Abstract
Focal cartilage defects caused by joint injury have a limited capacity to self-repair and, if left untreated, can lead to the early onset of osteoarthritis. The current standard of care, microfracture surgery, induces an endogenous repair response, but typically results in poorly integrated fibrocartilage, rather than native hyaline cartilage. The objective of this study was to test the hypothesis that a self-assembling peptide hydrogel functionalized with the proanabolic growth factor heparin-binding insulin-like growth factor-1 (HB-IGF-1) may improve integration between native cartilage and neotissue when combined with a brief enzymatic pretreatment to the defect site. This enzymatic pretreatment releases proteoglycans from the walls of the surrounding native cartilage in a controlled manner and, thereby, creates space for newly synthesized repair tissue to anchor and integrate with adjacent host cartilage. We used an in vitro model in which a cylindrical annulus of native cartilage was pretreated with trypsin over a 2-min period and then filled with a chondrocyte-seeded [KLDL]3 hydrogel functionalized with proanabolic HB-IGF-1 that had been premixed into the gel. This procedure was deemed to be clinically tractable in the context of ongoing parallel animal studies as a method to augment the microfracture procedure. The trypsin pretreatment depleted proteoglycan content of adjacent cartilage in a controlled manner without inducing cell death. The addition of HB-IGF-1 was found to stimulate matrix biosynthesis both in the surrounding cartilage and the chondrocyte-seeded KLD scaffold, and to enhance mechanical integration of neotissue into native matrix.
Impact Statement
A critical attribute for the long-term success of cartilage defect repair is the strong integration between the repair tissue and the surrounding native tissue. Current approaches utilized by physicians fail to achieve this attribute, leading to eventual relapse of the defect. This article demonstrates the concept of a simple, clinically viable approach for enhancing tissue integration via the combination of a safe, transient enzymatic treatment with a locally delivered, retained growth factor through an in vitro hydrogel/cartilage explant model.
Keywords: cartilage repair, HB-IGF-1, scaffolds, trypsin
Introduction
Focal cartilage defects, often caused by traumatic joint injuries, worsen with time due to the inability of avascular cartilage tissue to self-repair. To overcome this lack of vasculature, microfracture surgery attempts to divert the underlying blood supply and progenitor cells to the defect site by perforating the subchondral bone.1 While this temporarily relieves pain, it commonly results in poorly integrated, fibrotic repair tissue that degrades in 3–5 years due to biological and mechanical discontinuities at the defect/native interface.2,3
To withstand compressive and shear forces and provide a lasting fix, the repair tissue must integrate with underlying bone and surrounding native cartilage and have strong equilibrium modulus, contributing to the integrative potential. Achieving successful integration remains a challenge in neotissue-generating methods such as microfracture surgery and in surgical implantation techniques using allografts, autografts, or engineered tissue constructs.3–6 Lack of integration may be attributed to a variety of factors, including decreased cellularity or cell viability at the defect boundary, and breakdown of mechanically dissimilar neotissue. Also, the dense extracellular matrix (ECM) of the native tissue may hinder cellular ingress from the neotissue. Without a proper integration strategy, micromotions due to joint loading can generate stress concentrations at the defect interface and inhibit repair.7
One strategy toward improving lateral integration between engineered neotissue and surrounding native cartilage is an enzymatic pretreatment to enhance cellularity and anabolic activity of cells at the interface.3,8–12 Studies using collagenase and hyaluronidase pretreatments demonstrated significant extracellular matrix biosynthesis, remodeling, and subsequent enhanced integration,8–10 although other studies have demonstrated that disruption of the collagen network can lead to irreparable damage.13–15 Targeting proteoglycans instead of collagen via a chondroitinase pretreatment has also been shown to increase cellularity at the defect interface in vitro11 and provide a safe method of matrix depletion in an in vivo rabbit model, where no side effects were observed in the treated or contralateral joint.5 Studies using the broader acting enzyme trypsin have also demonstrated a capacity to enhance integration without harmful side effects.11,16,17
To support the growth of hyaline repair tissue and ultimately tissue integration, scaffold and growth factor (GF) combinations can be implanted into the defect site. The scaffold environment determines the ability of cells to differentiate and produce hyaline cartilage, rather than fibrocartilage. In vitro and in vivo studies have shown that augmenting microfracture through the addition of cell-free scaffolds functionalized with GFs may help foster chondrogenesis of endogenous progenitor cells, neotissue production by these cells, and subsequent integration of neotissue with host tissue.17–25
Self-assembling peptide hydrogels, such as [RADA]4, [KLDL]3, and peptide-amphiphile nanofibers, which assemble on exposure to physiological pH and ionic strength,26,27 have been shown to foster chondrogenesis of bone marrow progenitor cells,28,29 matrix production by primary chondrocytes in vitro,23,30 and enhanced mechanical integration as measured by a push-out test,31 as well as a biocompatible environment for repair in vivo.24,29,32
Recent studies have also shown that the addition of a single dose of the anabolic fusion protein heparin-binding insulin-like growth factor-1 (HB-IGF-1)33 to chondrocyte-seeded RAD is retained in the hydrogel and enhances matrix biosynthesis compared with the cell-seeded scaffold alone or with a single dose of regular insulin-like growth factor-1 (IGF-1).23 Cartilage tissue also retains HB-IGF-1 compared with IGF-1 if delivered by RAD in vitro23 and intra-articularly injected in vivo.34 We hypothesize that this sustained delivery of HB-IGF-1 to cartilage may stimulate improved integration through enhanced matrix biosynthesis of cells at the defect periphery.
In the present study, we hypothesized that a controllable trypsin pretreatment combined with a single dose of HB-IGF-1 administered in a hydrogel may enhance integration between neotissue and native tissue through proteoglycan depletion and subsequent matrix biosynthesis in an augmented microfracture approach. To test this hypothesis, we (1) developed a clinically relevant trypsin pretreatment strategy, (2) characterized the ability of a trypsin pretreatment combined with HB-IGF-1 to stimulate matrix biosynthesis in an in vitro cartilage defect model, and (3) examined the impact of this combination on integration through histology and mechanical testing.
Methods
Materials
The self-assembling peptide hydrogel, [KLDL]3, with the sequence AcN-(KLDL)3-CNH2, hereafter known as KLD, was donated by 3-D Matrix (Waltham, MA). KLD hydrogels were prepared at a final concentration of 0.35% (w/v) in 10% sterile sucrose for all experiments.28,35 HB-IGF-1 was provided by Dr. Richard Lee (Brigham and Women's Hospital, Boston, MA).
