Abstract
A common approach to measuring binding constants involves combining receptor and ligand and measuring the distribution of bound and free states after equilibration. For class I major histocompatibility (MHC-I) proteins, which bind short peptides for presentation to T cells, this approach is precluded by instability of peptide-free protein. Here we develop a method wherein a weakly-binding peptide covalently attached to the N-terminus of the MHC-I β2m subunit is released from the peptide binding site after proteolytic cleavage of the linker. The resultant protein is able to bind added peptide. A direct binding assay and method for estimation of peptide binding constant (Kd) are described, in which fluorescence polarization is used to follow peptide binding. A competition binding assay and method for estimation of inhibitor binding constant (Ki) using the same principle also are also described. The method uses a cubic equation to relate observed binding to probe concentration, probe Kd, inhibitor concentration, and inhibitor Ki under general reaction conditions without assumptions relating to relative binding affinities or concentrations. We also delineate advantages of this approach compared to the Cheng-Prusoff and Munson-Rodbard approaches for estimation of Ki using competition binding data.
Keywords: Major histocompatibility protein, Competition assay, Fluorescence polarization, Peptide exchange, IC50, Equilibrium binding constant
1. Introduction
Presentation of antigenic peptides to T cells is both requisite for initiation of an adaptive immune response and necessary for tolerance to self. The affinity and stability of antigenic peptides binding to MHC molecules are intrinsic aspects of T cell activation and have been used as predictors of immunogenicity and immunodominance [1,2]. Defining the molecular interactions between MHC molecules and peptide ligands thus has implications in effective vaccine design, in diagnostic capability, and in understanding basic immunological processes. In the last several decades, considerable progress has been made in characterizing these interactions, and peptide affinity for MHC has been shown to be a critical determinant of the T cell response in infection, autoimmunity, and tumor models [3–7]. Despite development of many assays to quantify or characterize MHC-peptide affinities, these methodologies are often encumbered by laborious and time-consuming experimental steps, such that substantial effort has been made to establish more efficient and high-throughput epitope screening methods.
Assays to evaluate the affinities of putative and known MHC-I epitopes have been developed over the last 30 years using both cell-based and cell-free platforms. Perhaps the earliest work on quantifying peptide affinity employed cell-free biochemical methods to measure binding of iodinated peptides to MHC-I [8–10]. Using this technique, MHC-I molecules are purified from cell lysates and solubilized in detergent, and affinity is measured by quantifying binding of radiolabeled peptides by gel filtration of MHC-I complexes vs. free peptide. Later methods employed fluorescence labeling rather than iodination, thereby eliminating the radioactive waste and hazard, but this assay still necessitated purification of each peptide-MHC-I complex by chromatographic or electrophoretic separation and was therefore labor-intensive and low-throughput [11,12]. A high-throughput scintillation proximity assay based on radioactive peptide exchange using purified native MHC-I protein has since been reported [13]. Cell-based methods have also been used to evaluate MHC-I-peptide interactions, including a cell-surface stabilization assay, in which surface MHC-I of TAP-deficient T2 cells is stabilized by the addition of iodinated or fluorescently-labeled peptides [14,15], as well as cell-surface binding assays, in which endogenous peptides are exchanged in situ [16,17] or partially removed by acid treatment of surface MHC-I-bound peptides followed by addition of fluorescent peptides [18,19]. Surface plasmon resonance has been used to measure peptide binding in indirect assays monitoring β2-microglobulin dissociation [20], and in direct assays following MHC-I binding to covalently-coupled peptides [21]. The peptide dependence of in vitro MHC-I folding reactions can serve as the basis for MHC-I-peptide binding assays, with detection using conformation-specific antibodies or pairs of antibodies specific for MHC-I heavy-chain and β2-microglobulin [22,23].
