Abstract
OX40 agonists have potent immunotherapeutic effects against a variety of murine tumors, yet it is unclear the role that age-related immune senescence plays on their efficacy. We found that middle-aged and elderly tumor-bearing mice (12 and 20 months old, respectively) treated with anti-OX40 were less responsive compared to young mice six-months or less of age. Decreased tumor-free survival was observed in both male and female mice, and was not due to changes in the surface expression of OX40 on T cells in older animals. Enumeration of cytokine-producing effector T cells in tumor-bearing mice revealed a significant decline in these cells in the older mice treated with anti-OX40 compared to their younger counterparts. The decrease of this critical T cell population in middle-aged mice was not a result of inherent T cell deficiencies, but was revealed to be T cell extrinsic. Finally, combining IL-12, an innate cytokine, with anti-OX40 boosted levels of differentiated effector T cells in the older anti-OX40 treated mice and partially restored the defective anti-tumor responses in the middle-aged mice. Our data show that the anti-OX40-enhancement of tumor immunity and effector T cell numbers is decreased in middle-aged mice and was partially reversed by co-administration of the proinflammatory cytokine IL-12.
Keywords: T cells, tumor immunity, costimulation
Introduction
The immune system of aging animals is characteristically described to be both poorly responsive and functionally dysregulated, a condition known as immune senescence (1). Virtually every aspect of the immune system is in some way affected by age-associated immune senescence, including cellular immune responses where age-associated alterations in CD8 and CD4 T cell function have been well documented (2). For example, CD8 T cells from aged mice exhibit decreased cytotoxicity, IL-2 production, and expansion, and naïve CD4 T cells from aged mice proliferate with less rigor and generate poor effector T cells due to decreased IL-2 production (3, 4). These age-related effects on T cells lead to decreased immunity against pathogens, and may also complicate the design and efficacy of cancer immunotherapies. Tumor-specific immunotherapeutic strategies are often developed in models using young animals, which neglect the potential impact of immune senescence on efficacy. Thus, due to the age-related changes in T cell function, cancer immunotherapies may also need to be adjusted for optimal translational therapeutic value.
Stimulation of certain TNFR family members has been shown to lead to effective anti-tumor immunotherapy and in some cases restored the function of immune cells in aged animals. For instance, engagement of 4-1BB mediated significant anti-tumor protection against a number of tumors and reversed priming defects related to age (5-7). Another member of the TNFR family, OX40, is expressed on both CD4 and CD8 T cells within tumors (8) and when activated confers significant tumor protection in mice (9). In vivo stimulation of OX40 also greatly enhances a number of antigen-specific T cell functions, including proliferation, cytokine production, and memory T cell development in young animals (10-14). These characteristics suggest OX40 could overcome potential immune senescence of T cells and enhance anti-tumor immunity in old hosts. In fact, activating OX40 during tumor priming was initially observed to increase immune responses to a pre-B cell lymphoma in aged (18+ months of age) animals (15). In these studies, vaccinating aged mice with tumors expressing CD80 and eGFP and then treating these mice with anti-OX40 resulted in long-term protection from a secondary challenge using the parental tumor. These data showed that anti-OX40 enhanced “epitope spreading” to tumor-specific antigens in the older mice. However the anti-tumor response relied on the older mice first responding to increased CD80 signaling as well as to a foreign protein (eGFP) associated with the tumor vaccine, prior to rejecting the parental tumor.
Thus, we sought to further investigate whether systemic administration of an OX40 agonist (anti-OX40) in the absence of an immunogenic foreign protein or vaccine, would enhance anti-tumor immunity in aged animals. Mice aged two, six, 12, and 20 months were challenged with sarcoma (MCA205) tumor cells and treated with anti-OX40. Systemic administration of anti-OX40 in the youngest tumor-bearing mice generated significant tumor-free survival, yet OX40-mediated tumor-free survival among the 12- and 20-month old mouse cohorts treated was severely diminished. To identify a possible explanation for the diminished tumor-free survival in the older anti-OX40-treated mice, T cells from tumor-bearing mice were assessed, revealing a significant decrease in cytokine-producing T cells in the 12-month old anti-OX40-treated mice and a coincident increase in CD4+CD25+FoxP3+ T cells. The decrease in the cytokine-producing effector T cells appeared to not be the result of an inherent defect in the aging T cells, but was found to likely originate from defects in cells important for the promotion and support of OX40-enhanced immune responses. Finally, IL-12 is a potent promoter of effector T cell differentiation and when administered in combination with OX40 engagement, partially restored OX40-mediated tumor survival in middle-aged mice (12 month old). These findings suggest that during aging, critical immunological defects occur by middle age, rendering OX40-mediated anti-tumor responses insufficient for effective tumor clearance.
Materials and Methods
Mice
Four to six week old male and female C57BL/6 mice were purchased from Charles River Laboratory (Boston, MA) and used at eight weeks of age. For the aging mice, 6-8 week old, 6 and 10 month old mice were purchased from Charles River Laboratory, Harlan (Indianapolis, IN), and the NIA (Washington, DC) and housed till reaching the mean ages of 6, 12 and 20 months. DO11.10 were bred and housed till reaching a mean age of two or 12 months. All mice were maintained at the EARCI animal facility and all animal studies were approved by the Earle A Chiles Research Institute IACUC committee.
Tumor models
H12 is derived from the original 3-methylcholanthrene-induced parental tumor MCA205 and can be passaged in culture. The tumor cells were suspended in HBSS (4-5 × 105 or 1 × 106) and injected s.c. into female or male C57BL/6 mice. The CT26 colon carcinoma cell line was suspended in HBSS (1 × 105) and injected into female BALB/c mice. Three and seven days following tumor challenge mice were injected i.p. with 250-750 μg anti-OX40 (OX86) or rat IgG. In some experiments rmIL-12 (100 ng) (R&D Systems, Minneapolis, MN) was administered i.p daily 4-9 days after tumor challenge. Mice were then monitored for tumor growth and sacrificed if the tumor became ulcerated or growth reached 150 mm2.
