Abstract
Background:
The human papillomavirus (HPV) E6 oncoprotein enhances the oncogenic potential of ErbB proteins in HPV-related malignancies. This phase I study evaluates the addition of afatinib, an ErbB family inhibitor, and ribavirin to paclitaxel and carboplatin induction chemotherapy in HPV-associated, locally advanced oropharyngeal squamous cell carcinoma (SCC).
Methods:
This dose escalation study included 2 doses of oral afatinib: 30 and 40 mg daily. Ribavirin dosing was weight based. Paclitaxel (80 mg/m2) and carboplatin (area under the curve [AUC] 1.5) were administered on days 1 and 8 of each 21-day cycle. After 3 cycles, patients were removed from protocol to receive definitive treatment.
Results:
Among 10 patients, there were no dose-limiting toxicities. Six patients (67%) had unconfirmed objective partial responses. The 2-year progression-free survival rate was 75%.
Conclusion:
Afatinib, ribavirin, paclitaxel, and carboplatin induction chemotherapy is safe and well tolerated. The phase II recommended dose of afatinib is 40 mg oral daily in this combination regimen.
Keywords: afatinib, human papillomavirus, oropharyngeal cancer, ribavirin, squamous cell carcinoma
1 |. INTRODUCTION
Induction chemotherapy in the management of patients with locally and/or regionally advanced head and neck squamous cell carcinoma (HNSCC) offers several theoretical advantages. Primary administration of systemic therapy allows for the treatment of micrometastatic disease and allows for improved locoregional disease control.1 Further, the response to induction chemotherapy can potentially be applied to tailor subsequent definitive therapy. In cases with favorable responses to induction chemotherapy, lower dosages of concurrent chemotherapy can potentially be administered with definitive radiotherapy to decrease the associated toxicity of chemoradiation and allow for improved organ preservation.
Docetaxel, cisplatin, and 5-fluorouricil is the standard induction chemotherapy regimen after demonstrating superiority over cisplatin and 5-flurouracil but can cause serious toxicities.2,3 An induction chemotherapy regimen consisting of weekly carboplatin (area under the curve [AUC] 2), paclitaxel (135 mg/m2), and cetuximab (400 mg/m2 loading dose, followed by 250 mg/m2 weekly) for 6 treatments has yielded encouraging response rates in a phase II trial, suggesting an alternative path away from high-dose induction chemotherapy in HNSCC clinical research.4 Further development of induction chemotherapy regimens that combine weekly carboplatin and paclitaxel with targeted therapies is needed. A regimen of deintensified weekly carboplatin (AUC 1.5), paclitaxel (80 mg/m2), and temsirolimus (25 mg flat dose) on days 1 and 8 of a 21-day cycle achieved an overall radiologic response rate of 43% in a single arm study for patients with recurrent/metastatic HNSCC, despite the unfavorable prognostic features of the study group.5 The observation that the deintensified weekly carboplatin and paclitaxel-based regimen had relatively high activity in the advanced disease setting presents an opportunity to develop this less toxic approach as initial therapy for favorable prognosis for patients with human papillomavirus (HPV)-associated oropharyngeal cancer who can experience significant toxicities with current standards of care.
The study concept involves combining deintensified induction chemotherapy plus 2 orally administered agents, afatinib and ribavirin, with compelling biologic rationale in this disease. The HPV infection has been associated with enhanced phosphorylation and activity of ErbB proteins in preclinical models. Normally, protein tyrosine phosphatase PTPN13 reduces ErbB2 activity via dephosphorylation and inhibits epidermal growth factor receptor (EGFR)/ErbB2 signaling that allows for activation of the oncogenic mitogen-activated protein kinase signaling pathway.6,7 However, the HPV E6 oncoprotein triggers the degradation of PTPN13, and PTPN13 loss allows for EGFR/ErbB2 signaling.7,8 Preclinical data supports that coexpression of ErbB2 and HPV oncoproteins E6/E7 is the mechanism for tumorigenesis in HPV-driven malignancies.9 Afatinib (BIBW 2992) is a potent and selective tyrosine kinase inhibitor of members of the ErbB family.10 In a randomized phase II crossover study11 in patients with platinum-refractory recurrent/metastatic disease, comparable response rates were achieved with afatinib versus cetuximab, the latter being a Food and Drug Administration approved EGFR targeting agent for HNSCC, on independent central review.