Due to the inherent instability of peptide-free “empty” MHC-I molecules, assays that measure peptide-binding affinity generally include some type of peptide exchange as a necessary step in the reaction. Full-length MHC-I proteins purified in detergent from mammalian cells are largely occupied with endogenous peptides, and while peptide exchange can be measured for these preparations, the efficiency is low. Peptides can be removed from MHC-I preparations by partial denaturation, and assays based on this approach have been reported [24]. As an alternate approach, MHC-I subunits can be folded from heavy chain and β2-microglobulin subunits expressed in E. coli inclusion bodies [25,26]. Folding in vitro is highly peptide-dependent, and this provides a means to prepare MHC-I complexes with defined peptides. MHC-I proteins preloaded with weakly-binding, easily exchangeable peptides potentially could be used as starting material for peptide-exchange assays. This requires identification of peptides with MHC-I–peptide-binding affinity sufficiently high to allow for in vitro folding and purification but sufficiently low to allow for efficient exchange with test peptides. Dipeptides have been identified for some MHC-I proteins that could serve this purpose [27], but a “conditional ligand” strategy has proven more useful, wherein MHC-I complexes are folded with full-length peptides carrying photocleavable [28] or chemically-labile [29] amino acid residues that upon cleavage produce easily-exchangeable peptide fragments. Similar assays have been developed for evaluating affinities of MHC-II epitopes [11,30–34], facilitated in many cases by the increased stability of peptide-free MHC-II relative to MHC-I [35], and by the availability of recombinant MHC-II proteins expressed in E. coli or insect cells with empty peptide-binding sites or carrying only weakly-associating peptides [35–38].
Here, we describe a novel technique to measure peptide affinities, which incorporates a different mode of peptide exchange based on a previously described covalent peptide approach. In that approach, a tight-binding peptide is tethered to the N-terminus of the β2-microglobulin subunit [39], where it is positioned near the peptide-binding site, similar to covalent peptide approaches conventionally used for MHC-II molecules [40]. This allows for production of essentially homogenous MHC-I peptide complexes in mammalian cells [41]. Here, epitope-linked β2-microglobulin (ELBM) is expressed in mammalian cells with a weakly binding peptide tethered to the β2m subunit via a linker that contains a thrombin cleavage site, so that upon addition of protease, the peptide is released. This design circumvents issues that may be encountered with MHC molecules that are difficult to fold in vitro and avoids complications with handling MHC-I proteins folded with suboptimal peptides. Following recent studies [42,43], we monitored MHC-I-peptide binding using fluorescence polarization, a technique wherein plane-polarized light is employed to distinguish between bound and free ligands without the need for physical separation [34,42]. The degree of polarization is measured by excitation of a fluorophore and calculated by measurement of fluorescence intensities both parallel and perpendicular to the plane of polarized light. In an MHC-peptide binding assay, the labeled peptide will exhibit high fluorescence polarization when bound to MHC-I due to decreased molecular mobility but will tumble freely in solution and display low polarization when unbound. Titration of unlabeled competitor peptides allows for computation of binding affinities, and this assay is also highly amenable to high-throughput screening. Using this approach, we developed direct binding and competition assays to evaluate binding affinities for peptides binding to the common human MHC-I allele HLA-A*02:01 (HLA-A2). The direct binding assay can be used to estimate binding affinities for labeled peptides binding to MHC-I. The competition binding assay, using unlabeled test peptides and a single labeled probe peptide, is suitable for high-throughput analyses and can be used to give half-maximal inhibition (IC50) values that report relative binding affinity of test peptides and estimates of inhibitor peptide-binding constant (Ki).