Cartilage explant harvest
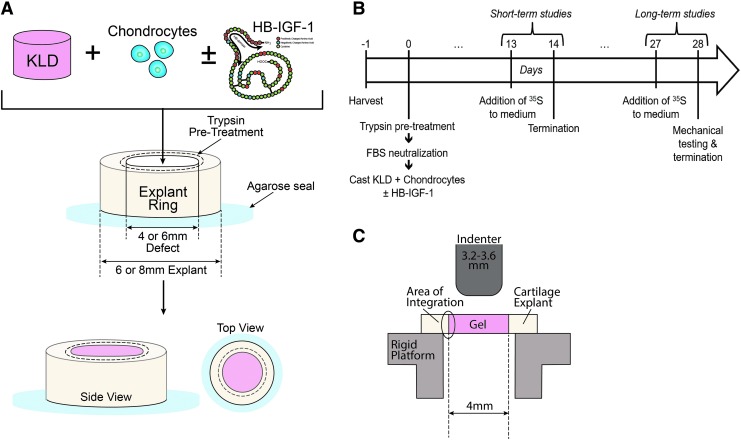
Middle-to-deep-zone cartilage explant disks (8 or 6 mm diameter, 1.4 mm thick) were obtained from the femoropatellar grooves of immature bovine calves (Research 87, Boylston, MA) using a biopsy punch. Six and 4 mm concentric circular holes were punched in the 8 and 6 mm disks, respectively, to create annuli of cartilage tissue similar to previous studies17,20,22,36 (Fig. 1A).
FIG. 1.
In vitro cartilage defect model. (A) Trypsin pretreatment: cartilage explant disks 6 mm (or 8 mm) in diameter were cored with a 4 mm (or 6 mm) dermal punch to form annuli and sealed to the bottom of a 24-well plate. The inner surface of the annuli was treated with trypsin, neutralized with FBS, and rinsed with PBS. Hydrogel/explant construct: KLD self-assembling peptide was cast into cartilage annuli with or without trypsin pretreatment to the tissue. To assess the release of HB-IGF-1 from the construct, HB-IGF-1 alone was premixed into KLD before casting. To assess neotissue production and integration, chondrocyte-seeded KLD was premixed with and without HB-IGF-1 before casting. Schematic of HB-IGF-1 molecular configuration adapted from Tokunou et al.33 (B) Time line of long-term and short-term studies. (C) A bevel-edged cylindrical indenter (3.2–3.6 mm in diameter) was used to shear neotissue cores from constructs placed on a rigid platform with an opening below the core. The peak failure force was normalized to the interfacial surface area to determine the failure stress. FBS, fetal bovine serum; HB-IGF-1, heparin-binding insulin-like growth factor-1; PBS, phosphate-buffered saline.
These explant annuli were equilibrated for 24–48 h in serum-free, basal medium consisting of high-glucose Dulbecco's modified Eagle's medium (Mediatech, Inc., Manassas, VA) supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin (PSA), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Invitrogen, Carlsbad, CA), 0.4 mM proline, 20 μg/mL ascorbate, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids (Sigma-Aldrich). Annuli with an 8 mm outer diameter were used for enzyme depletion characterization (Supplementary Fig. S1) and short-term (≤15 days) in vitro culture models, while 6 mm outer diameter annuli were used for long-term (4 weeks) in vitro culture models.
Chondrocyte isolation
Chondrocytes were harvested from the same immature bovine calves from which the cartilage explants were harvested. Cartilage tissue from the femoral condyles was collected aseptically and digested with proteinase for 1 h and then with collagenase overnight.30,37 The resulting cells were then encapsulated in KLD for culture on the same day, as described below.
Controlled depletion of sulfated glycosaminoglycan
Following equilibration in basal medium, the annuli were sealed to the bottom of a 24-well plate using 3% agarose.38 Fifty microliters of 50 μg/mL39 trypsin derived from bovine pancreas (Sigma-Aldrich) in phosphate-buffered saline (PBS; pH 7.4) was applied to the center of the annulus for 2 min, aspirated, and followed by a 2-min application of 50 μL of fetal bovine serum (FBS; Thermo Scientific, Logan, UT) to neutralize the trypsin. The annulus was then thoroughly flushed with PBS and transferred to basal medium before further use. This trypsin pretreatment dose and duration were chosen for subsequent culture studies after extensive characterization (see the Results section and trypsin dose/time responses in Supplementary Fig. S1).
In vitro integration model
To test the impact of the trypsin pretreatment and HB-IGF-1-loaded KLD on neotissue/host tissue integration, KLD premixed with 30 × 106 chondrocytes/mL, with or without 615 nM HB-IGF-1, was cast into cartilage annuli (8 mm outer diameter) that had either been pretreated with trypsin as previously described or left untreated. These four conditions of constructs—untreated control, trypsin pretreatment alone, HB-IGF-1 alone, and HB-IGF-1 plus trypsin pretreatment, were cultured in basal medium. After 15 days (short-term culture), the rate of sulfated glycosaminoglycan (sGAG) synthesis and the overall sGAG content were measured to assess basal tissue growth in the defect model.
To enhance macro-scale matrix production sufficient for mechanical testing, a separate set of constructs was cultured in ITS/FBS medium (basal medium +1% ITS+Premix (BD Biosciences, San Jose, CA) and 0.2% FBS40) for 4 weeks (long-term culture). To overcome technical challenges due to swelling and warping of constructs during the long-term culture, the smaller cartilage annuli (6 mm outer diameter) were used, and constructs were allowed to rest freely in the well of a six-well plate (n = 2–4 per well) instead of seating with agarose. The same four conditions as in the short-term culture experiments were tested (and the same tissue growth assays were performed), with only 20 μL of volume needed for trypsin pretreatment.
Assessment of cell migration
To assess whether the trypsin pretreatment enhances migration of KLD-encapsulated chondrocytes into explant annuli or explant chondrocytes into KLD cores, each cell source was stained with a distinct fluorescent dye and constructs were analyzed by confocal microscopy after 12 days of culture. Briefly, explant annuli were submerged in 20 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Thermo Fisher), at 37°C for 2 h, rinsed with PBS, and washed twice with medium containing 10% FBS for 30 min. Isolated chondrocytes were stained with PKH26 (Sigma) at a concentration of 10 μM.41 Explant annuli stained with CFSE were then exposed to the trypsin pretreatment protocol described above (or left untreated as a control), and PKH26-stained chondrocytes were then encapsulated in KLD with no added HB-IGF-1 and cast into the annuli cores, as above. Confocal images of the constructs were obtained using 488 and 594 nm excitation lasers following 12 days of culture in ITS/FBS medium.
