Abstract
Mammalian embryos initially develop progenitor tissues for both male and female reproductive tract organs, known as the Wolffian ducts and the Müllerian ducts, respectively. Ultimately, each individual develops a single set of male or female reproductive tract organs. Therefore, an essential step for sex differentiation is the regression of one duct and growth and differentiation of the other duct. In males, this requires Müllerian duct regression and Wolffian duct growth and differentiation. Müllerian duct regression is induced by the expression of Amh, encoding anti-Müllerian hormone, from the fetal testes. Subsequently, receptor-mediated signal transduction in mesenchymal cells surrounding the Müllerian duct epithelium leads to duct elimination. The genes that induce Amh transcription and the downstream signaling that results from Amh activity form a pathway. However, the molecular details of this pathway are currently unknown. A set of essential genes for AMH pathway function has been identified. More recently, transcriptome analysis of male and female Müllerian duct mesenchyme at an initial stage of regression has identified new genes that may mediate elimination of the Müllerian system. The evidence taken together can be used to generate an initial gene regulatory network describing the Amh pathway for Müllerian duct regression. An Amh gene regulatory network will be a useful tool to study Müllerian duct regression, sex differentiation, and its relationship to environmental influences.
Keywords: sex differentiation, anti-Müllerian hormone, transcription
Introduction
Classic experiments by Alfred Jost in fetal rabbits identified a Müllerian inhibitor associated with the testis that was required for the regression of the Müllerian ducts [1, 2]. The Müllerian inhibitor was subsequently identified as anti-Müllerian hormone (AMH), also known as Müllerian inhibiting substance (MIS) or factor (MIF) [3]. Molecular studies led to the cloning of the genes encoding AMH and its type II receptor (AMHR2) [4–7]. Human studies have identified mutations in the AMH and AMHR2 genes, leading to a condition known as persistent Müllerian duct syndrome (PMDS), a rare recessive intersex condition [8]. Males with PMDS have a uterus and fallopian tubes and can have testicular descent abnormalities. Mutations in these two genes have been shown to result in PMDS in human, mouse, and dog [9]. In addition, gene knockout studies in mice have led to the identification of Amh, Amhr2, and other genes required for Müllerian duct regression (Table 1). Together, there is now sufficient information to describe the first gene regulatory network (GRN) for Müllerian duct regression. This GRN should provide a useful framework to understand the genetic interactions that lead to Müllerian duct regression, sex differentiation, and its relationship to environmental influences.
Table 1:
genes that participate in AMH GRN for Müllerian duct regression
| Gene | Mutation | Phenotype | Reference |
|---|---|---|---|
| Acvr1 | Conditional KO | Normal MD regression | Orvis et al. [36] |
| Amh | Null | PMDS | Behringer [21]; Arango et al. [22] |
| Amhr2 | Null | PMDS | Mishina et al. [30]; Jamin et al. [31]; Arango et al. [17] |
| Bmpr1a | Conditional KO | PMDS | Jamin et al. [31]; Orvis et al. [36] |
| Ctnnb1 (beta-catenin) | Conditional KO | PMDS | Kobayashi et al. [41] |
| Gata4 | Binding site mutant or deletion | Normal MD regression | Bouchard et al. [26] |
| Mmp2 | Null | Normal MD regression | Roberts et al. [44] |
| Smad1/5/8 | Conditional KO | PMDS | Orvis et al. [36] |
| Sp7 (Osterix) | Null | Delayed MD regression | Mullen et al. [42] |
| Wif1 | Null | Normal MD regression | Park et al. [46] |
| Wnt7a | Null | PMDS | Parr and McMahon [34] |
Conditional KO, Müllerian duct mesenchyme-specific knockout; MD, Müllerian duct; null, full body knockout; PMDS, persistent Müllerian duct syndrome.
Müllerian Ducts Form in Both Male and Female Embryos
During fetal development, the Wolffian and Müllerian ducts form in both males and females. The Müllerian ducts can differentiate into the oviduct, uterus, and a portion of the vaginal canal. The Wolffian ducts can differentiate into vasa deferentia, epididymides, seminal vesicles, and the ejaculatory ducts. The Müllerian duct is a mesoepithelial tissue that requires the Wolffian duct for its development [10, 11]. Müllerian duct formation begins shortly after the Wolffian duct forms, taking place in three phases: initiation, invagination, and elongation [11]. Initiation consists of the specification of mesonephric epithelial cells to become Müllerian duct cells, characterized by Lhx1 expression, at about E11.75 in mice. During the invagination phase, the Lhx1+ cells form an invagination that makes contact with the Wolffian duct. The Müllerian duct then elongates along the Wolffian duct [12]. During Müllerian duct elongation, cell proliferation is observed throughout the duct with more proliferating cells at the growing tip. The Müllerian duct crosses the Wolffian duct at E12.5 to gain a medial position and subsequently fuses with the urogenital sinus at E13.5 [13]. Wnt9b is expressed in the Wolffian duct [14]. Wnt9b knockout mice form Wolffian ducts but do not elongate the Müllerian ducts, suggesting a molecular mechanism for the requirement of the Wolffian duct for Müllerian duct development [14]. Shortly after the Müllerian ducts form, sex differentiation proceeds. In females, the Müllerian system continues to develop into the uterus, oviducts and a portion of the vagina. However, in males the Müllerian ducts are actively eliminated.