Traditionally, inhibition binding assays are interpreted in terms of the IC50 value, i.e. the concentration of unlabeled test peptide that results in 50% inhibition of binding of a probe peptide. Because IC50 values vary with experimental conditions, measurements of Ki, the equilibrium binding constant of the inhibitor peptide, are more useful in quantification of inhibitor peptide-binding affinity. Under some experimental conditions, IC50 and Ki values are similar. Requirements for this are that the probe peptide concentration is low relative to that of the test peptide, the probe peptide has a low Kd relative to the concentration used, and MHC concentration is low relative to peptide concentration. For experimental conditions in which these parameters deviate from concentration or binding constant limits, corrections have been proposed to calculate Ki from IC50 values. The most commonly used of these is the Cheng-Prusoff correction, which corrects for probe peptide concentration and binding affinity effects. However, the MHC concentration must remain low relative to the binding constants for this correction to be accurate. Munson and Rodbard proposed an exact correction for the Cheng-Prusoff approach when y0, the ratio of bound to free probe peptide in the absence of inhibitor, is available. However, in practice, this approach can lead to large errors if the relevant parameters are not precisely known. To circumvent these issues, we developed an analysis relating Ki to IC50 without assumptions about concentrations or binding affinities, using a cubic equation. The cubic equation describes the amount of probe peptide bound in terms of concentrations and binding constants for both probe peptide and inhibitor peptide, and this approach is suitable for curve fitting inhibition binding data.
2. Materials and methods
2.1. Peptide synthesis and labeling
All test peptides and probe peptide (shown in Table 1) were synthesized by 21st Century Biochemicals (Marlborough, MA). HIV-RT (ILKEPVCGV) was labeled with Alexa Fluor 488 C5-maleimide (Thermo Fisher Scientific, Waltham, MA) via the thiol of C7. Labeling was performed for 2 h at RT, and separation of labeled peptide from free fluorophore was performed using Jupiter C18 reverse-phase chromatography (Phenomenex, Torrance, CA).
Table 1.
Peptide sequences.
| Peptide | Sequence |
|---|---|
| ML9 | MLQEKPFQ |
| FL9 | FIALWIPDL |
| HIV-RT | ILKEPVCGVa |
| A2_P1061 | GLFDFVNFV |
| MVA013 | RLYDYFTRV |
| MVA165 | KVDDTFYYV |
| VACWR082_Ag | ILDDNLYKV |
| VACWRSPI2_Ag | HVDGKILFV |
| GGV | GAGGGVGGV |
Cysteine residue introduced in place of histidine for addition of fluorescent label.
2.2. HLA-A*0201 expression and purification
The heavy chain of epitope-linked β2m (ELBM) consisted of the HLA-A2*0201 extracellular domain, a linker, the basic half of a heterodimeric leucine zipper [44], the biotinylation signal peptide 85 [45], and a His6 tag [46], while the light chain encoded an N-terminal stuffer peptide (ML9 [MLQEKPFQL] or FL9 [FIALWIPDL]), a 20-amino-acid linker containing a thrombin cleavage site, β2m, an additional 15-amino-acid linker, and the acidic half of the leucine zipper. A signal sequence (SS) derived from human ceruloplasmin was also used, with a 2-amino-acid modification to incorporate an SbfI restriction site, (shown in bold: MKILILGIFLFLCSTPAWA → MKILILGIFLFLCSTPLQA). Constructs are depicted in Fig. 1. For generation of ELBM-ML9 recombinant protein, lentivirus was produced via the calcium phosphate method by co-transfection of HEK293T/17 cells (293, ATCC #CRL-11268) using a second-generation lentiviral vector based on pWPI (Addgene #12254), which encoded the marker Thy1.1 and for which expression was driven by a CMV promoter, together with the packaging plasmids pMD2.G (Addgene #12259) and psPAX2 (Addgene #12260). 293 cells were cultured in complete DMEM media supplemented with 10% FCS, 1% antibiotic-antimycotic (Thermo Fisher) and 50 μM d-biotin (complete d-biotin medium, CDM), and transfections were performed with 45 μg each of the heavy and light chain expression constructs, 60 μg of psPAX2, and 30 μg of pMD2.G. Medium was replaced within 4–6 h following transfection and was harvested at 2.5 days. Supernatants were centrifuged at 3000 g for 30 min, filtered through a 0.2 μm filter, and lentivirus was pelleted by ultracentrifugation (18000 rpm for 3 h). Concentrated lentivirus was used to transduce GnTI-HEK293S cells [47] previously transduced with BirA and EGFP and seeded at 2 × 104 cells/well the day before in a 96-well plate in CDM. Fresh CDM was added after 24 h, and cells were expanded for a further 4–5 days with media changes. High expression of EGFP and Thy1.1 was confirmed post-transduction, and cells were expanded for protein production in CELLine 1000 flasks (DWK Life Sciences, Mill-ville, NJ). Supernatant was collected once per week for 5–15 weeks, and the bimolecular complex was affinity-purified from culture super-natants with the anti-zipper mAb 2H11 (a gift from Ellis Reinherz) using AKTAprime systems (GE, Chicago, IL), followed by concentration and buffer exchange using Amicon centrifugation filters (Millipore Sigma, Burlington MA). ELBM-FL9 was produced similarly, with the following modifications; ELBM vectors expressed both Thy1.1 and DsRed, HEK293T cells were transduced for protein expression, and cells were sorted for marker expression following transduction. Protein yield was determined to be ~10 mg/L. For generation of pre-loaded MHC-peptide complexes for use as controls and in ThermoFluor experiments, we refolded E. coli-produced HLA-A2 heavy chain and β2m with Alexa488-RT(cys) or indicated peptides as described [25].