Viability staining and histology
To assess the viability of the annuli and KLD-encapsulated chondrocytes, sections of the explant and gels were stained with 4 μg/mL fluorescein diacetate (FDA; live cells) and 35 μg/mL propidium iodide (PI; dead cells). The sections were viewed using a Nikon Eclipse TE-300 fluorescence microscope at the commencement and completion of culture. Additional samples fixed in 10% neutral-buffered formalin were prepared for histological assessment. Samples were stained with toluidine blue to visualize GAG content,28 or examined for collagen type II content by immunohistochemistry, as described previously.28
Analysis of matrix production and cell proliferation after short- and long-term culture
One day before the end of short-term and long-term cultures, 5 μCi of 35S-sulfate (Perkin Elmer, Waltham, MA) was added to the medium to enable assessment of sGAG synthesis rates.42 At the end of the cultures, cartilage annuli and neotissue cores were separated and digested overnight in 0.1 mg/mL proteinase-K (Roche Applied Science, Indianapolis, IN).28 Total sGAG accumulation was measured by DMMB dye binding, and DNA content was measured by Hoechst dye binding, as described previously.43–45 Total sGAG accumulation and 35S-incorporation rates were each normalized to the maximum value for their respective animal before data were pooled among animals.
Mechanical assessment of neotissue/host tissue integration
Before digestion for biochemical analysis, the strength of integration between the hydrogel-neotissue core and the cartilage explants following 4 weeks of culture was measured using a push-out test.17,20,22,36 The hydrogel-neotissue was pushed out from the explant with a bevel-edge cylindrical indenter, while the cartilage annulus was supported on a rigid, annular platform with a 4 mm inner diameter (Fig. 1C). Indenters of diameters ranging from 3.2 to −3.6 mm were selected on a per-construct basis to fit the final inner diameter of the construct as closely as possible, due to swelling and growth of the samples over the extended culture time.
The tests were performed using a Dynastat mechanical spectrometer (IMASS, Hingham, MA) by driving the push-out rod on top of the hydrogel disc through the cartilage explant at 10 μm/s, with displacement and load monitored at a sample frequency of 20 Hz. The maximum force achieved before separation of the tissues was normalized by the lateral area of the core, with the resulting value considered representative of the interfacial strength.20
Statistical analyses
Values are reported as mean ± standard error of the mean. Comparisons use general linear mixed effects models with animal as a random variable and Tukey's HSD post hoc test (p < 0.05; JMP 11; SAS, Inc., Cary, NC).
Results
Trypsin pretreatment induces controlled removal of sGAG from medial surface of cartilage annuli
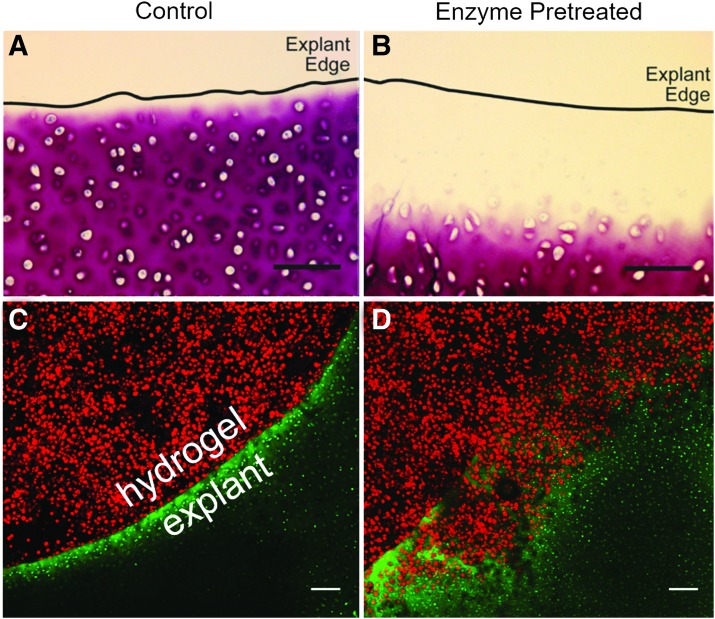
Histomorphological analysis of the cartilage annuli subjected to trypsin pretreatment indicated that GAG content could be selectively removed from the edge of the defect (Fig. 2A, B). Control annuli that were not exposed to trypsin showed no signs of GAG depletion. At 50 and 500 μg/mL (Supplementary Fig. S1), trypsin induced sGAG depletion that increased with longer incubation times, with faster kinetics at 500 μg/mL than 50 μg/mL. After 2 min, 50 μg/mL of trypsin consistently depleted ∼200 μm of sGAG, and was selected as our final trypsin pretreatment protocol for further studies.
FIG. 2.
Trypsin pretreatment creates space for cell invasion. (A) Control and (B) trypsin pretreated cartilage annuli stained with toluidine blue show clearance of proteoglycan content on trypsin pretreatment. (C, D) Intratissue chondrocytes of cartilage annuli were stained with CFSE (green) and isolated chondrocytes were stained with PKH26 (red) before KLD encapsulation and casting into cartilage annuli. (C) No cells from the KLD core invaded the untreated control explant annuli, nor did any intratissue chondrocytes depart the untreated control cartilage annuli for the KLD annuli following 12 days of culture. (D) Proteoglycan content of cartilage annuli subjected to 2 min of 50 μg/mL trypsin pretreatment was sufficiently depleted at the annuli interface to allow invasion of chondrocytes from the KLD core (red cells) by 12 days of culture, while chondrocytes initially in the cartilage explant (green) remained in the cartilage tissue. Scale bars = 100 μm. CFSE, carboxyfluorescein diacetate succinimidyl ester.
Trypsin pretreatment stimulates migration of KLD-encapsulated cells into surrounding cartilage annuli
Intratissue chondrocytes and KLD-encapsulated chondrocytes were stained with the fluorescent dyes CFSE (green) and PKH26 (red), respectively, to determine if the trypsin pretreatment impacted the ability of cells to cross the defect interface. Confocal analysis of constructs cultured for 12 days showed no translocation of either cell source across the interface in the nontrypsin-treated control (Fig. 2C). In the trypsin pretreated constructs, however, cells originally encapsulated in KLD (red) penetrated the explant annuli after 12 days, although chondrocytes initially in cartilage (green) remained in the annuli (Fig. 2D). This suggests that proteoglycan depletion by the trypsin created space for hydrogel-encapsulated cells to infiltrate the cartilage tissue. However, chondrocytes initially in cartilage remained encapsulated within their pericellular and ECM and were not yet able to leave the explant by 12 days.
