Abstract
The understanding of the intrinsic and extrinsic causes of longevity variation has deservedly received much attention in evolutionary ecologist. Here we tested the association between longevity and spawning-site groups across 38 species of Chinese anurans. As indicators of group-spawning we used spawning-site group size and spawning-site density, which we measured at 152 spawning sites in the field. We found that both spawning-site density and group size were positively associated with longevity. Male group-spawning (e.g., male spawning-site density and male spawning-site group size) was also positively correlated with longevity. A phylogenetic path analysis further revealed that longevity seems directly associated with spawning-site density and group size, and that the association in part depend on the ‘groups-spawning-age at first reproduction’ association. Our findings suggest that the increased group-spawning are likely to benefit in declining extrinsic mortality rates and living longer through improving total anti-predator behaviour under predation pressure.
Subject terms: Evolutionary ecology, Evolutionary developmental biology
Introduction
Longevity varies greatly both within and among species and populations in animals1–9 and understanding the proximate and ultimate causes of this variation has deservedly received much attention in evolutionary biologists10–18. The proximate causes are the results of hazards from the environments, such as predator risk, diseases, parasitism, famine, competition, drought or accidents19,20. Meanwhile, the ultimate causes result from intrinsic processes of physical and functional degradation originating within the body, such as spontaneous chemical reactions, replication errors and accumulation of metabolic waste products20.
Senescence can be explained by a decline in the power of natural selection with age21,22. The senescence theory states that members of populations exposed to lower degree of extrinsic mortality will evolve longer potential longevity2,17. In particular, animals suffering lower extrinsic mortality rates can postpone age at first reproduction, placing organisms under additional stress, which can, in turn, decrease intrinsic mortality23. This can select for a longer longevity so that potential reproduction is maximized2. From those preceding studies, it is evident that age at first reproduction is important predictor of longevity24. For instance, maximum longevity is positively correlated with age at first reproduction in taxa14,16,25–27.
Most studies have found longevity to be strongly associated with both intrinsic and extrinsic mortality rates in animal kingdoms2,4,17,28–31. For example, the use of poison and nocturnality, which ascribe to reduced predation pressure (low extrinsic mortality rate) are associated with longer longevity in amphibians4. Stark et al. found reptilian, that live on islands, and in colder and more seasonal environments, presumably suffer lower rates of predation, live longer18. Additionally, group size is an important life-history trait that is expected to affect longevity12,26. Species living in larger groups should exhibit lower extrinsic mortality rate because aggregations in animals typically decrease per capita predation risk32,33, and thereby possessing longer longevity. However, there are evidences that longevity is not correlated with group-foraging and sociality in North American birds14,15 and group size in mammals27,31. For anurans, group spawners typically aggregate in ponds where the density of individuals varies across species34. The measure can be used as proxy for extrinsic mortality rate where denser aggregations indicate lower predation risk because denser or larger aggregations have been shown to (i) yield survival benefits to individuals, (ii) to increase total anti-predator behaviour, and (iii) to reduce the costs of anti-predator behaviours35–42. In addition, competition is the other force that drives the evolution of life history43. According to life history theory, increasing population size would enhance competition, the K-selected strategy would be favored in this scenario that eventually shapes the whole life history, such as prolonged longevity12.Although the factors (e.g., chemical protection, activity period, microhabitat preferences and annual temperature) influencing longevity have been tested formally in amphibians4,29,44,45, the relationship between group-spawning and longevity in anurans remains untested.
Here, we evaluated the association between longevity and group-spawning (e.g., spawning-site density and spawning-site group size) among 38 species of anurans when controlling the associated variables (e.g., altitude, latitude, age at first reproduction and SVL: snout-vent length) which may affect longevity variation. Larger group-spawners have lower predation pressures, which result in lower extrinsic mortality rate12. Hence, we predict that frogs with denser group-spawning should mature later and live longer.
Results
Group-spawning (e.g., spawning-site group density and spawning-site group size) tended to be correlated with SVL in our sample of 38 frog species (Table S1). Group-spawning was further closely correlated with age at first reproduction (Table S1). PGLS revealed that longevity was positively correlated with age at first reproduction and SVL (P < 0.001 and P = 0.022, respectively), and tended to be positively correlated with altitude and latitude, respectively (P = 0.093 and P = 0.057; Table S2).
