Abstract
Background:
Despite known genetic variation across races, studies examining pharmacogenetics of a single nucleotide polymorphism (SNP) of the mu-opioid receptor gene (OPRM1) on clinical response to naltrexone have been conducted in predominantly Caucasian samples. Evidence is mixed for pharmacogenetic OPRM1 and naltrexone effects on neural responses to alcohol cues. The current study tests the pharmacogenetic effects of naltrexone and OPRM1 on neural responses to alcohol taste cues in heavy drinkers of East Asian descent.
Methods:
Participants (N=41) completed two double-blinded and counterbalanced functional magnetic resonance imaging (fMRI) sessions: one after taking naltrexone (50 mg/day) for four days and one after taking placebo for four days. Following titration, participants completed an fMRI alcohol taste-cues task. Analyses tested effects of naltrexone, OPRM1, and their interaction in whole-brain and region of interest (ROI) analyses of functional activation and functional connectivity in response to alcohol versus water taste cues.
Results:
We found no effects of naltrexone or OPRM1 on neural activation in whole-brain and ROI analyses, which included left and right ventral striatum (VS), anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC). Naltrexone increased functional connectivity between left VS and clusters in medial prefrontal cortex, posterior cingulate gyrus, as well as right VS and occipital cortex, compared to placebo.
Conclusions:
Naltrexone treatment enhanced functional connectivity in a key reinforcement-related pathway during alcohol versus water taste cues, corroborating neuroimaging work with other substances. Null medication and pharmacogenetics effects on functional activation add to a mixed naltrexone literature and may underscore the modest size of these effects in East Asians.
Keywords: naltrexone, pharmacogenetics, fMRI
1. INTRODUCTION
Endogenous opioid transmission mediates acute hedonic and subjective rewarding effects of alcohol consumption. Naltrexone, which functions predominately as an opioid receptor antagonist, attenuates endogenous opioid activity to reduce these motivationally salient effects of alcohol (Donoghue et al., 2015). Naltrexone reduces alcohol administration within the laboratory (O’Malley et al., 2002), neural responses to alcohol consumption and craving (Myrick et al., 2008; Schacht et al., 2017) and drinking behavior in real-world settings (Anton et al., 2006). Meta-analyses of naltrexone, however, have identified relatively modest effect sizes for relapse rates in treating alcohol use disorder (AUD), with variability in its effectiveness across individuals (Donoghue et al., 2015; Jonas et al., 2014). For this reason, efforts to identify potential moderators of naltrexone treatment response are underway to individualize and improve naltrexone pharmacotherapy.
Genetic contributions to variability in endogenous opioid transmission may be one moderator of naltrexone pharmacotherapy response (Krishnan-Sarin et al., 2007; Ray et al., 2012; Rubio et al., 2005). Given naltrexone’s high affinity for the mu-opioid receptor, studies have focused on a single nucleotide polymorphism (SNP) that encodes the binding affinity of this receptor (OPRM1; rs1799971). Individuals with at least one Asp40 allele (Asp40 carriers) exhibit up to three times greater binding affinity for beta endorphins compared to Asn40 homozygotes, and are posited to be more responsive to and experience better clinical outcomes when treated with naltrexone. However, evidence for this pharmacogenetic effect is mixed; meta-analyses of retrospective pharmacogenetic trials have found that the Asp40 allele may be associated with reduction in heavy drinking related to naltrexone pharmacotherapy (Chamorro et al., 2012; Jonas et al., 2014), though multiple laboratory studies (Anton et al., 2012; Ehlers et al., 2008; McGeary et al., 2006; Ziauddeen et al., 2016) and prospective pharmacogenetic trials have failed to replicate these effects (Oslin et al., 2015; Schacht et al., 2017).
The inconsistency of OPRM1 and naltrexone pharmacogenetic findings may be attributable to multiple causes, including heterogeneity in phenomenology of AUD, and the likely overall small effect size of OPRM1 on naltrexone treatment response (Donoghue et al., 2015). Relatedly, most studies examining pharmacogenetic effects have been limited to Caucasian samples due to concerns about population stratification effects. The OPRM1 Asp40 allele frequency varies across ethnicities, such that the minor allele frequency is approximately 20% in Caucasians, 5% in individuals of African ancestry, and up to 50% among individuals of East Asian descent (i.e., Chinese, Korean, or Japanese; Arias et al., 2006). In light of mixed findings regarding the Asn40Asp SNP in predominantly Caucasian samples with AUD, further study is needed to examine the role of OPRM1 variation in naltrexone-related outcomes within ethnically diverse populations.
Despite the high prevalence of the Asp40 allele in East Asian populations, only three studies have examined naltrexone pharmacogenetics in East Asian individuals. A small clinical trial in 32 Korean alcohol dependent patients found that Asp40 carriers who were medication-compliant had a longer time to relapse than Asn40 homozygotes (Kim, 2009). In a randomized, crossover laboratory pilot study from our group, 35 heavy drinkers of East Asian descent completed an intravenous alcohol (up to 0.06 g/dl) administration session after taking naltrexone or placebo for four days. Asp40 carriers, relative to Asn40 homozygotes, reported greater alcohol-induced sedation and subjective intoxication, and lower alcohol craving on naltrexone compared with placebo (Ray et al., 2012). However, a follow-up to that pilot study which included 77 heavy drinkers of East Asian descent found no pharmacogenetic effects for alcohol-induced stimulation, sedation, craving for alcohol, or alcohol self-administration in the laboratory. Asp40 carriers exhibited a longer latency to first drink and consumed fewer total drinks relative to Asn40 homozygotes across medication conditions (Ray et al., 2018). Further exploration of neural modulators of the pharmacogenetic effects of naltrexone in this population may help to elucidate the cause of this variability observed across studies.
Neuroimaging methods have been used to study neural substrates of Asn40Asp SNP effects on alcohol phenotypes, as evidence indicates that cue-induced neural activation may be an important predictor of treatment response (Courtney et al., 2016; Schacht et al., 2017). In a seminal study, Filbey and colleagues employed an fMRI-based alcohol taste-cue paradigm to activate the mesocorticolimbic circuitry underlying craving among heavy drinkers (Filbey et al., 2008a; Filbey et al., 2008b). This study found that among individuals homozygous for the short allele of the DRD4 exon 3 VNTR, Asp40 carriers had greater blood oxygenation level dependent (BOLD) response in mesocorticolimbic areas before and after a priming dose of alcohol, relative to control cues, compared to Asn40 homozygotes (Filbey et al., 2008b). Notably, however, a limitation of this study was the small sample of Asp40 carriers (n=11). A separate translational study combined intravenous alcohol administration with positron emission tomography (PET) to examine striatal dopamine (DA) response to alcohol in social-drinking men (Ramchandani et al., 2011); Asp40 carriers displayed greater striatal DA release in response to alcohol, compared to Asn40 homozygotes.