Müllerian Duct Regression Occurs in Male Embryos
A major event in male sex differentiation is Müllerian duct regression. Mesenchyme–epithelia interactions mediate this process to ensure that oviducts and a uterus do not develop within the male body [15]. The cells of the Müllerian duct have a mesoepithelial character during regression [11]. Regression begins shortly after the Müllerian duct connects to the urogenital sinus at E13.5 in the mouse and is completed by birth. Early stages of regression can be observed by tightly condensed mesenchymal cells surrounding the Müllerian duct with intercellular spaces located more radially [16, 17]. Müllerian duct regression is proposed to occur through at least three mechanisms: epithelial cell migration, epithelial to mesenchymal transformation, and apoptosis [18–20]. Apoptosis is initially detected in the rostral region of the Müllerian duct and subsequently in intermediate and caudal regions [18]. Disruptions in the Müllerian duct basement membrane are followed by epithelial cell entrance into the mesenchymal compartment [18]. The regression of the Müllerian duct is proposed to occur in a rostral to caudal manner [15].
Genes That Regulate Müllerian Duct Regression
Anti-Müllerian Hormone (Amh)
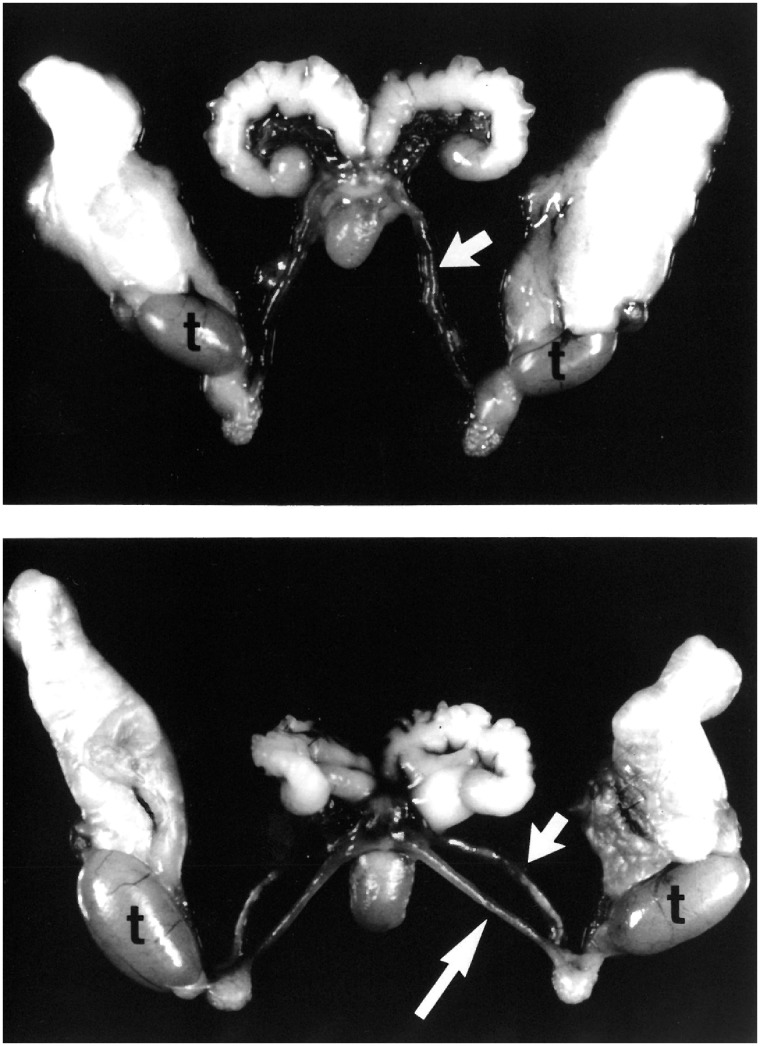
The first gene knockout in the AMH signaling pathway was in the Amh locus [21]. Males homozygous for the targeted Amh mutation did not regress the Müllerian ducts, resulting in the development of a uterus, oviducts, and vaginal tissue (Fig. 1). These mutant males had correctly descended testes and Wolffian duct derivatives, including seminal vesicles, vasa deferentia, and epididymides. Only ∼10% of the mutant males were fertile. However, sperm from the mutant epididymides were competent to fertilize oocytes in vitro. These genetic findings demonstrate that Amh produced by the fetal testes is essential for Müllerian duct regression.