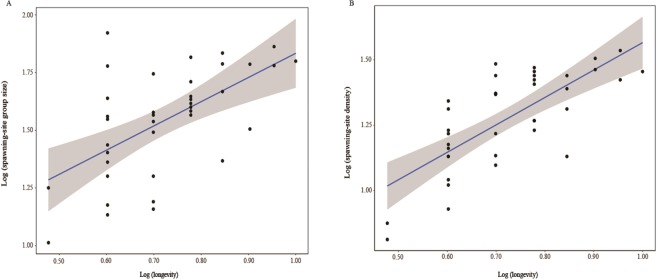
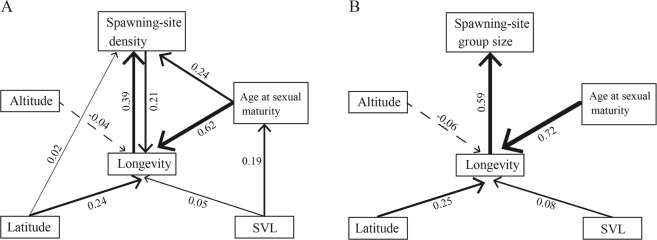
Longevity was positively correlated with group-spawning (Fig. 1; spawning-site density: β = 0.52, t = 6.54, P < 0.001, λ < 0.0011, <0.001; spawning-site group size: β = 0.33, t = 4.34, P < 0.001, λ < 0.0011, <0.001). Longevity sample size did not affect this relationship between spawning-site density and longevity (β = −0.002, t = −0.233, P = 0.818, λ < 0.0011, <0.001) and between longevity and spawning-site group size (β = 0.005, t = 0.453, P = 0.654, λ < 0.0011, <0.001). Group-spawning was positively correlated with longevity when controlling the effects of age at first reproduction, altitude, latitude and SVL (Table 1). The association between group-spawning and longevity was dependent on the ‘spawning groups-age at first reproduction’ association which was supported by our phylogenetic confirmatory path analyses (Figs 2 and S1, S2; Tables S3, S4). When using mean age (average age of all individuals for every species), instead of maximum lifespan as proxy for longevity and relating it to group-spawning, our results remained qualitatively similar as frogs with denser group-spawning were older (Table 1).
Figure 1.
Relationships between group-spawning (e.g., spawning-site density and spawning-site group size) and longevity across 38 species of frogs. The shaded areas indicated 95% confidence interval and the blue line indicated significant correlation between group-spawning and longevity.
Table 1.
PGLS model of relationships between lifespan/mean age and group-spawning for 38 species of anurans.
| Predictors | Longevity | Mean age | ||||||
|---|---|---|---|---|---|---|---|---|
| λ | β | t | P | λ | β | t | P | |
| Spawning-site density | <0.0011,<0.001 | 0.308 | 4.385 | <0.001 | <0.0011,0.002 | 0.213 | 2.551 | 0.016 |
| SVL | 0.040 | 0.498 | 0.622 | 0.201 | 2.100 | 0.044 | ||
| Altitude | −0.008 | −0.235 | 0.816 | 0.070 | 1.711 | 0.097 | ||
| Latitude | 1.076 | 2.587 | 0.014 | 1.218 | 2.453 | 0.020 | ||
| Age at sexual maturity | 0.350 | 4.265 | <0.001 | 0.284 | 2.879 | 0.007 | ||
| Spawning-site group size | <0.0011,<0.001 | 0.167 | 2.827 | 0.008 | <0.0011,0.012 | 0.115 | 1.795 | 0.082 |
| SVL | 0.053 | 0.580 | 0.566 | 0.209 | 2.089 | 0.045 | ||
| Altitude | −0.011 | −0.290 | 0.774 | 0.071 | 1.658 | 0.107 | ||
| Latitude | 1.130 | 2.401 | 0.022 | 1.266 | 2.448 | 0.020 | ||
| Age at sexual maturity | 0.424 | 4.790 | <0.001 | 0.337 | 3.445 | 0.002 | ||
Significant predictors are marked in bold. Phylogenetic scaling parameters (superscripts following λ denote P-values of likelihood ratio tests against models with λ = 0 and λ = 1, respectively).
Figure 2.
Visual representation of the averaged best-fitting path models (ΔCICc ≤ 2) for the anurans. Arrows reflect the direction of the path, and their line width is proportional to their standardized regression coefficients (adjacent to arrows).
Longevity was positively correlated with male group-spawning (male spawning-site density: β = 0.44, t = 6.14, P < 0.001, λ < 0.0011, <0.001; male spawning-site group size: β = 0.39, t = 5.78, P < 0.001, λ < 0.0011, <0.001). Longevity and mean age were positively correlated with male group-spawning when controlling the effects of age at first reproduction, altitude, latitude and SVL (Table S5). Hence, when using male group-spawning instead of group-spawning, we found a same result.
Discussion
We first time investigated the relationship between group-spawning and longevity outside of homeothermic taxa. We found a positive association between longevity and group-spawning after controlling for several potential confounding factors and phylogenetic non-independence, which is inconsistent with what has previously been shown in birds14,15 and mammals27. Spawning aggregations of anurans with longer longevity were denser than spawning aggregations with shorter longevity. Our findings suggest that evolving a longer longevity is beneficial when denser group-spawning may result in lower extrinsic mortality by declining predation risk36,37. Below we discuss what may underlie this consistent pattern of longevity variation across vertebrate taxa.