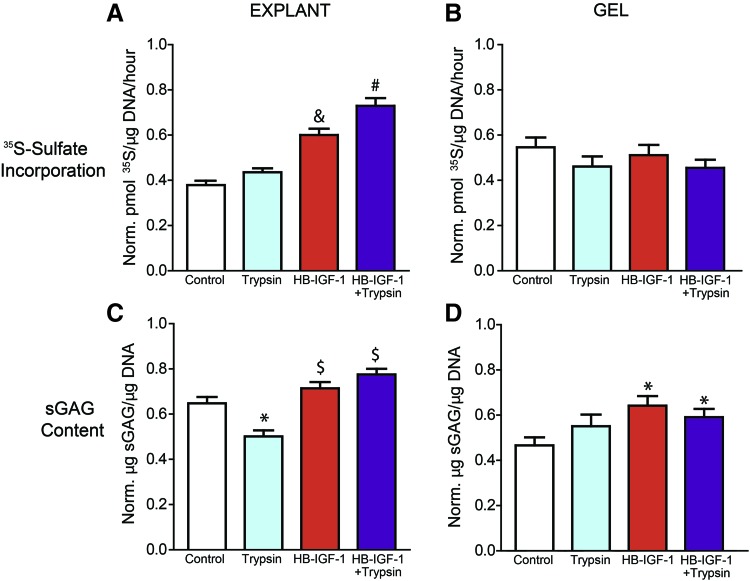
Premixed HB-IGF-1 and trypsin pretreatment increased GAG content in chondrocyte-seeded hydrogel cores and increased biosynthesis in cartilage annuli over 15 days in basal medium
Chondrocyte-seeded, KLD hydrogels were cast into the center of cartilage annuli to investigate the capacity of the combination of trypsin pretreatment and a single dose of HB-IGF-1 premixed into KLD to stimulate matrix deposition. Following 15 days of culture, cartilage annuli showed a significant increase in sulfated proteoglycan synthesis rate in constructs that had received HB-IGF-1 compared with those that had not, as well as a difference between those that had received the combination of HB-IGF-1+trypsin pretreatment compared with those receiving only HB-IGF-1 (Fig. 3A). Cartilage annuli exposed to trypsin pretreatment alone showed a decrease in overall sGAG content compared with the untreated control (as expected, since trypsin removed sGAG), although the addition of HB-IGF-1 restored cartilage annulus sGAG content to that of untreated controls (Fig. 3C).
FIG. 3.
A single dose of HB-IGF-1 premixed into KLD stimulates proteoglycan biosynthesis in explant annuli and GAG deposition in hydrogel cores over 15 days of culture in basal medium. Chondrocyte-seeded KLD hydrogels, premixed with or without HB-IGF-1 and cast into cartilage annuli with or without trypsin pretreatment, were separated from their corresponding explant at day 15 of culture following 1 day of 35S supplementation of the medium. Explants and gels were digested in proteinase K and separately assessed for proteoglycan synthesis rates via 35S incorporation (A, B) and sGAG deposition via DMMB (C, D). N = 3 animals, n = 6–8 per animal. *Indicates significantly different from the control group. $Indicates significantly different from the trypsin group. &,#Indicate significantly different from all other groups. sGAG, sulfated glycosaminoglycan. Color images are available online.
Biochemical analysis of KLD-neotissue cores showed no differences in 35S-incorporation rate between conditions (Fig. 3B), but did show significantly higher sGAG deposition in constructs that received HB-IGF-1 compared with those that did not (Fig. 3D). Additional experiments using acellular KLD showed that a significant portion of the HB-IGF-1 premixed into the scaffold was delivered laterally to the cartilage annuli (Supplementary Fig. S2).
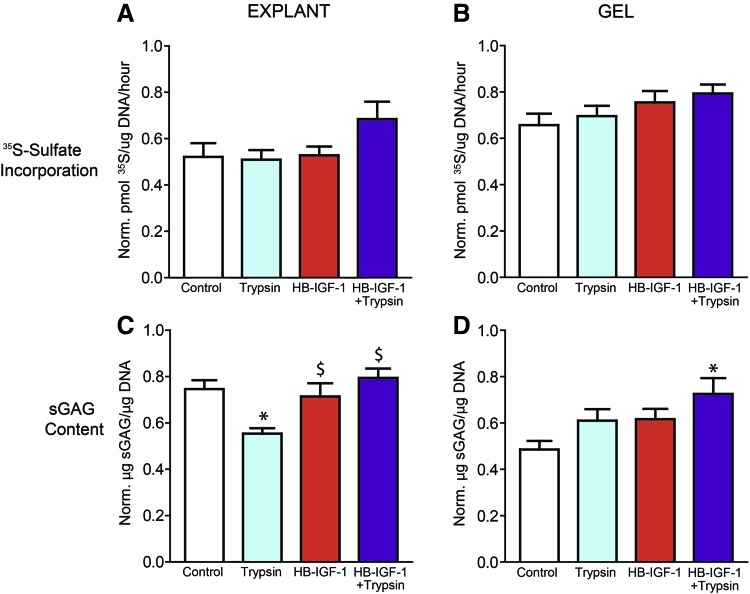
HB-IGF-1 stimulates GAG deposition in chondrocyte-seeded KLD neotissue cores and surrounding cartilage annuli over 4 weeks
To quantify the integration strength between the cartilage annuli and the neotissue core via mechanical push-out tests after long-term culture, constructs were maintained in ITS/FBS medium for 4 weeks to increase neotissue production.40 While live/dead staining revealed dead cells at the cut edges of explant annuli, as previously shown by Redman et al.,46 we observed that the complete tissue cross-section showed high cell viability in all treatment conditions at 4 weeks, indicating that trypsin-pretreatment did not induce cell death (Supplementary Fig. S3).
HB-IGF-1 restored the trypsin-induced sGAG depletion in the cartilage annuli following trends observed at 15 days in basal medium (Fig. 4C), although no significant differences were observed in the sulfated proteoglycan synthesis rate between conditions at 4 weeks (Fig. 4A). This suggests that by this time point, the effect of HB-IGF-1 had subsided. Biochemical analysis of KLD-neotissue cores showed no differences in 35S-incorporation rate between conditions at 4 weeks (Fig. 4B), but it did show significantly higher sGAG deposition between conditions that received HB-IGF-1+trypsin pretreatment compared with the untreated control (Fig. 4D). These results demonstrate that the initial effects of HB-IGF-1 and trypsin pretreatment were still observable after 4 weeks of culture in an enhanced medium.
FIG. 4.