Figure 1:
PDMS in the mouse. Dissected reproductive tract organs from control (top) and Amh homozygous mutant (bottom) males. In the mutant, the uterine horns (long arrow) and vas deferens (short arrow) parallel each other down to the testes (t) because of a common connective tissue. In this dissection, the connective tissue has been cut to reveal the dual nature of the reproductive tract. Note that because of the physical constraints imposed by the vas deferens, the uterine horns project caudally instead of rostrally. Images from Behringer [21]
The cis-regulation of Amh transcription was studied in vitro and in vivo [22, 23]. In vitro studies identified interactions of the nuclear hormone receptor steroidogenic factor 1 (SF1) also known as Ad4-binding protein (Ad4BP) or NR5A1 with a 20-bp sequence just upstream of the TATAA motif that is required for Amh transcription [23]. About 50-bp upstream of the SF1-binding site is a conserved binding site for the high-mobility group transcription factor SOX9 for activation of Amh transcription [24]. SF1- and SOX9-binding site mutations were introduced into the endogenous Amh locus by gene targeting in embryonic stem (ES) cells [22]. Surprisingly, males homozygous for the SF1-binding site mutation had normal Müllerian duct regression. Molecular studies showed that Amh transcript levels in fetal and postnatal testes were 3-fold lower in comparison to wild type. Thus, the SF1-binding site regulates Amh transcript levels. In contrast, males homozygous for the SOX9-binding site mutation were a phenocopy of the Amh-null male phenotypes, i.e. PMDS. These findings suggest that SF1 regulates Amh transcript levels and SOX9 is essential for the activation of Amh transcription.
There are also multiple GATA-binding sites 5′ of the Amh transcriptional start site [25]. Recently, male mice homozygous for a 2-bp mutation in one of the GATA-binding sites that abolishes GATA binding (termed GATAmut) were generated, using CRISPR genome editing [26]. GATAmut homozygotes were found to have a 50% reduction in Amh transcripts in their testes [26]. In addition, a 40-bp deletion that included the GATA-binding site and an adjacent SF1-binding site resulted in a 90% reduction in testicular Amh transcripts. Although there was a dramatic reduction in Amh transcripts, both types of adult mutant males did not retain Müllerian duct derivatives. Apparently, there are still sufficient levels of AMH for Müllerian duct regression in these mouse mutants.
Anti-Müllerian Hormone Receptor 2 (Amhr2)
A 21-day-old rat Sertoli cell cDNA, encoding the type II receptor for AMH, Amhr2, was first reported by Baarends et al. [5]. Their conclusion was based on protein domain structure, indicating a transmembrane serine/threonine kinase receptor and expression localized in the Müllerian duct of male and female fetuses. Amhr2 transcripts were detected in the mesenchyme adjacent to the Müllerian duct epithelium during embryogenesis, suggesting that the target cell for AMH action is the Müllerian duct mesenchyme. Amhr2 is also expressed in the fetal and postnatal gonads, specifically in Sertoli and granulosa cells [5, 22, 27–29].
A targeted mutation in the mouse Amhr2 gene was generated by gene targeting in ES cells [30]. Approximately 4.4 kb of Amhr2, including exons 1–6, was deleted, replacing these sequences with a neomycin resistance gene expression cassette. Homozygous mutant males were normal in size, had correctly descended testes, and differentiated derivatives of the Wolffian ducts. All of the homozygous mutant males also developed a uterus, oviducts, and partial vagina in addition to their male reproductive organ system. This was a phenocopy of Amh-null male mice. Some of the homozygous mutant males were fertile. In addition, two other Amhr2 loss-of-function alleles have been generated, including Cre and lacZ knock-ins [17, 31]. Both alleles result in a persistence of Müllerian duct derivatives in homozygous mutant males. These findings demonstrate that Amhr2 is required for Müllerian duct regression.
The transcriptional regulation of Amhr2 has been explored [32, 33]. Wt1 encodes a zinc finger transcription factor. A microarray analysis of ∼E11.0 Wt1 wild-type and null urogenital ridges identified Amhr2 as a candidate gene regulated by Wt1. Wt1 and Amhr2 were found to be co-expressed in the urogenital ridge. Three WT1-binding sites are within 100-bp of the Amhr2 transcriptional start site. Biochemical and in vitro studies showed that these sequences bind WT1 and act together to regulate Amhr2 transcription. More studies are required to determine if these sequences are required for Amhr2 transcription in vivo for Müllerian duct regression.