Selection for larger body size leads to better predator avoidance46. Larger-bodied species experience fewer predators than smaller-bodied species, thereby reducing mortality risk47–49. In the case where larger-bodied species can increase survival, thereby living longer. Alternatively, larger-bodied species have lower basal metabolic rate which is correlated with increased longevity in endotherms50. In particular, there are evidences that body size is positively correlated with longevity across taxa in both endotherms and ectotherms 2,4,5,27,30,51. Body size is positively associated with longevity across amphibians and within the large amphibian orders4. The trade-off between growth and reproduction can the general pattern because the growth of a large size need take longer development time and delay reproduction, and this selects for a longer longevity31. We found that longevity was positively correlated with SVL across 38 species of anurans, similar with previous studies on amphibians30,51–54.
Most studies have shown that longevity is positively correlated with age at first reproduction in anurans1,25,55. This positive correlation can be explained by life-history trade-off12 that growing to larger body and longer longevity need delays reproduction, thus selecting for prolonged age at first reproduction. Path analyses also suggested the positive relationship between group-spawning and longevity depending on the positive relationship between group-pawning and age at first reproduction. Moreover, we found that longevity sample size did not affect relationship between longevity and group-spawning. It appears that increasing sample size does not strongly increase the probability of finding older individuals in our study.
Previous studies have shown the different relationships between social groups (e.g., group-foraging, complex sociality, and colony size) and longevity29,56–58). In comparative studies, species living in larger social groups do not display longer longevity when controlling for other factors known to affect longevity in birds14,15,29,56 and in mammals27,31. However, social breeding was positively linked to increased longevity and survival rates in birds when phylogenetic effect and sampling effort was not controlled57,58. The findings result from the argument that species living in social groups confers benefits to group members, consequently reducing mortality rates and increasing longevity12,59. Our anurans results showed a positive effect of spawning-group on longevity, suggesting that frogs with denser group-spawning faced to lower predation risk, and thereby decreasing mortality rates and living longer. By contrast, Kamilar et al. found a weak and negative correlation between group size and longevity in artiodactyls27. The shorter longevity may result from higher rates of extrinsic mortality in larger groups where they are more conspicuous to predators in open habitats27.
The cognitive buffer hypothesis (CBH) predicts that the increased cognitive abilities provided by a larger brain can facilitate appropriate behavioral responses toward uncommon or complex socioecological alterations60–63. The role of sexual selection64, especially mate choice65 in brain evolution has recently been addressed. For instance, humans evolved such large brains because the increased cognitive abilities associated with large brains are attractive to females66. For males, relatively larger brains and better cognitive abilities can likewise result in better male mate competition in the guppy67. According to life history theory, as population size or density increases, males will encounter comparatively more other males, and thus increasing intensity of competition for mating opportunities68,69. In this case, population size or density eventually shapes the whole life history based on the K-selected strategy12. This was also candidate causation to the positive association between spawning-site group size and longevity in this study. As a result, larger-brained anurans with better cognitive abilities were expected to experience denser spawning aggregations and stronger male mate competition than smaller-brained species. Previous studies have suggested that a larger brain facilitate the evolution of a longer lifespan via increasing individual survival probability in unpredictable situations70,71. Likewise, Yu et al. revealed that larger-brained anurans live longer in the wild25 because the developmental costs hypothesis predicts that a larger brain occurs as a by-product of a generally slower life history such as longer lifespan72. Hence, anurans displayed a positive association between spawning aggregations and longevity based on cognitive abilities and developmental costs.
Additionally, larger groups are shown to associate with higher levels of parasite infection73. However, there is no association between parasite species richness and host longevity in a large sample of anthropoid primates74 while a negative association is observed for 23 mammal species75. Additional studies are therefore needed to investigate the effect of parasite infection in different group size on longevity in anurans.
In conclusion, we found that frogs with denser spawning groups mature later and live longer and interpret this as the evidences for group-spawning increase resulting in decrease in mortality through improving anti-predator behaviour and cognitive adaptations to predation pressure35–42.
Materials and Methods
Ethical approval
All experimental methods were carried out in accordance with the current laws of China concerning animal experimentation, and permission to collect amphibians was received from the ethical committee for animal experiments in China Council on Animal Care (CCAC) guidelines. All experimental protocols were approved by the Animal Ethics Committee (AEC) at China West Normal University.
