Abstract
Although bioaccessibility assessment of ambient particulate matter (APM) is recently receiving increasing attention, limited research has been undertaken into standardising methodologies. The fraction of APM that is most relevant to a human inhalation scenario is PM10 (particles with <10μm in aerodynamic diameter), which may potentially enter and deposit in the lung lining fluid with a neutral pH. Contradictory suggestions exist in the literature regarding which assay parameters should be adopted, e.g. solid to liquid ratio (S/L), maximum extraction time, and the composition of simulated lung fluid (SLF). Particularly, 13 different SLF compositions have been used in the literature and their extraction efficiencies have not been investigated. Additionally, 90% of the PM10 depositing in the lung may be cleared within 24 hours by mucociliary mechanisms and pass through the gastro-intestinal (GIT) tract, with potential continuation of metal(loid) dissolution.
The objective of this study was to standardise a conservative inhalation-ingestion bioaccessibility assay (IIBA) by simulating the inhalation of PM10, including its transition into the ingestion pathway. To achieve this aim, the bioaccessibility of arsenic (As) and lead (Pb) in PM10 from three Australian mining/smelting impacted regions was used to investigate the effect of S/L (1:100–1:5000), extraction time (1–120 hours), agitation (occasional, orbital, magnetic stirring and end over end rotation) and five major SLF compositions. Using the biologically relevant parameters that resulted in the most conservative bioaccessibility outcome, the IIBA was developed by leaching PM10 in SLF, followed by simulated GIT solutions.
Results from this study revealed that fluid composition and solid to solution ratio most significantly (p<0.05) affected metal(loid) dissolution. The highest cumulative bioaccessibility of Pb was obtained using SLF + simulated gastric solution, while that of As was using SLF + simulated gastric + intestinal solutions. Compared to SLF alone, cumulative metal(loid) dissolution using the IIBA was significantly higher (p < 0.05) for both metal(loid)s for all PM10 samples.
Keywords: Bioaccessibility, PM10, Simulated lung fluid, Gamble, Hatch, Inhalation
Graphical Abstract
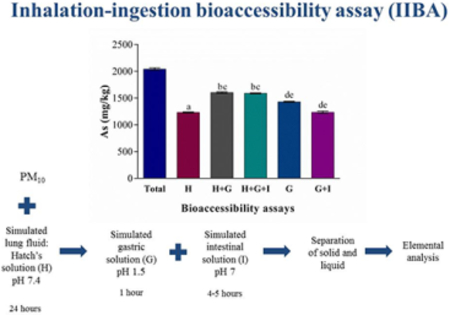
1. Introduction
Metal(loid) inhalation from ambient particulate matter (APM) is increasingly becoming recognised as a significant exposure pathway associated with potentially adverse human health outcomes. According to the USEPA, the fraction of APM that enters the respiratory system and deposits in the fluid lining the human lung has an aerodynamic diameter (Dae) of <10μm (PM10). High metal(loid) concentrations in PM10 are often reported near areas with current or historic industrial activities, such as mining, smelting and manufacturing. Manganese (Mn), nickel (Ni), zinc (Zn), chromium (Cr) and iron (Fe) in PM10 near industrial sites have recently been correlated to deteriorating respiratory health in Italy (Rosa et al., 2016). Inhalation of PM10 associated high concentrations of arsenic (As), lead (Pb), copper (Cu) and cadmium (Cd) in blood and urine have also been documented in the United States (Landrigan and Baker, 1981), Zambia (Ndilila et al., 2014), Africa (Nriagu, 1992) and Mexico (Díaz-Barriga et al., 1997). Additionally, inhalation of metal(loid)s from fly ash has been linked to acute and systemic pulmonary and cardiovascular toxicity in mice and rat (Wallenborn et al., 2007, Kodavanti et al., 1998, Costa and Dreher, 1997). Furthermore, a reduction of inflammatory responses in airway epithelial cells and rats by the removal of metal cations from PM10 extracts indicated that the metal(loid) component of PM10 was responsible for inflammation (Molinelli et al., 2002). Inhalation of PM10 associated metal(loid)s can therefore be considered a significant exposure pathway in many countries with industrial activities such as mining and smelting or traffic related air pollution (Zereini et al., 2012, Sysalová et al., 2014).
Upon inhalation, PM10 comes in contact with surfactants and fluid lining the human lung, which has a neutral pH (7.2–7.8) (Utembe et al., 2015). Although a fraction of the particles with Dae <2.5μm (PM2.5) are phagocytized by macrophages and 90% of particles with Dae 0.1–1μm are exhaled in subsequent breath according to the International Commission of Radiological Protection) (Carvalho et al., 2011), for the refinement of an inhalation bioaccessibility protocol, 100% deposition of PM10 in the fluid lining the human lung can be assumed. Instead of using total metal(loid) content, bioaccessibility (fraction that is dissolved in a simulated body fluid) and bioavailability (fraction that is absorbed in the systemic blood circulation) based assessment approaches are increasingly being adopted (Kastury et al., 2017). Particularly, bioaccessibility based approaches can be a rapid and cost effective way to assess exposure and are often preferable over animal dosing because of reduced ethical concerns.
However, in contrast to oral bioaccessibility, (metal(loid) dissolution in gastrointestinal (GIT) fluid), where several protocols have been developed and validated (Juhasz et al., 2009, Bradham et al., 2011, Smith et al., 2011b), inhalation bioaccessibility protocols using simulated lung fluid (SLF) remains inconsistent (Kastury et al., 2017, Wiseman, 2015). For example, the maximum suggested extraction time during inhalation bioaccessibility studies have ranged from 24 hours using certified reference materials in (Julien et al., 2011) to 96 hours using mine tailings in (Wragg and Klinck, 2007). Similarly, (Julien et al., 2011) suggested using a solid to liquid ratio (S/L) between 1/500 – 1/50,000, while (Sysalová et al., 2014) reported that bioaccessibility of As, Cr, Mn, Ni, Pb and Zn increased 37–141% as S/L is increased from 1/500 to 1/1000. Additionally, at least thirteen different SLF compositions have been reported in the literature exhibiting significant differences in the amount of proteins, surfactants, enzymes and antioxidants (Kastury et al., 2017, Boisa et al., 2014). Investigation into their comparative extraction efficiencies remain an important knowledge gap preventing the standardisation of an inhalation bioaccessibility method. Approximately 90% of the deposited particles are transported to the pharynx by mucociliary action in the tracheobronchial region and swallowed within 24 hours. Metal(loid)s from PM10 potentially undergo further solubilisation in the gastric and intestinal fluids, which has received little attention.
The overarching aim for this study was to develop a conservative bioaccessibility assay that provides the maximum metal(loid) dissolution using an SLF simulating a human inhalation scenario. To achieve this aim, PM10 from three environmental matrices were used to study the effect of S/L ratio, type of agitation, fluid composition and extraction time on the bioaccessibility. The parameters that provide the highest metal(loid) solubility were used in conjunction with simulated gastro-intestinal fluids to reflect the passage of PM10 via the gut. This novel approach is the first investigation to study metal(loid) bioaccessibility combining both inhalation and oral exposure pathways.
2. Materials and methods
2.1. Sample collection and size fractionation
Surface soil samples (0–20 cm) containing elevated concentrations of multiple metal(loid)s due to anthropogenic activities were collected from three Australian locations: Port Pirie (PP) with elevated Pb from smelting, York Peninsula (SH15) with historic nonferrous slag application, and calcinated mine waste (CMW) from the golden triangle region of Victoria with high concentrations of As resulting from thermal processing of gold ores. All samples were dried at 40°C. PP and SH15 samples were sieved to <2 mm (total soil), <250 μm (ingestible fraction) and <53 μm (stock sample), while CMW sample was sieved to <53 μm due to limited supply. The <53 μm fraction was further sieved to <10 μm to represent the coarse inhalable fraction or PM10 using an Endecotts Octagon digital shaker. All fractions were homogenised by end over end rotation (45 rpm) for 24 hours and stored at 20°C. While all size fractions were used for physico-chemical characterisation only, PM10 was used during the development of the IIBA.
2.2. Physico-chemical characterization
PM10 fractions (50 mg) were dispersed in 0.1M NaCl overnight and the particle size distribution analysed using Particle Sizer 380 ZLS (NICOPM). Approximately 50–100 mg of each fraction was thermally degassed (200°C) overnight using Vac Prep 061 (Micrometritics), followed by BET surface area analysis using ASAP 2420 (Micrometritics).