The antiviral agent ribavirin, which the Food and Drug Administration approved as part of the treatment of hepatitis C, is a guanine ribonucleotide analog that has activity against an HNSCC xenograft model (FaDu) due to targeting of eukaryotic initiation factor 4E (eIF4E), the key regulator of mammalian cap-dependent translation.12,13 We propose the clinical development of ribavirin in treatment in HPV-related cancers because eIF4E can function as an oncogene and is commonly expressed at elevated levels in these tumors.14–18 Preclinically, the HPV16 E6 viral oncoprotein can increase the eIF4E transcription and can stimulate cap-dependent translation.18–20 The HPV16 E6 is involved in coopting the host cell protein translational apparatus to favor viral production partially through stimulation of the transcription of eIF4E.18 In preclinical models, ribavirin was shown to have anticancer activity directly by targeting eIF4E.12 In xenograft experiments with an eIF4E-dependent HNSCC cell line, tumors significantly responded to ribavirin treatment.12 Speculatively, a positive feedback loop may exist between the E6 oncoprotein and eIF4E because both proteins are translated from capped mRNA.21 Proof of principal for the clinical anticancer activity of ribavirin was achieved in a pilot study in acute myeloid leukemia tumors that express high levels of eIF4E.22
Although the synergistic or additive effects of the combination of afatinib and ribavirin have not been previously evaluated, there is strong mechanistic rationale for this combination. Ribavirin should provide direct antitumor activity in oropharyngeal squamous cell carcinoma (SCC) through eIF43 inhibition and indirect suppression of HPV E6 function, which, in turn, should theoretically lead to prevention of PTPN13 loss to augment the efficacy of pan-ErbB inhibition by afatinib.
We designed a phase I study to define the phase II recommended dose of afatinib when added to ribavirin, along with deintensified paclitaxel and carboplatin induction chemotherapy for patients with HPV-associated, locally advanced oropharyngeal SCC. The induction regimen included paclitaxel 80 mg/m2 and carboplatin AUC 1.5 administered on days 1 and 8 of 21-day cycles for a total of 3 cycles. Ribavirin was given at standard weight-based dosing (1000 mg or 1200 mg total daily dose), as supported by findings from a window of opportunity study23 that showed reduction in the levels of phosphorylated eIF4E in patients with HPV-positive, locally advanced oropharyngeal SCC who received ribavirin at the standard weight-based dose for 2 weeks before definitive treatment. Dose exploration was for afatinib only. The higher dose level for afatinib was 40 mg daily, the dosage found to be clinically active per LUX-Head and Neck 1,24 a study that compared afatinib to methotrexate in patients with recurrent and/or metastatic HNSCC and resulted in favor of afatinib.
2 |. MATERIALS AND METHODS
2.1 |. Patient eligibility criteria
This was a single-institution phase I study that was approved by our hospital’s institutional review board. All patients provided written informed consent. Eligible patients had previously untreated HPV-associated stage IVA or IVB SCC of the oropharynx (palatine tonsil or base of tongue). Confirmation of HPV-associated disease was per p16 immunohistochemistry +/− HPV in situ hybridization positivity. Enrolled patients were aged ≥18 years, had a Karnofsky Performance Status ≥80%, and had the ability to swallow. Baseline laboratory requirements were an absolute neutrophil count ≥1.5 × 109/L, platelets ≥160 × 109/L, hemoglobin ≥12 g/dL, serum creatinine ≤1.3 mg/dL, or a creatinine clearance ≥55 mL/min, per the standard Cockcroft and Gault formula, and serum bilirubin ≤1.0 mg/dL. Aspartate or alanine aminotransferase levels were required to be ≤1.5 times the upper limit of normal.
Patients were excluded if they had an active infection or serious underlying medical condition that would interfere with the ability to receive treatment on study. Further, patients were ineligible if they had a history of hemolytic anemia or thalassemia, transient ischemic attack or cerebrovascular accident, and preexisting interstitial lung disease, as well as active hepatitis B or C virus, New York Heart Association grade II or greater congestive heart failure, clinically significant peripheral vascular disease, and poorly controlled gastrointestinal disorders that could affect the absorption of afatinib and ribavirin (for example, Crohn disease, ulcerative colitis, malabsorption, or Common Terminology Criteria for Adverse Events grade ≥2 diarrhea of any etiology). Patients who received current or prior treatment with ribavirin or current therapeutic anticoagulation with Warfarin (Bristol-Myers-Squibb, New York, USA) were excluded, in addition, patients who were unable to discontinue potent P-glycoprotein inhibitors (cyclosporine, erythromycin, ketoconazole, itraconazole, quinidine, phenobarbital salt with quinidine, ritonavir, Valspodar (Sandoz Pharmaceutical Corp, New Jersey, USA), and verapamil) or inducers (St John’s wort and rifampicin).