Data collection
Spawning-site group size and density for 38 species was determined in breeding season (April to June) in the field between 2008 and 2017 according to the following procedure. Firstly, we located breeding ponds (four per species) and captured all individuals at each pond at night using a 12-V flashlight. The number of individuals was then counted and their sexes were determined by their secondary sexual characteristics (e.g., nuptial pad in males and the eggs readily visible through the skin of the abdomen in adult females). A red string was used to leg-marked individuals where they were released in their respective breeding ponds. The next day all individuals (including previously marked individuals) were recaptured, and we counted their numbers. All new individuals were leg-marked using yellow string and released in the pond. The third day, we again captured all individuals. We estimated the group size of each pond based on average number of individuals on the three days. In field, we selected all ponds where the shape did not vary and was always near rectangular to collect individuals. We then measured greatest length and width of pond to determine pond sizes and calculate surface area: A = length * width. Spawning-site group size was the number of individuals per pond and we calculated spawning-site density as the ratio between present individuals and the area of pond. For every species we determined average spawning-site group size, spawning-site density based on data on three days of all four ponds. Moreover, we estimated the male group size and density of each species. The estimated group-spawning size may change with the weather condition (e.g., raining or sunny day), and we collected group size of all species at raining day in their breeding peak. This case can control the effects of weather condition on the group-size data collection among different species. We also collected breeding season length of each species based on four spawning sites38.
Age determination
Longevity data were the maximum lifespan (years) reported for each species. We obtained age at sexual maturity, mean age, and longevity for 38 species from published literature25 (based on skeletochronology, Table S6). Because the estimate of longevity could be based on unequal numbers of individuals for different species, sample size may affect estimate of maximum lifespan. In this study, with an increasing number of sampled individuals the likelihood of sampling a particularly old individual should increase. As sample size per species ranged between 20 and 141 individuals we corrected for some of the potential biases inherent in the use of maxima resulting from the longevity sample size when longevity was estimated for each species. In particular, we treated longevity as the response variable, group-spawning as the predictor variable, and longevity sampling size as a covariate to correct the effect of sampling size on the relationship between longevity and group-spawning.
Associated variables
Other traits may co-vary with longevity and group-spawning. It is therefore important to investigate their relationship with group-spawning and longevity due to the correlative nature of comparative analyses. Covariation of life-history traits is common across species76,77, we therefore extracted information on life-history traits (e.g., breeding season length and age at sexual maturity) that are typically correlated with longevity from our own previous studies25 (Table S6). Moreover, environmental factors (mean annual temperatures and rainfall) affect longevity variation78. We collected everyday average temperature and rainfall at each location and calculated mean annual temperatures and rainfall from Chinese Meteorological Stations (http://www.lishi.tianqi.com) between 2011 and 201579. Also, longevity can be affected by geography44,80. For instance, latitude is a strong predictor for longevity in birds81 while altitude is positively correlated with longevity in anurans44. We therefore included altitude and latitude in our analyses (see below).
Phylogeny
We reconstructed a molecular phylogeny with a full coverage of 38 species following the methods of the reference25 (Fig. S3). We obtained the GenBank accession numbers (Table S7). BEAUTi and BEAST v.1.8.382 with unlinked substitution models, a relaxed uncorrelated lognormal clock, a Yule speciation process, and no calibration points due to a lack of fossil dates, were used to constructed phylogenies (Fig. S3). We made the Markov Chain Monte Carlo (MCMC) simulation to run for 100 million generations and sampled a tree every 5000th generation. The satisfying convergence of the Bayesian chain and adequate model mixing for each of the tree statistics was shown the effective sample size (ESS) in the program Tracer v.1.6.083. We used TreeAnmtator V.1.8.382 to generate a maximum clade credibility tree with mean node heights and a 10% burn-in.
Data analyses
All statistical analyses were conducted in the R statistical environment version 3.3.184, and all data was log10-transformed to meet normal distribution. We accounted for the phylogenetic structure of the model residuals using phylogenetic generalized least-squares (PGLS) models in the R package caper85 and our reconstructed phylogeny. Using a maximum-likelihood approach, we evaluated the phylogenetic effect on relationships through phylogenetic scaling parameter λ86,87. These λ values cover a scale raging from 0 (phylogenetic nonindependence) to 188,89 (complete phylogenetic dependence). To investigate the relationships between group-spawning, environmental factors (e.g., altitude, latitude, temperature and rainfall) and life-history traits (breeding season length, age at sexual maturity and body size) we first used a PGLS models treating group-spawning as the response variable, and environmental factors or life-history traits as the predictor variable. To then investigate the factors of longevity variation, we examined the relationships between longevity and environmental factors (e.g., altitude, latitude, temperature and rainfall) and life-history traits in another PGLS approach, treating longevity as the response variable, environmental factors or life-history traits as predictor variables. Finally, for the analysis of the relationship between group-spawning and longevity we used the PGLS models treating longevity as the response variable, group-spawning as the predictor variable, and age at first reproduction, altitude, latitude and SVL as covariates.