Speciation of metal(loid)s of interest (As, Fe and Pb) was determined using X-ray absorption spectroscopy (XAS) (MRCAT beamlines 10-ID (Pb) and 10-BM (As and Fe), Sector 10 (Segre et al., 2000; Kropf et al., 2010), at the Advanced Photon Source of the Argonne National Laboratory, U.S) according to methodologies detailed in (Ollson et al., 2016).
Total metal(loid) content was analysed by pre-digesting 0.1 g of <2 mm (SH15 and PP), <250 μm (SH15 and PP) and <10 μm samples (SH15, PP and CMW) (n=3) overnight using 5ml aqua regia (1:3 70% HNO3: 36.5% HCl) and digested in a MARS-6 microwave (CEM) following USEPA method 3051 (USEPA, 1998). Dissolved metal(loid)s were separated from the solid residue by syringe-filtering (0.45 μm) and stored at 4°C. Metal(loid) analysis was undertaken using inductively coupled plasma mass spectrometry (ICP-MS) (ASX-500 series) following EPA Method 6020A (EPA, 1998). Accuracy of digestion was confirmed using the recovery of Standard Reference Material (SRM) 2710a (n=3) from National Institute of Standards and Technology (NIST). The average recovery (n=3) of metal(loid)s of interest were As (107±0.4 %), Cd (98±1.4 %), Fe (79±1.2 %), Mn (83±1.0 %), Pb (107±1.3 %) and Zn (128±1.8 %).
2.3. The effect of assay parameters on metal(loid) bioaccessibility using SLF
2.3.1. The effect of solid to liquid (S/L) ratio
The range of S/L ratio tested in this assay were 1:100, 1:500, 1:1000, 1:5000 to represent the most widely used S/L ratios used in inhalation bioaccessibility assays. Gamble’s solution (100 ml) (Moss, 1979) was used in this assay as it is the most commonly used SLF. To prevent the pH from drifting upwards with time, at the start of the assay and during each sampling period, carbogen (95% oxygen and 5% carbon dioxide) was bubbled through the solution according to (Moss, 1979) and the pH was maintained between 7.2–7.8 (Utembe et al., 2015), while magnetic stirring was used to keep the particles from agglomerating. Samples were collected at 1, 8, 24, 48, 72, 96 and 120 h, ensuring that at least 90% of the fluid remained at the end of the assay. Separation of solid from solution was achieved by centrifugation at 13,000 rpm (18 g) (Wragg and Klinck, 2007) for 3 minutes. The supernatant was acidified with 0.1 M HNO3 and stored at 4° C until analysis by ICP-MS using matrix matched calibration standards. Metal(loid) bioaccessibility was calculated using the following equation:
2.3.2. The effect of agitation
The effect of agitation methods was assessed using occassional shaking (150 rpm by orbital rotation, once a day for 15 minutes), orbital rotation (150 rpm), magnetic stirring (speed of 1.5 using 3 cm flea) and end over end rotation (45 rpm). Based on the results of the previous experiment a S/L of 1:5000 was used, keeping other conditions and processes idential to 2.3.1.
2.3.3. The effect of fluid composition
Five SLFs representing the major categories of SLF based on their composition (Kastury et al., 2017) were used in this assay: original Gamble’s solution (Moss, 1979), modified serum simulant (Julien et al., 2011), modified Gamble’s solution (Twining et al., 2005), simulated epithelial lung fluid (SELF) (Boisa et al., 2014) and Hatch’s solution (Berlinger et al., 2008) (Table 1). Preliminary results showed that when headspace was minimised, the pH can be maintained between 7.2–7.8 using end over end shaking without carbogen bubbling. pH was adjusted between 7.2–7.8 at the start of the experiment and during each sampling. Assays were performed for up to 120 hours with collected samples centrifuged at 13,000 rpm (18g) for 3 minutes. Supernatants from Gamble’s solutions, modified serum solution and SELF were diluted using 0.1M HNO3 prior to analysis by ICP-MS. To avoid the formation of precipitates due to the high protein content in the modified Gamble’s and Hatch’s solution, supernatants were diluted using a biological extract solution (Technologies, 2006) (1-butanbol (2%), EDTA (0.5%), Triton-X 100 (0.5%), NH4OH (1%) prepared in MilliQ water). All samples were stored at 4°C and filtered using 0.45 μm syringe filters immediately before analysis using ICP-MS. Matrix matched calibration standards for each SLF were used during analysis by ICP-MS to avoid matrix bias.
Table 1:
Composition of simulated lung fluids
| References | Moss 1979 | Julien et al. 2011 | Berlinger et al. 2008 | Twining et al. 2005 | Boisa et al. 2014 |
|---|---|---|---|---|---|
| Name of solution | Original Gamble’s solution |
Serum simulant (SS) | Hatch’s solution | Simulated Lung Fluid (SLF) | Simulated Epithelial Lung Fluid (SELF) |
| Inorganic component | |||||
| Sodium chloride NaCl | 6.02 | 6.40 | 7.00 | 6.43 | 6.02 |
| Sodium hydrogen carbonate NaHCO3 | 2.60 | 2.70 | 2.27 | 2.60 | 2.70 |
| Potassium chloride KCl | 0.30 | 0.37 | 0.30 | ||
| Disodium hydrogen phosphate Na2HPO4 | 0.14 | 0.15 | 0.12 | 0.15 | |
| Sodium sulfate Na2SO4 | 0.07 | 0.07 | |||
| Magnesium sulfate MgSO4 | 0.03 | ||||
| Calcium chloride dihydrate CaCl2.2H2O | 0.40 | 0.255 | |||
| Calcium chloride CaCl2 | 0.23 | 0.37 | 0.26 | ||
| Magnesium chloride hexahydrate MgCl2.6H2O | 0.20 | 0.21 | |||
| Magnesium chloride MgCl2 | 0.20 | 0.20 | |||
| Ammonium chloride NH4Cl | 118.00 | ||||
| Sulphuric acid H2SO4 | |||||
| Potassium dihydrogen phosphate KH2PO4 | 0.03 | 0.27 | |||
| Dipotassium sulfate K2SO4 | 0.17 | ||||
| Organic components | |||||
| Sodium citrate dihydrate C6H5O7Na3.2H2O | 0.10 | 0.160 | |||
| Citric acid C6H8O7 | 0.07 | ||||
| Sodium acetate 3H2O) C2H3O2Na.3H2O | 0.95 | ||||
| Magnesium acetate Mg(C2H3O2)2.4H2O | 0.210 | ||||
| Calcium acetate Ca(C2H3O2)2 | 0.400 | ||||
| Glucose | 1.00 | ||||
| Ascorbic acid C6H8O6 | 0.05 | 0.02 | |||
| Uric acid C5H4N4O3 | 0.03 | 0.02 | |||
| Glutathione C10H17N3O6S | 0.05 | 0.03 | |||
| Proteins | |||||
| Bovine serum albumin | 10.00 | 0.20 | 0.26 | ||
| Lysozyme | 2.50 | ||||
| α-tocopherol | 0.00 | ||||
| apo-transferrin | 0.20 | ||||
| Cysteine | 0.12 | ||||
| Glycine | 0.19 | 0.38 | |||
| Mucin | 0.50 | ||||
| Surfactant/lipids | |||||
| Dipalmitoylphosphatidycholine (DPPC) | 0.20 | 10.00 | 0.10 | ||
| Antibacterial/fungal or preservative | |||||
| Benalkonium chloride | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
2.4. Development of a two stgae inhalation-ingestion bioaccessibility assay (IIBA)
Approximately 90% of PM10 is carried to the pharynx by the mucociliary action of the airways and swallowed within 24 hours. A two-stage IIBA was developed to simulate the dissolution of metal(loid)s in lung fluid, followed by the passage through the gastrointestinal (GIT) tract. In stage 1, dissolution in lung fluid was simulated by leaching 8 mg PM10 in 40 ml of Hatch solution (S/L = 1:5000) at 37°C using end over end rotation (45 rpm) for 24 hours (n=3). The solution was centifuged at 3500 g for 20 minutes at room temperature to separate the solid from liquid. The supernatant was reserved after filtering using 0.45μm filters, diluted using biological extract diluent solution (Technologies, 2006) and stored at 4°C until analysis.