2.2 |. Treatment plan
Patients received an induction chemotherapy regimen consisting of oral daily afatinib (per the dose-escalation scheme) in combination with fixed doses of oral daily ribavirin and concurrent paclitaxel (80 mg/m2 intravenously) and carboplatin (AUC 1.5 intravenously with a maximum flat dose 225 mg) both on days 1 and 8 of each 21-day cycle. Ribavirin was administered according to standard weight-based dosing. Patients who weighed ≤75 kg received ribavirin 1000 mg/day orally (400 mg in the morning and 600 mg in the evening) and patients who weighted >75 kg received 1200 mg/day orally (600 mg twice daily). The study regimen is provided in Table 1. Three 21-day cycles of induction chemotherapy were planned for each patient. After induction chemotherapy, patients underwent cross-sectional imaging of the primary tumor and involved neck lymph nodes before removal from the study. Definitive management was at the discretion of the treating physician, in accord with standard practice.
TABLE 1.
Dose escalation scheme
| Dose level | Afatinib, oral on d 1-21 of each 21-d cycle | Ribavirin, oral on d 1-21 of each 21-d cycle | Paclitaxel, intravenously on d 1 and 8 of each 21-d cycle | Carboplatin, intravenously on d 1 and 8 of each 21-d cycle |
|---|---|---|---|---|
| 1 | 30 mg/d | Weight baseda | 80 mg/m2 | AUC 1.5b |
| 2 | 40 mg/d | Weight baseda | 80 mg/m2 | AUC 1.5b |
Abbreviation: AUC, area under the curve.
Patients who weighed ≤75 kg received ribavirin 400 mg orally in the morning and 600 mg orally in the evening and patients who weighted >75 kg received 600 mg orally twice daily.
The dose of carboplatin could not exceed a flat dose of 225 mg.
2.3 |. Dose escalation and definition of dose-defining toxicity
A standard 3 + 3 dose escalation plan for 2 dose levels of afatinib was followed. The period of evaluation for dose-limiting toxicity (DLT) was through the completion of cycle 1 for each patient and there was no intrapatient dose escalation. The maximum tolerated dose was defined as the highest dose level at which ≤ 1 of 6 patients experienced a DLT during cycle 1. Patients who experienced a DLT during cycle 1 were removed from the study with subsequent management off-protocol, per the standards of care for HNSCC.
A DLT was defined as all toxicities of grade 3 or higher, felt to be possibly, probably, or definitely related to afatinib. Regarding immunosuppression, the laboratory parameter for DLT determination was absolute neutrophil count (not total white blood cell count or absolute lymphocyte count). The following were not considered to be DLTs: grade 3 fatigue for ≤24 hours; grade 3 hypomagnesemia for ≤24 hours; grade 3 diarrhea lasting ≤24 hours; grade 3 nausea and/or grade 3 vomiting lasting ≤24 hours; uncomplicated grade 3 or 4 neutropenia lasting ≤7 days; grade 3 thrombocytopenia without bleeding; grade 3 anemia, even if treatment was given with stimulators of erythropoiesis or packed red blood cell transfusions; uncomplicated grade 3 hyperglycemia for ≤72 hours; and uncomplicated grade 4 hyperglycemia for ≤24 hours.
2.4 |. Safety evaluations
On day 1 of each cycle before the administration of treatment, patients underwent clinical assessments and comprehensive laboratory evaluations that included a complete blood count, basic metabolic panel with magnesium, and liver function tests. A complete blood count was required on day 8 before administration of carboplatin and paclitaxel. During cycle 1, patients underwent an additional clinical assessment during week 2. Only 1 clinical assessment was required during cycles 2 and 3.
Adverse events (AEs) were assessed according to the National Cancer Institute’s Common Toxicity Criteria version 4.0. The AEs that occurred on-treatment were considered to be those events that occurred within the period of the first administration of afatinib through 28 days after the last administration of afatinib. Therefore, patients were followed for toxicity for 28 days after the last dose of afatinib. If patients had AEs that persisted beyond 28 days after the last dose of afatinib, they were followed until the AEs resolved or had been sufficiently characterized.