Wnt7a in the Müllerian Duct Epithelium Induces Amhr2 Expression in Adjacent Mesenchyme
Wnt7a was identified as a gene required for Müllerian duct regression [34]. Wnt7a is expressed in the Müllerian duct epithelium in both male and female mice from E12.5 to E14.5 [34]. Expression continues in the Müllerian ducts of females as they differentiate into the oviducts and uterus. Wnt7a-null males are born with Müllerian duct derivatives [34]. In-situ hybridization analysis of the mutant males showed that Amhr2 expression was detected in testes but absent in the Müllerian duct mesenchyme [34]. This suggests that the essential function of Wnt7a in the Amh pathway is to activate Amhr2 transcription in the Müllerian duct mesenchyme [34]. Thus, Wnt7a expressed in the Müllerian duct epithelium signals to the adjacent mesenchyme that induces Amhr2 transcription making the Müllerian ducts competent to respond to AMH for regression [34].
Bmpr1a and Acvr1 Encode Type I Receptors That Mediate AMH Signaling
The transforming growth factor (TGF)-beta superfamily consists of >30 cytokines [35]. However, only seven type I receptors have been identified. Thus, TGF-beta family members must share type I receptors to mediate their signal transduction. Two TGF-beta type 1 superfamily receptor genes, Acvr1 and Bmpr1a, have been identified as AMH receptors for Müllerian duct regression [36]. Acvr1- and Bmpr1a-null mice are embryonic lethal before the Müllerian ducts form [37, 38]. Thus, tissue-specific knockouts were generated. When Bmpr1a was knocked out in the Müllerian duct mesenchyme of male mice, Müllerian duct retention was observed in ∼50% of the mutants [31, 36]. This Müllerian duct retention phenotype was identical to the phenotype observed for Amh and Amhr2 knockout males. All males with a conditional knockout of Acvr1 in the Müllerian duct mesenchyme had Müllerian duct regression [36]. However, when both Acvr1 and Bmpr1a were both knocked out in the Müllerian duct mesenchyme, 100% of the male mutants retained the Müllerian ducts, forming the uterus and oviducts [36]. These results suggest that Acvr1 and Bmpr1a act redundantly in the Amh-induced Müllerian duct regression pathway.
Smad1, Smad 5, and Smad8 Act Redundantly to Mediate AMH Signaling
Smad activity has been shown to contribute to Müllerian duct regression within the Amh pathway. Smad1, Smad5, and Smad8 (also known as Smad9) are all expressed in the Müllerian duct mesenchyme [39, 40]. A conditional knockout of Smad1 in the Müllerian duct mesenchyme resulted in proper Müllerian duct regression, as did a conditional Smad1/Smad8 knockout [36]. Conditional knockouts of Smad1 or Smad8 combined with a Smad5 conditional knockout resulted in partial Müllerian duct retention [36]. This consisted of only part of the Müllerian duct being retained: caudally, rostrally, and/or on one side. The triple conditional knockout of Smad1/Smad5/Smad8, however, resulted in fully retained Müllerian duct derivatives [36]. Therefore, the three Smad genes function redundantly within the pathway, likely downstream of Acvr1 and Bmpr1a.
Beta-Catenin Is Required for MD Regression
Multiple Wnt genes are expressed in the mesonephros, including Wnt4, Wnt5a, Wnt7a, and Wnt9b (gudmap.org). To determine if the canonical WNT pathway was required for Müllerian duct regression, a Müllerian duct mesenchyme-specific knockout of β-catenin was performed [41]. Loss of beta-catenin in the Müllerian duct mesenchyme resulted in the persistent Müllerian duct phenotype in all male mutants. Additionally, AMH was found to be expressed in the Sertoli cells of the mutant testes, implying that loss of β-catenin disrupts the pathway downstream of AMH expression [41]. These results suggest that β-catenin functions specifically in the Müllerian duct mesenchyme to mediate Müllerian duct regression downstream of AMH signaling [41].