DO11.10 transgenic TCR adoptive transfer
DO11.10 transgenic TCR CD4 T cell are specific for a peptide of the ovalbumin protein (357-364) and were identified by an antibody designated KJ-126. Spleens and LNs were harvested and processed by crushing between two frosted glass microscope slides and red blood cells lysed with ACK (Lonza, Walkersville, MD). The percentage of DO11.10 T cells was identified by anti-KJ-126 prior to transfer and a total of 2-3 × 106 transgenic TCR T cells were adoptively transferred i.v. into BALB/c recipients. One day later mice were immunized s.c. with 500 μg ovalbumin (Sigma, St. Louis, MO) and 50 μg of anti-OX40 (OX86) or IgG control (Sigma, St. Louis, MO). The following day mice were given a second injection of anti-OX40 or rat IgG. IL-12 (100 ng) or PBS (0.05% mouse serum) was administered at the time of adoptive transfer and then daily for the next three days.
Isolation of leukocytes from tumors
Tumors were surgically removed from mice 12 days after challenge and tumor was disrupted by dicing and processing between frosted glass slides. Disrupted tumors were then digested for at least 1.5 hours using a triple enzyme mixture (collagenase, DNAse, hyluronidase; Sigma, St. Louis, MO) under constant stirring. The digested tumor was washed and the lymphocytes isolated by gradient centrifugation (Ficoll; GE Health Care, Uppsala, Sweden). Lymphocytes were isolated from the interface and washed.
FACS analysis of T cells from lymph nodes and tumors
T cells were liberated from lymph nodes by disruption between two frosted glass slides. Cells from lymph nodes and tumors were stained with various combination of the following antibodies: FITC-CD4, APC-CD25, PE Cy7-CD8, APC-CD62L, PE-CD25, PE Cy7-CD25 and biotinylated-KJ-126 and in some experiments made permeable with fixation/permeablization buffers and stained with PE-FoxP3 (eBioscience, San Diego, CA). Harvested samples, isotype controls, and single stain controls were run on the FACSCalibur (Becton Dickinson, Franklin Lakes, NJ).
Intracellular cytokine staining
T cells from the tumor-draining lymph nodes (TDLNs) were obtained as described above and stimulated for six hours in vitro with 1 μg/mL anti-CD3 in RPMI containing 10% fetal bovine serum and 1 μg/mL Golgi stop (BD Biosciences, Pharmingen, San Diego, CA). In addition, antigen-draining lymph nodes (axillary) were harvested from adoptively transferred recipients four days after Ag challenge and liberated cells stimulated for six hours in vitro with 5 μg/ml ovabumin323-339 and 1 μg/mL Golgi stop. Cells were collected and stained with FITC-CD4, KJ-126 and/or APC Cy7-CD8 (eBioscience, San Diego, CA). Cells were made permeable with CytoPerm/PermWash buffers and stained with APC-IFNγ, PE-IL-2 and PE Cy7-TNFα (BD Biosciences, Pharmingen, San Diego, CA). T cells were analyzed on the BD LSRII or FACSCalibur (Becton Dickinson, Franklin Lakes, NJ).
Statistical Analysis
For all experiments, a Student's t test (two-tailed) was used to compare means of selected groups. For analysis, values of p≤0.05 were considered significant and expressed as follows: *p≤0.05 and **p≤0.001, if not specifically stated.
Results
OX40-mediated tumor immune responses were less effective in aged mice.
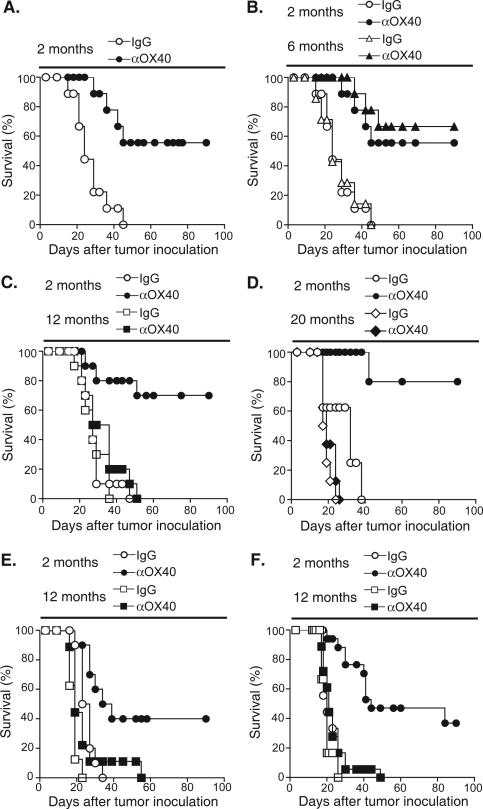
The effect of OX40 engagement in tumor-bearing mice of various ages was addressed in two different tumor models. Two, six, 12 and 20 month old female C57BL/6 mice were challenged with MCA205 tumor (s.c.). Three and seven days after tumor challenge mice were treated with anti-OX40 or IgG control and monitored for tumor growth. As expected, anti-OX40 improved tumor-free survival in the two-month old mice compared to IgG controls (Fig. 1A). Improved tumor-free survival following OX40 engagement was also observed in six-month old mice, but not in the 12- or 20-month old mice (Fig. 1B, C and D). In these older mice, tumor-free survival was significantly reduced or completely abrogated. In addition, anti-OX40 failed to significantly delay tumor growth in the older mice (data not shown). To determine whether this was a dose-dependent effect, we increased the dose of anti-OX40 three-fold (750 μg). The substantial increase in anti-OX40 failed to increase tumor free survival of tumor-bearing 12-month old mice (data not shown). Sex-specific effects could also not account for the age-related differences, as survival of 12-month old male MCA205 tumor-bearing mice did not improve following anti-OX40 treatment (Fig. 1E). These data demonstrate that aged mice are less responsive to OX40 agonist administration when tested in tumor-bearing hosts.
FIGURE 1.
The age of the tumor-bearing host affects MCA205 tumor free survival following systemic OX40 stimulation. A-D, Female C57BL/6 mice, ages two, six, 12 and 20 months, were injected s.c. with 4-5 × 105 MCA205 tumor cells. Three and seven days later 250 μg anti-OX40 or rat IgG were injected i.p. E, Male C57BL/6 mice, ages two and 12 month, were injected with 4 × 105 MCA205 tumor cells. Three and seven days later 250 μg anti-OX40 or rat IgG were injected i.p. F. Female BALB/C mice, ages two and 12 month, were injected with 1 × 105 CT26 tumor cells. Three and seven days later 250 μg anti-OX40 or rat IgG were injected i.p. Mice were then monitored for tumor growth.