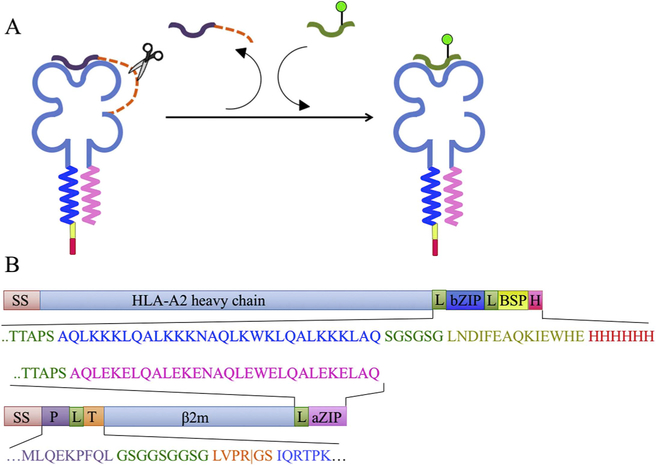
Fig. 1. Peptide epitope-linked β2-microglobulin HLA-A2 constructs.
(A) Schematic of peptide exchange using epitope-linked β2-microglobulin. (B) Schematic of constructs used to express ELBM (SS: secretion signal sequence; bZIP: basic leucine zipper; BSP: BSP85 peptide; H: His6 tag; P: peptide: T: thrombin cleavage site; aZIP: acidic leucine zipper). Linker sequences shown in green. Vertical line in thrombin sequence shows expected cleavage site. The peptide epitope sequence shown is from the ML9 peptide, while in some experiments other sequences were used (see below). Not drawn to scale. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.3. Fluorescence polarization (FP) assay and IC50 measurements
An FP assay using 500 nM ELBM-ML9, 25 nM Alexa488-RT(cys), and 0.01 unit/μl thrombin (MP Biomedicals, Santa Ana, CA) was performed in PBS containing 0.1% octylglucoside (Sigma Aldrich, St. Louis, MO) in 96-well black polypropylene microtiter plates (Greiner Bio-One, Monroe, NC) in a total volume of 120 μl. After 16 h incubation at room temperature (RT), FP was measured as millipolarization (mP) units in a VICTOR X5 plate reader (PerkinElmer, Waltham, MA) at 495 excitation and 520 emission. SDS-PAGE using a 16.5% Tris-Tricine gel (Bio-Rad, Hercules, CA) was used to confirm cleavage of ELBM-ML9. To measure competition binding, test peptides were added at 2-fold dilutions ranging from 5 μM to ~40 nM. For equilibrium experiments, MHC concentration was varied from 4 μM to 7.8125 nM (2-fold dilutions) with a fixed probe peptide concentration of 25 nM, or probe peptide concentration was varied from 4 μM to 62.5 nM (2-fold dilutions) with a fixed MHC concentration of 1 μM. mP measurements in the absence of MHC (mPfree) as well as mP measurements of A2 refolded with Alexa488-RT(cys) (mPbound) were also determined to calculate concentration of MHC bound to probe peptide. For kinetic analyses, FP values were measured every 7 min for ~20 h at RT with MHC concentrations ranging from 4 μM to 62.5 nM (2-fold dilutions).