In stage 2, passage through the GIT was simulated by adding simulated gastric and intestinal fluids (40 ml) to the residual solid from step 1, according to the SBRC assay (Kelley et al., 2002, Juhasz et al., 2009). Briefly, to simulate the gastric phase after ingestion of particles, gastric solution (0.4 M glycine, pH adjusted to 1.5 using concentrated HCl) (40ml) was added to each sample. The pH of the solution was adjusted to 1.5±0.5, if necessary. Samples were rotated end over end (45 rpm) for one hour, after which gastric phase solution (4 ml) was collected, syringe filtered (0.45 μm), diluted with 0.1M HNO3 and stored at 4°C until analysis. To simulate the intestinal phase, bovine bile (70 mg) and porcine pancreatin (20 mg) was dissolved in 3 ml MilliQ water and added to the gastric solution. The pH was adjusted to 7±0.1 using 5 and 50% NaOH and rotated end over end (45 rpm) for 4 hours. After the end of the intestinal phase period, samples were collected by syringe filtering (0.45 μm), diluted with 0.1M HNO3 and stored at 4°C until analysis.
Additionally, to compare metal(loid) dissolution in simulated gastro-intestinal fluids only and dissolution following the IIBA, PM10 was leached in simulated gastric and intestinal fluids only following the the same conditions. Metal(loid) analysis was performed using ICP-MS using matrix matching calibration standards for each fluid.
3. Results and discussion
Although bioaccessibility of metal(loid)s from APM is increasingly being used as an inexpensive and rapid assay to measure inhalation exposure in humans, the lack of consistency in the methodologies is a significant impediment to its standardisation. Most studies to date have mainly focused on metal(loid) dissolution from a single matrix or individual metal(loid)s (Kastury et al., 2017). However, co-contamination of multiple metal(loid)s in PM10 is a common occurrence (Tsai et al., 2015, Kurt-Karakus, 2012), particularly in mining/smelting impacted dust. Recent oral bioavailability studies have suggested that metal(loid) co-contamination, particularly As, Cd and Pb, as well as the soil matrix significantly influences bioavailability (Ollson et al., 2017b, Ollson et al., 2017a). For this reason, multiple environmental matrices with co-contaminants at elevated levels were used during the development of the IIBA. Particular focus was given to Pb and As in the discussion of the results because of their ranking as the number 1 and 2 in the Priority List of Hazardous Substances by Agency for Toxic Substances and Disease Registry (ATSDR).
3.1.1. Physico-chemical characteristics: relationship between metal(loid) concentration, particle size distribution and BET surface area.
Soil matrix, contaminant source, and metal(loid) speciation may influence contaminant bioaccessibility (Kastury et al., 2017, Julien et al., 2011). Total metal(loid) concentrations in the three dust samples (Table 2) varied depending on the source of the contamination and its associated industrial activity. Total Pb concentration (mg/kg ± SE) in PM10 of SH15 and CMW were similar (1266±20 and 1302±85 mg/kg respectively), but it was 5 times higher in PM10 of PP (6968 ± 498 mg/kg). The elevated Pb concentration in PP can be attributed to historic Pb smelting activities near the site. In addition to Pb, the concentration of As in all three dust samples were higher than Health Based Investigation (HIL) levels according to standards proposed by the National Environment Protection Measure (NEPM). However, average As concentration varied considerably among the three sites (157 ± 2.6 mg/kg in PP, 2041 ± 24 mg/kg in SH15 and 18494 ± 834 mg/kg in CMW). The high As concentration in CMW PM10 is consistent with materials associated with thermal processing of gold (Ollson et al., 2016, Meunier et al., 2010) and may pose health risk via the inhalation pathway.
Table 2:
Total metal(loid) concentration of major and minor elements, BET surface area and particle size distribution of mining and smelting impacted materials according to three size fractions.
| SH15 | PP | CMW | ||||
|---|---|---|---|---|---|---|
| < 10 μm | < 10 μm | < 10 μm | ||||
| Mean | SE | Mean | SE | Mean | SE | |
| Minor elements | ||||||
| As (mg/kg) | 2042* | 24.1 | 157* | 2.6 | 18494* | 834 |
| Cd (mg/kg) | 49.8* | 0.3 | 37.7* | 0.2 | 3.6 | 0.2 |
| Co (mg/kg) | 14.2 | 0.3 | 16.8 | 0.6 | 181* | 9.6 |
| Cr (mg/kg) | 39.5 | 0.5 | 43.6 | 1.2 | 58.5 | 3.1 |
| Cu (mg/kg) | 166 | 1.4 | 375 | 25.3 | 407 | 27.7 |
| Mn (mg/kg) | 1162 | 13.3 | 1087 | 84.6 | 602 | 22.7 |
| Ni (mg/kg) | 23.0 | 0.1 | 25.9 | 0.8 | 425 | 26.5 |
| Pb (mg/kg) | 1267* | 20.9 | 6968* | 498 | 1302* | 85.2 |
| Zn (mg/kg) | 16847* | 180 | 9411* | 357 | 1092 | 91.4 |
| Major elements | ||||||
| Al (mg/kg) | 22618 | 200 | 32873 | 2045 | 10818 | 184 |
| Ca (mg/kg) | 107496 | 854 | 60004 | 2227 | 7753 | 567 |
| Fe (mg/kg) | 41043 | 446 | 33646 | 822 | 271480 | 21979 |
| K (mg/kg) | 5312 | 93 | 9832 | 485 | 3525 | 48.9 |
| Mg (mg/kg) | 26061 | 292 | 15062 | 581 | 7331 | 109 |
| P (mg/kg) | 3113 | 29.4 | 1264 | 23.1 | 571 | 9.8 |
| Mean particle diameter (μm) | 1.0 | 0.12 | 1.95 | 0.35 | 1.06 | 0.04 |
| BET surface area (m2/g) | 16.6 | 24. 1 | 9.8 | |||
Metal(loid) exceeding Health Based Investigation (HIL) level in residential areas (including child day care centres, preschool and primary schools) with accessible soil (having no poultry and < 10% of fruit and vegetable intake from garden) NEPM (2011).
According to the USEPA, particles with aerodynamic diameter <10 μm are considered inhalable (USEPA, 2016) and may potentially deposit in the human respiratory system. However, a recent review of the literature revealed that particles up to 250 μm are sometimes used in inhalation bioaccessibility assays (Kastury et al., 2017). In this study, an increase in metal(loid) concentration was observed in SH15 and PP as particle size decreased from <250 μm to <10 μm (Table 2). For example, As in SH15 was 1135 ± 12 mg/kg in the <250 μm fraction and 2042 ± 24 mg/kg in the < 10 μm fraction, exhibiting a 1.8 fold increase. Similarly, Pb increased from 805 ± 26 mg/kg in <250 μm fraction to 1267 ± 21 mg/kg, which is a 1.6 fold enrichment in the < 10 μm fraction. In SH15, BET surface area (Table 2) also increased from 11.8 m2/g in the <250 μm fraction to 16.6 m2/g in the < 10 μm fractions (1.5 fold increase), which suggests that metal(loid) enrichment may be related to the increase in surface area. Likewise, As and Pb concentrations in PP increased 4.3 and 3.9 fold respectively when particle size decreased from <250 μm to <10 μm, which may also be related to the 5.1 fold increase in BET surface area. A similar trend of metal(loid) enrichment was also found for Al, Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Ni, P and Zn, which have been depicted in Figure S1. An increase in metal(loid) concentration with decreasing particle size is similar to previous studies by (Juhasz et al., 2011), who reported a 2 fold increase in Pb concentration in the <50 μm fraction compared to particles in the < 2 mm fraction.
Surface area of the three dust samples ranged from 9.8 m2/g (CMW) to 24.1 m2/g (PP). Metal(loid)s associated with particles having high surface area are prone to becoming rapidly solubilised and absorbed into the systemic circulation (Barltrop and Meek, 1979, Ruby et al., 1999). The relationship between metal(loid) concentration, particle size and BET surface area suggests that if particles larger than PM10 are used in inhalation bioaccessibility assays, it may yield lower bioaccessibility results due to a lower initial concentration.