Osterix Is an AMH-Induced Regulator of Müllerian Duct Regression
A transcriptome analysis of RNA-seq data generated from purified E14.5 male and female Müllerian duct mesenchyme identified Osterix (Osx), also known as Sp7, as a male-enriched gene [42]. Osx was originally identified as a gene required for osteoblast differentiation [43]. Osx is expressed in a sexually dimorphic manner in the Müllerian duct mesenchyme. At E14.5, Osx expression is detected in the Müllerian ducts of male fetuses, whereas no expression was detected in the Müllerian ducts of female fetuses [42]. This male-specific expression continues throughout the remaining regression process [42]. In addition, Osx expression is lost in male mice lacking Amhr2 [42]. In contrast, overexpression of human AMH in females, using an MT-hAMH transgene stimulates Osx transcription [42]. This Amh- and Amhr2-dependent, sex-specific expression implies a role for Osx in the Amh signaling pathway. Additionally, loss of ß-catenin expression leads to a reduction in Osx transcripts, implying that Osx is downstream of ß-catenin in the regression pathway [42]. Osx knockout males have a 24-h delay in Müllerian duct regression [42]. When compared to wild-type males, Osx knockout males showed longer and thicker segments of the Müllerian duct at E15.5, E16.5, and E17.5 but complete regression by E18.5 [42]. Taken together, the data suggest that Osx is an AMH-induced gene that contributes to Müllerian duct regression.
Other Genes That May Be Involved in Müllerian Duct Regression
Mmp2 (matrix metallopeptidase 2) transcripts are detected in the male Müllerian duct mesenchyme [44]. Mmp2 expression in the Müllerian duct mesenchyme is lost in Amh−/− male mouse fetuses at E13 and E14 [44]. Pharmacological inhibition of Mmp2 in vitro blocks Müllerian duct regression in male urogenital organ culture [44]. Knockdown of Mmp2 using morpholino oligonucleotides also resulted in Müllerian duct regression defects in male urogenital organ culture. In contrast, activation of MMPs resulted in Müllerian duct regression in female urogenital organ culture. However, male Mmp2 mutant mice have normal Müllerian duct regression [44], suggesting that Mmp2 is not essential for Müllerian duct regression in vivo or may act redundantly perhaps with other Mmp genes.
Wif1 (WNT inhibitory factor 1) is a secreted frizzled-related protein that inhibits WNT signaling by binding WNT, blocking binding to receptors [45]. Wif1 expression is detected in the male Müllerian duct but not the female at E13.5 and E14.5 [46]. Wif1 transcripts are not detected in Amhr2−/− male fetuses at E13.5 [46]. Exogenous AMH can induce Wif1 expression in the Müllerian duct mesenchyme in female urogenital organ culture. Knockdown of Wif1 expression by siRNA in vitro inhibits Müllerian duct regression [46]. However, newborn and 4-week-old Wif1 knockout male mice did not have residual Müllerian tissues, suggesting that Wif1 is not essential for Müllerian duct regression in vivo or there is gene redundancy.
GRNs Describe Pathways That Regulate Biological Processes
GRNs can be described as control systems, at the genetic level, for living creatures. They can include, but are not limited to, DNA sequence-specific transcription factors and downstream genes. The network consists of the processes by which gene products and sequences function collectively to fulfill a biological task. The gene sequences in this case are targets of transcription factors, including enhancers, insulators, and silencers [47]. GRNs are particularly paramount in development. GRNs, for example, specify morphological structures in organisms by dictating the timing and development of cells and tissues that make up a structure [48]. GRNs, therefore, can be uncovered and visualized to illuminate the proper development and function of an organism. When mapped, a GRN can display nodes, feedback loops, enhancement, inhibition, and much more to represent genetic regulation. Regulatory information placed into GRNs is first uncovered and supported through experimentation. Nodes are often defined from knockout or knockdown experiments, which show that a gene is required for function of the network. Further experimentation then specifies genes that encode regulators of those required genes or regulatory sequences within the genome. Once a GRN has begun to be mapped, it can be used to generate hypotheses for the GRN or biological process affected by the GRN. A GRN from one species can be compared to other GRNs across species for evolutionary study, as GRNs hold the modifications that differentiate one species from another [48]. Medically, GRNs dictate normal bodily function and are, therefore, effective aids in finding causes and solutions for disease. Considering sexual development particularly, GRNs provide information needed to understand how organisms become sexually differentiated. In the case of this review, we will define our GRN using the studies by Eric Davidson as a guide [49].