With respect to naltrexone neuroimaging studies, there is evidence that naltrexone attenuates alcohol cue-elicited activation of VS, anterior cingulate cortex (ACC), medial prefrontal cortex, and orbitofrontal cortex (OFC) - brain regions implicated in reward processing, decision making, and selective attention (Myrick et al., 2008; Schacht et al., 2013b; 2017). Some studies, however, have either not found injectable, extended-release naltrexone effects (XR-NTX) on cue-elicited VS activation (Lukas et al., 2013), or found that naltrexone increased VS activation (Spagnolo et al., 2014) in response to alcohol. Lukas and colleagues (2013), however, did find that XR-NTX reduced cue-elicited activation of the orbitofrontal and medial prefrontal cortex. Fewer studies have examined naltrexone’s effects on functional connectivity measures. One study of methamphetamine users found that naltrexone decreased functional connectivity between precuneus and sensorimontor regions and increased functional connectivity between dorsal striatum and precuneus with frontal regions, suggesting that naltrexone may alter communication between brain reward regions and those involved in executive function and effortful decision making (Courtney et al., 2016).
Results from neuroimaging studies of naltrexone and OPRM1 pharmacogenetic effects remain relatively mixed. Some studies have found that OPRM1 does not moderate the effects of naltrexone on alcohol infusion- and cue-elicited activation of VS among both alcohol-dependent treatment seekers (Spagnolo et al., 2014) and non-treatment seekers (Schacht et al., 2013b; Ziauddeen et al., 2016). In contrast, one study found that relative to Asn40 homozygotes, Asp40 carriers exhibited less OFC activation in response to alcohol cues (Schacht et al., 2013b), and that Asp40 carriers more quickly escalated to heavy drinking after discontinuing naltrexone (Schacht et al., 2017). Overall, these mixed results suggest a potential OPRM1 pharmacogenetic effect, but imply that mechanisms underlying this effect, particularly for localized functional activation, are less reliably replicated.
In light of the mixed literature on naltrexone and OPRM1 pharmacogenetics and the need to extend these findings to diverse populations, this study examined the pharmacogenetic effects of naltrexone on neural responses to alcohol taste cues in a sample of heavy drinking individuals of East Asian descent. The present study is an extension of our previous trial (Ray et al., 2018), whereby a subset of participants from our laboratory study completed a task involving the presentation of alcohol and water taste cues during fMRI. Specifically, we examined the pharmacogenetic effects on functional activation using both whole-brain and regions of interest (ROI) analyses, using a priori-defined anatomical ROIs (VS, ACC, OFC) that have been shown to be attenuated by naltrexone during alcohol craving (Mann et al., 2014; Schacht et al., 2013b; 2017). We also examined the pharmacogenetic effects on functional connectivity during alcohol taste cue presentation, using the left and right VS as seed regions that correspond to reward-related neural circuitry. Based on previous studies, we hypothesized that naltrexone, compared with placebo, would attenuate neural response to alcohol relative to water taste cues in the mesocorticolimbic pathway, and that naltrexone would do so to a greater extent in Asp40 carriers relative to Asn40 homozygotes. For functional connectivity, we anticipated that naltrexone would decrease VS connectivity with sensorimotor regions, and increase connectivity with precuneus and/or prefrontal cortex (Courtney et al., 2016); though largely exploratory, we hypothesized that naltrexone would produce greater such functional connectivity changes in Asp40 carriers relative to Asn40 homozygotes.
2. MATERIALS AND METHODS
2.1. Participants & Screening Procedures
Participants were recruited between July 2013 and December 2016 from the community through fliers, advertisements, and social media. Inclusion criteria were: 1. Alcohol-Use Disorders Identification Test (AUDIT; Allen et al., 1997) score ≥ 8; 2. East Asian ethnicity (i.e., self-identified as Chinese, Korean, Japanese, or Taiwanese); and 3. age 21–55 years old. Exclusion criteria were: 1. history of depression with suicidal ideation; 2. lifetime psychotic disorder; 3. current non-alcohol substance use disorder (except cannabis); 4. >10 on the Clinical Institute Withdrawal Assessment-revised (CIWA-R) (Sullivan et al., 1989); 5. currently seeking AUD treatment; 6. history of epilepsy, seizures, or severe head trauma; 7. nonremovable ferromagnetic objects in body; 8. claustrophobia; and 9. pregnancy. All participants were required to have a breath alcohol concentration (BrAC) of 0.00 g/dL before each neuroimaging session. The study was approved by the University of California, Los Angeles Institutional Review Board.
Initial assessment of the eligibility criteria was conducted through a telephone interview. Eligible participants were invited to the laboratory for additional screening. Upon arrival, participants completed informed consent procedures and provided a saliva sample for DNA analyses (see supplementary materials). Participants then completed a series of measures and interviews, including the 30-day Timeline Follow-back (TLFB; Sobell et al., 1986). All participants were required to test negative on a 10-panel urine drug test (except for marijuana). This panel assesses for amphetamines, methadone, tetrahydrocannabinol (marijuana), benzodiazepines, barbiturates, methamphetamine, phencyclidine, cocaine, opiates, and oxycodone. Prospective genotyping was not utilized in this study due to the anticipated allele frequency of nearly 50% and the previously successful utilization of this approach by our group (Ray, Lara A et al., 2012). Eligible participants completed a physical examination at the UCLA Clinical and Translational Research Center (CTRC) to determine medical eligibility. A total of 199 participants were screened in the laboratory, and 106 completed the physical exam, 5 of whom were ineligible for medical reasons and 14 of whom declined participation in the parent laboratory study. Of the 87 individuals randomized to the parent study, 7 individuals reported MRI contraindications and 6 declined to participate in the neuroimaging study. The study scanner was upgraded during the end of the study; due to concerns related to changes in scanner parameters and image quality, scanning data were not collected for 12 MRI-eligible participants at the end of the study. Therefore, 62 participants were randomized for the current study, 48 of whom completed both neuroimaging sessions. Of these 48 participants, we excluded 7 participants due to excessive motion (>2 mm translation) and/or poor registration. The final analyzed sample consisted of 41 participants. See Figure 1 for a CONSORT Diagram for this trial.
Figure 1.
CONSORT Diagram
*The scanner utilized for the study was upgraded towards the end of the study. Due to parameter compatibility concerns, scanning data was not collected from 12 MRI-eligible participants.
2.2. Medication Procedures
Participants were assigned to a medication sequence based on randomization pattern of ABBA. Participants completed one fMRI session after taking naltrexone for 4 days (25 mg for days 1–2, 50 mg for days 3–4) and one fMRI session after taking a matched placebo for 4 days (minimum 7-day wash-out between conditions). Active medication and placebo were delivered in a counterbalanced and double-blinded fashion. Participants were asked to report any side effects to the study physician. A series of non-parametric Fisher’s exact tests, accounting for small cell sizes (Fisher, 1922), were conducted to examine 24 possible side effects from the medication (Levine and Schooler, 1986). Five participants dropped out of the study as a result of anticipated medication side effects. Active medication and placebo capsules were packaged with 50mg of riboflavin allowing for medication compliance to be visually examined via urine samples collected prior to each lab visit. As analyzed under ultraviolet light (Del Boca et al., 1996), all samples tested positive for riboflavin content.