3.1.2. The effect of solid to liquid (S/L) ratio on metal(loid) bioaccessibility
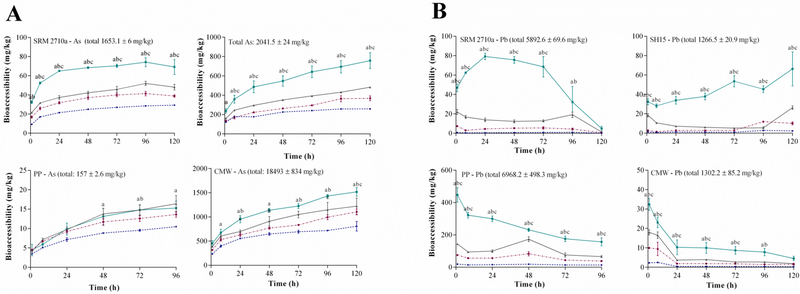
As particle loading during bioaccessibility studies may significantly affect metal(loid) dissolution (Sysalová et al., 2014, Julien et al., 2011) the S/L ratio used in an in-vitro bioaccessibility assay should be representative of a human inhalation scenario. Particularly, for mining/smelting impacted dust with elevated total metal(loid) content, the concentration of chelating agents in solution may become a limiting factor for dissolution. Figure 1A demonstrates that compared to a S/L ratio of 1:100–1:1000, As dissolution after 120 hours was significantly higher (p < 0.05) when a S/L ratio of 1:5000 was used (69.2 ± 7.8 mg/kg in SRM2710a, 758 ± 82 mg/kg in SH15 and 1517 ± 89 mg/kg in CMW). In PP, As bioaccessibility using 1:5000 (15.3 ± 1.8 mg/kg) was significantly higher (p < 0.05) than 1:100 and 1:500 only. Because PP samples contained lower total As concentration (157 mg/kg), it is possible that saturation of chelating agents did not occur at an S/L of 1:1000 and 1:5000. Increasing S/L ratio from 1:100 to 1:5000 increased As dissolution 44–136% in SRM 2710a, 57–194% in SH15 and 24–88% in CMW. Similar to As, Pb bioaccessibility in all four matrices was also the highest when a S/L ratio of 1:5000 was used (Figure 1B). This trend was also observed for Al, Cd, Fe, Mn and Zn (Figure S2), which suggests that in samples with high total metal(loid) concentration, S/L ratio plays an important role in metal(loid) solubility and therefore bioaccessibility.
Figure 1.
Effect of solid to liquid (S/L) ratio (1:100, 1:500, 1:1000& 1:5000) on A) As and B) Pb bioaccessibility (mg/kg) (37 °C, original Gamble’s solution, magnetic stirring) (mean±SEM, n=3). SRM2710a=standard reference material from the National Institute of Standards and Technology, SH15=non-ferrous slag impacted PM10, PP=smelter impacted PM10 and CMW=calcinated mine waste impacted PM10. Significant difference (ANOVA, α = 0.05) between 1:5000 & 1:100= a, 1:5000 & 1: 500= b, 1:5000 & 1:1000= c.
However, in contrast to As, Pb dissolution peaked within the first hour in PP and CMW, within 24 hours in SRM 2710a, while it fluctuated near the maximum in SH15 after 72 hours. While Pb bioaccessibility in SH15 did not decrease over time, the percentage of solubilised Pb at the end of the assay was the highest (5.2%), followed by PP (2.3%), CMW (0.4%) and SRM2710a (0.08%). The trend of decreasing Pb bioaccessibility with time in SRM 2710a, PP and CMW suggests that Pb may form insoluble compounds when incubated in Gamble’s solution that precipitates over time. Analysis of Pb speciation in the residual dust after 24-hour incubation in Gamble’s solution (Figure 3) revealed that 22% of Pb in PP was converted to leadhillite (Pb4SO4(CO3)2(OH)2). Pb sulphate (anglesite), which is similar to leadhillite, has been reported to exhibit low solubility at a similar pH to Gamble’s solution (Ruby et al., 1993), which may explain the decrease in Pb solubility over time in PP.
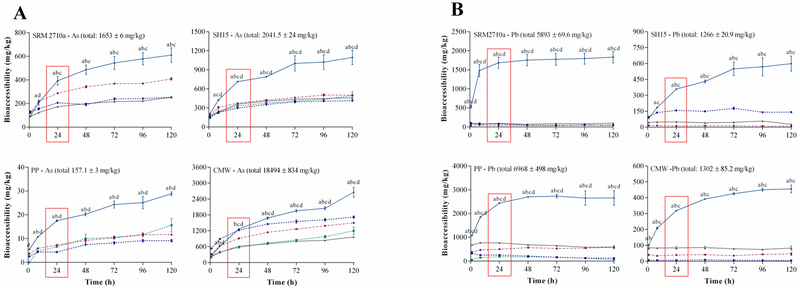
Figure 3.
Effect of fluid composition (original Gamble’s solution, modified serum simulant, modified Gamble’s solution, simulated epithelial lung fluid (SELF), Hatch’s solution) on A) As and B) Pb bioaccessibility (mg/kg) (37 °C, S/L ratio of 1:5000 and end-over-end rotation: 45 rpm) (mean±SEM, n=3). SRM2710a=standard reference material from the National Institute of Standards and Technology, SH15=non-ferrous slag impacted PM10, PP=smelter impacted PM10 and CMW=calcinated mine waste impacted PM10. Significant differences (two-way ANOVA, α=0.05) in bioaccessibility among the SLFs are indicated as: a=Hatch’s solution vs Original Gamble’s solution; b=Hatch’s solution vs modified Gamble’s solution; c=Hatch’s solution vs modified serum simulant; and d = Hatch’s solution vs SELF. As 90% of the PM10 are cleared from the lung within 24 h, bioaccessibility at this time was considered the most biologically significant (highlighted using a red rectangle). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
During 24 hours, 85–90% of PM10 is believed to be cleared from the lungs by mucociliary action (Hofmann and Asgharian, 2003), which suggests that an extraction time of 24 hours is the most biologically relevant for inhalation bioaccessibility assays. After 24 hours, As and Pb bioaccessibility using a S/L ratio of 1:5000 from all matrices (except As in PP) was significantly higher (p<0.05) than 1:100–1:1000. An increase in metal(loid) bioaccessibility with increasing S/L ratio is supported by studies conducted by (Julien et al., 2011) and (Sysalová et al., 2014). However, (Julien et al., 2011) did not observe a statistically significant difference above and S/L of 1:500. The important difference between the results of (Julien et al., 2011) and this study is the sample matrix. (Julien et al., 2011) used four certified reference materials to test the effect of S/L ratio on Pb bioaccessibility. Among the four matrices used in this study, the certified reference material, SRM 2710a, demonstrated the lowest Pb bioaccessibility (0.08%), indicating that the effect of bioaccessibility using certified reference materials may not be reflective of bioaccessibility trends observed using environmental samples. Similar to this study, (Sysalová et al., 2014) who used environmental matrices and assessed bioaccessibility of multiple metal(loid)s observed an increase in bioaccessibility when the S/L ratio was increased from 1: 500 and 1: 1000. Furthermore, a more complex formulation of SLF containing a higher number of chelating agents (Hatch’s solution) was used by (Sysalová et al., 2014), which may have aided in increasing metal(loid) bioaccessibility.
A wide range of S/L ratios (1:20 to 1:20,000) are currently employed in bioaccessibility studies, the most commonly used value being 1:100 (Kastury et al., 2017). According to USEPA, the maximum PM10 concentration in the air should not exceed 150 μg/m3 in a 24 hour period more than once every three years (EPA, 2013). However, during 2008–2012 a world average of 200 μg/m3 was reported by the World Health Organisation (WHO), the maximum being 540 μg/m3 in Peshawar, Pakistan (WHO, 2014). Therefore, using a concentration range of 200–500 μg/m3, an average air intake rate of 20 m3 (Julien et al., 2011), the mass of PM10 that may be inhaled in a 24 hour period may fall within 4–10 mg. Using 20 ml as the average lung fluid volume (Julien et al., 2011), the corresponding S/L ratio (g/100ml) can be calculated as 1:2000–1:5000. Results from this study suggest that metal(loid) bioaccessibility is maximised when an S/L ratio of 1:5000 is used, which corresponds to the S/L ratio relevant to the world average PM10 concentration. Therefore, 1:5000 was used in subsequent experiments as the most optimised S/L ratio for inhalation bioaccessibility studies.