The failure of OX40 agonist administration to mediate tumor-free survival in the 12-month old mice was particularly intriguing, as these mice might not be considered elderly. Although, 18- to 24 -month old mice are typically used in studies investigating the effects of age on immunological function, the results here suggest that immunologic deficiencies associated with immune senescence may be apparent much earlier (12 months of age). There are potential advantages to studying animals at this earlier time point as they may represent a population with fewer accumulated immunological defects, which may allow us to more readily identify the most relevant cause of OX40 non-responsiveness. For this reason, the ensuing experiments predominately tested 12-month old (middle-aged) mice.
To verify further that the decrease of anti-OX40 mediated tumor free survival seen in animals 12 months of age was not a tumor- or strain-specific effect, BALB/C mice (two- and 12- months old) were challenged with the colon carcinoma, CT26. These mice were then treated with anti-OX40 or control IgG at three and seven days post tumor transplantation and monitored for tumor growth. Similar to the results with MCA205, anti-OX40 treatment improved tumor free survival in the two-month old CT26 tumor-bearing mice compared to control IgG treatment, but failed to improve tumor free survival in the 12-month old mice (Fig. 1F). The decrease in OX40-mediated survival, in both the CT26 and the MCA205 tumor models, suggests that the age-related differences in OX40-mediated tumor-free survival are universal and not tumor model-specific.
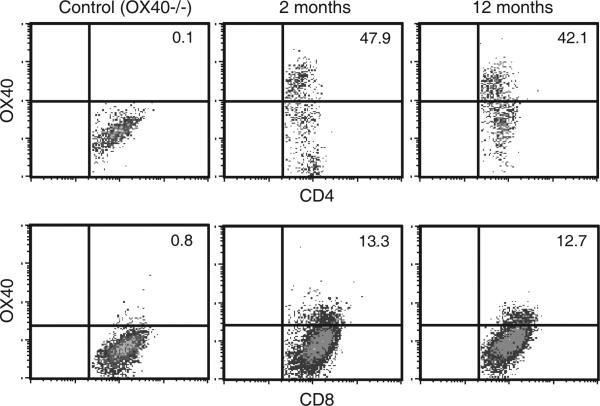
The response of T cells to OX40 stimulation is related to expression of functional OX40 protein on the cell surface (16). Expression of OX40 has been previously observed on both CD4 and CD8 T cells infiltrating tumors (17), and any age-related changes in this expression may explain the differences in anti-OX40-mediated tumor free survival. To test this possibility, mice (two- and 12-month old) were injected with MCA205 and the tumor infiltrating lymphocytes (TILs) were assessed for OX40 expression. Tumors developed 12 days after tumor inoculation (9-25 mm2) at which time they were harvested and the TILs isolated. Infiltrating CD4 or CD8 T cells harvested at this time from two- and 12-month old mice tumor-bearing mice as well as T cells from TDLNs had comparable levels of OX40 expression (Fig. 2 and data not shown).
FIGURE 2.
Surface expression of OX40 on T cells infiltrating the tumors of two- and 12- month old mice. C57BL/6 mice ages two and 12 months and OX40−/− mice two months of age were challenge with 1 × 106 MCA205 tumor cells. Tumors were surgically resected 12 days following challenge and the lymphocytes infiltrating the tumors were harvested. CD4 and CD8 T cells were then analyzed by FACS for the expression of OX40. Gates for OX40 staining were based on data from OX40−/− mice. The data are representative of two independent experiments.
Age-related differences in the accumulation of differentiated effector T cells
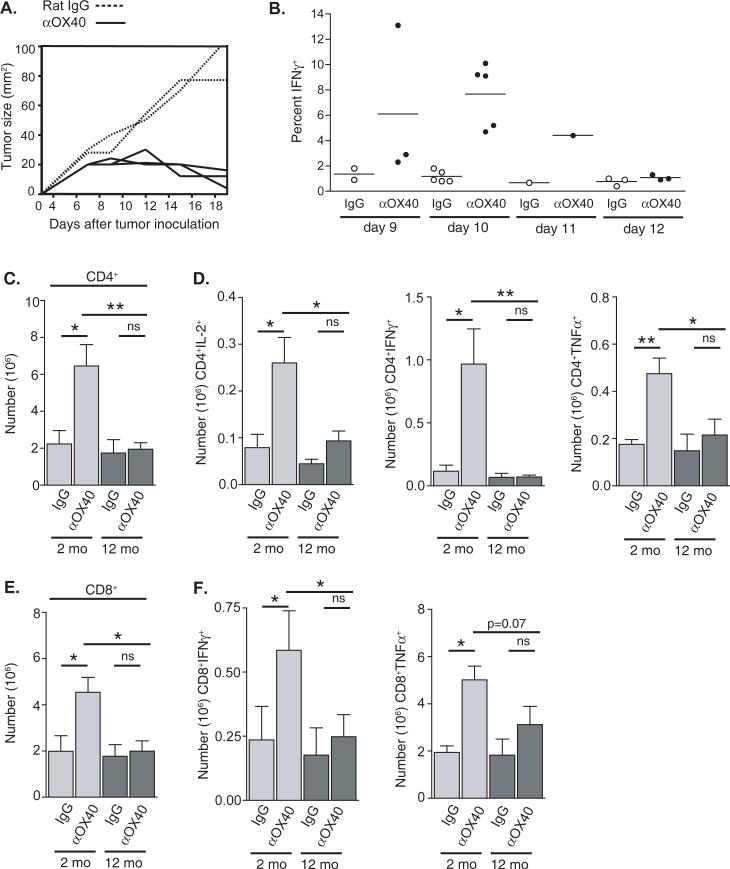
We next sought to identify a potential age-related mechanism underlying the differences in OX40-mediated tumor-free survival, by analyzing the responding effector T cells following anti-OX40 treatment. To assess the acquisition of an effector phenotype in anti-OX40-treated tumor-bearing mice, we analyzed T cells from TDLNs when the tumors in the anti-OX40 treated mice start to regress (days of 9 and 13 days after tumor challenge), and found CD8 T cells from these animals exhibited peak IFNγ production ten days after tumor challenge (Fig. 3A and 3B). When TDLNs from tumor-bearing two- and 12-month old mice treated with anti-OX40 or control IgG, were harvested on day 10 the total number of CD4 and CD8 T cells present in the TDLNs from 12-month old anti-OX40-treated animals was significantly lower than the numbers from two-month old anti-OX40 treated animals (Fig. 3C and 3E). The number of CD4 T cells from the TDLNs of two-month old tumor-bearing mice that produced IL-2, IFNγ, and TNFα after re-stimulation was significantly increased following anti-OX40-treatment compared to IgG controls, as well as 12-month old mice treated with anti-OX40 (Fig. 3D). The observed OX40-mediated increases in cytokine production appeared to occur primarily in the TDLN, as levels of cytokine production in T cells from distant LNs were similar to T cells from IgG-treated TDLNs or LNs from non-tumor-bearing mice treated with anti-OX40 (data not shown). The number of CD8 T cells producing IFNγ from anti-OX40 treated mice was also significantly increased in the two-month old animals when compared to age-matched IgG controls, and also were significantly greater than 12-month old anti-OX40 treated mice (Fig. 3F). These results suggested a potential age-related deficiency or defect in the accumulation of differentiated effector T cells, following anti-OX40 administration.