Longevity and group-spawning may be directly linked; they may also be indirectly associated via changes in age at first reproduction, altitude, latitude and SVL. Hence, phylogenetic confirmatory path analyses90 on the basis of pre-specified candidate path models in the R package phylopath v1.0.091 were performed to test the associations between longevity, age at first reproduction, altitude, latitude and SVL and group-spawning. To evaluate the models, we compared a total of 20 models with different configurations of these variables (Supplementary Information: Figs S1, S2) using the C-statistic Information Criterion (CICc) corrected for small sample sizes. A path analysis approach in the R package phylopath v1.0.091 ranked all candidate models based on their C-statistic Information Criterion (CICc) and averaged those with ΔCICc ≤ 2 from the top model was used to examine the conditional independences of each model. Path coefficients are averaged only over models where the path exists90.
Supplementary information
Acknowledgements
The study was supported by the National Natural Sciences Foundation of China (31772451; 31970393), the Science and Technology Youth Innovation Team of Sichuan Province (19CXTD0022), the Key Cultivation Foundation of China West Normal University (17A006) and the Talent Project of China West Normal University (17YC335).
Author Contributions
C.L.M. and Y.L.C. conceived the analysis, analyzed data and wrote methods and results. Y.L.C. and C.L.M. collected the data. W.B.L. wrote the introduction and discussion.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yun Lin Cai and Chun Lan Mai contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50368-w.
References
- 1.Liao WB, Lu X. Age structure and body size of the Chuanxi Tree Frog Hyla annectans chuanxiensis from two different elevations in Sichuan (China) Zool. Anz. 2010;248:255–263. doi: 10.1016/j.jcz.2009.10.002. [DOI] [Google Scholar]
- 2.Healy K, et al. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. B. 2014;281:20140298. doi: 10.1098/rspb.2014.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao WB, Luo Y, Lou SL, Lu D, Jehle R. Geographic variation in life-history traits: growth season affects age structure, egg size and clutch size in Andrew’s toad (Bufo andrewsi) Front. Zool. 2016;13:6. doi: 10.1186/s12983-016-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stark G, Meiri S. Cold and dark captivity: drivers of amphibian longevity. Global Ecol. Biogeogr. 2018;27:1384–1397. doi: 10.1111/geb.12804. [DOI] [Google Scholar]
- 5.Holm S, et al. A comparative perspective on longevity: The effect of body size dominates over ecology in moths. J. Evol. Biol. 2016;29:2422–2435. doi: 10.1111/jeb.12966. [DOI] [PubMed] [Google Scholar]
- 6.Munch SB, Salinas S. Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. Proc. Natl. Acad. Sci. USA. 2009;106:13860–13864. doi: 10.1073/pnas.0900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharf I, et al. Late bloomers and baby boomers: Ecological drivers of longevity in squamates and the tuatara. Global Ecol. Biogeogr. 2015;24:396–405. doi: 10.1111/geb.12244. [DOI] [Google Scholar]
- 8.Lou SL, et al. Altitudinal variation in age and body size in Yunnan Pond Frog (Pelophylax pleuraden) Zool. Sci. 2012;29:493–498. doi: 10.2108/zsj.29.493. [DOI] [PubMed] [Google Scholar]
- 9.Liao WB, Lu X, Jehle R. Altitudinal variation in reproductive investment and trade-off between egg size and clutch size in the Andrew’s Toad (Bufo andrewsi) J. Zool. 2014;293:84–91. doi: 10.1111/jzo.12122. [DOI] [Google Scholar]
- 10.Williams SB. Life history variation in the black swallowtail butterfly. Oecologia. 1981;48:116–122. doi: 10.1007/BF00347956. [DOI] [PubMed] [Google Scholar]
- 11.Stearns SC. Life history evolution: success, limitations, and prospects. Naturwissenschaften. 2000;87:476–486. doi: 10.1007/s001140050763. [DOI] [PubMed] [Google Scholar]
- 12.Roff, D. A. Life-history evolution. (Sunderland, Massachusetts, USA: Sinauer Associates, 2002).
- 13.Reznick DN, Bryga H, Endler JA. Experimentally induced life-history evolution in a natural population. Nature. 1990;346:357–359. doi: 10.1038/346357a0. [DOI] [Google Scholar]
- 14.Blumstein DT, Møller AP. Is sociality associated with high longevity in North American birds? Biol. Lett. 2008;4:146–148. doi: 10.1098/rsbl.2007.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beauchamp G. Group-foraging is not associated with longevity in North American birds. Biol. Lett. 2010;6:42–44. doi: 10.1098/rsbl.2009.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shattuck MR, Williams SA. Arboreality has allowed for the evolution of increased longevity in mammals. Proc. Natl. Acad. Sci. USA. 2010;107:4635–4639. doi: 10.1073/pnas.0911439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valcu M, Dale J, Griesser M, Nakagawa S, Kempenaers B. Global gradients of avian longevity support the classic evolutionary theory of ageing. Ecography. 2014;37:930–938. doi: 10.1111/ecog.00929. [DOI] [Google Scholar]
- 18.Stark G, Tamar K, Itescu Y, Feldman A, Meiri S. Cold and isolated ectotherms: drivers of reptilian longevity. Biol. J. Linn. Soc. 2019;125:730–740. doi: 10.1093/biolinnean/bly153. [DOI] [Google Scholar]
- 19.Wachter, K. W. & Finch, C. E. Between Zeus and the salmon: the biodemography of longevity. (Washington: The National Academies Press, 1997). [PubMed]
- 20.Koopman JJ, Wensink MJ, Rozing MP, van Bodegom D, Westendorp RG. Intrinsic and extrinsic mortality reunited. Exp. Gerontol. 2015;67:48–53. doi: 10.1016/j.exger.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Medawar, P. B. An unsolved problem of biology. (London: H. K. Lewis, 1952).