3.1.3. The effect of agitation on metal(loid) bioaccessibility
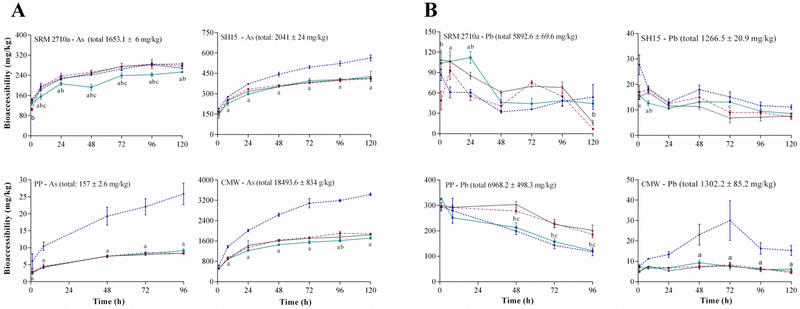
According to (Julien et al., 2011), certain methods of agitation that cause particle agglomeration in-vitro may reduce the surface area available for metal(loid) dissolution over time, e.g. orbital rotation. When PM10 enters the respiratory system, particles are displaced from the air into the fluid lining the lung (Geiser and Kreyling, 2010). Because PM10 concentrations in the air are low, and the lung surface area large (e.g. 100 m2) (Julien et al., 2011), it can be expected that particles deposit as a dispersed coating with minimal agglomeration. On the contrary, when dust is added to the SLF in-vitro, agglomeration occurs unless dispersed using agitation. However, because mechanical agitation is absent in the lung, strenuous mechanical mixing should be avoided when simulating an inhalation scenario. The methods of agitation applied in the literature varies with respect to both the type and frequency, e.g. stirring few times a day, orbital rotation (30–150 rpm), end over end rotation and ultra-sonication (Kastury et al., 2017). To investigate the role of agitation, four agitation approaches were tested, including occasional shaking to represent minimal mixing, magnetic stirring to represent robust mechanical shaking, orbital shaking to represent a method that causes agglomeration and end over end rotation to represent a method that promotes fine dispersion.
Visual inspection demonstrated that both magnetic stirring and end over end rotation kept particles suspended throughout the assay, while during occasional and orbital shaking particles deposited at the centre of flasks. The results of this assay (Figure 2) suggest that magnetic agitation provides the maximum bioaccessibility for As in environmental matrices (564 ± 22 mg/kg in SH15, 25.9 ± 3.2 mg/kg in PP and 3432 ± 50.7 mg/kg in CMW). However, for the purposes of simulating metal(loid) dissolution upon inhalation, end over end rotation, which also achieved fine particle dispersion, was deemed more biologically relevant than magnetic agitation. Using end over end rotation, As bioaccessibility at the end of the assay was 253 ± 3.3 mg/kg in SRM 2710a, 414 ± 8.7 mg/kg in SH15, 9.1 ± 0.5 mg/kg in PP and 1716 ± 43.5 mg/kg in CMW. Similar to Figure 2A, Pb dissolution decreased over time using end over end rotation, with Pb bioaccessibility at the end of the assay of 44.4 ± 4.1 mg/kg in SRM2710a, 8.5 ± 0.3 mg/kg in SH15, 122 ± 3.8 mg/kg in PP and 5.1 ± 1.1 mg/kg in CMW. Results from a two-way ANOVA demonstrated that other than magnetic stirring, As and Pb bioaccessibility using end over end rotation did not significantly differ from occasional stirring or orbital shaking at 24 hours (p > 0.05). Similar trends were observed for Al, Cd, Fe, Mn and Zn (Figure S3). It is possible that at a S/L ratio of 1:5000, the ratio of chelators to metal(loid)s was sufficiently high that particle agglomeration did not affect metal(loid) dissolution in the Gamble’s solution. The use of end over end rotation was therefore suggested as it visibly achieved dispersion and kept the pH from drifting.
Figure 2.
Effect of agitation (magnetic stirring, orbital rotation, occasional stirring, end-over-end) on A) As and B) Pb bioaccessibility(mg/kg) (37 °C, original Gamble’s solution, S/L ratio of 1:5000) (mean±SEM, n=3). SRM2710a=standard reference material from the National Institute of Standards and Technology, SH15=non-ferrous slag impacted PM10, PP=smelter impacted PM10 and CMW=calcinated mine waste impacted PM10. Significant difference between end over end vs magnetic stirring=a, end over end vs occasional stirring=b and end-over-end vs orbital rotation = c.
3.1.4. Effect of fluid composition on metal(loid) bioaccessibility
The human respiratory system is lined by two layers of extracellular secretions: a ‘sol’ layer which covers the cilia and a thicker ‘gel’ layer of mucous on top of the “sol” layer, where inhaled particles first deposit (Hatch, 1992). In addition to protecting the lung from dehydration, the mucous layer accumulates dust particles, toxins and microorganisms and prevents these from reaching the underlying ‘sol’, the lung epithelium and finally blood. The majority of SLFs used currently are based on the composition of the extracellular fluid of the skeletal muscle described by Gamble in (1967). The composition of Gamble’s solution is close to the sol layer found in the lung (Hatch, 1992). Although the original Gamble’s solution has undergone at least thirteen modifications, there are only four major categories (Kastury et al., 2017): original Gamble’s solution (Moss, 1979), modified serum simulant with NH4Cl for pH stabilisation and lung surfactants (Kanapilly et al., 1973) (latest adaptation by (Julien et al., 2011), modified Gamble’s solution with proteins (Twining et al., 2005) and simulated epithelial lung fluid (SELF) modified with proteins, surfactants and organic acids (Boisa et al., 2014). A second major type of SLF that has been in used in a limited number of studies is known as “Hatch’s solution” (Berlinger et al., 2008, Sysalová et al., 2014). This SLF formulation takes into account the composition of the mucous layer described in (Hatch, 1992) and contains elevated concentrations of protein, surfactant, complex organic molecules and enzymes. The differences in the extraction efficiencies of these five categories of SLFs are crucial in order to select an SLF that gives the most conservative bioaccessibility result.
Figure 3A illustrates As bioaccessibility and Figure 3B illustrates Pb bioaccessibility using five different SLFs, a S/L ratio of 1:5000 and end over end shaking (45 rpm). A 24 hour “exposure” was utilised for comparison as it was considered the most biologically significant extraction time for inhalation bioaccessibility assays. Across all three environmental matrices and SRM, metal(loid) dissolution was significantly higher (p<0.05) in Hatch’s solution compared to the other four SLFs inspired from the Gamble’s solution. Arsenic bioaccessibility in Hatch’s solution after 24 hours was up to 4 fold higher compared to the other four SLFs. For Pb bioaccessibility, values were 2.3–47 fold higher in Hatch’s solution compared to original and modified Gamble’s solution, modified serum simulant and SELF. Similar trends were also observed for Cd, Fe and Mn which are depicted in Figure S4.
This result is similar to that observed by (Berlinger et al., 2008) who determined that at 24 hours Pb bioaccessibility in Hatch’s solution increased up to 6.5 fold compared to Gamble’s solution. The higher extraction efficiency of Hatch’s solution compared to the original and modified Gamble’s solution (including SELF) may be attributed to its more complex composition. For example, Hatch’s solution contains 10 g/L albumin, which is 38 times higher than in SELF and 50 times higher than in the modified Gamble’s solution (Table 1). Both original Gamble’s solution and modified serum solution contained glycine as a substitute for albumin. Albumin is well-known for its capacity to bind metals, particularly using a 24 amino acid fragment known as the Asp fragment (Peters and Blumenstock, 1967). One of the main roles of albumin in human serum has been attributed to its capacity to sequester metals (Fasano et al., 2005). Therefore, the high albumin content may play a role into higher metal(loid) extraction as observed for Hatch’s solution.