A single dose of HB-IGF-1 premixed into KLD GAG deposition in explant annuli and hydrogel cores over 4 weeks of culture in ITS/FBS medium. Chondrocyte-seeded KLD hydrogels, premixed with or without HB-IGF-1 and cast into cartilage annuli with or without trypsin pretreatment, were separated from their corresponding explant following 4 weeks of culture with 35S added to the medium for the final day. Explants and gels were digested in proteinase K and separately assessed for proteoglycan synthesis rates via 35S incorporation (A, B), and sGAG deposition via DMMB (C, D). N = 2 animals, n = 4–6 per animal. *Indicates significantly different from the control group. $Indicates significantly different from the trypsin group. Color images are available online.
HB-IGF-1 stimulates integration of chondrocyte-seeded KLD neotissue cores and surrounding cartilage annuli over 4 weeks by histological and mechanical assessment
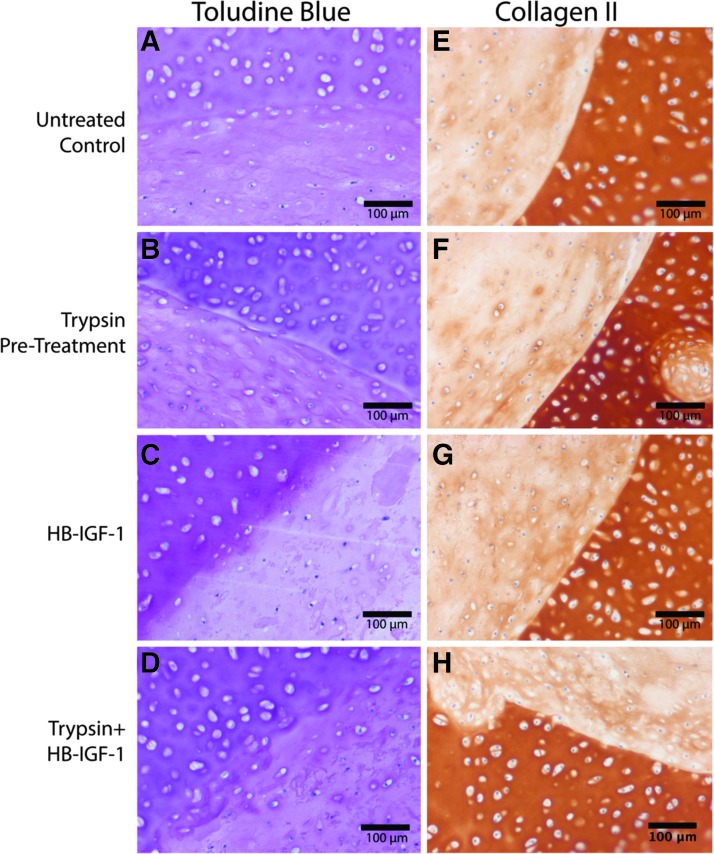
Histological assessment of constructs cultured in ITS/FBS medium for 4 weeks revealed a clear recovery of trypsin-depleted proteoglycan content with the addition of HB-IGF-1 (Fig. 5B, D), while untreated control samples showed a clear demarcation of the explant/core interface with little trans-interfacial staining (Fig. 5A). Constructs treated with both trypsin and HB-IGF-1 (Fig. 5D) showed a clear recovery of the proteoglycan content lost due to trypsin pretreatment alone (Fig. 5B), and the highest degree of trans-interfacial staining of toluidine blue. Collagen type II immunohistochemistry of constructs cultured in ITS/FBS medium for 4 weeks showed little variance between conditions (Fig. 5E–H). All conditions showed a sharp interface between the explant annuli and hydrogel core, indicating that the trypsin pretreatment did not disrupt the collagen network of the native tissue.
FIG. 5.
The combination of HB-IGF-1 and trypsin pretreatment enhances toluidine blue staining across the explant annuli/hydrogel interface following 4 weeks of culture in ITS/FBS medium. Chondrocyte-seeded KLD hydrogels, premixed with or without HB-IGF-1 and cast into cartilage annuli with or without trypsin pretreatment, were fixed in formalin and processed for toluidine blue staining (A–D) and collagen type II immunohistochemistry (E–H) as described in the Methods section. The bulge into and hole in the explant in (F, H), respectively, are due to the previous existence of a blood vessel in the native tissue.
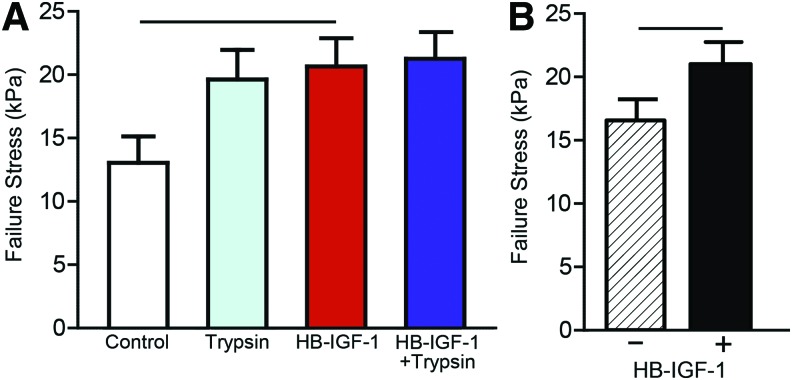
Mechanical push-out tests to assess integration between neotissue cores and surrounding cartilage annuli after 4 weeks in ITS/FBS were performed as shown in Figure 6A. Overall values of failure stress were higher for these constructs than those cultured for 15 days in basal medium (data not shown). Push-out tests showed a statistically significant effect of HB-IGF-1 on integration strength but did not show differences between specific groups (Fig. 6B and Table 1). These results suggest that delivery of HB-IGF-1 via KLD is critical to enhancing mechanical integration in our in vitro model.
FIG. 6.
HB-IGF-1 stimulates enhanced mechanical integration. Groups receiving HB-IGF-1 had a significantly higher failure stress than groups that did not (p = 0.0430) (A), and the HB-IGF-1-alone group had significantly higher failure stress than the control group (p = 0.0480) (B). See Figure 1C for schematic. Color images are available online.
Table 1.
Push-Out Test p-Values of All Pairwise Comparisons from a Tukey Post Hoc Test Following a Linear Mixed Effects Model with Animal as a Random Variable
| Condition | Control | Trypsin | HB-IGF-1 | HB + T |
|---|---|---|---|---|
| Control | — | |||
| Trypsin | 0.1098 | — | ||
| HB-IGF-1 | 0.0480 | 0.9901 | — | |
| HB + T | 0.0755 | 0.9786 | 0.9992 | — |
HB-IGF-1, heparin-binding insulin-like growth factor-1.