2.5 |. Efficacy assessments
Pretreatment cross-sectional imaging of the neck with CT with contrast or MRI with gadolinium was performed within 6 weeks before the initiation of treatment on-study. Interval assessment imaging was performed during cycle 3. Radiologic response was assessed using the revised Response Evaluation Criteria in Solid Tumors guidelines version 1.125 to obtain the objective response rate among patients treated with afatinib, carboplatin, paclitaxel, and ribavirin. Confirmatory radiologic assessments were not obtained, as patients subsequently received definitive locoregional therapy off-protocol that would confound further imaging. Progression-free survival was estimated per the method of Kaplan-Meier.
2.6 |. Human papillomavirus assay
Eligible patients were required to have oropharyngeal SCC that exhibited p16 expression per immunohistochemistry and/or HPV in situ hybridization positivity. The immunohistochemistry analysis for p16 (K5334 clone E6H4, dilution 1:75; Dako) was performed in all cases.26 The presence of high-risk HPV strains was confirmed using the Ventana HPV III family 16 probe (Ventana Medical Systems, Tucson, AZ).27
3 |. RESULTS
3.1 |. Patient characteristics
Ten patients were enrolled on the study from March 15, 2013, through March 27, 2014. Baseline characteristics of the entire enrolled study population are summarized in Table 2. All enrolled patients were men and the median age was 59.5 years (range 46-77 years). The median Karnofsky performance status was 90% (range 80%−100%). Of the 10 patients, the primary site of oropharyngeal cancer was the palatine tonsil in 4 patients and base of tongue in 6 patients. The majority of patients had clinical stage IVA disease.
TABLE 2.
Characteristics of the study population (10 patients)
| Characteristic | No. of patients |
|---|---|
| Sex | |
| Men | 10 |
| Women | 0 |
| Age, years: median (range) | 59.5 (46–77) |
| Karnofsky performance status: median (range) | 90% (80–100) |
| Primary site of oropharyngeal cancer | |
| Palatine tonsil | 4 |
| Base of tongue | 6 |
| Stage, clinical | |
| IVA | 8 |
| IVB | 2 |
| Tobacco history | |
| Never smoked | 7 |
| <1 pack-year | 2 |
| ≥1 and <10 pack-years | 0 |
| ≥10 and <20 pack-years | 0 |
| ≥20 pack-years | 1 |
| Alcohol use | |
| Never | 4 |
| Social, <1 drink/d | 3 |
| 1 drink/d | 2 |
| 2 drinks/d | 1 |
| ≥3 drinks/d | 0 |
Among the 10 patients, 7 were never smokers and 3 were former smokers, 2 for <1 pack-year and 1 for >20 pack-years. Additionally, 4 patients did not drink alcohol, 3 drank socially with <1 drink daily, 2 consumed 1 drink daily, and 1 consumed 2 drinks daily.
3.2 |. Dose escalation and dose-limiting toxicities
Four patients were enrolled at dose level 1, afatinib 30 mg/day. Among the initial 3 patients who were treated at dose level, 1 patient experienced a severe reaction to paclitaxel on day 8 of cycle 1 exhibited by facial flushing, hypotension, hypoxia, and chest pain, and was removed from the study and replaced. There were no DLTs among the 3 patients treated at dose level 1 who completed cycle 1 of induction treatment and were evaluable for safety.
Six patients were enrolled at dose level 2, afatinib 40 mg/day, and all were evaluable for safety. There were no DLTs among the 6 patients treated at dose level 2. Given that the maximum tolerated dose was not exceeded among the 6 patients treated at dose level 2, the phase II recommended dose of afatinib is 40 mg/day in combination with ribavirin, carboplatin, and paclitaxel induction therapy.
3.3 |. Adverse events associated with induction chemotherapy
All 10 enrolled patients were evaluable for toxicity. Any AEs were reported for those experienced while on-study treatment with induction chemotherapy and not for off-protocol definitive treatment. The only patient who did not complete 1 cycle of treatment was the patient in dose level 1 who was removed for a severe paclitaxel-associated toxicity. The other 9 patients all received at least 2 cycles of induction chemotherapy for a median treatment duration of 54 days that ranged from 46 to 71 days. Eight patients received treatment into cycle 3. Table 3 lists all AEs, regardless of attribution. Thus, there were no DLTs observed during cycle 1 of induction chemotherapy. Among the 3 patients with a decreased white blood cell count during induction chemotherapy, 1 patient was found to have grade 2 neutropenia. One patient was found to have lymphopenia with a normal white blood cell count. There were no cases of febrile neutropenia.