Additionally, Hatch’s solution contains 10 g/L lung surfactant in the form of Dipalmitoyl phosphatidyl choline (DPPC), which is 50 times higher than in the modified serum simulant and 100 times higher than in SELF (Table 1). Surfactants are also known metal chelators as they aggregate at the solid/solution interphase, thereby directly interacting with metal(loid)s bound to the surface of particles (Banat et al., 2000). Figure 3B also illustrates that when Hatch’s solution was used, Pb remained in solution up to 120 hours compared to Figures 1B and 2B, where Pb solubility in Gamble’s solution decreased with time. An increased concentration of albumin and surfactants in Hatch’s solution may have promoted metal(loid) dissolution by binding to and removing free metal(loid)s from solution (Banat et al., 2000). Because of the biological similarity to human lung lining fluid and the highest extraction efficiency, Hatch’s solution was utilised for subsequent bioaccessibility studies.
3.2. Development of the inhalation-ingestion bioaccessibility assay (IIBA)
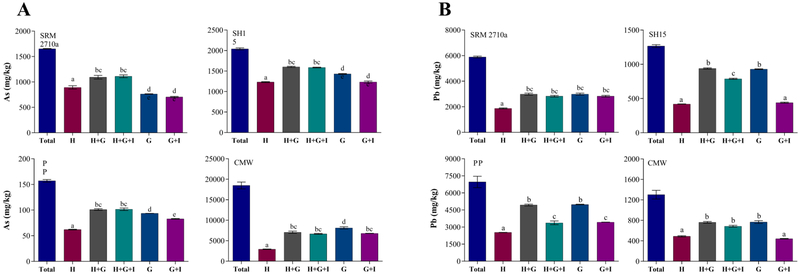
The inhalation exposure pathway is intricately linked to the ingestion pathway as an estimated 85–90% of particles that deposit in the respiratory system are cleared from the lung within 24 hours and reach the GIT (Hofmann and Asgharian, 2003). Several studies have attempted to address this by leaching inhalable particles directly in simulated gastrointestinal fluids (Puls et al., 2012). However, because of the low pH, leaching in synthetic gastric solutions may overestimate bioaccessibility of metal(loid)s from PM10. To simulate this dual exposure, PM10 was first leached in Hatch’s solution for 24 hours, followed by synthetic gastric and intestinal solutions. The results demonstrate (Figure 4) that a significantly higher concentration of metal(loid)s are solubilised when dual exposure is assessed compared to dissolution in Hatch’s solution only (p < 0.5). For example, As bioaccessibility (Figure 4A) increased significantly (p < 0.05) when using Hatch’s + gastrointestinal solution compared to when using Hatch’s solution alone (24.4% from SRM 2710a; 30.1% from SH15, 63.7% from PP and 41.6% from CMW). Similar to As, compared to Hatch’s solution alone, when Hatch’s solution + gastric solution was used, Pb bioaccessibility increased by 48.8% (PP) to 124% (SH15). Similar trends were observed for Cd, Mn and Fe as illustrated in Figure S5.
Figure 4.
Comparison of A) As and B) Pb bioaccessibility (mg/kg) in PM10when assessed using Hatch’s solution (H;), Hatch’s and gastric solution (H+G;), Hatch’s and gastric and intestinal solution (H + G + I;), gastric solution alone (G;) and gastric and intestinal solution (I;) (mean ± SEM, n = 3). SRM2710a = standard reference material from the National Institute of Standards and Technology, SH15 = non-ferrous slag impacted PM10, PP = smelter impacted PM10 and CMW = calcinated mine waste impacted PM10. PM10 was assessed in Hatch’s solution (H) for 24 h, followed by gastric (G) and intestinal (I) solutions (SBRC method). Additionally, PM10 was assessed in gastric (G) and intestinal (I) solutions only to investigate the difference between inhalation-ingestion and ingestion only pathways. Statistically significant differences (ANOVA, α = 0.05) among As bioaccessibility in simulated biological solutions were indicated by dissimilar letters.
Another notable result from this assay demonstrated that for three of the four matrices, As bioaccessibility in Hatch’s + gastrointestinal solution was significantly higher than results obtained for gastrointestinal solution alone (p < 0.05) (up to 48.8% higher in SRM 2710a, 10.5% in SH15, 4.4% in PP). It was expected that the acidic pH of the simulated gastric solution (pH 1.5±0.5) would solubilise the maximum concentration of As, as was obserevd for CMW. One of the reasons for increased As bioaccessibility may be attributed to the length of the IIBA, e.g. 24 hours in Hatch’s solution + 5 hours in gastrointestinal solution (1 hr gastric + 4 hr intestinal extraction periods), compared to 5 hours in gastrointestinal solution alone. However, unlike As, there was no significant difference (p > 0.05) between Pb bioaccessibility in Hatch’s + gastric solution versus gastric solution alone for either of the environmental matrices. Similar to As, Figure S5 also reveals that Fe and Mn bioaccessibility increased in Hatch’s + gastrointestinal solutions compared to gastrointestinal solution alone. Speciation analysis of Fe (Table 3) revealed that after 24 hours of incubation in Gamble’s solution, 20% of the Fe has been converted into organic bound Fe. It is possible that during the dissolution of Fe and subsequent sorption onto organic molecule, As may have been released from the dust particles in the Hatch’s solution, thereby increasing As concentration when assessed using IIBA.
Table 3:
Speciation of As, Fe and Pb in PM10 samples before and after incubation in original Gamble’s solution
| Sample | SH15 | PP | CMW | ||||
|---|---|---|---|---|---|---|---|
| Untreated | 24 hrs in Gamble’s solution | Untreated | 24 hrs in Gamble’s solution | Untreated | 24 hrs in Gamble’s solution | ||
| Lead (Pb) | Mineral sorbed Pb | 59 | 59 | 64 | 63 | 48 | 43 |
| Tert. Pb phosphate | 27 | 7 | |||||
| Hydroxy pyromorphite (Pb5(PO4)3OH) | 10 | ||||||
| Pb Hydroxyapatite | 8 | ||||||
| Litherage (PbO) | 4 | ||||||
| Plumbojarosite (PbFe3+6(SO4)4(OH)12) | 9 | ||||||
| Leadhillite (Pb4SO4(CO3)2(OH)2) | 22 | ||||||
| Organic bound Pb | 41 | 28 | 52 | 57 | |||
| Arsenic (As) | Arsenopyrite (FeAsS) | 10 | |||||
| As (III) sorbed | 15 | ||||||
| As(V) Sorbed | 77 | 50 | 75 | 67 | |||
| Jarosite-As(V) | 14 | 14 | |||||
| Scorodyte (FeAsO4·2H2O) | 19 | 35 | 12 | 15 | 10 | 20 | |
| Beudantite (PbFe3(OH)6SO4AsO4) | 5 | 16 | |||||
| Iron (Fe) | Ferryhydrite | 59 | 30 | no data | 18 | 61 | 59 |
| Geothite | 10 | 17 | 25 | ||||
| Lepidocrocite | 15 | 6 | 10 | ||||
| Hematite | 6 | 6 | 8 | 7 | |||
| Magnetite | 13 | 6 | 10 | 12 | 11 | ||
| Fe metal | 10 | 9 | |||||
| Organic bound Fe | 20 | 14 | 3 | 4 | |||
| Scorodyte | 3 | ||||||
| Clay | 9 | 27 | 11 | ||||
After leaching in Hatch’s + gastric solution, when the pH was increased from 1.5 to 7 during the simulation of the intestinal phase, Pb bioaccessibility decreased significantly (p < 0.05) in SH15 (939 ± 8.6 to 789 ± 7.8 mg/kg) and in PP (4937 ± 163 to 3375 ± 25.5 mg/kg). When the pH was modified from 1.5 to 7 using gastrointestinal solutions alone, the decrease in Pb bioaccessibility in the environmental matrices was more pronouced (929 ± 5.6 to 440 ± 8.2 mg/kg in SH15, 4987 ± 25.5 to 3431 ± 10.1 mg/kg in PP and 768 ± 26.1 to 441 ± 6 mg/kg in CMW). A sharp decrease in Pb bioaccessibility when the gastric phase is modified to intestinal phase conditions has also been widely reported for various matrices (mining/smelting, incinerator, shooting range and urban impacted soil) using multiple oral bioaccessibility methods including the SRBC used in this study (Juhasz et al., 2011, Juhasz et al., 2009), in vitro gastrointestinal (IVG) method (Schroder et al., 2004) and physiologically based extraction test (PBET) (Ruby et al., 1996). During oral bioaccessibility studies when bioaccessible Pb concentrations decrease in response to pH modification, a corresponding decrease in Fe concentration is often observed, indicating that Pb may sorb to Fe precipitating out of solution (Juhasz et al., 2011, Smith et al., 2011a). In this study, a decrease in Fe bioaccessibility was only significant (p < 0.05) in simulated gastro-intestinal solution alone in environmental samples and only for SH15 and CMW using IIBA (Figure S5). This suggests that when a dual exposure assay (simulated lung and gastrointestinal solution) is used, Pb may not be precipitating solely as a result of sorption to Fe. Table 3 shows that when PP was incubated in Gamble’s solution with a pH of 7.4 for 24 hours, leadhillite (Pb4SO4(CO3)2(OH)2) was formed, which is also sparingly soluble and may explain the decrease in soluble Pb concentration when pH is increased to 7.