2.4. Differential scanning fluorimetry (ThermoFluor assay)
The ThermoFluor assay was performed using 2.5 μM A2 complexes refolded with test peptides and a 500-fold dilution of SYPRO orange (Thermo Fisher Scientific) in PBS pH 7.4 in a total solution volume of 20 μl in optically clear 96-well plates. Temperatures were increased from 20 °C to 95 °C at 1 °C/min in a Bio-Rad C1000 Thermal Cycler RT-PCR instrument (Bio-Rad). To determine the Tm or thermal stability of each complex, the temperature derivative of the melting curve was computed.
2.5. Curve fitting
Prism (version 7.0c, GraphPad Software, San Diego, CA) was used for curve fitting with ordinary unweighted nonlinear least-squares regression.
3. Results
3.1. Class I MHC produced as epitope-linked β2-microglobulin constructs
Epitope-linked β2m (ELBM) was prepared by co-expressing in mammalian cells a soluble HLA-A2 heavy chain construct and a β2-microglobulin construct carrying an N-terminal “stuffer” epitope peptide, a thrombin cleavage site, complementary leucine zippers, and purification tags (Fig. 1). Both constructs have signal sequences, and so the resultant heterodimeric protein is secreted into the culture medium where it can be collected by affinity chromatography. The protein is designed such that with addition of thrombin, the linker peptide can be proteolytically cleaved, and a peptide of interest can be added to bind to the MHC-I complex (Fig. 1). The ELBM protein was then used to develop a fluorescence polarization assay to measure direct binding and optimize a competitive inhibition strategy for affinity measurements for a panel of peptides.
3.2. Fluorescence polarization discriminates MHC-bound and free forms of an Alexa488-labeled peptide
To evaluate the dynamic range of this assay and to determine parameters needed to convert polarization values to fractional binding, we first measured mP values for free and fully MHC-bound peptide. FP values for free labeled peptide (25 nM Alexa488-RT[cys]) were 21 ± 4.5 mP (mean ± standard deviation, n = 8), while recombinant HLA-A2 refolded with Alexa488-RT(cys) displayed FP values of 134 ± 4.2 mP, independent of concentration in the range ~30 nM to 2 μM (Fig. 2A). We evaluated practical limits on labeled peptide concentrations resulting from non-specific binding of peptide to the assay plate at low concentrations as well as the sensitivity of the instrument used to measure FP. Based on titrations of peptide in glycerol to simulate peptide binding (Supplementary Fig. 1), ~25 nM peptide appears to be the lower limit for this assay, as FP values increased at lower peptide concentrations. Although mP values are reliably consistent when up to 400 nM peptide is used (Supplementary Fig. 1), higher peptide concentrations are not ideal for quantitative work (please see Section 3.4). 25 nM probe peptide was therefore chosen as the concentration to be used for direct binding and competition assays.
Fig. 2. Optimization of peptide and assay conditions.
(A) Fluorescence polarization of free peptide (25 nM Alexa488-RT[cys]) and bound peptide (HLA-A2 refolded with Alexa488-RT[cys]) were measured at 21 ± 4.5 mP (free) and 134 ± 4.2 mP (bound). Bound mP values were independent of MHC concentration. (B) Addition of 0.01 unit/μl thrombin to 300 nM ELBM-ML9 and 25 nM Alexa488-RT(cys) demonstrated an increase in FP, suggesting cleavage of the linker, release of the ML9 peptide, and binding of the Alexa488-RT(cys) peptide, while ELBM-FL9 displayed little polarization under the same conditions, with or without thrombin. (C) SDS-PAGE analysis of ELBM-ML9 before and after thrombin cleavage using a 16.5% Tris-Tricine gel demonstrates cleavage of the linker with the addition of thrombin. HC: heavy chain; pep-β2m: peptide-β2m; T: thrombin.
