZF ablation for right-sided ablation procedures in pediatric populations. Scaglione et al.15 reported the use of ZF with a CARTO3 system in 26 pediatric patients with right-sided AP ablations. However, the cryo-catheter could not be visualized during cryoenergy delivery with the CARTO3 system. In addition, high profile catheters were needed, which can increase the risk of vascular complications in small children. This limitation increased the risk and uncertainty of ablation procedures. In our study, the procedural duration was comparable between the control group and the study group, while no fluoroscopy was used in any patient in the study group. Furthermore, none of our patients in the study group experienced serious complications requiring interventions. Thus, our study demonstrates that reducing the fluoroscopy to zero in right-sided supraventricular tachyarrhythmia substrates, including AVNRT and right-sided AP, in pediatric populations is feasible.
A previous literature reported an initial success rate of 93% for all SVT substrates in a pediatric population. In further classifications, the AP ablation success rate was 94% (right free wall: 90%; right septal: 89%), and 99% for AVNRT.2 Recurrence at 12 months was related to the substrate and was highest for right-sided APs (right free wall: 15.8%; right septal: 24.6%).2 A more recent multicenter report from three Czech Republic centers included 708 procedures in 633 pediatric patients, and reported acute/long-term success rates for AP ablation and AVNRT of 91.1/83% and 98.9/90.6%, respectively.23 For specific localizations of AP in septal AP and right-free wall AP, the results were 76.9/62.8% and 92.2/72.2%, respectively. Some single center studies have reported ablation of AVNRT in pediatric populations under near zero or ZF with recurrence rates ranging from 0.9% to 13.7%.24-26 However, some of these studies treated multiple cryo-lesions and some delivered cryo-energy during AVNRT in the procedures. The safety and possibility of late AV blocks are of great concern. In another small series involving AP-mediated AVRT, Scaglione et al.15 reported the results of ZF ablation procedures performed in patients at a mean age of 13.1 ± 3.3 years under CARTO3 electroanatomic mapping system guidance. The percentage of recurrent arrhythmias was 15.9% (7/44 patients), and the mean elapsed time to recurrence was 7.0 ± 15.2 months. In our study, the overall acute procedural success rates were 98.6% in the control group and 97.6% in the study group. In addition, the overall recurrence rates were 11.5% in the control group and 7.5% in the study group at follow-up durations of 22.7 ± 15 months and 10.7 ± 4.4 months, respectively. These results are similar to data reported worldwide regardless of the arrhythmia mechanisms and AP localizations in both the control and study groups at our institution. These results further support that ZF guidance during ablation in a pediatric population is as effective as conventional fluoroscopic guidance procedures. Therefore, this technique should be considered as a recommended procedure when dealing with right-sided SVT substrates, including AVNRT and AVRT, especially in a pediatric population.
Study limitations
Our study has some limitations. First, this is a retrospective case-control study, and selection bias may have occurred. However, we enrolled all consecutive patients to eliminate this potential confounding. Second, AVNRT patients comprised a significantly large portion of the study group. This may have been because cryoablation was introduced to our institution in February 2015. Many AVNRT patients diagnosed before this time were waiting for this treatment, and thus there were more AVNRT patients in the control group than in the study group. This may also have affected the average age, height and weight. However, we performed subgroup analysis to minimize such bias. Third, the long-term results of the ZF-guided ablation procedures are not known due to the short follow-up period. However, our follow-up duration in the study group was longer than the average recurrence time reported in the literature. Further follow-up and comparisons of the clinical conditions of the study group are required to clarify the long-term follow-up results.
CONCLUSIONS
Using the ZF technique to diminish radiation exposure during pediatric transcatheter ablation of right-sided SVT substrates, including AVNRT and AP-related AVRT is safe and feasible. The acute success and arrhythmia recurrence rates at mid-term follow-up were also comparable to the conventional fluoroscopic guidance approach. We suggest that the ZF technique should be considered as a recommended procedure when dealing with right-sided SVT substrates, including AVNRT and AP-related AVRT in pediatric populations.
Acknowledgments
This cardiac mapping system is supported by the Paujar Charity Foundation.
CONFLICT OF INTEREST
All the authors declare no conflicts of interest.