TABLE 3.
Summary of adverse events, regardless of attribution
| All toxicities | No. of AEs (%) | |
|---|---|---|
| Any grade | Grade 3 | |
| Fatigue | 10 (100) | 0 (0) |
| Diarrhea | 9 (90) | 2 (20) |
| Rash maculopapular | 8 (80) | 0 (0) |
| Anemia | 7 (70) | 0 (0) |
| Cough | 6 (60) | 0 (0) |
| Hypertension | 6 (60) | 0 (0) |
| Nausea | 6 (60) | 0 (0) |
| Alopecia | 5 (50) | 0 (0) |
| Anorexia | 5 (50) | 0 (0) |
| Dizziness | 5 (50) | 0 (0) |
| Hypoalbuminemia | 5 (50) | 0 (0) |
| Pain | 5 (50) | 0 (0) |
| Bilirubin elevation | 4 (40) | 0 (0) |
| Hypocalcemia | 4 (40) | 0 (0) |
| Mucositis oral | 4 (40) | 0 (0) |
| Decreased white blood cell count | 3 (30) | 1 (10) |
| Dyspepsia | 3 (30) | 0 (0) |
| Dyspnea | 3 (30) | 0 (0) |
| Epistaxis | 3 (30) | 0 (0) |
| Pruritus | 3 (30) | 0 (0) |
| Sinus tachycardia | 3 (30) | 0 (0) |
| Vomiting | 3 (30) | 0 (0) |
| Weight loss | 3 (30) | 0 (0) |
| Syncope | 1 (10) | 1 (10) |
Abbreviation: AEs, adverse events.
At dose level 1, there was 1 patient who required a dose reduction of afatinib for grade 2 anemia. Three patients at dose level 2 required a dose reduction of afatinib. The reasons for dose reduction included grade 2 hyperbilirubinemia, grade 2 anemia, and grade 3 diarrhea. All dose reductions occurred after cycle 1, beyond the evaluable period for DLTs.
3.4 |. Subsequent definitive locoregional therapy
After induction chemotherapy on-protocol, patients were removed from the study. Subsequent management was per the discretion of each patient’s treating physician. All 10 patients enrolled received definitive radiotherapy with concurrent chemotherapy at Memorial Sloan Kettering Cancer Center. All but 1 patient received concurrent chemotherapy with cisplatin. Cisplatin was administered weekly in 6 patients and every 3 weeks in 3 patients. The patient who did not receive cisplatin received cetuximab concurrently with radiation. Table 4 lists the definitive treatment received by each patient.
TABLE 4.
Additional patient and tumor characteristics with definitive locoregional treatment and response assessments
| Patient | Dose level | Age, years | Sex | Cigarette history, pack-years | HPV status | Primary tumor site, TNM classification | Definitive chemoradiation | Response rate |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 71 | M | Never | p16-positive | BOT, T1N2cM0 | Cisplatin 35 mg/m2 weekly × 7, 6040 cGy | −29% |
| 2 | 1 | 59 | M | <1 | p16-positive | Tonsil, T2N2cM0 | Cisplatin 100 mg/m2 q3 wk × 3, 7000 cGy | −36% |
| 3 | 1 | 56 | M | 21 | p16-positive, ISH HPV-positive | Tonsil, T4bN0M0 | Cisplatin 100 mg/m2 q3 wk × 3, 7000 cGy | Removed in cycle 1 due to paclitaxel reaction |
| 4 | 1 | 62 | M | Never | p16-positive | BOT, T1N2bM0 | Cisplatin 40 mg/m2 weekly × 4, 5412 cGy | −37% |
| 5 | 2 | 60 | M | <1 | p16-positive | Tonsil, T3N3N0 | Cisplatin 40 mg/m2 weekly × 5, 5412 cGy | −64% |
| 6 | 2 | 77 | M | Never | p16-positive | BOT, T3N2cM0 | Cetuximab 400 mg/m2 × 1, 20 mg/m2 × 6, 5940 cGy | −10% |
| 7 | 2 | 46 | M | Never | p16-positive | Tonsil, T2N2bM0 | Cisplatin 40 mg/m2 weekly × 5, 5412 cGy | −78% |
| 8 | 2 | 77 | M | Never | p16-positive, HPV-ISH+ | BOT, T4aN2cM0 | Cisplatin 35 mg/m2 weekly × 5, 5940 cGy | −50% |
| 9 | 2 | 47 | M | Never | p16-positive, HPV-ISH+ | BOT, T2N2bM0 | Cisplatin 40 mg/m2 weekly × 7, 6010 cGy | −54% |
| 10 | 2 | 50 | M | Never | p16, ISH HPV-positive | BOT, T4aN2bM0 | Cisplatin 50 mg/m2 × 2 d, q3 wk × 2.5, 6400 cGy | 100% |
Abbreviations: BOT, base of tongue; HPV, human papillomavirus; ISH, in situ hybridization.