- 22.Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Proc. R. Soc. B. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- 23.Quinlan RJ. Extrinsic mortality effects on reproductive strategies in a Caribbean community. Hum. Nat. 2010;21:124–139. doi: 10.1007/s12110-010-9085-1. [DOI] [Google Scholar]
- 24.Gaillard JM, et al. An analysis of demographic tactics in birds and mammals. Oikos. 1989;56:59–76. doi: 10.2307/3566088. [DOI] [Google Scholar]
- 25.Yu X, et al. Large-brained frogs mature later and live longer. Evolution. 2018;72:1174–1183. doi: 10.1111/evo.13478. [DOI] [PubMed] [Google Scholar]
- 26.Møller AP. Sociality, age at first reproduction and senescence: comparative analyses of birds. J. Evol. Biol. 2006;19:682–689. doi: 10.1111/j.1420-9101.2005.01065.x. [DOI] [PubMed] [Google Scholar]
- 27.Kamilar JM, Bribiescas RG, Bradley BJ. Is group size related to longevity in mammals? Biol. Lett. 2010;6:736–739. doi: 10.1098/rsbl.2010.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austad SN. Comparative aging and life histories in mammals. Exp. Gerontol. 1997;32:23–38. doi: 10.1016/S0531-5565(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 29.Blanco MA, Sherman PW. Maximum longevities of chemically protected and non-protected fishes, reptiles, and amphibians support evolutionary hypotheses of aging. Mech. Ageing. Dev. 2005;126:794–803. doi: 10.1016/j.mad.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Li ST, et al. Body size variation of Odorous Frog (Odorrana grahami) across altitudinal gradients. Herpetol. J. 2013;23:187–192. [Google Scholar]
- 31.Wilkinson GS, South JM. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124–131. doi: 10.1046/j.1474-9728.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 32.Beauchamp, G. Social predation: How group living benefits predators and prey. (Academic Press, London, UK, 2014).
- 33.Bednekoff PA, Lima SL. Re–examining safety in numbers: interactions between risk dilution and collective detection depend upon predator targeting behaviour. Proc. R. Soc. B. 1998;265:2021–2026. doi: 10.1098/rspb.1998.0535. [DOI] [Google Scholar]
- 34.Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- 35.Hoogland JL, Sherman PW. Advantages and disadvantages of bank swallow (Riparia riparia) coloniality. Ecol. Monogr. 1976;46:33–58. doi: 10.2307/1942393. [DOI] [Google Scholar]
- 36.Krause, J. & Ruxton, G. D. Living in Groups. (Oxford University Press, Oxford, UK, 2002).
- 37.Wiklund CG, Andersson M. Natural selection of colony size in a passerine bird. J. Anim. Ecol. 1994;63:765–774. doi: 10.2307/5254. [DOI] [Google Scholar]
- 38.Arroyo B, Mougeot F, Bretagnolle V. Colonial breeding and nest defence in Montagu’s harrier (Circus pygargus) Behav. Ecol. Sociobiol. 2001;50:109–115. doi: 10.1007/s002650100342. [DOI] [Google Scholar]
- 39.Serrano D, Oro D, Ursua E, Tella JL. Colony size selection determines adult survival and dispersal preferences: allee effects in a colonial bird. Am. Nat. 2005;166:E22–E31. doi: 10.1086/431255. [DOI] [PubMed] [Google Scholar]
- 40.Krams I, Krama T, Igaune K, Mänd R. Experimental evidence of reciprocal altruism in the pied flycatcher. Behav. Ecol. Sociobiol. 2007;62:599–605. doi: 10.1007/s00265-007-0484-1. [DOI] [Google Scholar]
- 41.Fei, L., Ye, C. Y. & Jiang, J. P. Colored atlas of Chinese amphibians. (Sichuan Publishing House of Science and Technology, Chengdu, 2010).
- 42.Jungwirth A, Josi D, Walker J, Taborsky M. Benefits of coloniality: communal defence saves anti-predator effort in cooperative breeders. Funct. Ecol. 2016;29:1218–1224. doi: 10.1111/1365-2435.12430. [DOI] [Google Scholar]
- 43.Andersson, M. Sexual selection. (Princeton University Press, Princeton, 1994).