FIGURE 3.
Age-related differences in the numbers of CD4 and CD8 T cells in the tumor draining lymph nodes of anti-OX40 and rat IgG treated tumor-bearing mice ten days after MCA205 tumor challenge. Two- and 12-month old mice were challenged with 1 × 106 MCA205 s.c. and then treated i.p. with 250 μg anti-OX40 or rat IgG. A. After tumor challenge two-month old mice were measured over time for tumor growth. Each curve represents an individual animal. B. Tumor-draining lymph nodes from two-month old tumor-bearing mice were harvested on various days after tumor challenge. Cells from the tumor draining lymph nodes of anti-OX40-treated mice (closed circles) or rat IgG-treated mice (open circles) were restimulated with anti-CD3 (1 μg/mL) for six hours and CD8 T cells were analyzed for intracellular IFNγ production. Each circle represents one animal. C-F. Tumor draining lymph nodes were harvested ten days after tumor challenge and CD4 C. and CD8 E. T cells enumerated. T cells were then restimulated with anti-CD3 for six hours and analyzed for cytokine production via intracellular cytokine staining. D. Number of CD4 T cell producing IL-2, IFNγ, and TNFα F. Number of CD8 T cell producing of IFNγ and TNFα.
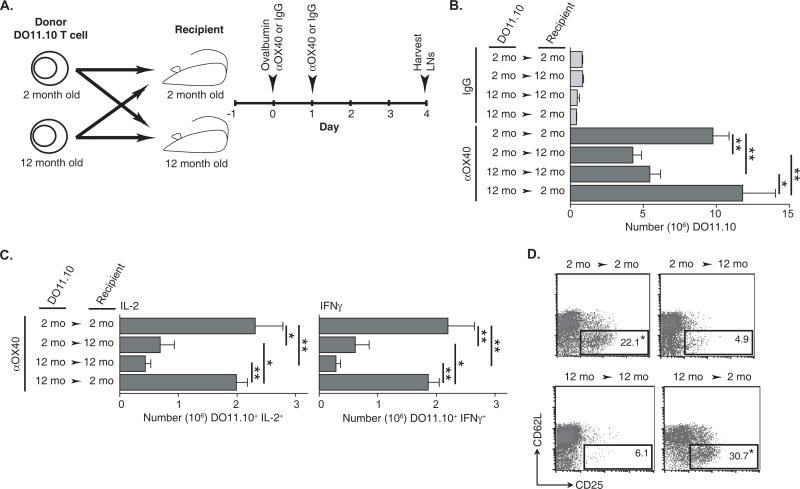
To more specifically explore the potential age-related deficiencies in antigen-specific T cells, experiments were undertaken in the DO11.10 transgenic TCR model. DO11.10 T cells from two-month old donors were transferred to two- or 12-month old wild type recipients and alternatively, DO11.10 T cells from 12-month old donors were transferred to two- or 12-month old recipients (Fig. 4A). The recipient mice were then immunized with antigen (ovalbumin) and anti-OX40, followed by a second administration of anti-OX40 one day later. Antigen-draining LNs were harvested four days after antigen and anti-OX40 administration, the peak of DO11.10 T cell expansion (18), and transgenic T cells were enumerated and analyzed. The crisscross design allowed for the comparison of the responses of two-month old T cells to that of 12-month old T cells in the same aged host environment. Overall numbers of antigen-specific T cells after antigen immunization and anti-OX40 administration were significantly greater in the young recipients compare to older recipients regardless of the age of the transferred DO11.10 T cells (Fig. 4B). It can be expected that in addition to the DO11.10 T cells, endogenous T cells may express OX40 to be engaged by anti-OX40 and could contribute to the expansion of the DO11.10 T cells through a bystander mechanism, but in other transgenic T cell models we have failed to see this phenomenon play a role in the anti-OX40-mediated expansion of transferred transgenic TCR T cells (data not shown). The difference in antigen-specific T cells was reflected in the numbers of peptide re-stimulated IL-2- and IFNγ-producing DO11.10 T cells, which were also significantly decreased in the older 12-month old recipients regardless of the age of transferred transgenic TCR T cells (Fig. 4C). Additional, phenotypic analysis of the DO11.10 T cells from anti-OX40-treated young two-month old recipients also revealed a large increase in the frequency of an activated effector phenotype defined by CD62Llow CD25+ compared to DO11.10 T cells from 12-month old recipients, with no overt change in CD25+FoxP3+ T cells (Fig. 4D and data not shown). These results suggest first, that 12-month old T cells can respond in a comparable fashion to younger T cells after agonist OX40 treatment when transferred into a younger recipient and secondly, the decrease in the levels of cytokine-producing T cells in 12-month old tumor-bearing anti-OX40-treated mice may likely be due to T cell extrinsic deficiencies and not intrinsic defects.
FIGURE 4.
The age of the recipient and not the adoptively transferred transgenic TCR CD4 T cell determines the extent of an OX40-enhanced antigen-specific T cell immune response. A. Schematic of the crisscross adoptive transfer of two- or 12-month old DO11.10 CD4 T cells into two- or 12-month old BALB/c recipients and immunization (500 μg ovalbumin, 50 μg anti-OX40 or rat IgG s.c.) schedule. B. Enumeration of the antigen-specific DO11.10 T cells and C. cytokine (IL-2 and IFNγ)-producing DO11.10 T cells from the draining LNs four days after challenge, D. Representative FACs plots of DO11.10 T cells (CD4+KJ-126+) with an activation phenotype (CD62LlowCD25+) from the antigen-draining LNs four days after challenge. Statistical representation is measured between age-matched adoptively transferred DO11.10 and described in the Materials and Methods.