Discussion
Therapeutic techniques to stimulate cartilage regeneration, including microfracture surgery, the current standard of care, often yield poor lateral integration between neotissue at the repair site and the surrounding native cartilage.1,47 Efforts have been made to engineer improved repair using GF-functionalized scaffolds to stimulate tissue growth, or enzymatic roughening of the defect wall to enhance integration,3,47–49 but neither has been successful alone. Here, motivated by an augmented microfracture strategy for cartilage repair, we tested the effects of the proanabolic GF HB-IGF-1 and a trypsin pretreatment, separately and in combination, on integration between native cartilage and neotissue produced in a chondrocyte-seeded KLD hydrogel. We demonstrated that a single, premixed dose of HB-IGF-1 in KLD stimulates matrix biosynthesis, both in the surrounding cartilage and the hydrogel scaffold, over both short-term and long-term cultures (Figs. 3 and 4). Furthermore, mechanical assessment via push-out tests showed that HB-IGF-1 increased integration strength compared with conditions that did not receive the GF (Fig. 6). Taken together, these data suggest that a single dose of HB-IGF-1 premixed into KLD can be delivered to surrounding cartilage where it stimulates improved lateral integration via matrix biosynthesis, and that such biosynthesis is complemented by the addition of a trypsin pretreatment.
A variety of enzyme pretreatments have been studied for their impact on cartilage repair integration, including, but not limited to, chondroitinase,11,13,50 hyaluronidase,36 and trypsin.11,16 They have been shown to increase cell adhesion to native tissue11,12,16,50–52 and cell density at the defect edge9,36,53 without negatively affecting proteoglycan deposition.5 A critical aspect of implementing an enzymatic pretreatment is controlling the intended degradation, as irreparable structural damage can occur if molecules such as collagen are disrupted.13–15 Chondroitinase is highly targeted, as it restricts degradation to chondroitin sulfate (CS) GAG chains. While it has a very specific target, the duration of chondroitinase exposure to tissue is difficult to control since it cannot be neutralized. Furthermore, at a high molecular weight of 120 kDa, its size limits its ability to diffuse through tissue. The more broadly acting serine protease, trypsin, is only 23 kDa in size, and cleaves at the C-terminal side of lysines and arginines. As this cleavage is more nonspecific than chondroitinase, it will generally deplete both GAG-containing proteoglycans, but importantly, bovine trypsin (unlike human trypsin-2) will leave collagen intact.54
Another key advantage of trypsin is that it can be neutralized by serum, so the duration of the enzyme exposure to the tissue is much more controllable. Taken together, trypsin's small size and broad enzymatic activity allow it to rapidly clear out ECM proteoglycans yet leave the native collagen network intact, while its ability to be neutralized by serum provides a high level of control for in vitro and potential clinical application. The results presented here demonstrate that cartilage exposed to 2 min of a 50 μg/mL dose of bovine trypsin followed by 2 min of serum neutralization resulted in an ∼200 μm of at least partial sGAG depletion. This short-duration treatment lends itself to clinical translation.
Confocal images of cartilage/hydrogel constructs at 12 days also clearly showed that this trypsin pretreatment created space for cells to traverse the defect interface from the hydrogel into the native cartilage (Fig. 2). The addition of HB-IGF-1 to the KLD may further promote cell migration of neotissue cells into the native cartilage. Overall, endogenous cells in an augmented microfracture setting may have the ability to cross the interface of defects subjected to the trypsin pretreatment, potentially stimulating enhanced integration.
The pronounced effect of trypsin and HB-IGF-1 on sulfated proteoglycan synthesis rate and deposition over both short-term and long-term culture provide insight into their role in enhancing neotissue/native tissue integration. As hypothesized, cartilage annuli exposed only to a trypsin pretreatment showed reduced sGAG content over both 15 days and 4 weeks in culture. The addition of a single premixed dose of HB-IGF-1 to the KLD core in the HB-IGF-1+trypsin condition, however, was capable of stimulating a full recovery of overall sGAG content in the cartilage annuli to the level of the untreated control (Figs. 3C and 4C). This result demonstrates that the HB-IGF-1 delivered to the cartilage annuli from KLD (Supplementary Fig. S2) stimulates the anabolic growth of a new matrix that had been depleted of sGAGs by a trypsin pretreatment.
Further insight into the kinetics of this process can be inferred when comparing the short-term and long-term cultures. The increased 35S-incorporation rate at 15 days (Fig. 3A) but not 4 weeks (Fig. 4A) in cartilage annuli exposed to HB-IGF-1 indicates that GF's ability to restore sGAG content lost during trypsin pretreatment (Figs. 3C and 4C) occurs at earlier time points rather than later ones.
While a high percentage of the HB-IGF-1 in the system was delivered to the surrounding cartilage annuli, it still had a measurable effect on the matrix production within the chondrocyte-seeded KLD hydrogel cores. Although 35S-incorporation rate in the hydrogel core did not vary among conditions at 15 days or 4 weeks, conditions, including HB-IGF-1, had a significantly higher amount of sGAG deposition (Figs. 3D and 4D). These data suggest that a single dose of HB-IGF-1, premixed into a chondrocyte-seeded KLD hydrogel and cast into a cartilage annulus, has a positive effect on matrix synthesis and accumulation within the hydrogel core, but it likely occurs before the 15-day time point. This is consistent with previous work demonstrating the ability of a single, premixed dose of HB-IGF-1 in a chondrocyte-seeded RAD hydrogel to stimulate matrix production over 6 and 10 days.23 Importantly, the trypsin pretreatment had no negative effects on the system in terms of matrix biosynthesis (Figs. 3 and 4) or cell viability (Supplementary Fig. S3).
Histological assessment of constructs cultured for 4 weeks in the ITS/FBS medium did not demonstrate significant visual differences between conditions. A slight increase in toluidine blue staining across the interface under the trypsin+HB-IGF-1 condition was observed, although little difference in collagen type II deposition was seen (Fig. 5). While overall histological staining for proteoglycan content and type II collagen in the hydrogel core was significantly lower than the surrounding native tissue, this early time result has been previously observed in a similar in vitro defect model that demonstrated good mechanical integration.22 Furthermore, as these constructs were only cultured for 4 weeks, compared with months-long development process of the native tissue, weaker histological staining for matrix components in the hydrogel core compared with the native tissue is reasonable. It is also important to note that these studies were performed using tissue from juvenile tissue and tissue from adult animals may yield a different response.