2.3. fMRI Scanning Visit
At the start of the scanning visit, participants were required to have a BrAC of 0.00 g/dL, a negative urine toxicology screen for all drugs (excluding marijuana), and a negative pregnancy screen for female participants. Participants who smoked cigarettes were allowed to smoke 30 minutes prior to the scan to prevent cigarette craving. To assess for pre-scan alcohol craving, participants completed the Alcohol Urges Questionnaire (AUQ) immediately before entering the scanner (Bohn et al., 1995).
2.4. fMRI Task
The taste cues task employed was a modification of the Alcohol Taste Cues Task (Filbey et al., 2008a; Filbey et al., 2008b), which has been previously used in our laboratory (Courtney et al., 2014; Courtney et al., 2015; Courtney and Ray, 2014; Ray et al., 2014). Each trial began with the presentation of a visual cue such that the words Alcohol or Water were visually presented to participants (2 second duration). This was followed by a fixation cross (duration jittered using an exponential distribution with a mean of 3 seconds and a range of 0.5 to 6 seconds), presentation of the word Taste upon which corresponding liquid was delivered (2 mL alcohol or water; 5 seconds), and a second fixation cross (duration jittered as above). All visual cues corresponded with the delivered liquid for that trial. Alcohol and water tastes were delivered through Teflon tubing using a computer-controlled delivery system (Infinity Controller) as described by Filbey and colleagues (Filbey et al., 2008a). Participants were instructed to press a button on a response box to indicate the point at which the bolus of liquid was swallowed. Alcohol tastes consisted of participants’ preferred wine (either red or white), which has been effective in eliciting alcohol-cue related activation in previous studies from our group (Ray et al., 2014). Beer could not be administered due to incompatibility of the alcohol administration device with carbonated liquids. A total of 16 participants from the final analyzed sample chose white wine and 25 participants chose red wine, and 5 total participants overall reported wine as their preferred alcohol. Visual stimuli and response collection were programmed using MATLAB (Mathworks, Natick, MA) and the Psychtoolbox (www.psychtoolbox.org), and visual stimuli were presented using MRI-compatible goggles (Resonance Technologies, Van Nuys, CA). The taste cues task was administered over the course of two runs with 50 trials per run.
2.5. Analytic Plan
Information regarding image acquisition parameters and preprocessing steps are available in Supplementary Materials. The main contrast of interest was difference in activation corresponding to alcohol taste delivery relative to water delivery, across the two task runs (Alcohol > Water); however, all variations of this contrast were modeled (i.e., Water > baseline, Alcohol > baseline, Water > Alcohol), as well as time periods corresponding with the visual text prior to taste delivery. These analyses were conducted for each within-subject medication condition. Group-level analyses utilized FSL’s FLAME 1 (Woolrich et al., 2004) with outlier deweighting (Woolrich, 2008); Z-statistic images were thresholded with cluster-based corrections for multiple comparisons based on the theory of Gaussian Random Fields with a cluster-forming threshold of Z > 2.3 and a cluster-probability threshold of p < 0.05 (Worsley, 2001).
Pre-test comparisons were conducted to determine whether OPRM1 groups differed on demographic and drinking variables using t-tests and chi-square tests with a significance threshold of p < 0.05. To ensure that activation from the main contrast of Alcohol > Water was not broadly driven by genetic differences in neural activation, OPRM1 effects were examined for Alcohol Taste and Water Taste separately. Multilevel mixed models were used to test group level aims, specifically to assess the effects of medication, OPRM1 genotype, medication × OPRM1 genotype interaction on task-related activation for whole-brain and ROI analyses. The primary dependent variable was the contrast of Alcohol > Water. Medication was a two-level within-subjects factor [naltrexone (NTX) and placebo (PLAC)] and OPRM1 genotype was a two-level between-subjects factor (Asp40 carriers and Asn40Asn). A 3-level genotype analysis (Asp40Asp, Asp40Asn, Asn40Asn) was not conducted due to small cell sizes. Pre-scan AUQ scores, AUDIT total scores TLFB number of drinking days and days since last drink, gender, age, and cigarette and marijuana use status were examined as potential covariates in separate whole brain and ROI functional activation analyses. To further validate that medication effects were not impacted by alcohol metabolizing genes, all analyses examined ALDH2 (rs671) and ADH1B (rs1229984) markers as potential covariates, but these genotypes were ultimately not significantly associated with activation for any of the primary analyses.
2.6. ROI Analyses
Based on previous studies examining alcohol and cue-induced craving (Aalto et al., 2015; Ray et al., 2015; Schacht et al., 2013a; Schacht et al., 2017), four anatomically-defined a priori regions of interest were utilized to examine pharmacogenetic effects on functional activation, including left and right VS, bilateral ACC, and bilateral OFC. ROIs were anatomically defined using the Harvard-Oxford atlas (in standard MNI space) and transformed into individual participants’ native space using FSL’s FLIRT (see Figure S1). Mean contrast estimate values from the Alcohol > Water contrast were extracted from these regions for each subject and submitted to mixed models for group-level analyses.
2.7. PPI Analyses
Functional connectivity analyses were conducted in FSL 5.0 using psychophysiological interaction (PPI) analyses which examines the interaction of task conditions and functional connectivity between the time course of activation for specific seed regions with the rest of the brain (O’Reilly et al., 2012). Based on previous work that utilized anatomically-defined left and right VS as primary regions of interest (Schacht et al., 2017), PPI analyses were conducted to examine the interaction of the Alcohol > Water contrast and the left and right VS seed regions for the comparisons: NTX > PLAC and PLAC > NTX. The first-level PPI models included four regressors: 1) Alcohol - Water; 2) Alcohol + Water; 3) “physiological” regressor modeling the seed time course; and 4) interaction regressor (regressor 1 multiplied by regressor 3). Whole-brain contrast images were generated separately for the left and right VS seed regions, with cluster-forming thresholds of Z>2.3 and cluster-probability thresholds of p<0.05.
3. RESULTS
3.1. Baseline and Demographic Comparisons
The pre-test comparisons on demographic and drinking variables revealed no significant OPRM1 genotype group differences across demographic variables (p’s ≥ 0.12; see Table 1). Results revealed a trend for a genotype difference in drinking days and days since last drink over the past 30 (p’s = 0.06–0.07), although no other alcohol or substance use variables approached significance (p’s ≥ 0.15). There were no significant differences in pre-scan craving or reported side effects between conditions (p’s > 0.18). There were also no significant differences in dropout or reported side effects by genotype (p’s > 0.46).
Table 1.