REFERENCES
- 1.Chiu SN, Lu CW, Chang CW, et al. Radiofrequency catheter ablation of supraventricular tachycardia in infants and toddlers. Circ J. 2009;73:1717–1721. doi: 10.1253/circj.cj-09-0123. [DOI] [PubMed] [Google Scholar]
- 2.Philip Saul J, Kanter RJ, Writing C, et al. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease: developed in partnership with the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American Academy of Pediatrics (AAP), the American Heart Association (AHA), and the Association for European Pediatric and Congenital Cardiology (AEPC). Heart Rhythm. 2016;13:e251–e289. doi: 10.1016/j.hrthm.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Brugada J, Blom N, Sarquella-Brugada G, et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace. 2013;15:1337–1382. doi: 10.1093/europace/eut082. [DOI] [PubMed] [Google Scholar]
- 4.Kovoor P, Ricciadello M, Collins L, et al. Risk to patients from radiation associated with radiofrequency ablation for supraventricular tachycardia. Circulation. 1998;98:1534–1540. doi: 10.1161/01.cir.98.15.1534. [DOI] [PubMed] [Google Scholar]
- 5.Limacher MC, Douglas PS, Germano G, et al. ACC expert consensus document. Radiation safety in the practice of cardiology. J Am Coll Cardiol. 1998;31:892–913. doi: 10.1016/s0735-1097(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H, el-Atassi R, Kalbfleisch SJ, et al. Effect of operator experience on outcome of radiofrequency catheter ablation of accessory pathways. Am J Cardiol. 1993;71:1104–1105. doi: 10.1016/0002-9149(93)90581-v. [DOI] [PubMed] [Google Scholar]
- 7.Gaita F, Guerra PG, Battaglia A, Anselmino M. The dream of near-zero X-rays ablation comes true. Eur Heart J. 2016;37:2749–2755. doi: 10.1093/eurheartj/ehw223. [DOI] [PubMed] [Google Scholar]
- 8.Journy N, Ancelet S, Rehel JL, et al. Predicted cancer risks induced by computed tomography examinations during childhood, by a quantitative risk assessment approach. Radiat Environ Biophys. 2014;53:39–54. doi: 10.1007/s00411-013-0491-8. [DOI] [PubMed] [Google Scholar]
- 9.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700–707. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baysson H, Rehel JL, Boudjemline Y, et al. Risk of cancer associated with cardiac catheterization procedures during childhood: a cohort study in France. BMC Public Health. 2013;13:266. doi: 10.1186/1471-2458-13-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith G, Clark JM. Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing Clin Electrophysiol. 2007;30:510–518. doi: 10.1111/j.1540-8159.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- 13.Clark BC, Sumihara K, McCarter R, et al. Getting to zero: impact of electroanatomical mapping on fluoroscopy use in pediatric catheter ablation. J Interv Card Electrophysiol. 2016;46:183–189. doi: 10.1007/s10840-016-0099-4. [DOI] [PubMed] [Google Scholar]
- 14.Casella M, Dello Russo A, Pelargonio G, et al. Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: the NO-PARTY multicentre randomized trial. Europace. 2016;18:1565–1572. doi: 10.1093/europace/euv344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaglione M, Ebrille E, Caponi D, et al. Zero-fluoroscopy ablation of accessory pathways in children and adolescents: CARTO3 electroanatomic mapping combined with RF and cryoenergy. Pacing Clin Electrophysiol. 2015;38:675–681. doi: 10.1111/pace.12619. [DOI] [PubMed] [Google Scholar]
- 16.Lu CW, Wu MH, Chen HC, et al. Epidemiological profile of Wolff-Parkinson-White syndrome in a general population younger than 50 years of age in an era of radiofrequency catheter ablation. Int J Cardiol. 2014;174:530–534. doi: 10.1016/j.ijcard.2014.04.134. [DOI] [PubMed] [Google Scholar]
- 17.Razminia M, Manankil MF, Eryazici PL, et al. Nonfluoroscopic catheter ablation of cardiac arrhythmias in adults: feasibility, safety, and efficacy. J Cardiovasc Electrophysiol. 2012;23:1078–1086. doi: 10.1111/j.1540-8167.2012.02344.x. [DOI] [PubMed] [Google Scholar]
- 18.Razminia M, Willoughby MC, Demo H, et al. Fluoroless catheter ablation of cardiac arrhythmias: a 5-year experience. Pacing Clin Electrophysiol. 2017;40:425–433. doi: 10.1111/pace.13038. [DOI] [PubMed] [Google Scholar]
- 19.Justino H. The ALARA concept in pediatric cardiac catheterization: techniques and tactics for managing radiation dose. Pediatr Radiol. 2006;36 Suppl 2:146–153. doi: 10.1007/s00247-006-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Hare GF, Javitz H, Carmelli D, et al. Prospective assessment after pediatric cardiac ablation: demographics, medical profiles, and initial outcomes. J Cardiovasc Electrophysiol. 2004;15:759–770. doi: 10.1046/j.1540-8167.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- 21.Wan G, Shannon KM, Moore JP. Factors associated with fluoroscopy exposure during pediatric catheter ablation utilizing electroanatomical mapping. J Interv Card Electrophysiol. 2012;35:235–242. doi: 10.1007/s10840-012-9701-6. [DOI] [PubMed] [Google Scholar]
- 22.Drago F, Silvetti M, Di Pino A, et al. Exclusion of fluoroscopy during ablation treatment of right accessory pathway in children. J Cardiovasc Electrophysiol. 2002;13:778–782. doi: 10.1046/j.1540-8167.2002.00778.x. [DOI] [PubMed] [Google Scholar]
- 23.Kubus P, Vit P, Gebauer RA, et al. Long-term results of paediatric radiofrequency catheter ablation: a population-based study. Europace. 2014;16:1808–1813. doi: 10.1093/europace/euu087. [DOI] [PubMed] [Google Scholar]
- 24.Backhoff D, Klehs S, Muller MJ, et al. Long-term follow-up after catheter ablation of atrioventricular nodal reentrant tachycardia in children. Circ Arrhythm Electrophysiol. 2016;9(11):pii: e004264. doi: 10.1161/CIRCEP.116.004264. [DOI] [PubMed] [Google Scholar]
- 25.Karacan M, Celik N, Akdeniz C, Tuzcu V. Long-term outcomes following cryoablation of atrioventricular nodal reentrant tachycardia in children. Pacing Clin Electrophysiol. 2018;41:255–260. doi: 10.1111/pace.13277. [DOI] [PubMed] [Google Scholar]
- 26.Balli S, Kucuk M, Bulut MO, et al. Transcatheter cryoablation procedures without fluoroscopy in pediatric patients with atrioventricular nodal reentrant tachycardia: a single-center experience. Acta Cardiol Sin. 2018;34:337–343. doi: 10.6515/ACS.201807_34(4).20180326A. [DOI] [PMC free article] [PubMed] [Google Scholar]