The impact of HB-IGF-1 and trypsin on matrix biosynthesis provides insight into the underlying mechanism of cartilage defect repair, but successful integration of neotissue with native tissue can only truly be assessed with mechanical testing. The push-out test configuration and analysis implemented in the present study have been used in previous studies (Fig. 6A).17,20,22,36,55 The results presented here demonstrate that HB-IGF-1 induced significantly improved integration at 4 weeks compared with GF-free conditions, as measured by failure stress (Fig. 6B). The magnitudes of the failure stresses are also comparable with those of previous studies conducted for 3 weeks55 and 6 weeks.22
While the effect of combining a trypsin pretreatment with HB-IGF-1 did not reveal an increased level of mechanical integration in these studies, it did have a positive effect on early matrix biosynthesis, and it did not decrease mechanical integration strength. Previous in vivo studies have clearly demonstrated that acellular or cell-seeded hydrogels alone implanted into cartilage defects do not integrate well.24,32,47,56,57 Thus, since there was a positive early matrix biosynthesis and no negative effect in our in vitro model attributable to trypsin, we believe combining trypsin pretreatment remains a viable prointegrative approach that may be revealed in longer term in vivo studies.
While beyond the scope of the work presented here, two such studies are indeed now underway utilizing our previously developed 12-week rabbit defect model24 and a 1-year equine defect model,32 but now incorporating trypsin pretreatment combined with KLD-HB-IGF-1 defect fill. As components of native synovial fluid may neutralize the enzymatic effect of trypsin, it is important to note that such clinically relevant in vivo studies include a brief preflush with saline. In addition, the concentration of HB-IGF-1 utilized here (615 nM) is approximately 30–600 times higher than the reported synovial fluid concentrations of IGF-1,58 providing a window that may be large enough to see a detectable effect of very local delivery to immediately adjacent tissue for integration in vivo.
Conclusion
This work has demonstrated that a single, premixed dose of HB-IGF-1 in a chondrocyte-seeded KLD hydrogel, in combination with a trypsin pretreatment, improves neotissue/native tissue integration in an in vitro cartilage model. This novel approach of combining an enzymatic pretreatment with a proanabolic GF that is retained over time has generated significant progress toward a viable integrative repair strategy. This approach is currently being applied in vivo in an animal model of augmented microfracture.
Supplementary Material
Acknowledgments
Research funded by NIH-NIAMS Grant AR060331. HB-IGF-1 kindly provided by Dr. Richard Lee (Brigham and Women's Hospital). The authors thank Dr. Parth Patwari for discussion on HB-IGF-1. KLD kindly donated by 3-D Matrix.
Disclosure Statement
Dr. Grodzinsky has equity in 3-D Matrix, Ltd., Japan.
Supplementary Material
References
- 1. Steadman J.R., Briggs K.K., Rodrigo J.J., Kocher M.S., Gill T.J., and Rodkey W.G. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 19, 477, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Stanish W.D., McCormack R., Forriol F., et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am 95, 1640, 2013 [DOI] [PubMed] [Google Scholar]
- 3. Khan I.M., Gilbert S.J., Singhrao S.K., Duance V.C., and Archer C.W. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater 16, 26, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Pabbruwe M.B., Esfandiari E., Kafienah W., Tarlton J.F., and Hollander A.P. Induction of cartilage integration by a chondrocyte/collagen-scaffold implant. Biomaterials 30, 4277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quinn T.M., and Hunziker E.B. Controlled enzymatic matrix degradation for integrative cartilage repair: effects on viable cell density and proteoglycan deposition. Tissue Eng 8, 799, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Hoemann C.D., Sun J., McKee M.D., et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage 15, 78, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Guo H., Chen T., Warren R.F., and Maher S.A. Inhomogeneous aggregate modulus of the native cartilage causes micromotion between native and tissue-engineered cartilage under loading. Trans. 60th Orthopaedic Res Soc, New Orleans, LA, March 15–18, 2014 [Google Scholar]
- 8. Riley K.N., and Herman I.M. Collagenase promotes the cellular responses to injury and wound healing in vivo. J Burns Wounds 4, 112, 2005 [PMC free article] [PubMed] [Google Scholar]
- 9. Bos P.K., DeGroot J., Budde M., Verhaar J.A., and van Osch G.J. Specific enzymatic treatment of bovine and human articular cartilage: implications for integrative cartilage repair. Arthritis Rheum 46, 976, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Lee D.A., Bentley G., and Archer C.W. Proteoglycan depletion alone is not sufficient to stimulate proteoglycan synthesis in cultured bovine cartilage explants. Osteoarthritis Cartilage 2, 175, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Hunziker E.B., Kapfinger E., and Müller M.E. Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells. J Bone Joint Surg Br 80, 144, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Lee M.C., Sung K.L., Kurtis M.S., Akeson W.H., and Sah R.L. Adhesive force of chondrocytes to cartilage. Effects of chondroitinase ABC. Clin Orthop Relat Res 370, 286, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Buckwalter J.A., Mankin H.J., and Grodzinsky A.J. Articular cartilage and osteoarthritis. Instr Course Lect 54, 465, 2005 [PubMed] [Google Scholar]
- 14. Grenier S., Bhargava M.M., and Torzilli P.A. An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. J Biomech 47, 645, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iqbal J., Bird J.L., Hollander A.P., and Bayliss M.T. Effect of matrix depleting agents on the expression of chondrocyte metabolism by equine chondrocytes. Res Vet Sci 77, 249, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hunziker E.B., and Rosenberg L.C. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am 78, 721, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Obradovic B., Martin I., Padera R.F., Treppo S., Freed L.E., and Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res 19, 1089, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Yu Y., Brouillette M.J., Seol D., Zheng H., Buckwalter J.A., and Martin J.A. Use of recombinant human stromal cell-derived factor 1α-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol 67, 1274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisher M.B., Henning E.A., Söegaard N.B., Dodge G.R., Steinberg D.R., and Mauck R.L. Maximizing cartilage formation and integration via a trajectory-based tissue engineering approach. Biomaterials 35, 2140, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunter C.J., and Levenston M.E. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng 10, 736, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Tognana E., Chen F., Padera R.F., et al. Adjacent tissues (cartilage, bone) affect the functional integration of engineered calf cartilage. Osteoarthritis Cartilage 13, 129, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Vinardell T., Thorpe S.D., Buckley C.T., and Kelly D.J. Chondrogenesis and integration of mesenchymal stem cells within an in vitro cartilage defect repair model. Ann Biomed Eng 37, 2556, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Florine E.M., Miller R.E., Liebesny P.H., et al. Delivering heparin-binding insulin-like growth factor 1 with self-assembling peptide hydrogels. Tissue Eng Part A 21, 637, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller R.E., Grodzinsky A.J., Vanderploeg E.J., et al. Effect of self-assembling peptide, chondrogenic factors, and bone marrow-derived stromal cells on osteochondral repair. Osteoarthritis Cartilage 18, 1608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Im G.I. Endogenous cartilage repair by recruitment of stem cells. Tissue Eng Part B Rev 22, 160, 2016 [DOI] [PubMed] [Google Scholar]