Pretest Differences Between Genotype Groups
| Variablea | Asn40Asn (n=18) | Asn40Asp/Asp40Asp (n=23) | Test for Difference |
|---|---|---|---|
| Gender | χ2 (1) = .146, p = 0.702 | ||
| Female (%) | 6 (33%) | 9 (39%) | |
| Male (%) | 12 (67%) | 14 (61%) | |
| Ethnicity | Fisher’s exact test, p = 0.20 | ||
| Chinese (%) | 8 (44%) | 7 (30%) | |
| Japanese (%) | 0 (0%) | 3 (13%) | |
| Korean (%) | 7 (39%) | 12 (52%) | |
| Taiwanese (%) | 3 (17%) | 1 (4%) | |
| ALDH2b | Fisher’s exact test, p = 0.21 | ||
| *I/*I (%) | 17 (94%) | 18 (78%) | |
| *I/*2 (%) | 1 (6%) | 5 (22%) | |
| *2/*2 (%) | 0 (0%) | 0 (0%) | |
| ADH1Bb | Fisher’s exact test, p = 0.99 | ||
| *I/*I (%) | 8 (44%) | 11 (48%) | |
| *I/*2 (%) | 8 (44%) | 9 (39%) | |
| *2/*2 (%) | 2 (11%) | 3 (13%) | |
| Agec | 30.17 (8.61) | 26.78 (5.00) | t(39) = 1.58, p = 0.12 |
| AUDd | Fisher’s exact test, p = 0.57 | ||
| None | 9 (50%) | 9 (39%) | |
| Mild | 5 (28%) | 11 (48%) | |
| Moderate | 2 (11%) | 2 (9%) | |
| Severe | 2 (11%) | 1 (4%) | |
| AUDITe | 15.39 (4.89) | 13.74 (5.41) | t(39) = 1.01, p = 0.32 |
| Drinking Daysf | 15.78 (7.49) | 12.00 (5.33) | t(39) = 1.89, p = 0.07 |
| Drinks/Drinking Dayf | 5.38 (2.78) | 4.32 (1.75) | t(39) = 1.48, p = 0.15 |
| Marijuana Daysf | 1.50 (2.64) | 2.17 (4.91) | t(39) = −0.53, p = 0.60 |
| PLAC Days since Drinkf | 1.83 (1.10) | 2.78 (1.78) | t(39) = 1.98, p = 0.06 |
| NTX Days since Drinkf | 2.33 (1.53) | 3.26 (1.66) | t(39) = −1.84, p = 0.07 |
| PLAC pre-scan AUQ | 8.11 (6.06) | 7.53 (5.39) | t(39) = 0.33, p = 0.77 |
| NTX pre-scan AUQ | 5.94 (6.46) | 5.53 (5.64) | t(39) = 0.20, p = 0.84 |
Standard deviations appear within parentheses for continuous variables.
*I/*I = GG, *I/*2= AG, *2/*2 = AA.
Assumption of homogeneity of variance not met, adjusted degrees of freedom, t-statistic, and significance level accounted for within table.
Current (past 3 months) Alcohol Use Disorder (AUD) assessed by the Structure Clinical Interview for Alcohol Use Disorder (DSM-5).
Alcohol Use Disorder Identification Test (AUDIT) score > 8 indicates hazardous drinking pattern; possible range of scale: 0 – 40.
Assessed by Timeline Follow Back (TLFB) interview for the past 30 days
3.2. Main Effect of Task (Alcohol > Water Contrast)
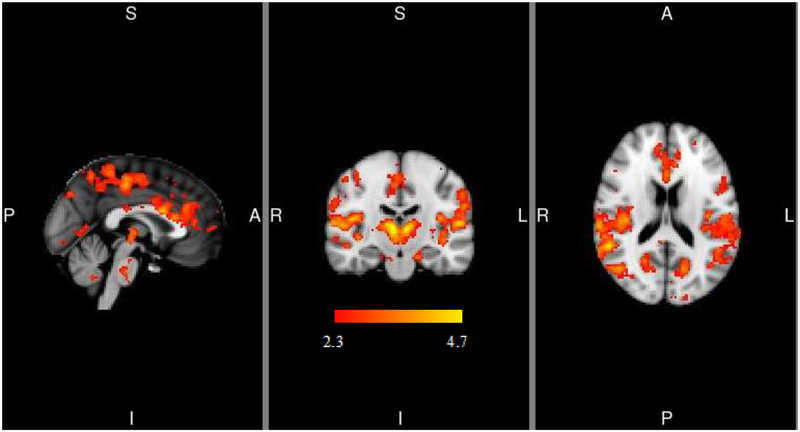
Within the placebo condition, alcohol taste cues, compared to water taste cues, elicited eight clusters of activation at the whole-brain level, including the thalamus, precuneus, occipital cortex, parietal operculum cortex, and temporal and angular gyri, and central opercular cortex (see Figure 2 and Table 2). Whole brain activation clusters did not differ as a function of medication condition.
Figure 2.
Alcohol > Water Taste task-related activation. MNI coordinates for depicted slices are X = 0, Y = −18, Z = 18. Color bar represents z-values. L=left, R=right, S=superior, I=inferior, A=anterior, P=posterior
Table 2.
Alcohol > Water contrast cluster peaks.
| Peak MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Cluster region | X | Y | Z | # Voxels | Max-Z | p-value |
| Left thalamus | 0 | −20 | −4 | 560 | 21.9 | 1.79E-07 |
| Right Parietal operculum cortex | 62 | −20 | 12 | 479 | 17.6 | 1.13E-06 |
| Right Inferior temporal gyrus | 44 | −68 | −18 | 396 | 18.1 | 8.17E-06 |
| Precuneus | 6 | −78 | 44 | 222 | 19.7 | 0.0009 |
| Left Middle temporal gyrus | −48 | −60 | 8 | 155 | 10 | 0.0078 |
| Precentral gyrus | 0 | −26 | 46 | 154 | 15.2 | 0.0081 |
| Right Angular gyrus | 64 | −52 | 18 | 147 | 13.1 | 0.0103 |
| Left Central opercular cortex | −58 | −20 | 12 | 138 | 13.5 | 0.0141 |
3.3. Naltrexone and Genotype Effects: Whole Brain Analyses
There were no significant effects of medication condition on whole-brain activation for the Alcohol > Water contrast. Activation related to the Alcohol > Water contrast was also not found to significantly differ by OPRM1 genotype. Finally, there was no pharmacogenetic effect (OPRM1 × Medication) on Alcohol > Water activation. Controlling for age, sex, pre-scan AUQ, AUDIT, number of drinking days and days since last drink, cigarette smoking and marijuana status, and ALDH2 and ADH1B genotypes did not alter these results. Of note, there were also no significant differences between medication conditions or OPRM1 genotype groups on activation in response to the alcohol taste or water taste alone relative to baseline. Uncorrected medication effects for the Alcohol > Water contrast and by OPRM1 genotype are depicted in Figures S2 and S3.
3.4. ROI Analyses
For left VS, there was no significant medication effect [F(1,39) = .05, p = 0.82] or medication by OPRM1 genotype interaction [F(1,39) = .12, p = 0.73]. There was, however, a significant main effect of OPRM1 genotype [F(1,39) = 4.26, p = 0.05, ηp2= .10], such that Asp40 carriers exhibited higher left VS activation than Asn homozygotes (parameter estimate M(SD) = 4.11(15.49) and −1.66(13.15), respectively). For the right VS, there was no significant medication effect [F(1,39) = 1.20, p = 0.28], OPRM1 effect [F(1,39) = .67, p = 0.42], or pharmacogenetic effect by OPRM1 genotype [F(1,39) = 1.02, p = 0.32].