3.5 |. Efficacy
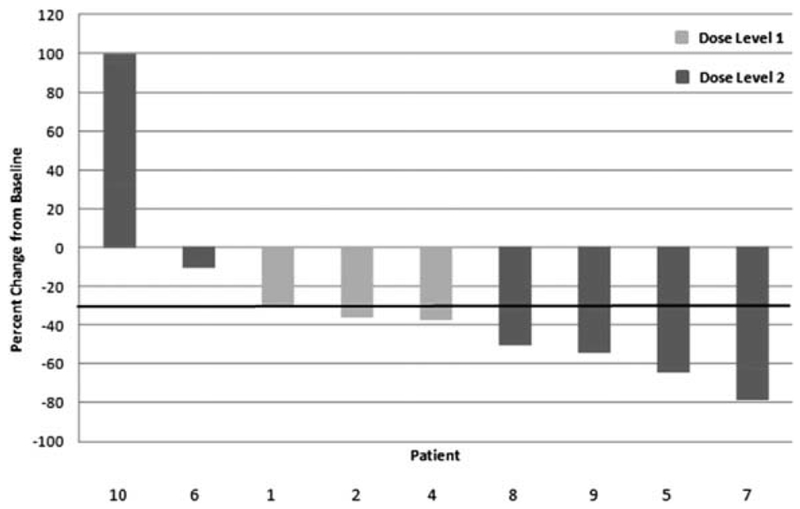
Nine patients received at least 2 cycles of induction chemotherapy and were evaluable for best overall response per radiologic evaluation. The best overall response per Response Evaluation Criteria in Solid Tumors version 1.1 criteria included an unconfirmed partial response in 6 patients (67%), stable disease in 2 patients (22%), and progression of disease in 1 patient (11%). The patient with disease progression was removed from the study after 2 cycles of induction chemotherapy. Table 4 includes the response rate for each patient on-study and Figure 1 shows a waterfall plot of the best response rate.
FIGURE 1.
Waterfall plot of best response during induction treatment
Survival analysis for the study is descriptive only. The survival cutoff date was December 16, 2016. One of the 9 patients evaluable for best response was immediately lost to follow-up after definitive treatment and did not have posttreatment imaging. Among these 8 patients, who were evaluable for best response and received continued follow-up, the median follow-up was 34.8 months (2.9 years). The progression-free survival rate at 2 years from the start of treatment was 75%. The patient who was removed from the study due to disease progression on induction chemotherapy experienced a complete response with definitive chemoradiation and had a 12.4-month disease-free interval before developing recurrent distant metastatic disease. Among the 7 patients who completed induction chemotherapy on-study and underwent posttreatment imaging after definitive treatment, the median duration of disease-free survival is 29.4 months (2.5 years; range 11.0-37.3 months). The disease-free survival rate remains 86%. One of the 7 patients developed a locoregional recurrence with subsequent distant metastatic disease after an 11-month disease-free interval.
4 |. DISCUSSION
This phase Ib trial demonstrates that the addition of afatinib and ribavirin to weekly deintensified paclitaxel and carboplatin (2 weeks on, 1 week off) induction chemotherapy is safe and well tolerated among patients with HPV-associated, locally advanced oropharyngeal SCC. The most common grade 3 AE was diarrhea, occurring in 20% of patients but not meeting criteria for DLT. The recommended dose of afatinib is 40 mg oral daily when combined with the study regimen. This induction chemotherapy regimen had an overall response rate of 67% (unconfirmed) among 9 evaluable patients.
The AE profile of the regimen is consistent with that which has been previously reported for the individual components of the regimen, and there was no obvious evidence of intensification of any of the expected toxicities with the combination. Diarrhea and rash were expected for afatinib, and were readily manageable by following established toxicity-management procedures for this agent. There was no evidence of intensification of worsening of bone marrow suppression with the addition of afatinib and ribavirin to low-dose carboplatin and paclitaxel, but a larger sample size would be needed to exclude the possibility of such an effect. The potential for ribavirin to intensify anemia would require close scrutiny in any follow-up studies.