The previous results suggest the potential age-related immunological dysfunction underlying the decreased accumulation of differentiated effector T cells resides in the cells that support T cell differentiation, which would include cells of the innate immune response (e.g. DCs). The inflammatory cytokine, IL-12, is produced by innate immune cells and is important for T cell differentiation, function, and survival (19, 20) and may positively affect T cell differentiation in our model. To investigate any IL-12 effects in OX40-mediated T cell responses in 12-month old mice, the previously used DO11.10 model was modified. Two- and 12-month-old T cells were transferred into age-matched recipients and challenged with antigen, anti-OX40, and with or without IL-12. The addition of IL-12 increased the frequency of both two- and 12-month old transgenic T cells producing IFNγ (Fig. 5A). More importantly, IL-12 in combination with anti-OX40 significantly boosted the number of the 12-month old IFNγ-producing DO11.10 T cells in the older 12-month old recipients close to the levels of those found in the younger mice (Fig. 5B). Interestingly, the overall numbers of the young DO11.10 T cell from mice following anti-OX40 and IL-12 were less than numbers from mice treated with anti-OX40 alone, resulting in nearly equal levels of IFNγ-producing T cells (Fig. 5B and data not shown).
FIGURE 5.
Co-administration of exogenous IL-12 and anti-OX40 increases the acquisition of an effector CD4 T cell phenotype and accumulation of differentiated effector CD4 T cells. Two- or 12-month old DO11.10 CD4 T cells were adoptively transferred into age-matched BALB/c recipients, and immunized s.c. with ovabumin (500 μg) and anti-OX40 (50 μg) or IL-12 (100 ng). A second dose of anti-OX40 was administered the next day and IL-12 was injected s.c. daily for the next three days. Antigen-draining LNs were harvested four days after challenge and analyzed. A. Representative FACs plots showing the frequency of DO11.10 T cells (CD4+KJ-126+) producing IFNγ. B. Enumeration of IFNγ-producing DO11.10 T cells.
Differences in the accumulation of CD8 T cells and CD4+CD25+FoxP3+ T cells within the tumors of anti-OX40-treated two- and 12-month old mice
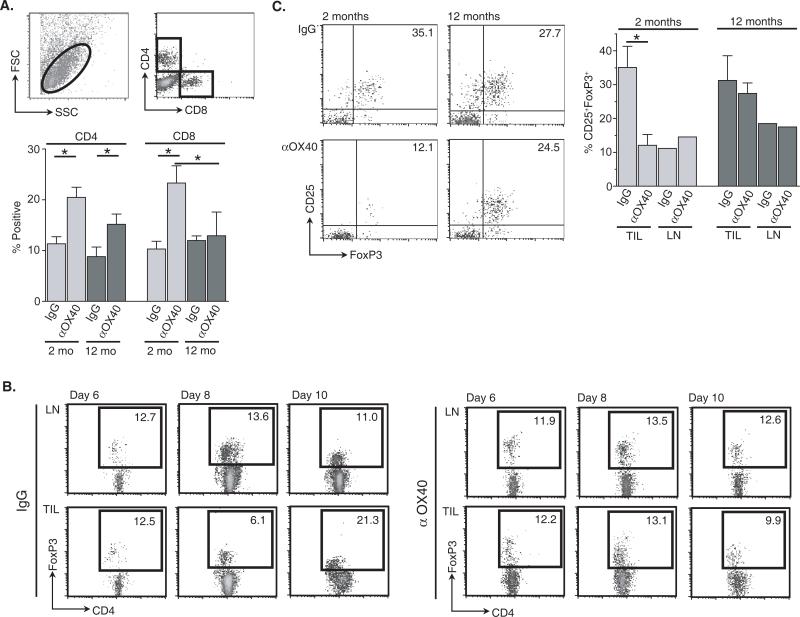
We next analyzed changes in TILs from two- and 12-month old tumor-bearing animals following anti-OX40 treatment. MCA205 tumors were harvested from two- and 12-month old mice treated with anti-OX40 or control IgG 12 days after tumor challenge, and TILs were isolated. There was no significant difference in the total number of TILs per mm2 obtained from two- or 12-month old mice regardless of treatment (data not shown), but there was a greater frequency of CD4+ T cells found in the TILs of anti-OX40 treated mice compared to IgG controls, regardless of age (Fig. 6A). The percentage of CD8+ T cells within the tumors of two-month old mice was increased following anti-OX40 treatment, as opposed to CD8 T cell levels in anti-OX40 treated 12-month old mice, which remained at a similar level to that observed in the IgG control group (Fig. 6A).
FIGURE 6.
Age-related increase of CD4+CD25+FoxP3+ T cells within the tumors of 12-month old anti-OX40 treated animals. Two- and 12-month old mice were challenged with 1 ×106 MCA205 s.c. and then treated i.p. with 250 μg anti-OX40 or rat IgG, as previously described. Tumors were resected 12 days after tumor challenge and TILs harvested (Materials and Methods). A. The frequency of CD4 and CD8 T cells from processed tumors was determined using the gate described. B. Time course of CD4+FoxP3+ T cell accumulation in the distal LNs and TILs of IgG- and anti-OX40-treated MCA205 tumor-bearing mice (two-months old). C. The frequency of CD4+ T cells expressing a CD25+FoxP3+ phenotype from TILs and pooled distal LNs from the same mice were assessed by FACs 12 days after tumor challenge.
To further dissect age-related differences in OX40-mediated tumor regression, CD4+ TIL isolated from anti-OX40 or IgG-treated mice were assessed for changes in regulatory T cell percentages (CD25 and forkhead box protein 3 expression). An extensive body of literature has shown that a CD4+ T cell subpopulation identified by CD25 and FoxP3 negatively influences normal immune responses and, more importantly, anti-tumor responses via immunosuppressive cytokine release and/or direct cell-cell contact (21, 22). In this tumor model (MCA205), CD4+CD25+FoxP3+ T cells begin to accumulate within the tumors of untreated mice at greater levels than that found in non-tumor tissue (distal LNs) about ten days after tumor challenge and reach a plateau between days 12 and 20, however in anti-OX40-treated mice the level of this T cell population remained constant (days 6-10) (Fig. 6B, 6C, and data not shown). Moreover, the level of CD4+CD25+FoxP3+ T cells (as a percentage of total CD4+ T cells) infiltrating the tumors of anti-OX40 treated two-month old mice was significantly lower than in control IgG controls 12 days after tumor challenge (Fig. 6C). In contrast, the percentage of CD25+FoxP3+ T cells in the TILs of anti-OX40- and control IgG-treated 12-month old mice was similar. These results also suggest a correlation between the level of accumulated CD4+CD25+FoxP3+ T cells in the tumor and the effectiveness of OX40 therapy in two-month versus 12-month old tumor-bearing mice.