For ACC, there was no significant medication effect [F(1,38) = .45, p = 0.51] or medication by OPRM1 genotype interaction [F(1,34) = .10, p = 0.75]. There was, however a significant OPRM1 effect [F(1,38) = 5.82, p = 0.02, ηp2= .13], such that Asp40 carriers exhibited higher ACC activation than Asn40 homozygotes (parameter estimate M(SD) = 8.27(13.40) and 4.24(16.00), respectively). Significant covariates included 30-day TLFB drinks per drinking day [F(1,38) = 4.20, p = 0.04].
For OFC, there was no significant medication effect [F(1,37) =.07, p = 0.79] or medication by OPRM1 genotype interaction [F(1,37) = 2.13, p = 0.15]. There was, however, a significant OPRM1 effect [F(1,37) = 6.20, p = 0.02, η p2= .14], such that Asp40 carriers exhibited higher OFC activation than Asn40 homozygotes (parameter estimate M(SD) = 4.00(8.32) and 0.29(9.60), respectively). Drinks per drinking day in the last 30 days (as measured by TLFB) [F(1,37) = 5.99, p = 0.02] showed a significant relationship with OFC activation, and there was a trending effect of sex [F(1,37) = 3.36, p = 0.08].
3.5. PPI Analyses
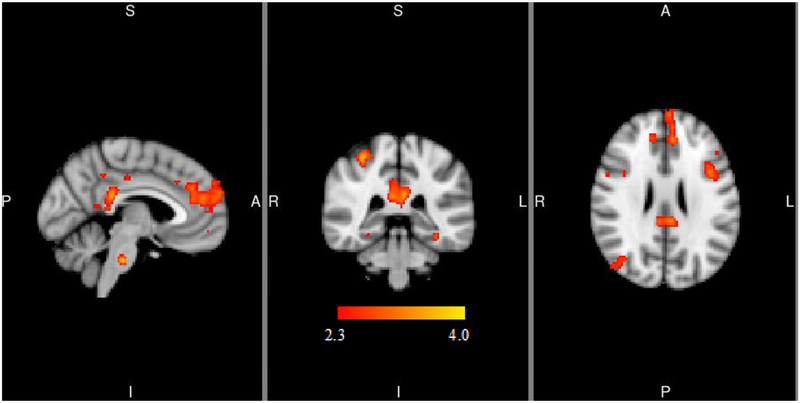
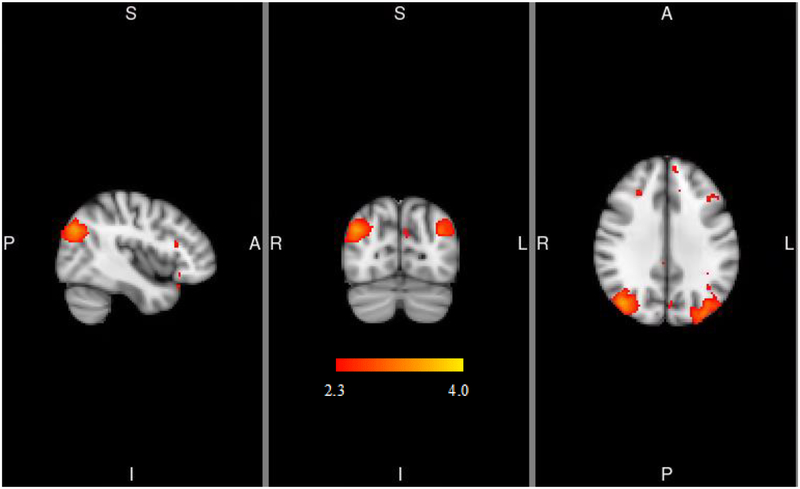
For the left VS seed, PPI analyses indicated that, relative to placebo, naltrexone elicited stronger connectivity with the frontal pole and cingulate gyrus within the Alcohol > Water contrast (see Figure 3A and Table 3). For the right VS seed, PPI results indicated that naltrexone relative to placebo elicited stronger connectivity with the clusters in the lateral occipital cortex within the Alcohol > Water contrast (see Figure 3B and Table 3). There were no differences in functional connectivity or the “physiological” regressor maps by OPRM1 genotype, nor was there a pharmacogenetic effect of naltrexone and OPRM1 on functional connectivity for either the right or left VS. Controlling for age, sex, pre-scan AUQ, AUDIT, number of drinking days and days since last drink, cigarette smoking and marijuana status, and ALDH2 and ADH1B genotypes did not alter these results.
Figure 3.
PPI analyses indicating functional connectivity of left (3a) and right (3b) ventral striatum during alcohol cue presentations. MNI coordinates for depicted slices are X = −4 (left), Y = −36 (middle), Z = 24 (right) in 3a and X = 42 (left), Y = −74 (middle), Z = 32 (right) in 3b. Color bar represents z-values. L=left, R=right, S=superior, I=inferior, A=anterior, P=posterior
Table 3.
Significant clusters for psychophysiological interaction analyses using the Alcohol > Water contrast
| Peak coordinates | ||||||
|---|---|---|---|---|---|---|
| Cluster region | X | Y | Z | # Voxels | Max-Z | p-value |
| Left Ventral Striatum PPI | ||||||
| Frontal pole | −2 | 58 | 20 | 971 | 3.28 | 0.006 |
| Cingulate gyrus | −2 | −36 | 24 | 662 | 3.33 | 0.045 |
| Right Ventral Striatum PPI | ||||||
| Right Lateral occipital cortex | 42 | −74 | 32 | 865 | 4.07 | 0.02 |
| Left Lateral occipital cortex | −34 | −84 | 32 | 704 | 3.67 | 0.04 |
4. DISCUSSION
In light of the mixed literature on naltrexone and OPRM1 pharmacogenetic effects, the current study examined neural pharmacogenetic effects of naltrexone and OPRM1 within a sample of heavy drinkers of East Asian ancestry. Relative to Asn40 homozygotes, Asp40 carriers exhibited increased activation in VS, ACC, and OFC during alcohol versus water taste cues. Overall, we did not find a significant medication or pharmacogenetic effect on functional activation during alcohol taste cues in this sample. Naltrexone did, however, increase functional connectivity between left VS and posterior cingulate cortex and medial prefrontal cortex, as well as increase functional connectivity between right VS and occipital cortex. Similar to the localized functional activation results, there was no pharmacogenetic effect on functional connectivity.