The phase Ib study was not powered to establish efficacy, but the response rate that was observed is not dissimilar to response rates seen with induction chemotherapy regimens with full-dose cytotoxic chemotherapy.1–3 The concept that lower-dose induction chemotherapy in combination with a biologic agent can be associated with a high response rate has been previously demonstrated in a phase II study of carboplatin + paclitaxel + cetuximab.4 The current study advances this strategy by further deintensifying the carboplatin + paclitaxel, and incorporating 2 biologically targeted agents. One obvious advantage of this approach is decreased risk of febrile neutropenia, which was not observed in the current study. Further study with greater patient numbers would be necessary to more fully describe safety and to establish if the efficacy of the current regimen is comparable to that of more dose-intense induction chemotherapy regimens.
As this study neared completion, the results of the LUX-Head and Neck study were reported.24 In this randomized phase III comparison of afatinib versus methotrexate among patients with recurrent or metastatic HNSCC, afatinib achieved superior progression-free survival (2.6 vs 1.7 months). Subgroup analysis indicated that the benefit of afatinib was associated with p16-negative status. The response rate for afatinib in p16-positive versus p16-negative was 0/31 (0%) versus 19/141 (13.5%), respectively. Benefit with EGFR targeting agents in p16-negative recurrent/metastatic HNSCC has been described in some, but not all, studies.28 Among patients with locally or regionally advanced disease, the potential impact of HPV status on efficacy of EGFR or ERBB-family targeted therapy has not been rigorously studied but would require close attention in subsequent studies that may seek to incorporate these agents into the management of newly diagnosed patients with HNSCC with locally/regionally advanced disease.
Further development of the study regimen would be aided by better understanding of potential biomarkers of resistance to exclude patients unlikely to benefit from these agents. In regard to mechanisms of resistance to ribavirin, preclinical studies with the FaDu HNSCC cell line indicate that this may be mediated by aberrant activation of the sonic hedgehog pathway, particularly the transcript factor glioma-associated protein 1.29 A pilot study of high-dose ribavirin for recurrent/metastatic HPV-related cancers is now exploring if resistance to ribavirin is associated with glioma-associated protein 1 expression in the clinic (). One potential limitation of ribavirin is that it exerts its clinical effects at micromolar concentrations, and has numerous effects other than eIF4E targeting. More potent and specific eIF4E-targeting agents are entering the clinic,30 which may be well suited for clinical development against HPV-related cancers.
In conclusion, this phase Ib study demonstrates that afatinib is safe and well tolerated when combined with ribavirin, in addition to deintensified paclitaxel and carboplatin induction chemotherapy, in patients with locally advanced HPV-associated oropharyngeal SCC. Although the role of induction chemotherapy in the treatment of HNSCC remains controversial, this study defined a well-tolerated induction chemotherapy regimen that can provide insights into treatment deintensification strategies through incorporation of HPV-directed therapy for patients with HPV-associated oropharyngeal cancer. With advances in novel immunotherapies targeting HPV oncoproteins, this study regimen can provide a paradigm for integration of HPV-directed therapy into a well-tolerated induction strategy.
Acknowledgments
Funding information
This work was supported by a grant from the National Comprehensive Cancer Network Oncology Research Program, National Institutes of Health (NIH)/National Cancer Institute (NCI) Cancer Center Support (grant P30 CA008748).