Co-administration of IL-12 and anti-OX40 increased tumor-free survival of 12-month old mice
To boost immune responses against the MCA205 tumor in 12-month old anti-OX40-treated tumor-bearing mice, we investigated the efficacy of IL-12. The results in the DO11.10 model (Fig. 5) suggest the combination of an OX40 agonist and IL-12 may represent an effective means to improve tumor-free survival and restore OX40-specific anti-tumor responses in 12-month old mice. Although, we did not see any detectable changes in IL-12 secretion in tumor-bearing mice treated with anti-OX40 (data not shown), we have described a relationship between IL-12 and OX40 that was shown to be essential for the survival of CD4 T cells after antigen priming and that co-administration of IL-12 and anti-OX40 synergized to eradicate tumors in various models (23, 24). The effects of IL-12 on tumor immunity in two- and 12-month old MCA205 tumor-bearing mice were assessed. IL-12, in these experiments, was administered starting four days after tumor challenge and then daily for an additional six days. IL-12 alone has significant anti-tumor effects (25) and it appeared to slow MCA205 tumor growth in young mice, but not in the older mice. However, IL-12 ultimately failed to generate prolonged tumor-free survival in either the two- or 12-month old mice (Fig. 7A). Co-administration of IL-12 (administered daily, days 4-9) and anti-OX40 (day 3 and 7) to MCA205 tumor-bearing twelve-month old mice, induced greater tumor-free survival compared to mice treated with anti-OX40 alone (Fig. 7B). The combination of anti-OX40 and IL-12 did not increase the extent of tumor-free survival in anti-OX40-treated two-month old mice in this model. When TILs were obtained from MCA205 tumors harvested from co-treated 12-month old mice (anti-OX40 and IL-12), there was a decrease in the accumulation of CD25+FoxP3+ T cells compared to age-matched rat IgG-, IL-12- or anti-OX40-treated mice and corresponded to the increase in tumor-free survival (Fig. 7C). These results suggest that IL-12 helps to reverse the negative impact of age on OX40-mediated tumor immune responses.
FIGURE 7.
Systemic administration of IL-12 to anti-OX40-treated 12-month old mice increased MCA205 tumor-free survival and reduces the accumulation of CD4+CD25+FoxP3+ in the tumors mice treated anti-OX40 and IL-12. Female C57BL/6 mice, two- and 12-months of age, were injected s.c. with 4-5 × 105 MCA205 tumor cells. A. IL-12 (100 ng) or PBS (control) was injected i.p. daily four-to-nine days after tumor challenge. B. Three and seven days after tumor challenge 250 μg anti-OX40 was injected i.p. IL-12 (100 ng) or PBS (control) was injected i.p. daily four-to-nine days after tumor challenge. Mice were then monitored for tumor growth. C. Three and seven days after tumor challenge 250 μg anti-OX40 was injected i.p. IL-12 (100 ng) or PBS (control) was injected i.p. daily four-to-nine days after tumor challenge. Tumors were resected 12 days after tumor challenge and TILs harvested (Materials and Methods). The frequency of CD4+ T cells expressing CD25+ and FoxP3+ from the tumors and pooled contra-lateral lymph nodes from the same mice were assessed by FACs.
Discussion
The results reported here demonstrate the negative impact of immune senescence on an effective tumor immunotherapy using systemic OX40 agonist administration. Elderly (20 month old) mice and surprisingly, middle-aged (12 month old) mice experienced a dramatic loss of therapeutic benefit from agonist OX40 stimulation, compared to younger mice (two and six months old). At least one deficiency underlying the loss of therapeutic benefit appeared to be related to the decrease in the OX40-mediated accumulation of cytokine-producing effector T cells in older tumor-bearing mice. This deficiency at first glance looked as if it was caused by defects inherent in the aging T cell component, but crisscross adoptive transfer experiments with young and middle-aged antigen-specific T cells suggest the defect was not T cell intrinsic. Instead the decreased levels of effector T cells following agonist OX40 treatment in middle-aged mice may originate from the cells that promote and support T cell activation, including those of the innate immune response. Indeed, administration of the innate cytokine IL-12 boosted levels of antigen-specific cytokine-producing T cells and partially restored OX40-mediated tumor-free survival in middle-aged mice.
Tumor-specific effector T cells are considered to be necessary to produce effective anti-tumor immune responses. In cancer patients, the generation and accumulation of effector T cells are critical for effective anti-tumor responses, as the presence of these cells within tumors in large numbers represents a positive clinical prognosis (26, 27). The acquisition of effector function and accumulation of effector T cells in tumor-bearing mice are also hallmarks of tumor regression following OX40 engagement in mice (17, 28-30). These studies directly link the anti-tumor properties of OX40 co-stimulation to an increase in the function and accumulation of differentiated effector T cells. In the experiments presented here, we observed a significant age-related decrease in the number of cytokine-producing effector T cells following OX40 agonist administration (Fig. 3) and the presence of fewer CD8 T cells infiltrating the tumors of the older anti-OX40-treated mice (Fig. 5A). Additionally, transfer of young and middle-aged DO11.10 T cells into young and middle-aged recipients respectively, also demonstrated an age-related decrease in the number of responding antigen-specific effector T cells following agonist OX40 administration. The diminished response in these mice was due in part to an overall decrease in numbers, but also an impaired ability to acquire OX40-enhanced effector function (Fig. 4B and 6A). Taken together, these results suggest the inability to generate sufficient numbers of differentiated effector T cells in older tumor-bearing mice contributes to the dramatic loss of anti-OX40-mediated tumor immunity.
Defects in various cell types and immunological systems could account for the age-related deficiency following OX40 engagement, but defects in the T cell component initially appeared to be the most probable. Previous studies have established that aged CD4 T cell contain significant intrinsic age-related T cell defects (31-33) and we observed decreased numbers of differentiated effector CD4 T cells in older mice. Crisscross adoptive transfer experiments using young and middle-aged transgenic TCR CD4 T cells, however, showed the aged-related deficiencies following OX40 engagement resided in the aged recipient, and were likely not inherent in the responding older T cells. These data agree with several other reports that show age-related deficiencies in T cell responses are not entirely intrinsic and potential defects in the host environment also contribute to the overall T cell dysfunction (34-36). While the exact age-related deficiency accounting for the loss of OX40-mediated anti-tumor immunity remains unknown, middle-aged cells of the innate immune response may harbor a crucial defect and therefore requires further study.