Several researchers have argued that because PM10 may remain in the lung for longer than 24 hours that the maximum extraction time should be longer than this time period (Li et al., 2016). The results of this study demonstrated that leaching for 24 hours in Hatch’s solution followed by simulated gastrointestinal solutions yields the most conservative bioaccessibility values for multiple matrices. Moreover, metal(loid)s that tend to precipitate at neutral pH, e.g. Pb, leaching in simulated lung solution for 24 hours followed by gatsrointestinal solutions may result in the most conservative bioaccessibility estimate.
4. Conclusions
In this study, a certified reference material and PM10 from three environmental matrices with elevated concentrations of multiple metal(loid)s due to mining/smelting activities were used to investigate the effects of methodological parameters on inhalation bioaccessibility. Additionally, the assay was extended to include gastrointestinal solutions to simulate a scenario where PM10 is cleared from the lung and swallowed. The main findings were used to develop an optimised, two-stage inhalation-ingestion bioaccessibility assay (IIBA), which provides the most conservative exposure via this pathway. During stage one, PM10 should be leached in Hatch’s solution for 24 hours at 37°C using an S/L ratio of 1:5000 and end over end rotation (45 rpm). After 24 hours, PM10 should then be separated from SLF by centrifugation, reserving the solution for metal(loid) analysis. During step two, PM10 should be further leached in simulated gastrointestinal solutions according to (Juhasz et al., 2009). The highest cumulative metal(loid) dissolution should be used as the final inhalation bioaccessibility result.
Supplementary Material
Highlights.
Exposure to metal(loid)s from PM10 is a significant risk to human health
Current inhalation bioaccessibility tests vary in methodological parameters
The influence of solid/liquid ratio, agitation and simulated lung fluid composition was assessed
A conservative assay linking inhalation and ingestion was proposed
5. Acknowledgements
The authors would like to acknowledge University of South Australia for supporting Farzana Kastury with the Vice Chancellor and President’s Scholarship and the MF & MH Joyner Scholarship in Science. This project was supported in part for Ranju Karna by an appointment to the Internship/Research Participation Program at the National Risk Management Research Laboratory, U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA. Although EPA contributed to this article, the research presented was not performed by or funded by EPA and was not subject to EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in this article are solely those of the authors and do not necessarily reflect or represent EPA’s views or policies. MRCAT operations are supported by the Department of Energy and the MRCAT member institutions. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357.
6. References
- (ATSDR), A. F. T. S. A. D. R. ATSDR’s Substance Priority List.
- BANAT IM, MAKKAR RS & CAMEOTRA SS 2000. Potential commercial applications of microbial surfactants. Applied microbiology and biotechnology, 53, 495–508. [DOI] [PubMed] [Google Scholar]
- BARLTROP D & MEEK F 1979. Effect of particle size on lead absorption from the gut. Archives of Environmental Health: An International Journal, 34, 280–285. [DOI] [PubMed] [Google Scholar]
- BERLINGER B, ELLINGSEN DG, NÁRAY M, ZÁRAY G & THOMASSEN Y 2008. A study of the bio-accessibility of welding fumes. Journal of Environmental Monitoring, 10, 1448–1453. [DOI] [PubMed] [Google Scholar]
- BOISA N, ELOM N, DEAN JR, DEARY ME, BIRD G & ENTWISTLE JA 2014. Development and application of an inhalation bioaccessibility method (IBM) for lead in the PM 10 size fraction of soil. Environment international, 70, 132–142. [DOI] [PubMed] [Google Scholar]
- BRADHAM KD, SCHECKEL KG, NELSON CM, SEALES PE, LEE GE, HUGHES MF, MILLER BW, YEOW A, GILMORE T & SERDA SM 2011. Relative bioavailability and bioaccessibility and speciation of arsenic in contaminated soils. Environmental health perspectives, 119, 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARVALHO TC, PETERS JI & WILLIAMS III RO 2011. Influence of particle size on regional lung deposition – What evidence is there? International Journal of Pharmaceutics, 406, 1–10. [DOI] [PubMed] [Google Scholar]
- COSTA DL & DREHER KL 1997. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environmental health perspectives, 105, 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DíAZ-BARRIGA F, BATRES L, CALDERÓN J, LUGO A, GALVAO L, LARA I, RIZO P, ARROYAVE MAE & MCCONNELL R 1997. The El Paso Smelter 20 Years Later: Residual Impact on Mexican Children. Environmental Research, 74, 11–16. [DOI] [PubMed] [Google Scholar]
- EPA 1998. Method 6020A (SW-846): Inductively Coupled Plasma-Mass Spectrometry, Revision 1.
- EPA. 2013. Particulate Matter (PM) Standards [Online]. Available: http://www3.epa.gov/ttn/naaqs/standards/pm/s_pm_history.html [Accessed 15/01/2016].
- FASANO M, CURRY S, TERRENO E, GALLIANO M, FANALI G, NARCISO P, NOTARI S & ASCENZI P 2005. The extraordinary ligand binding properties of human serum albumin. IUBMB life, 57, 787–796. [DOI] [PubMed] [Google Scholar]
- GAMBLE JL 1967. Chemical anatomy, physiology and pathology of extracellular fluid: a lecture syllabus, Harvard University Press. [Google Scholar]
- GEISER M & KREYLING WG 2010. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol, 7, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATCH G 1992. Comparative biochemistry of airway lining fluid. Comparative biology of the normal lung, 1, 617–632. [Google Scholar]
- HOFMANN W & ASGHARIAN B 2003. The effect of lung structure on mucociliary clearance and particle retention in human and rat lungs. Toxicological Sciences, 73, 448–456. [DOI] [PubMed] [Google Scholar]
- JUHASZ AL, WEBER J & SMITH E 2011. Impact of soil particle size and bioaccessibility on children and adult lead exposure in peri-urban contaminated soils. Journal of Hazardous Materials, 186, 1870–1879. [DOI] [PubMed] [Google Scholar]
- JUHASZ AL, WEBER J, SMITH E, NAIDU R, MARSCHNER B, REES M, ROFE A, KUCHEL T & SANSOM L 2009. Evaluation of SBRC-gastric and SBRC-intestinal methods for the prediction of in vivo relative lead bioavailability in contaminated soils. Environmental science & technology, 43, 4503–4509. [DOI] [PubMed] [Google Scholar]
- JULIEN C, ESPERANZA P, BRUNO M & ALLEMAN LY 2011. Development of an in vitro method to estimate lung bioaccessibility of metals from atmospheric particles. Journal of Environmental Monitoring, 13, 621–630. [DOI] [PubMed] [Google Scholar]
- KANAPILLY G, RAABE O, GOH C & CHIMENTI R 1973. Measurement of in vitro dissolution of aerosol particles for comparison to in vivo dissolution in the lower respiratory tract after inhalation. Health Physics, 24, 497–507. [DOI] [PubMed] [Google Scholar]
- KASTURY F, SMITH E & JUHASZ AL 2017. A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal (loid) s from ambient particulate matter or dust. Science of The Total Environment, 574, 1054–1074. [DOI] [PubMed] [Google Scholar]
- KELLEY ME, BRAUNING S, SCHOOF R & RUBY M 2002. Assessing oral bioavailability of metals in soil, Battelle Press. [Google Scholar]