- 44.Sinsch U, Dehling JM. Tropical anurans mature early and die young: Evidence from eight Afromontane Hyperolius species and a meta-analysis. PLoS One. 2017;12:e0171666. doi: 10.1371/journal.pone.0171666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang LX, Lu X. Amphibians live longer at higher altitudes but not at higher latitudes. Biol. J. Linn. Soc. 2012;106:623–632. doi: 10.1111/j.1095-8312.2012.01876.x. [DOI] [Google Scholar]
- 46.Kotrschal A, et al. Brain size affects female but not male survival under predation threat. Ecol. Lett. 2015;18:646–652. doi: 10.1111/ele.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werner EE, Gilliam JF. The ontogenetic niche and species interactions in size-structured populations. Annu. Rev. Ecol. Syst. 1984;15:393–425. doi: 10.1146/annurev.es.15.110184.002141. [DOI] [Google Scholar]
- 48.Wolff JO, Guthrie RD. Why are aquatic small mammals so large? Oikos. 1985;45:365–373. doi: 10.2307/3565572. [DOI] [Google Scholar]
- 49.Ebensperger LA, Blumstein DT. Sociality in New World hystricognath rodents is linked to predators and burrow digging. Behav. Ecol. 2006;17:410–418. doi: 10.1093/beheco/arj048. [DOI] [Google Scholar]
- 50.Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J. Gerontol. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.B47. [DOI] [PubMed] [Google Scholar]
- 51.Liu YH, Liao WB, Zhou CQ, Mi ZP. Altitudinal variation in body size in the Rice Frog (Rana limnocharis) in southwestern China. Acta Herpetol. 2012;7:57–68. [Google Scholar]
- 52.Liao WB, Zhou CQ, Yang ZS, Hu JC, Lu X. Age, size and growth in two populations of the Dark-Spotted Frog Rana nigromaculata at different altitudes in south-western China. Herpetol. J. 2010;20:77–82. [Google Scholar]
- 53.Liao WB, Lu X, Shen YW, Hu JC. Age structure and body size of two populations of the rice frog Rana limnocharis from different altitudes. Ital. J. Zool. 2011;78:215–221. doi: 10.1080/11250001003639590. [DOI] [Google Scholar]
- 54.Liao WB. Evolution of sexual size dimorphism in a frog obeys the inverse of Rensch’s rule. Evol. Biol. 2013;40:293–299. doi: 10.1007/s11692-012-9212-5. [DOI] [Google Scholar]
- 55.Morrison C, Hero JM. Geographic variation in life-history characteristics of amphibians: a review. J. Anim. Ecol. 2003;72:270–279. doi: 10.1046/j.1365-2656.2003.00696.x. [DOI] [Google Scholar]
- 56.Munshi-South J, Wilkinson GS. Diet influences life span in parrots (Psittaciformes) Auk. 2006;123:108–118. doi: 10.1093/auk/123.1.108. [DOI] [Google Scholar]
- 57.Jullien M, Clobert J. The survival value of flocking in Neotropical birds: reality or fiction? Ecology. 2000;81:3416–3430. doi: 10.1890/0012-9658(2000)081[3416:TSVOFI]2.0.CO;2. [DOI] [Google Scholar]
- 58.Wasser DE, Sherman PW. Avian longevities and their interpretation under evolutionary theories of senescence. J. Zool. 2010;280:103–155. doi: 10.1111/j.1469-7998.2009.00671.x. [DOI] [Google Scholar]
- 59.Taborsky M. Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim. Behav. 1984;32:1236–1252. doi: 10.1016/S0003-3472(84)80241-9. [DOI] [Google Scholar]
- 60.Allman, J. Evolving brains. (Scientific American Library, 2000).
- 61.Gonzalez-Voyer A, Kolm N. Sex, ecology and the brain: evolutionary correlates of brain structure volumes in Tanganyikan cichlids. PLoS ONE. 2010;5:e14355. doi: 10.1371/journal.pone.0014355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kotrschal A, et al. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 2013;23:168–171. doi: 10.1016/j.cub.2012.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buechel SD, Boussard A, Kotrschal A, van der Bijl W, Kolm N. Brain size affects performance in a reversal-learning test. Proc. R. Soc. B. 2018;285:20172031. doi: 10.1098/rspb.2017.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobs LF. Sexual selection and the brain. Trends Ecol. Evol. 1996;11:A82–A86. doi: 10.1016/0169-5347(96)81048-2. [DOI] [PubMed] [Google Scholar]
- 65.Boogert NJ, Fawcett TW, Lefebvre L. Mate choice for cognitive traits: a review of the evidence in nonhuman vertebrates. Behav. Ecol. 2011;22:447–459. doi: 10.1093/beheco/arq173. [DOI] [Google Scholar]
- 66.Miller, G. F. The mating mind: How sexual selection shaped the evolution of human nature. (London: William Hienemann, 2000).