Inflammatory signals mediated by the innate immune response have been recognized to overcome some of the effects of immune senescence and we considered their use to restore the agonist OX40 responses in the older mice (37). One inflammatory signal of great interest, IL-12, induces Th1 differentiation and enhances IFNγ production (19, 20), making it an attractive candidate to restore OX40-enhanced anti-tumor immunity in middle-age mice. In fact, our results demonstrate IL-12 administration in combination with anti-OX40 increased the number of IFNγ-producing effector DO11.10 CD4 T cells in middle-aged mice and achieved partial restoration of the beneficial OX40-specific anti-tumor immune responses in older tumor-bearing mice. While IL-12, in combination with anti-OX40, restored partial tumor rejection in the middle-aged mice and reduced the accumulation of regulatory T cells, the levels of rejection fell short of the levels seen in the younger mice. This difference could be the result of exogenous IL-12 reversing a defect not directly caused by an age-related IL-12 deficiency, and/or could be due to the influence of age on cytotoxic T cell responses even in the absence of regulatory T cells (38). Furthermore, the combination of IL-12 and agonist OX40 failed to increase tumor regression in younger mice compared to agonist OX40 alone (Fig. 7A). A potential explanation for the lack of enhancement may relate to the data that showed the numbers of differentiated effector antigen-specific CD4 T cells were unchanged between these two treatment groups (Fig. 6B), suggesting priming to the MCA205 tumor induces optimal IL-12 secretion in younger mice. Overall these data demonstrate the ability of IL-12 to partially restore OX40-mediated tumor regression in older mice.
Our findings are not the first to describe the role of OX40 in anti-tumor immune responses in aged mice; previous publications demonstrated that effective co-stimulation, including OX40 engagement, overcame immune senescence and enhanced anti-tumor immune responses in elderly mice (15, 39). Although these studies appear to contradict results described here, the tumor models used in these studies relied on an immunogenic surrogate tumor antigen (eGFP) or an in vitro generated DC-tumor lysate vaccine. In the absence of these vaccination strategies, OX40 engagement alone had little to no effect on tumor regression in young mice. Despite the differences in antigen-priming in the models used here and in the earlier studies, all the studies demonstrate T cells from aging mice can respond to systemic OX40 administration (15, 39) (Fig. 4).
In addition, the studies presented here also revealed the accumulation of CD4+CD25+FoxP3+ T cells in actively growing tumors was influenced by OX40 engagement. Levels of CD4+CD25+FoxP3+ T cells infiltrating the tumors of young mice began to accumulate at greater levels compared to peripheral tissue around 10-12 days after tumor challenge (Fig. 6B and 6C). In contrast, the accumulation of CD4+CD25+FoxP3+ T cells within the tumors isolated from young mice treated with anti-OX40 (day 12) was significantly less than IgG treated mice, and also less than anti-OX40-treated middle-aged mice. The increased infiltration of CD4+CD25+FoxP3+ T cells into the tumors of the older anti-OX40-treated mice correlated with ineffective tumor clearance and has been shown by others to correlate with poor clinical prognosis (40).
The question remains as to the cause or causes for the increased accumulation of the CD4+CD25+FoxP3+ regulatory T cells in the tumors of agonist OX40 treated middle-aged mice. It is understood that tumors can recruit regulatory T cells (41), expand residing regulatory T cells (42), and convert naïve T cells into FoxP3+ regulatory T cells (43). Balancing out the effects of the tumor on regulatory T cells is OX40, which not only delivers a potent co-stimulatory signal to recently primed effector T cells, but can also strongly affect regulatory T cells, including abrogation of TGFβ1-induced generation of regulatory T cells (44-46). Recently, a proposed mechanism responsible for preventing regulatory T cell conversion following OX40 engagement was found to be mediated by IFNγ production by an OX40-expressing memory T cell population (47). IFNγ production has been previously shown to prevent the conversion of naïve T cells to regulatory T cells, instead directing Th1 differentiation (48). Another potential mechanism comes from recent data generated in our laboratory, suggesting anti-OX40 induces the expansion of CD4+CD25+FoxP3+ regulatory T cells in the absence of IFNγ and other Th differentiation cytokines (manuscript in preparation). Taken together, the decrease in IFNγ-producing T cells seen in older tumor-bearing mice treated with anti-OX40, ten days after tumor challenge, may account for increased regulatory T cells within the tumors of these mice shortly thereafter (day 12). Currently studies are underway to determine the contribution of both of these proposed mechanisms regarding OX40-specific control of regulatory T cell conversion and expansion.
Theoretically, these results illustrate that critical age-related immunological defects occur by middle age. Both elderly and middle aged mice experienced a similar decrease in tumor-free survival following anti-OX40 treatment. In contrast, a number of previous studies showed that immunological responses and T cell function in middle aged mice tended to represent an intermediate effect, falling somewhere between the young and elderly (18+ months old) subjects. For example, characterization of immune cell subsets and the expression of important chemokines and chemokine receptors in mice over a spectrum of ages showed graded or incremental changes in these readouts (49, 50). Our results suggest that even at middle age crucial aspects of an anti-tumor response can be dysfunctional, which may help to explain why there is a significant increase in the incidence of a number of cancers in middle-aged individuals (51). More importantly, the middle age “breakdown” also creates an opportunity to study and devise strategies to reverse age-related immunological deficiencies prior to the accumulation of other defects during the aging process.
In conclusion, engagement of OX40 has been proven to be an effective form of tumor immunotherapy in a number of mouse tumor models, which have treated young mice. Since agonists for OX40 and other TNFR proteins are in clinical trials for the treatment of cancer, our results showing diminished effects as mice age may have important ramifications for future clinical trials. The OX40-mediated improvements in survival dramatically decreased in mice at middle age, which is surprising, as immune responses from mice at this age have been shown to be relatively intact compared to younger mice. Upon further investigation, the critical age-related defect in these OX40-mediated anti-tumor responses likely involves decreased generation and accumulation of differentiated effector T cells. Interestingly, the underlying age-related deficiency in the generation and accumulation of differentiated effector T cells did not reside in the T cells themselves, but rather cells that promote and support T cell function. As evidence, IL-12 an innate cytokine, helped restore effector T cells levels and effective anti-tumor immune responses in middle-aged mice. Therefore, overcoming immune senescence in the treatment of cancer may require treatments that utilize immunotherapeutic reagents that can restore both adaptive and innate responses lost during the aging process.