These results replicate previous studies that have found that OPRM1 Asp40 carriers exhibit greater VS, vmPFC, and OFC activation in response to alcohol taste cues among heavy drinkers (Filbey et al., 2008b; Ray et al., 2014); this study corroborates that the OPRM1 effect is likely small, as it has primarily been observed in ROI rather than whole-brain voxel-wise results, and this result has not been replicated in visual alcohol cue studies with alcohol dependent individuals (Schacht et al., 2013b). The results of the current study also suggest that these OPRM1 effects may be localized to reward processing regions, without significantly impacting functional interactions between VS and other brain regions. Notably, the lack of genotype differences in functional connectivity for left and right VS contrast earlier findings that, relative to Asn40 homozygotes, Asp40 carriers exhibit reduced cue-induced connectivity between VS and insula, frontal medial cortex, thalamus, putamen, and paracingulate gyrus (Ray et al., 2014). These differing results may in part be due to the higher average AUD severity in this previous study. As alcohol dependence severity is associated with weakened frontostriatal connectivity and dysregulated activity during effortful decision making (Courtney et al., 2013; Lim et al., 2017), these results suggest that Asp40 carriers with more severe AUD require increased recruitment of frontal systems to regulate striatal reward processing regions.
The present results suggest that naltrexone may affect communication between brain regions to a greater degree during alcohol relative to water tastes than localized region activation specifically, as there were no significant effects of naltrexone relative to placebo on localized functional activation during consumption of alcohol relative to water taste cues. Notably, nonsignificant naltrexone effects were found both at the whole-brain voxel-wise level and in ROI analyses of reward processing regions (namely, VS, ACC, and OFC) that have previously been shown to be attenuate with naltrexone during alcohol consumption and cue paradigms (Mann et al., 2014; Myrick et al., 2008; Schacht et al., 2013b; 2017),. These null findings do, however, corroborate and extend previous studies that have failed to observe significant naltrexone-induced changes in VS and in response to alcohol cues (Lukas et al., 2013) or during a monetary incentive delay task (Nestor et al., 2017).
Despite null localized functional activation results, naltrexone increased functional connectivity between left ventral striatum and medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC), regions implicated in coordinating attentional focus, decision making, and other executive functions (Hayden et al., 2009; Mashhoon et al., 2014). Intrinsic connectivity distribution analyses have indicated that individuals with AUD exhibit blunted cingulate connectivity with frontal regions, thalamus, and precuneus in response to both alcohol and stress cues, and PCC connectivity with frontoparietal regions specifically predicted a longer time to relapse in an AUD treatment study (Zakiniaeiz et al., 2017). With respect to mPFC, nucleus accumbens-mPFC connectivity during a monetary reward task has been shown to be negatively associated with drinking frequency and family history of AUD (Forbes et al., 2014). Altogether, these results suggest that connectivity among VS, mPFC, and PCC could be potential pathways of action for naltrexone.
The few naltrexone studies that have examined functional connectivity vary in analysis parameters, populations of interest, and study designs. These studies have shown that naltrexone modulates connectivity between ACC and hippocampus as a function of childhood adversity during an emotional priming task among alcohol-dependent individuals (Savulich et al., 2017), and that naltrexone improves local network efficiency in alcohol dependent individuals, reaching that of healthy controls (Morris et al., 2018). Most notably, in a study of methamphetamine users, naltrexone decreased connectivity between precuneus and sensorimontor regions and increased connectivity between dorsal striatum and precuneus with frontal regions (Courtney et al., 2016). This study’s results, therefore, go against our hypotheses and do not replicate these previous results regarding sensorimotor connectivity; future studies with both alcohol and methamphetamine-using populations are warranted to determine the reliability of such connectivity results. This study’s results do, however, corroborate a potential common effect of naltrexone across alcohol and methamphetamine through strengthened connections between frontal systems and reward processing regions. This result, in theory, may indicate greater activation of self-control networks in the brain over reward signals, following naltrexone treatment, and as compared to placebo.
Naltrexone also increased connectivity between right VS and occipital cortex. This is an unexpected finding, as most studies have either not observed or not examined an impact of naltrexone on this functional connectivity pattern or on occipital cortex activation (Mann et al., 2014; Schacht et al., 2013b; 2017). However, most visual alcohol cue studies find significant cue-elicited activation in occipital cortex (Hanlon et al., 2014), and one study found that naltrexone attenuates occipital cortex activation, thereby reducing salience of visual substance-related cues (Lukas et al., 2013). Interactive occipital cortex functional activation during cue and taste paradigms are not well-understood, and future functional connectivity studies may help to elucidate the significance and replicability of this particular finding.
There is a growing literature on the predictive value of cue-induced neural activation for real-world clinical outcomes in drug cessation (Courtney et al., 2016; Schacht et al., 2013b; 2017; Zakiniaeiz et al., 2017). Incorporating underrepresented groups in pharmacogenetics studies is critical for addressing health disparities in the context of personalized medicine (Cservenka et al., 2017). This study provides initial evidence that pharmacogenetic effects of naltrexone and OPRM1 are not supported in non-treatment seeking heavy drinkers of East Asian descent, with respect to alcohol taste-elicited neural activation. It is plausible that a robust effect in tightly controlled preclinical and experimental medicine models “fades” in the context of complex, real world clinical application and with heterogeneity of AUD. (Ray et al., 2012).
Importantly, these results should be interpreted in light of the human laboratory arm of the study, which found no support for pharmacogenetic effects of OPRM1 and naltrexone among individuals of East Asian descent (Ray et al., 2018) for alcohol-induced stimulation, sedation, craving for alcohol, or alcohol self-administration. There were no main effects of medication on those phenotypes, and the main effect of genetics on alcohol self-administration suggested that the Asp40 allele was protective for alcohol self-administration. In the context of significant naltrexone effects on functional connectivity in the absence of pharmacogenetic effects, these findings in East Asians add to the rather mixed literature on naltrexone pharmacogenetics in predominantly Caucasian samples and highlight the complexity of these effects and their overall limited replicability.
There were several notable study strengths, including a within-group, double-blind, randomized design, pharmacogenetic testing in a population that has a balanced OPRM1 allele frequency distribution, and consideration of multiple genetic and individual difference covariates. There were also several important study limitations. While the taste cues paradigm is based upon validated fMRI paradigms, the iteration utilized in this study increased the number of trials administered at the expense of reducing the duration of each individual trial. Future replication studies may be needed to further validate this taste paradigm, particularly as the main contrast of interest (Alcohol > Water) did not yield significant clusters of activation in the VS in the whole-brain analysis, and a post-scan AUQ was not conducted. Though this contrasts with other fMRI and PET alcohol taste studies (Oberlin et al., 2016; 2013; Schacht et al., 2013a), this lack of activation has been replicated in alcohol infusion studies with alcohol dependent treatment-seeking patients (Spagnolo et al., 2014) and alcohol olfactory cues studies (Lukas et al., 2013). It is possible that longer trial durations may be required to reliably recruit VS activation, though it is notable that naltrexone modulated functional connectivity despite this potential limitation. Drink choice was also limited to red or white wine, and these results warrant replication with other types of alcohol preference, particularly as only a minority of the sample reported wine as their preferred alcohol and this could potentially impact neural activation in response to a taste cue. Larger samples may also be required to identify effects of specific individual characteristics such as sex and cigarette smoking status that have been shown to moderate naltrexone response (Fridberg et al., 2014; King et al., 2012). Similarly, while pharmacogenetics effects are theoretically testable in absence of a main medication effect, it is possible that decreased variability and/or power of naltrexone-induced as well as general task-induced neural activation may have made it difficult to detect a pharmacogenetic effect; one potential explanation for a nonsignificant main effect may have been the relatively short duration of naltrexone treatment in the current study (4 days) relative to longer durations (7–14 days) reported in other studies (Lukas et al., 2013; Myrick et al., 2008; Schacht et al., 2017). Relatedly, riboflavin testing was conducted via visual inspection rather than quantitative testing; as riboflavin concentrations of 900 ng/mL have been established to visually classify positive samples 2 to 24 hours after ingestion (Herron et al., 2013), it is possible that the 100% adherence rate may refer to these more immediate periods rather than full compliance over the titration period. Additionally, while the sample consisted of heavy drinkers, approximately half of the sample did not meet criteria for an alcohol use disorder; future studies may benefit from examining these pharmacogenetic effects in individuals with more severe drinking, as higher alcohol dependence severity may be predictive of cue reactivity (Sjoerds et al., 2014).