3.3. Optimization of peptide binding assay
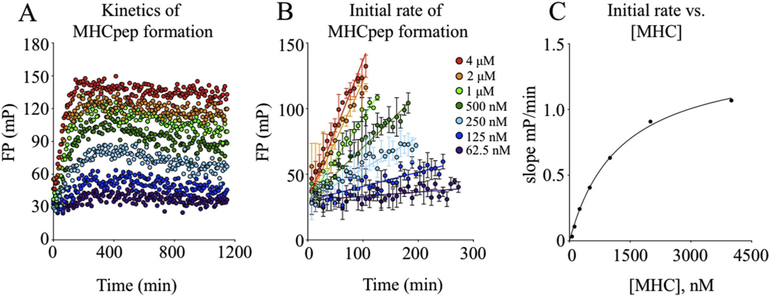
We initially tested MHC-ELBM constructed with two different epitopes (FL9 or ML9 [Table 1], Fig. 1), using different concentrations of MHC to determine the amount of MHC-ELBM necessary to bind 25 nM labeled Alexa488-RT(cys) peptide. Results indicated that ML9 was cleaved upon addition of thrombin, with increased binding of Alexa488-RT(cys) (Fig. 2B, shown at 300 nM MHC). ELBM constructed with the FL9 peptide, however, demonstrated little to no fluorescence polarization even with addition of thrombin, indicating that the FL9 peptide either had not been cleaved or was inefficiently exchanged for the probe peptide (Fig. 2B). All subsequent experiments were therefore performed with ELBM-ML9. SDS-PAGE of ELBM-ML9 further confirmed that the thrombin concentration used was effective in cleaving the ML9 peptide (Fig. 2C). Additional parameters tested and optimized included buffer composition, concentration of thrombin, and reaction volume. To examine the kinetics of probe peptide binding to ELBM-ML9 as well as to determine the optimal duration of the experiment, we performed an analysis using different concentrations of MHC with 25 nM Alexa488-RT(cys) and measured FP values over time (Fig. 3A). Results indicated that, for all concentrations of MHC tested, MHC binding to probe peptide increased rapidly for ~200 min with slower changes continuing for up to 1000 min. In subsequent work, reactions were analyzed after 16h incubation. The rate of binding was shown to saturate with increasing MHC concentration (Fig. 3B–C), indicating that the limiting step of this reaction - most likely cleavage or release of the cleaved fragment - occurs prior to peptide binding.
Fig. 3. Kinetics of MHC-peptide formation.
(A) Kinetics of fluorescence polarization using different concentrations of MHC show increased rates of probe peptide binding with greater MHC concentration. (B) Binding rate, as shown by initial rate of MHCpep formation, increases as MHC concentration increases. (C) Transformation of initial rate of MHCpep formation demonstrates saturation of rate of binding with increasing MHC concentration.