A GRN for Müllerian Duct Regression
Based on the genetic and molecular evidence presented above, we present the first GRN for AMH-induced Müllerian duct regression (Fig. 2). Sertoli cells of the fetal testes express Sf1/Nr5a1, Gata4, and Sox9, encoding transcription factors that directly bind the 5′ region of the Amh locus. These are among the very few direct interactions between transcription factors and cis-regulatory elements for genes in the GRN. Müllerian duct epithelial cells express and secrete WNT7A that interacts with the adjacent mesenchyme cells to induce the expression of Amhr2, making the mesenchyme competent to respond to AMH. AMH secreted by fetal Sertoli cells interacts with AMHR2/BMPR1A/ACVR1, resulting in phosphorylation of SMAD1/5/8. The transcriptional targets of these SMADs are currently unknown. The requirement of beta-catenin in mesenchyme for Müllerian duct regression suggests that canonical WNT signaling may be required. Beta-catenin regulates Osx transcript levels but there are other inputs for Osx transcription. Transcriptome comparisons between male and female Müllerian duct mesenchyme have identified numerous genes that are upregulated in males relative to females [42, 46]. These provide candidate genes to be investigated for their roles in Müllerian duct regression. The GRN indicates that environmental influences that alter Amh transcriptional regulators (SOX9, NR5A1, and GATA4) could alter Müllerian duct regression. However, more studies are required to investigate how environmental factors might alter transcriptional outputs within the Müllerian duct mesenchyme. In conclusion, this Amh-regulated GRN provides a tool to investigate Müllerian duct regression, male sex differentiation, and how it may relate to environmental influences.
Figure 2:
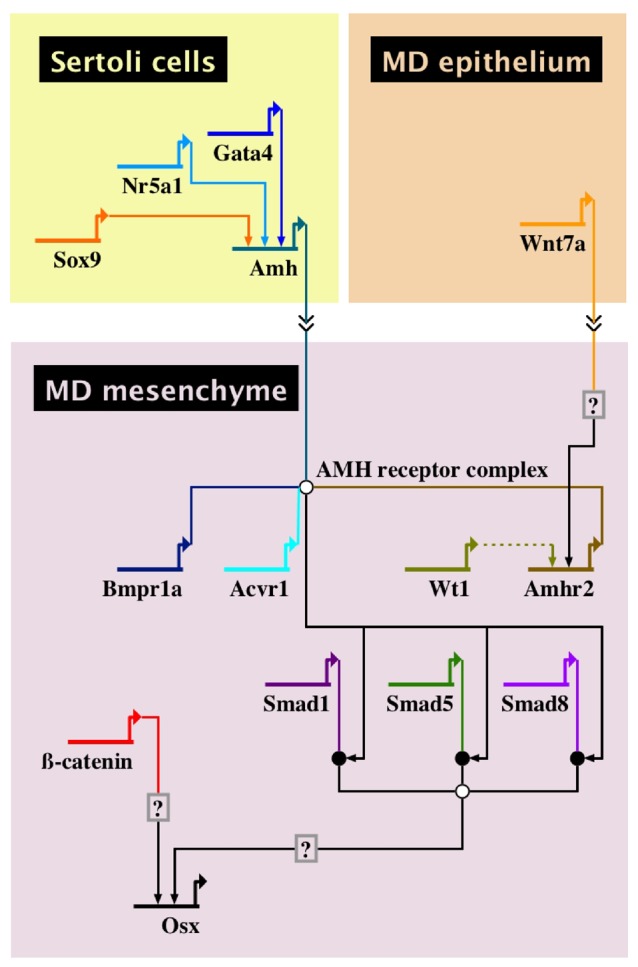
a GRN for AMH-induced Müllerian duct regression. The GRN is divided into domains, representing specific fetal cell types. The interactions contained within a domain occur within that cell type. Each gene in the GRN is depicted as a short horizontal line from which extends a bent arrow indicating transcription. The name of each gene is below the horizontal line. Arrows extending from the transcription arrow of one gene to the horizontal line of another gene indicate transcription factor binding to cis-regulatory elements to enhance transcription. Double arrows between cell-type domains indicate intercellular signaling of a protein. Circles in the GRN indicate intracellular protein activity. White circles indicate multiprotein complexes. Black circles indicate phosphorylation of a protein. Arrows formed by dotted lines indicate interactions that have been observed in vitro but not yet confirmed in vivo. Boxes containing question marks indicate a predicted site of regulation that has not yet been determined
Acknowledgements
We thank Rachel Mullen for helpful comments on the manuscript. R.R.B. was supported by National Institutes of Health (HD30284) and Ben F. Love Endowment.
Conflict of interest statement. None declared.