REFERENCES
- [1].Fury MG, Shah JP. Induction chemotherapy in the management of head and neck cancer. J Surg Oncol. 2010;101:292–298. [DOI] [PubMed] [Google Scholar]
- [2].Posner M, Hershok DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. [DOI] [PubMed] [Google Scholar]
- [3].Vermorken JB, Remenar E, Van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. [DOI] [PubMed] [Google Scholar]
- [4].Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010;28:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fury MG, Xiao H, Baxi SS, et al. A phase II trial of temsirolimus plus low-dose weekly carboplatin and paclitaxel for recurrent/metastatic HNSCC. J Clin Oncol. 2014;32:5s (suppl; abstract 6019). [Google Scholar]
- [6].Zhu JH, Chen R, Yi W, et al. Protein tyrosine phosphatase PTPN13 negatively regulates Her2/ErbB2 malignant signaling. Oncogene. 2008;27:2525–2531. [DOI] [PubMed] [Google Scholar]
- [7].Hoover AC, Strand GL, Nowicki PN, et al. Impaired PTPN13 phosphatase activity in spontaneous or HPV-induced squamous cell carcinomas potentiates oncogene signaling through the MAP kinase pathway. Oncogene. 2009;28:3960–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Spanos WC, Hoover A, Harris GF, et al. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. J Virol. 2008;82:2493–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Al Moustafa AE, Foulkes WD, Benlimame N, et al. E6/E7 proteins of HPV type 16 and ErbB-2 cooperate to induce neoplastic transformation of primary normal oral epithelial cells. Oncogene. 2004;23:350–358. [DOI] [PubMed] [Google Scholar]
- [10].Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Seiwert TY, Fayette J, Cupissol D, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25:1813–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kentsis A, Topisirovic I, Culojkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci U S A. 2004;101:18105–18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sonenberg N eIF4E, the mRNA cap-binding protein: from basic discovery to translational research. Biochem Cell Biol. 2008;86: 178–183. [DOI] [PubMed] [Google Scholar]
- [14].Wendel HG, de Stanchina E, Fridman JS, et al. Survival signaling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. [DOI] [PubMed] [Google Scholar]
- [15].Matthews-Greer J, Caldito G, De Benedetti A, et al. eIF4E as a marker for cervical neoplasia. Appl Immunohistochem Mol Morphol. 2005;13:367–370. [DOI] [PubMed] [Google Scholar]
- [16].Lee JW, Choi J, Lee KM, et al. eIF4E expression is associated with histologic grades in cervical neoplasia. Hum Pathol. 2005; 36:1197–1203. [DOI] [PubMed] [Google Scholar]
- [17].Fury MG, Drobnjak M, Sima C, et al. Tissue microarray analysis demonstrates association between p16 and phosphorylated eIF4E in tonsillar squamous cell carcinoma. Head Neck. 2011; 22:1340–1345. [DOI] [PubMed] [Google Scholar]
- [18].Wang S, Pang T, Gao M, Kang H, Ding W. HPV E6 induces eIF4E transcription to promote the proliferation and migration of cervical cancer. FEBS Lett. 2013;587:690–697. [DOI] [PubMed] [Google Scholar]
- [19].Spangle JM, Munger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol. 2010;84:9398–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spangle JM, Ghosh-Choudhury N, Munger K. Activation of cap-dependent translation by mucosal human papillomavirus E6 proteins in dependent on the integrity of the LXXLL binding motif. J Virol. 2012;86:7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stacey SN, Jordan D, Williamson AJK, Brown M, Coote JH, Arrand JR. Leaky scanning is the predominant mechanism for translation of human papillomavirus type 16 E7 oncoprotein from E6/E7 bicistronic mRNA. J Virol. 2000;74:7284–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Assouline S, Culjkovic B, Cocolakis E, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114: 257–260. [DOI] [PubMed] [Google Scholar]
- [23].Fury MG, Lou J, Katabi N, et al. The antiviral agent ribavirin inhibits eIF4E in HPV positive squamous cell cancer. Presented at the Fifth International Federation of Head and Neck Oncologic Societies (IFHNOS) World Congress 2014 Annual AHNS Meeting, July 26-30, 2014. [Google Scholar]
- [24].Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label randomized phase 3 trial. Lancet Oncol. 2015;16:583–594. [DOI] [PubMed] [Google Scholar]
- [25].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- [26].Hafkamp HC, Speel EJM, Haesevoets A, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer. 2003;107: 394–400. [DOI] [PubMed] [Google Scholar]
- [27].Guo M, Gong Y, Deavers M, et al. Evaluation of a commercialized in situ hybridization assay for detecting human papillomavirus DNA in tissue specimens from patients with cervical intraepithelial neoplasia and cervical carcinoma. J Clin Microbiol. 2008;46:274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pfister DG, Fury MG. New chapter in our understanding of human-papillomavirus-related head and neck cancer. J Clin Oncol. 2014;32:3349–3352. [DOI] [PubMed] [Google Scholar]
- [29].Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature. 2014;511:90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Prasad A, Shruvastava A, Papadopoulos E, et al. Combined administration of rituximab and ON 01305 induces apoptosis in mantle cell lymphoma cells and reduces tumor burden in a mouse model of mantle cell lymphoma. Clin Cancer Res. 2012; 19:85–95. [DOI] [PubMed] [Google Scholar]