- 26. Caplan M.R., Schwartzfarb E.M., Zhang S., Kamm R.D., and Lauffenburger D.A. Control of self-assembling oligopeptide matrix formation through systematic variation of amino acid sequence. Biomaterials 23, 219, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Hartgerink J.D., Beniash E., and Stupp S.I. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci U S A 99, 5133, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kopesky P.W., Vanderploeg E.J., Sandy J.S., Kurz B., and Grodzinsky A.J. Self-assembling peptide hydrogels modulate in vitro chondrogenesis of bovine bone marrow stromal cells. Tissue Eng Part A 16, 465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shah R.N., Shah N.A., Del Rosario Lim M.M., Hsieh C., Nuber G., and Stupp S.I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci U S A 107, 3293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kisiday J., Jin M., Kurz B., et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A 99, 9996, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maher S.A., Mauck R.L., Rackwitz L., and Tuan R.S. A nanofibrous cell-seeded hydrogel promotes integration in a cartilage gap model. J Tissue Eng Regen Med 4, 25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller R.E., Grodzinsky A.J., Barrett M.F., et al. Effects of the combination of microfracture and self-assembling peptide filling on the repair of a clinically relevant trochlear defect in an equine model. J Bone Joint Surg Am 96, 1601, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tokunou T., Miller R., Patwari P., et al. Engineering insulin-like growth factor-1 for local delivery. FASEB J 22, 1886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller R.E., Grodzinsky A.J., Cummings K., et al. Intraarticular injection of heparin-binding insulin-like growth factor 1 sustains delivery of insulin-like growth factor 1 to cartilage through binding to chondroitin sulfate. Arthritis Rheum 62, 3686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kopesky P.W., Lee H.Y., Vanderploeg E.J., et al. Adult equine bone marrow stromal cells produce a cartilage-like ECM mechanically superior to animal-matched adult chondrocytes. Matrix Biol 29, 427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van de Breevaart Bravenboer J., In der Maur C.D., Bos P.K., et al. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res Ther 6, R469, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ragan P.M., Chin V.I., Hung H.-H.K., et al. Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch Biochem Biophys 383, 256, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Enders J.T., Otto T.J., Peters H.C., et al. A model for studying human articular cartilage integration in vitro. J Biomed Mater Res 94, 509, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Bonassar L.J., Frank E.H., Murray J.C., et al. Changes in cartilage composition and physical properties due to stromelysin degradation. Arthritis Rheum 38, 173, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Kisiday J.D., Kurz B., DiMicco M.A., and Grodzinsky A.J. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng 11, 141, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Chawla K., Klein T.J., Schumacher B.L., et al. Tracking chondrocytes and assessing their proliferation with PKH26: effects on secretion of proteoglycan 4 (PRG4). J Orthop Res 24, 1499, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Sah R.L., Kim Y.J., Doong J.Y., Grodzinsky A.J., Plaas A.H., and Sandy J.D. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 7, 619, 1989 [DOI] [PubMed] [Google Scholar]
- 43. Kim Y.J., Sah R.L., Doong J.Y., and Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem 174, 168, 1988 [DOI] [PubMed] [Google Scholar]
- 44. Farndale R.W., Sayers C.A., and Barrett A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res 9, 247, 1982 [DOI] [PubMed] [Google Scholar]
- 45. Stegemann H., and Stalder K. Determination of hydroxyproline. Clin Chim Acta 18, 267, 1967 [DOI] [PubMed] [Google Scholar]
- 46. Redman S.N., Dowthwaite G.P., Thomson B.M., and Archer C.W. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthritis Cartilage 12, 106, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Dhinsa B.S., and Adesida A.B. Current clinical therapies for cartilage repair, their limitation and the role of stem cells. Curr Stem Cell Res Ther 7, 143, 2012 [DOI] [PubMed] [Google Scholar]
- 48. Ikada Y. Challenges in tissue engineering. J R Soc Interface 3, 589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doran P.M. Cartilage tissue engineering: what have we learned in practice? Methods Mol Biol 1340, 3, 2015 [DOI] [PubMed] [Google Scholar]
- 50. Lee J.-C, Min H.J., Lee S., Seong S.C., and Lee M.C. Effect of chondroitinase ABC on adhesion and behavior of synovial membrane-derived mesenchymal stem cells in rabbit partial-thickness chondral defects. J Orthop Res 31, 1293, 2013 [DOI] [PubMed] [Google Scholar]
- 51. Rich A.M., Pearlstein E., Weissmann G., and Hoffstein S.T. Cartilage proteoglycans inhibit fibronectin-mediated adhesion. Nature 293, 224, 1981 [DOI] [PubMed] [Google Scholar]
- 52. Jo C.H., Kim E.M., Ahn H.J., Kim H.J., Seong S.C., and Lee M.C. Degree of degeneration and chondroitinase ABC treatment of human articular cartilage affect adhesion of chondrocytes. Tissue Eng 12, 167, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Janssen L.M., In der Maur C.D., Bos P.K., Hardillo J.A., and van Osch G.J. Short-duration enzymatic treatment promotes integration of a cartilage graft in a defect. Ann Otol Rhinol Laryngol 115, 461, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Stenman M., Ainola M., Valmu L., et al. Trypsin-2 degrades human type II collagen and is expressed and activated in mesenchymally transformed rheumatoid arthritis synovitis tissue. Am J Pathol 167, 1119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reindel E.S., Ayroso A.M., Chen A.C., Chun D.M., Schinagl R.M., and Sah R.L. Integrative repair of articular cartilage in vitro: adhesive strength of the interface region. J Orthop Res 13, 751, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Stoop R. Smart biomaterials for tissue engineering of cartilage. Injury 39, 77, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Chen P., Tao J., Zhu S., et al. Radially oriented collagen scaffold with SDF-1 promotes osteochondral repair by facilitating cell homing. Biomaterials 39, 114, 2015 [DOI] [PubMed] [Google Scholar]
- 58. Matsumoto T., Gargosky S.E., Iwasaki K., and Rosenfeld R.G. Identification and characterization of insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP proteases in human synovial fluid. J Clin Endocrinol Metab 81, 150, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.