In sum, this study does not support a pharmacogenetic effect for naltrexone and OPRM1 on alcohol taste-induced neural activation in individuals of East Asian descent. There was no medication effect on localized functional activation, yet naltrexone increased functional connectivity during alcohol taste between regions involved in reward processing and frontal regions critical to executive function. On balance, these results add to a mixed naltrexone literature that has primarily been conducted in Caucasian individuals, and corroborate a potential common effect of naltrexone on functional connectivity across substances.
Supplementary Material
Highlights.
Among East Asian individuals, naltrexone relative to placebo increased functional connectivity between reward regions and anterior cingulate and orbitofrontal cortex.
There were no naltrexone or pharmacogenetic naltrexone by OPRM1 genotype effects on neural localized functional activation in response to alcohol versus water taste cues.
These results add to a mixed naltrexone literature and may underscore the modest size of these effects in East Asian individuals.
ACKNOWLEDGEMENTS
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (R01AA021744) and the UCLA Clinical and Translational Science Institute (CTSI) (grants UL1RR033176 and UL1TR000124). ACL, ENG, and RG have received funding from the National Institute on Drug Abuse as trainees (4T32 DA007272–25 (ACL) and T32DA024635–10 (ACL, ENG, and RG)). KEC is supported by a National Institute of Mental Health postdoctoral training grant (T32MH018399). LAR has received study medication from Pfizer and Medicinova and consulted for GSK. None of the authors have other conflicts of interest to disclose.
Role of Funding Source
Nothing declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
LAR has received study medication from Pfizer and Medicinova and consulted for GSK. None of the authors have other conflicts of interest to disclose.
REFERENCES
- Aalto S, Ingman K, Alakurtti K, Kaasinen V, Virkkala J, Nagren K, Rinne JO, Scheinin H, 2015. Intravenous ethanol increases dopamine release in the ventral striatum in humans: PET study using bolus-plus-infusion administration of [(11)C]raclopride. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 35(3), 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Litten RZ, Fertig JB, Babor T, 1997. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcoholism: Clinical and Experimental Research 21(4), 613–619. [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, 2006. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama 295(17), 2003–2017. [DOI] [PubMed] [Google Scholar]
- Anton RF, Voronin KK, Randall PK, Myrick H, Tiffany A, 2012. Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcoholism, clinical and experimental research 36(11), 2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A, Feinn R, Kranzler HR, 2006. Association of an Asn40Asp (A118G) polymorphism in the μ-opioid receptor gene with substance dependence: a meta-analysis. Drug and alcohol dependence 83(3), 262–268. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA, 1995. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism, clinical and experimental research 19(3), 600–606. [DOI] [PubMed] [Google Scholar]
- Chamorro AJ, Marcos M, Mirón-Canelo JA, Pastor I, González-Sarmiento R, Laso FJ, 2012. Association of μ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addiction biology 17(3), 505–512. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, London ED, Ray LA, 2014. The association between cue-reactivity in the precuneus and level of dependence on nicotine and alcohol. Drug and alcohol dependence 141, 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA, 2013. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addiction biology 18(3), 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA, 2015. The effect of alcohol priming on neural markers of alcohol cue-reactivity. The American journal of drug and alcohol abuse 41(4), 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ray LA, 2014. Subjective responses to alcohol in the lab predict neural responses to alcohol cues. Journal of studies on alcohol and drugs 75(1), 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJ, Ray LA, 2016. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addiction biology 21(1), 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Yardley MM, Ray LA, 2017. Review: Pharmacogenetics of alcoholism treatment: Implications of ethnic diversity. The American journal on addictions 26(5), 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF, 1996. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcoholism, clinical and experimental research 20(8), 1412–1417. [DOI] [PubMed] [Google Scholar]
- Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C, 2015. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction (Abingdon, England) 110(6), 920–930. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Lind PA, Wilhelmsen KC, 2008. Association between single nucleotide polymorphisms in the mu opioid receptor gene (OPRM1) and self-reported responses to alcohol in American Indians. BMC medical genetics 9, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE, 2008a. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 33(6), 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE, 2008b. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcoholism, clinical and experimental research 32(7), 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA, 1922. On the interpretation of χ 2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 85(1), 87–94. [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R, 2014. Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PloS one 9(5), e94640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberg DJ, Cao D, Grant JE, King AC, 2014. Naltrexone improves quit rates, attenuates smoking urge, and reduces alcohol use in heavy drinking smokers attempting to quit smoking. Alcoholism, clinical and experimental research 38(10), 2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, Cortese BM, 2014. Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug and alcohol dependence 143, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML, 2009. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America 106(14), 5948–5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron AJ, Mariani JJ, Pavlicova M, Parrinello CM, Bold KW, Levin FR, Nunes EV, Sullivan MA, Raby WN, Bisaga A, 2013. Assessment of riboflavin as a tracer substance: Comparison of a qualitative to a quantitative method of riboflavin measurement. Drug and Alcohol Dependence 128(1–2), 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Wines R, Shanahan E, Rowe CJ, Garbutt JC, 2014. Genetic polymorphisms and response to medications for alcohol use disorders: a systematic review and meta-analysis. Pharmacogenomics 15(13), 1687–1700. [DOI] [PubMed] [Google Scholar]
- Kim SG, 2009. Gender differences in the genetic risk for alcohol dependence--the results of a pharmacogenetic study in Korean alcoholics. Nihon Arukoru Yakubutsu Igakkai zasshi= Japanese journal of alcohol studies & drug dependence 44(6), 680–685. [PubMed] [Google Scholar]
- King AC, Cao D, O’Malley SS, Kranzler HR, Cai X, deWit H, Matthews AK, Stachoviak RJ, 2012. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. Journal of clinical psychopharmacology 32(5), 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS, 2007. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biological psychiatry 62(6), 694–697. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR, 1986. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology bulletin 22(2), 343–381. [PubMed] [Google Scholar]
- Lim AC, Cservenka A, Ray LA, 2017. Effects of alcohol dependence severity on neural correlates of delay discounting. Alcohol and alcoholism 52(4), 506–515. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C, Penetar DM, 2013. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. NeuroImage 78, 176–185. [DOI] [PubMed] [Google Scholar]