- 67.Corral-López Alberto, Kotrschal Alexander, Kolm Niclas. Selection for relative brain size affects context-dependent male preference for, but not discrimination of, female body size in guppies. The Journal of Experimental Biology. 2018;221(12):jeb175240. doi: 10.1242/jeb.175240. [DOI] [PubMed] [Google Scholar]
- 68.Kokko H, Rankin DJ. Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos. R. Soc. B. 2006;361:319–334. doi: 10.1098/rstb.2005.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lüpold S, Jin L, Liao WB. Population density drives differential investment in pre- and postmating sexual traits in frogs. Evolution. 2017;71:1686–1699. doi: 10.1111/evo.13246. [DOI] [PubMed] [Google Scholar]
- 70.Vincze O. Light enough to travel or wise enough to stay? Brain size evolution and migratory behavior in birds. Evolution. 2016;70:2123–2133. doi: 10.1111/evo.13012. [DOI] [PubMed] [Google Scholar]
- 71.Sayol F, et al. Environmental variation and the evolution of large brains in birds. Nat. Commun. 2016;7:13971. doi: 10.1038/ncomms13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Street SE, Navarrete AF, Reader SM, Laland KN. Coevolution of cultural intelligence, extended life history, sociality, and brain size in primates. Proc. Natl. Acad. Sci. USA. 2017;114:7908–7914. doi: 10.1073/pnas.1620734114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Altizer S, et al. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 2003;34:517–547. doi: 10.1146/annurev.ecolsys.34.030102.151725. [DOI] [Google Scholar]
- 74.Nunn CL, Altizer S, Jones KE, Sechrest W. Comparative tests of parasite species richness in primates. Am. Nat. 2003;162:597–614. doi: 10.1086/378721. [DOI] [PubMed] [Google Scholar]
- 75.Morand S, Harvey PH. Mammalian metabolism, longevity and parasite species richness. Proc. R. Soc. B. 2000;267:1999–2003. doi: 10.1098/rspb.2000.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harvey PH, Clutton-Brock TH. Life history variation in primates. Evolution. 1985;39:559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 77.Bielby J, Mace GM, Bininda-Emonds ORP, Cardillo M, Gittleman JL. The fast-slow continuum in mammalian life history: An empirical reevaluation. Am. Nat. 2007;169:748–757. doi: 10.1086/516847. [DOI] [PubMed] [Google Scholar]
- 78.Liao WB, Lu X. Adult body size = f (initial size + growth rate × age): explaining the proximate cause of Bergman’s cline in a toad along altitudinal gradients. Evol. Ecol. 2012;26:579–590. doi: 10.1007/s10682-011-9501-y. [DOI] [Google Scholar]
- 79.Luo Y, et al. Seasonality and brain size are negatively associated in frogs: evidence for the expensive brain framework. Sci. Rep. 2017;7:16629. doi: 10.1038/s41598-017-16921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minias P, Podlaszczuk P. Longevity is associated with relative brain size in birds. Ecol. Evol. 2017;7:3558–3566. doi: 10.1002/ece3.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Møller AP. Senescence in relation to latitude and migration in birds. J. Evol. Biol. 2007;20:750–757. doi: 10.1111/j.1420-9101.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 82.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;2:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rambaut, A. & Drummond, A. Tracer v1.6, http://tree.bio.ed.ac.uk/software/tracer/ (2014).
- 84.R Development Core Team R. A Language and Environment for Statistical Computing, http://www.R-project.org (2016).
- 85.Orme, C. D. L., Freckleton, R. P., Thomas, G. H., Petzoldt, T. & Fritz, S. A. caper: Comparative analyses of phylogenetics and evolution in R, http://R-Forge.R-project.org/projects/caper/ (2012).
- 86.Lynch M. Methods for the analysis of comparative data in evolutionary biology. Evolution. 1991;45:1065–1080. doi: 10.1111/j.1558-5646.1991.tb04375.x. [DOI] [PubMed] [Google Scholar]
- 87.Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 1999;48:612–622. doi: 10.1080/106351599260184. [DOI] [Google Scholar]
- 88.Freckleton RP, Harvey PH, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- 89.Cai YL, Mai CL, Yu X, Liao WB. Effect of population density on relationship between pre- and postcopulatory sexual traits. Anim. Biol. 2019;69:281–292. doi: 10.1163/15707563-20181057. [DOI] [Google Scholar]
- 90.von Hardenberg AV, Gonzalez-Voyer A. Disentangling evolutionary cause-effect relationships with phylogenetic confirmatory path analysis. Evolution. 2013;67:378–387. doi: 10.1111/j.1558-5646.2012.01790.x. [DOI] [PubMed] [Google Scholar]
- 91.van der Bijl W. phylopath: Easy phylogenetic path analysis in R. PeerJ. 2018;6:e4718. doi: 10.7717/peerj.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.