References
- 1. Jost A. Recherches sur la differenciation sexuelle de I'embryon de lapin. Arch Anat Microsci Morphol Exp 1947;36:271–315. [Google Scholar]
- 2. Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog Horm Res 1953;8:379–413. [Google Scholar]
- 3. Josso N, Cate RL, Picard JY, Vigier B, di Clemente N, Wilson C, Imbeaud S, Pepinsky RB, Guerrier D, Boussin L.. Anti-Müllerian hormone: the Jost factor. Recent Prog Horm Res 1993;48:1–59. [DOI] [PubMed] [Google Scholar]
- 4. Imbeaud S, Faure E, Lamarre I, Mattei MG, di Clemente N, Tizard R, Carre-Eusebe D, Belville C, Tragethon L, Tonkin C, Nelson J, McAuliffe M, Bidart JM, Lababidi A, Josso N, Cate RL, Picard JY.. Insensitivity to anti-Müllerian hormone due to a mutation in the human anti-Müllerian hormone receptor. Nat Genet 1995;11:382–8. [DOI] [PubMed] [Google Scholar]
- 5. Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, Uilenbroek JT, Karels B, Wilming LG, Meijers JH, et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Müllerian duct, Development 1994;120:189–97. [DOI] [PubMed] [Google Scholar]
- 6. Cate Rl, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP, Fisher RA, Bertonis JM, Torres G, Wallner BP, Ramachandran Kl, Ragin RC, Manganaro TF, MacLaughlin DT, Donahoe PK.. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell 1986;45:685–98. [DOI] [PubMed] [Google Scholar]
- 7. Picard JY, Benarous R, Guerrier D, Josso N, Kahn A.. Cloning and expression of cDNA for anti-Müllerian hormone. Proc Natl Acad Sci USA 1986;83:5464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Picard JY, Cate RL, Racine C, Josso N.. The persistent Müllerian duct syndrome: an update based upon a personal experience of 157 cases. Sex Dev 2017;11:109–25. [DOI] [PubMed] [Google Scholar]
- 9. Mullen RD, Ontiveros AE, Moses MM, Behringer RR.. AMH and AMHR2 mutations: a spectrum of reproductive phenotypes across vertebrate species. Dev Biol 2019. doi: 10.1016/j.ydbio.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobayashi A, Behringer RR.. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 2003;4:969–80. [DOI] [PubMed] [Google Scholar]
- 11. Orvis GD, Behringer RR.. Cellular mechanisms of Müllerian duct formation in the mouse. Dev Biol 2007;306:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang CC, Orvis GD, Kwan KM, Behringer RR.. Lhx1 is required in Müllerian duct epithelium for uterine development. Dev Biol 2014;389:124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masse J, Watrin T, Laurent A, Deschamps S, Guerrier D, Pellerin I.. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol 2009;53:411–24. [DOI] [PubMed] [Google Scholar]
- 14. Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP.. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 2005;9:283–92. [DOI] [PubMed] [Google Scholar]
- 15. Mullen RD, Behringer RR.. Molecular genetics of Müllerian duct formation, regression and differentiation. Sex Dev 2014;8:281–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dyche WJ. A comparative study of the differentiation and involution of the Müllerian duct and Wolffian duct in the male and female fetal mouse. J Morphol 1979;162:175–209. [DOI] [PubMed] [Google Scholar]
- 17. Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR.. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev 2008;75:1154–62. [DOI] [PubMed] [Google Scholar]
- 18. Allard S, Adin P, Gouedard L, Clemente ND, Josso N, Orgebin-Crist MC, Picard JY, Xavier F.. Molecular mechanisms of hormone-mediated Müllerian duct regression: involvement of beta-catenin. Development 2000;127:3349–60. [DOI] [PubMed] [Google Scholar]
- 19. Austin HB. DiI analysis of cell migration during Müllerian duct regression. Dev Biol 1995;169:29–36. [DOI] [PubMed] [Google Scholar]
- 20. Hutson JM, Fallat ME, Kamagata S, Donahoe PK, Budzik GP.. Phosphorylation events during Müllerian duct regression. Science 1984;223:586–9. [DOI] [PubMed] [Google Scholar]
- 21. Behringer RR. The in vivo roles of Müllerian-inhibiting substance. Curr Top Dev Biol 1994;29:171–87. [DOI] [PubMed] [Google Scholar]
- 22. Arango NA, Lovell-Badge R, Behringer RR.. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 1999;99:409–19. [DOI] [PubMed] [Google Scholar]
- 23. Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA.. Nuclear receptor steroidogenic factor 1 regulates the Müllerian inhibiting substance gene: a link to the sex determination cascade. Cell 1994;77:651–61. [DOI] [PubMed] [Google Scholar]
- 24. De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P.. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol 1998;18:6653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lasala C, Carre-Eusebe D, Picard JY, Rey R.. Subcellular and molecular mechanisms regulating anti-Müllerian hormone gene expression in mammalian and nonmammalian species. DNA Cell Biol 2004;23:572–85. [DOI] [PubMed] [Google Scholar]