- Mann K, Vollstadt-Klein S, Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN, 2014. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcoholism, clinical and experimental research 38(11), 2754–2762. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Czerkawski C, Crowley DJ, Cohen-Gilbert JE, Sneider JT, Silveri MM, 2014. Binge alcohol consumption in emerging adults: anterior cingulate cortical “thinness” is associated with alcohol use patterns. Alcoholism, clinical and experimental research 38(7), 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R Jr., 2006. Genetic moderators of naltrexone’s effects on alcohol cue reactivity. Alcoholism, clinical and experimental research 30(8), 1288–1296. [DOI] [PubMed] [Google Scholar]
- Morris LS, Baek K, Tait R, Elliott R, Ersche KD, Flechais R, McGonigle J, Murphy A, Nestor LJ, Orban C, Passetti F, Paterson LM, Rabiner I, Reed L, Smith D, Suckling J, Taylor EM, Bullmore ET, Lingford-Hughes AR, Deakin B, Nutt DJ, Sahakian BJ, Robbins TW, Voon V, 2018. Naltrexone ameliorates functional network abnormalities in alcohol-dependent individuals. Addiction biology 23(1), 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K, 2008. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Archives of general psychiatry 65(4), 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Murphy A, McGonigle J, Orban C, Reed L, Taylor E, Flechais R, Paterson LM, Smith D, Bullmore ET, Ersche KD, Suckling J, Tait R, Elliott R, Deakin B, Rabiner I, Lingford-Hughes A, Nutt DJ, Sahakian B, Robbins TW, 2017. Acute naltrexone does not remediate frontostriatal disturbances in alcoholic and alcoholic polysubstance-dependent populations during a monetary incentive delay task. Addiction biology 22(6), 1576–1589. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ, 2002. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology 160(1), 19–29. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H, 2012. Tools of the trade: psychophysiological interactions and functional connectivity. Social cognitive and affective neuroscience 7(5), 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Harezlak J, Kudela MA, Tran SM, Soeurt CM, Yoder KK, Kareken DA, 2016. Corticostriatal and Dopaminergic Response to Beer Flavor with Both fMRI and [(11) C]raclopride Positron Emission Tomography. Alcoholism, clinical and experimental research 40(9), 1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA, 2013. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 38(9), 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Leong SH, Lynch KG, Berrettini W, O’Brien CP, Gordon AJ, Rukstalis M, 2015. Naltrexone vs Placebo for the Treatment of Alcohol Dependence: A Randomized Clinical Trial. JAMA psychiatry 72(5), 430–437. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M, 2011. A genetic determinant of the striatal dopamine response to alcohol in men. Molecular psychiatry 16(8), 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Barr CS, Blendy JA, Oslin D, Goldman D, Anton RF, 2012. The role of the Asn40Asp polymorphism of the mu opioid receptor gene (OPRM1) on alcoholism etiology and treatment: a critical review. Alcoholism: Clinical and Experimental Research 36(3), 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Chin PF, Miotto K, 2012. Pharmacogenetics of naltrexone in asian americans: a randomized placebo-controlled laboratory study. Neuropsychopharmacology 37(2), 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED, 2015. Varenicline, naltrexone, and their combination for heavy-drinking smokers: preliminary neuroimaging findings. The American journal of drug and alcohol abuse 41(1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Hutchison KE, Mackillop J, Galvan A, Ghahremani DG, 2014. Initial evidence that OPRM1 genotype moderates ventral and dorsal striatum functional connectivity during alcohol cues. Alcoholism, clinical and experimental research 38(1), 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Green R, Roche DJO, Bujarski S, Hartwell EE, Lim AC, Rohrbaugh T, Ghahremani D, Hutchison K, Miotto K, 2018. Pharmacogenetic Effects of Naltrexone in Individuals of East Asian Descent: Human Laboratory Findings from a Randomized Trial. Alcoholism, clinical and experimental research 42(3), 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Ray LA, 2015. Subjective response as a consideration in the pharmacogenetics of alcoholism treatment. Pharmacogenomics 16(7), 721–736. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Rodriguez-Jimenez R, Jimenez-Arriero MA, Hoenicka J, Palomo T, 2005. Clinical predictors of response to naltrexone in alcoholic patients: who benefits most from treatment with naltrexone? Alcohol and Alcoholism 40(3), 227–233. [DOI] [PubMed] [Google Scholar]
- Savulich G, Riccelli R, Passamonti L, Correia M, Deakin JF, Elliott R, Flechais RS, Lingford-Hughes AR, McGonigle J, Murphy A, Nutt DJ, Orban C, Paterson LM, Reed LJ, Smith DG, Suckling J, Tait R, Taylor EM, Sahakian BJ, Robbins TW, Ersche KD, 2017. Effects of naltrexone are influenced by childhood adversity during negative emotional processing in addiction recovery. Translational psychiatry 7(3), e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H, 2013a. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addiction biology 18(1), 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Voronin KE, Randall PK, Li X, Henderson S, Myrick H, 2013b. Interacting effects of naltrexone and OPRM1 and DAT1 variation on the neural response to alcohol cues. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 38(3), 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, Anton RF, 2017. Predictors of Naltrexone Response in a Randomized Trial: Reward-Related Brain Activation, OPRM1 Genotype, and Smoking Status. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 42(13), 2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, van den Brink W, Beekman AT, Penninx BW, Veltman DJ, 2014. Cue reactivity is associated with duration and severity of alcohol dependence: an FMRI study. PloS one 9(1), e84560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E, 1986. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addictive Behaviors 11(2), 149–161. [DOI] [PubMed] [Google Scholar]
- Spagnolo PA, Ramchandani VA, Schwandt ML, Zhang L, Blaine SK, Usala JM, Diamond KA, Phillips MJ, George DT, Momenan R, Heilig M, 2014. Effects of naltrexone on neural and subjective response to alcohol in treatment-seeking alcohol-dependent patients. Alcoholism, clinical and experimental research 38(12), 3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, 1989. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐ Ar). British Journal of Addiction 84(11), 1353–1357. [DOI] [PubMed] [Google Scholar]
- Woolrich M, 2008. Robust group analysis using outlier inference. NeuroImage 41(2), 286–301. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM, 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage 21(4), 1732–1747. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, 2001. Statistical analysis of activation images, in: Jezzard P, Matthews PM, Smith SM (Eds.), Functional MRI: An Introduction to Methods. Oxford University Press. [Google Scholar]
- Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, Constable RT, 2017. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. NeuroImage. Clinical 13, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Nestor LJ, Subramaniam N, Dodds C, Nathan PJ, Miller SR, Sarai BK, Maltby K, Fernando D, Warren L, Hosking LK, Waterworth D, Korzeniowska A, Win B, Richards DB, Vasist Johnson L, Fletcher PC, Bullmore ET, 2016. Opioid Antagonists and the A118G Polymorphism in the mu-Opioid Receptor Gene: Effects of GSK1521498 and Naltrexone in Healthy Drinkers Stratified by OPRM1 Genotype. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 41(11), 2647–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.