- KODAVANTI UP, HAUSER R, CHRISTIANI DC, MENG ZH, MCGEE J, LEDBETTER A, RICHARDS J & COSTA DL 1998. Pulmonary responses to oil fly ash particles in the rat differ by virtue of their specific soluble metals. Toxicological Sciences, 43, 204–212. [DOI] [PubMed] [Google Scholar]
- KURT-KARAKUS PB 2012. Determination of heavy metals in indoor dust from Istanbul, Turkey: estimation of the health risk. Environment international, 50, 47–55. [DOI] [PubMed] [Google Scholar]
- LANDRIGAN PJ & BAKER EL 1981. Exposure of children to heavy metals from smelters: Epidemiology and toxic consequences. Environmental Research, 25, 204–224. [DOI] [PubMed] [Google Scholar]
- LI S-W, LI H-B, LUO J, LI H-M, QIAN X, LIU M-M, BI J, CUI X-Y & MA LQ 2016. Influence of pollution control on lead inhalation bioaccessibility in PM 2.5: A case study of 2014 Youth Olympic Games in Nanjing. Environment international, 94, 69–75. [DOI] [PubMed] [Google Scholar]
- MEUNIER L, WALKER SR, WRAGG J, PARSONS MB, KOCH I, JAMIESON HE & REIMER KJ 2010. Effects of soil composition and mineralogy on the bioaccessibility of arsenic from tailings and soil in gold mine districts of Nova Scotia. Environmental science & technology, 44, 2667–2674. [DOI] [PubMed] [Google Scholar]
- MOLINELLI AR, MADDEN MC, MCGEE JK, STONEHUERNER JG & GHIO AJ 2002. Effect of metal removal on the toxicity of airborne particulate matter from the Utah valley. Inhalation toxicology, 14, 1069–1086. [DOI] [PubMed] [Google Scholar]
- MOSS O 1979. Simulants of lung interstitial fluid. [PubMed] [Google Scholar]
- NDILILA W, CALLAN AC, MCGREGOR LA, KALIN RM & HINWOOD AL 2014. Environmental and toenail metals concentrations in copper mining and non mining communities in Zambia. International Journal of Hygiene and Environmental Health, 217, 62–69. [DOI] [PubMed] [Google Scholar]
- NRIAGU JO 1992. Toxic metal pollution in Africa. Science of The Total Environment, 121, 1–37. [DOI] [PubMed] [Google Scholar]
- OLLSON CJ, SMITH E, HERDE P & JUHASZ AL 2017a. Influence of co-contaminant exposure on the absorption of arsenic, cadmium and lead. Chemosphere, 168, 658–666. [DOI] [PubMed] [Google Scholar]
- OLLSON CJ, SMITH E, HERDE P & JUHASZ AL 2017b. Influence of sample matrix on the bioavailability of arsenic, cadmium and lead during co-contaminant exposure. Science of The Total Environment, 595, 660–665. [DOI] [PubMed] [Google Scholar]
- OLLSON CJ, SMITH E, SCHECKEL KG, BETTS AR & JUHASZ AL 2016. Assessment of arsenic speciation and bioaccessibility in mine-impacted materials. Journal of hazardous materials, 313, 130–137. [DOI] [PubMed] [Google Scholar]
- PETERS T & BLUMENSTOCK FA 1967. Copper-binding properties of bovine serum albumin and its amino-terminal peptide fragment. Journal of Biological Chemistry, 242, 1574–1578. [PubMed] [Google Scholar]
- PULS C, LIMBECK A & HANN S 2012. Bioaccessibility of palladium and platinum in urban aerosol particulates. Atmospheric Environment, 55, 213–219. [Google Scholar]
- ROSA MJ, BENEDETTI C, PELI M, DONNA F, NAZZARO M, FEDRIGHI C, ZONI S, MARCON A, ZIMMERMAN N & WRIGHT R 2016. Association between personal exposure to ambient metals and respiratory disease in Italian adolescents: a cross-sectional study. BMC pulmonary medicine, 16, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBY MV, DAVIS A, LINK TE, SCHOOF R, CHANEY RL, FREEMAN GB & BERGSTROM P 1993. Development of an in vitro screening test to evaluate the in vivo bioaccessibility of ingested mine-waste lead. Environmental science & technology, 27, 2870–2877. [Google Scholar]
- RUBY MV, DAVIS A, SCHOOF R, EBERLE S & SELLSTONE CM 1996. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environmental science & technology, 30, 422–430. [Google Scholar]
- RUBY MV, SCHOOF R, BRATTIN W, GOLDADE M, POST G, HARNOIS M, MOSBY D, CASTEEL S, BERTI W & CARPENTER M 1999. Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environmental science & technology, 33, 3697–3705. [Google Scholar]
- SCHRODER J, BASTA N, CASTEEL S, EVANS T, PAYTON M & SI J 2004. Validation of the in vitro gastrointestinal (IVG) method to estimate relative bioavailable lead in contaminated soils. Journal of environmental quality, 33, 513–521. [DOI] [PubMed] [Google Scholar]
- SMITH E, KEMPSON IM, JUHASZ AL, WEBER J, ROFE A, GANCARZ D, NAIDU R, MCLAREN RG & GRÄFE M 2011a. In vivo–in vitro and XANES spectroscopy assessments of lead bioavailability in contaminated periurban soils. Environmental science & technology, 45, 6145–6152. [DOI] [PubMed] [Google Scholar]
- SMITH E, WEBER J, NAIDU R, MCLAREN RG & JUHASZ AL 2011b. Assessment of lead bioaccessibility in peri-urban contaminated soils. Journal of Hazardous Materials, 186, 300–305. [DOI] [PubMed] [Google Scholar]
- SYSALOVÁ J, SZÁKOVÁ J, TREMLOVÁ J, KAŠPAROVSKÁ K, KOTLÍK B, TLUSTOŠ P & SVOBODA P 2014. Methodological Aspects of In Vitro Assessment of Bio-accessible Risk Element Pool in Urban Particulate Matter. Biological trace element research, 161, 216–222. [DOI] [PubMed] [Google Scholar]
- TECHNOLOGIES, A. 2006. Determination of heavy metals in whole blood by ICP-MS. Agilent Technologies; publication number 5988–0533EN [Online]. [Google Scholar]
- TSAI M-Y, HOEK G, EEFTENS M, DE HOOGH K, BEELEN R, BEREGSZÁSZI T, CESARONI G, CIRACH M, CYRYS J & DE NAZELLE A 2015. Spatial variation of PM elemental composition between and within 20 European study areas—results of the ESCAPE project. Environment international, 84, 181–192. [DOI] [PubMed] [Google Scholar]
- TWINING J, MCGLINN P, LOI E, SMITH K & GIERÉ R 2005. Risk ranking of bioaccessible metals from fly ash dissolved in simulated lung and gut fluids. Environmental science & technology, 39, 7749–7756. [DOI] [PubMed] [Google Scholar]
- USEPA 1998. Microwave assissted acid digestion of sediments, slidges, soils, and oils. [Google Scholar]
- USEPA. What is PM, and how does it get into the air? 2016 [Google Scholar]
- UTEMBE W, POTGIETER K, STEFANIAK AB & GULUMIAN M 2015. Dissolution and biodurability: Important parameters needed for risk assessment of nanomaterials. Particle and fibre toxicology, 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLENBORN JG, MCGEE JK, SCHLADWEILER MC, LEDBETTER AD & KODAVANTI UP 2007. Systemic translocation of particulate matter–associated metals following a single intratracheal instillation in rats. Toxicological Sciences, 98, 231–239. [DOI] [PubMed] [Google Scholar]
- WHO. 2014. Ambient (outdoor) air pollution in cities database 2014 [Online]. Available: http://www.who.int/phe/health_topics/outdoorair/databases/cities/en/ [Accessed 15/01/2016].
- WISEMAN CL 2015. Analytical methods for assessing metal bioaccessibility in airborne particulate matter: A scoping review. Analytica chimica acta, 877, 9–18. [DOI] [PubMed] [Google Scholar]
- WRAGG J & KLINCK B 2007. The bioaccessibility of lead from Welsh mine waste using a respiratory uptake test. Journal of Environmental Science and Health Part A, 42, 1223–1231. [DOI] [PubMed] [Google Scholar]
- ZEREINI F, ALSENZ H, WISEMAN CLS, PÜTTMANN W, REIMER E, SCHLEYER R, BIEBER E & WALLASCH M 2012. Platinum group elements (Pt, Pd, Rh) in airborne particulate matter in rural vs. urban areas of Germany: Concentrations and spatial patterns of distribution. Science of The Total Environment, 416, 261–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.