- 26. Bouchard MF, Bergeron F, Grenier Delaney J, Harvey LM, Viger RS.. In vivo ablation of the conserved GATA-binding motif in the Amh promoter impairs Amh expression in the male mouse. Endocrinology 2019;160:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baarends WM, Hoogerbrugge JW, Post M, Visser JA, De Rooij DG, Parvinen M, Themmen AP, Grootegoed JA.. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression during postnatal testis development and in the adult testis of the rat. Endocrinology 1995;136:5614–22. [DOI] [PubMed] [Google Scholar]
- 28. di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, Tizard R, Picard JY, Vigier B, Josso N, Cate R.. Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol Endocrinol 1994;8:1006–20. [DOI] [PubMed] [Google Scholar]
- 29. Teixeira J, He WW, Shah PC, Morikawa N, Lee MM, Catlin EA, Hudson PL, Wing J, Maclaughlin DT, Donahoe PK.. Developmental expression of a candidate Müllerian inhibiting substance type II receptor. Endocrinology 1996;137:160–5. [DOI] [PubMed] [Google Scholar]
- 30. Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR.. Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev 1996;10:2577–87. [DOI] [PubMed] [Google Scholar]
- 31. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR.. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet 2002;32:408–10. [DOI] [PubMed] [Google Scholar]
- 32. Shimamura R, Fraizer GC, Trapman J, Lau YfC, Saunders GF.. The Wilms' tumor gene WT1 can regulate genes involved in sex determination and differentiation: SRY, Müllerian-inhibiting substance, and the androgen receptor. Clin Cancer Res 1997;3:2571–80. [PubMed] [Google Scholar]
- 33. Klattig J, Sierig R, Kruspe D, Makki MS, Englert C.. WT1-mediated gene regulation in early urogenital ridge development. Sex Dev 2007;1:238–54. [DOI] [PubMed] [Google Scholar]
- 34. Parr BA, McMahon AP.. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 1998;395:707–10. [DOI] [PubMed] [Google Scholar]
- 35. Heldin CH, Moustakas A.. Signaling receptors for TGF-beta family members. Cold Spring Harb Perspect Biol 2016;8:a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orvis GD, Jamin SP, Kwan KM, Mishina Y, Kaartinen VM, Huang S, Roberts AB, Umans L, Huylebroeck D, Zwijsen A, Wang D, Martin JF, Behringer RR.. Functional redundancy of TGF-beta family type I receptors and receptor-Smads in mediating anti-Müllerian hormone-induced Müllerian duct regression in the mouse. Biol Reprod 2008;78:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mishina Y, Crombie R, Bradley A, Behringer RR.. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol 1999;213:314–26. [DOI] [PubMed] [Google Scholar]
- 38. Mishina Y, Suzuki A, Ueno N, Behringer RR.. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 1995;9:3027–37. [DOI] [PubMed] [Google Scholar]
- 39. Clarke TR, Hoshiya Y, Yi Se, Liu X, Lyons KM, Donahoe PK.. Müllerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol 2001;15:946–59. [DOI] [PubMed] [Google Scholar]
- 40. Visser JA. AMH signaling: from receptor to target gene. Mol Cell Endocrinol 2003;211:65–73. [DOI] [PubMed] [Google Scholar]
- 41. Kobayashi A, Stewart CA, Wang Y, Fujioka K, Thomas NC, Jamin SP, Behringer RR.. beta-Catenin is essential for Müllerian duct regression during male sexual differentiation. Development 2011;138:1967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mullen RD, Wang Y, Liu B, Moore EL, Behringer RR.. Osterix functions downstream of anti-Müllerian hormone signaling to regulate Müllerian duct regression. Proc Natl Acad Sci USA 2018;115:8382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B.. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002;108:17–29. [DOI] [PubMed] [Google Scholar]
- 44. Roberts LM, Visser JA, Ingraham HA.. Involvement of a matrix metalloproteinase in MIS-induced cell death during urogenital development. Development 2002;129:1487–96. [DOI] [PubMed] [Google Scholar]
- 45. Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J.. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 1999;398:431–6. [DOI] [PubMed] [Google Scholar]
- 46. Park JH, Tanaka Y, Arango NA, Zhang L, Benedict LA, Roh MI, Donahoe PK, Teixeira JM.. Induction of WNT inhibitory factor 1 expression by Müllerian inhibiting substance/antiMüllerian hormone in the Müllerian duct mesenchyme is linked to Müllerian duct regression. Dev Biol 2014;386:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oliveri P, Davidson EH.. Gene regulatory network analysis in sea urchin embryos. Methods Cell Biol 2004;74:775–94. [DOI] [PubMed] [Google Scholar]
- 48. Hinman VF, Nguyen AT, Cameron RA, Davidson EH.. Developmental gene regulatory network architecture across 500 million years of echinoderm evolution. Proc Natl Acad Sci USA 2003;100:13356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oliveri P, Davidson EH.. Gene regulatory network controlling embryonic specification in the sea urchin. Curr Opin Genet Dev 2004;14:351–60. [DOI] [PubMed] [Google Scholar]