Acknowledgements
We would like to thank Drs. Walter Urba and Janko Nikolich-Zugich for their critical review of this manuscript.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ershler WB. The influence of an aging immune system on cancer incidence and progression. J Gerontol. 1993;48:B3–7. doi: 10.1093/geronj/48.1.b3. [DOI] [PubMed] [Google Scholar]
- 2.Yung RL. Changes in immune function with age. Rheum Dis Clin North Am. 2000;26:455–473. doi: 10.1016/s0889-857x(05)70151-4. [DOI] [PubMed] [Google Scholar]
- 3.Linton PJ, Haynes L, Tsui L, Zhang X, Swain S. From naive to effector--alterations with aging. Immunol Rev. 1997;160:9–18. doi: 10.1111/j.1600-065x.1997.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 4.Effros RB, Walford RL. The immune response of aged mice to influenza: diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell Immunol. 1983;81:298–305. doi: 10.1016/0008-8749(83)90237-x. [DOI] [PubMed] [Google Scholar]
- 5.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 6.Ye Z, Hellstrom I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellstrom KE. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 7.Bansal-Pakala P, Croft M. Defective T cell priming associated with aging can be rescued by signaling through 4-1BB (CD137). J Immunol. 2002;169:5005–5009. doi: 10.4049/jimmunol.169.9.5005. [DOI] [PubMed] [Google Scholar]
- 8.Kjaergaard J, Peng L, Cohen PA, Drazba JA, Weinberg AD, Shu S. Augmentation versus inhibition: effects of conjunctional OX-40 receptor monoclonal antibody and IL-2 treatment on adoptive immunotherapy of advanced tumor. J Immunol. 2001;167:6669–6677. doi: 10.4049/jimmunol.167.11.6669. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, Urba WJ, Alvord G, Bunce C, Shields J. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 11.Evans DE, Munks MW, Purkerson JM, Parker DC. Resting B lymphocytes as APC for naive T lymphocytes: dependence on CD40 ligand/CD40. J Immunol. 2000;164:688–697. doi: 10.4049/jimmunol.164.2.688. [DOI] [PubMed] [Google Scholar]
- 12.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–2195. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaleeba JA, Offner H, Vandenbark AA, Lublinski A, Weinberg AD. The OX-40 receptor provides a potent co-stimulatory signal capable of inducing encephalitogenicity in myelin-specific CD4+ T cells. Int Immunol. 1998;10:453–461. doi: 10.1093/intimm/10.4.453. [DOI] [PubMed] [Google Scholar]
- 14.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8(+) memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37:157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 15.Lustgarten J, Dominguez AL, Thoman M. Aged mice develop protective antitumor immune responses with appropriate costimulation. J Immunol. 2004;173:4510–4515. doi: 10.4049/jimmunol.173.7.4510. [DOI] [PubMed] [Google Scholar]
- 16.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 17.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 18.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167:6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 19.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 20.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 21.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev. 2006;212:163–169. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 22.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 24.Kuriyama H, Watanabe S, Kjaergaard J, Tamai H, Zheng R, Weinberg AD, Hu HM, Cohen PA, Plautz GE, Shu S. Mechanism of third signals provided by IL-12 and OX-40R ligation in eliciting therapeutic immunity following dendritic-tumor fusion vaccination. Cell Immunol. 2006;243:30–40. doi: 10.1016/j.cellimm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 28.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 29.Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL Promote the Persistence of CD8 T Cells to Recall Tumor-Associated Antigen. J Immunol. 2005;175:3534–3541. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- 30.Song A, Song J, Tang X, Croft M. Cooperation between CD4 and CD8 T cells for anti-tumor activity is enhanced by OX40 signals. Eur J Immunol. 2007;37:1224–1232. doi: 10.1002/eji.200636957. [DOI] [PubMed] [Google Scholar]
- 31.Mittler JN, Lee WT. Antigen-specific CD4 T cell clonal expansion and differentiation in the aged lymphoid microenvironment. I. The primary T cell response is unaffected. Mech Ageing Dev. 2004;125:47–57. doi: 10.1016/j.mad.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J Exp Med. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J Immunol. 2007;178:1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- 35.Li SP, Cai Z, Shi W, Brunmark A, Jackson M, Linton PJ. Early antigen-specific response by naive CD8 T cells is not altered with aging. J Immunol. 2002;168:6120–6127. doi: 10.4049/jimmunol.168.12.6120. [DOI] [PubMed] [Google Scholar]
- 36.Linton PJ, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T. Intrinsic versus environmental influences on T-cell responses in aging. Immunol Rev. 2005;205:207–219. doi: 10.1111/j.0105-2896.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 37.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Win S, Uenaka A, Nakayama E. Immune responses against allogeneic and syngeneic tumors in aged C57BL/6 mice. Microbiol Immunol. 2002;46:513–519. doi: 10.1111/j.1348-0421.2002.tb02728.x. [DOI] [PubMed] [Google Scholar]
- 39.Sharma S, Dominguez AL, Lustgarten J. Aging affect the anti-tumor potential of dendritic cell vaccination, but it can be overcome by co-stimulation with anti-OX40 or anti-4-1BB. Exp Gerontol. 2006;41:78–84. doi: 10.1016/j.exger.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 42.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 43.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25 -T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 44.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 45.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Chang Li X. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao X, Kroemer A, Gao W, Ishii N, Demirci G, Li XC. OX40/OX40L costimulation affects induction of Foxp3+ regulatory T cells in part by expanding memory T cells in vivo. J Immunol. 2008;181:3193–3201. doi: 10.4049/jimmunol.181.5.3193. [DOI] [PubMed] [Google Scholar]
- 48.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connoy AC, Trader M, High KP. Age-related changes in cell surface and senescence markers in the spleen of DBA/2 mice: a flow cytometric analysis. Exp Gerontol. 2006;41:225–229. doi: 10.1016/j.exger.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Mo R, Chen J, Han Y, Bueno-Cannizares C, Misek DE, Lescure PA, Hanash S, Yung RL. T cell chemokine receptor expression in aging. J Immunol. 2003;170:895–904. doi: 10.4049/jimmunol.170.2.895. [DOI] [PubMed] [Google Scholar]
- 51.Macieira-Coelho A. Neoplastic disease through the human life span. Biogerontology. 2001;2:179–192. doi: 10.1023/a:1011552822076. [DOI] [PubMed] [Google Scholar]