3.4. MHC and peptide titration
To estimate a value for the Kd of the probe peptide, we assessed equilibrium binding by measuring FP with varying concentrations of either MHC-I or probe peptide. The amount of MHCpeptide complex formed was calculated using the equation:
| 1 |
where mP is the experimental FP value, mPfree and mPbound are values for free and MHC-bound probe peptide, determined as described above (Section 3.2), and Peptot is the total concentration of probe peptide, i.e. the amount added at the start of the reaction (this equation assumes additivity of fluorescence polarization, which strictly speaking is limited to fluorescence anisotropy, but for mPfree and mPbound values observed here the simplification leads to fractional binding errors of < 1%). With [peptide] fixed at 25 nM and [MHC] increased as in Fig. 3A above, a sigmoidal binding curve with apparent saturation at [MHCpep] ~25 nM was observed (Fig. 4A). Non-linear least-squares fitting to either a simple two-component binding equation:
| 2 |
where MHCtot is the variable [MHC] and Kd is the equilibrium binding constant, or to a quadratic equation that accounted for ligand-depletion effects (see Appendix A):
| 3 |
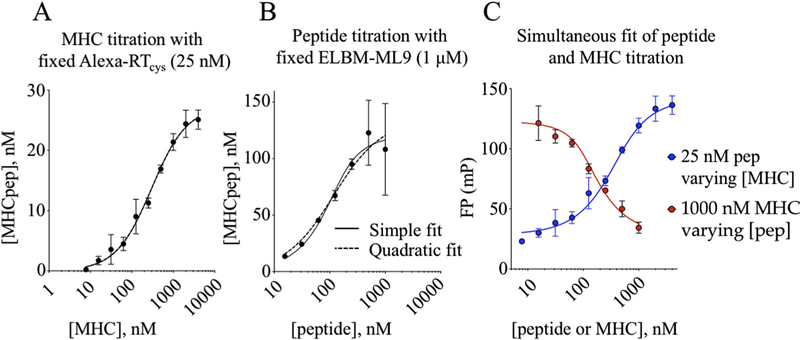
revealed an apparent inhibition constant Kd,app = 282 ± 31 nM (Fig. 4A). In a separate experiment with [MHC] fixed at 1000 nM and [peptide] increased, a sigmoidal binding curve again was observed (Fig. 4B). The curve appeared to reach maximum [MHCpep] in the vicinity of 100 nM, although there was substantial experimental uncertainty at high [peptide] due to low bound fraction for the probe peptide and small denominator in Eq. (1). Fitting this curve to simple or quadratic competition binding equations (Eqs. (2) and (3)) revealed Kd,app = 37 ± 38 nM. The 5- to 10-fold discrepancy between half-maximal concentrations of peptide and MHC in the two titrations, and between corresponding saturation levels, is unexpected for a simple binding reaction, but could occur if only a fraction of the MHC participates in the reaction. This could be due to incomplete thrombin cleavage of the ELBM construct, incomplete peptide release, or conversion to an inactive form of MHC as previously observed for MHC-II [48].
Fig. 4. Direct binding assay for estimation of Kd.
(A) With varying [MHC] and constant [Alexa488-RT(cys)], a curve is fit using a quadratic equation, or using an equation for one-site specific binding (simple fit). The Kd,app is calculated to be 283 ± 31 nM. (B) With varying [Alexa488-RT(cys)] and constant [MHC], curve fits are generated as above. The Kd,app is calculated to be 37 ± 18 nM. (C) Simultaneous fitting of MHC and peptide titrations using a quadratic equation gave a Kd value of 16 ± 4 nM for the Alexa488-RT(cys) probe peptide and an active MHC fraction of 8.2 ± 0.8%. Curves in (A) and (B) were fit using Eqs. (2) and (3); curves in (C) were fit using Eq. (4).
3.5. Estimation of Kd and MHC active fraction
To analytically evaluate the fraction of MHC protein that participates in the binding reaction, we simultaneously fit the MHC and peptide titrations, using a quadratic binding equation that includes a term fract that describes the active MHC fraction (Fig. 4C). To avoid transformations of the experimental data that result in non-linear amplification of experimental uncertainty, we recast the quadratic binding equation in terms of the experimentally observable fluorescence polarization mPobs, as well as mPfree, mPbound and the total reactant concentrations MHCtot and Peptot (Appendix A):
| 4 |
At fixed [peptide], mPobs increases as MHCtot is increased, reaching a saturating value at mPbound (Fig. 4C, blue symbols). At fixed [MHC], mPobs decreases as Peptot is increased, given that the fractional amount of peptide decreases (Fig. 4C, red symbols). Simultaneous fitting of both datasets revealed a Kd of ~16 nM with an active fraction of ~8%.
3.6. Competition binding assay for determination of IC50
Competition binding assays, in which binding of a fixed concentration of a probe binder is assessed as the concentration of an unlabeled competitor is varied, are useful in routine binding analysis given that they allow for testing of many unlabeled test peptides using a single preparation of labeled probe peptide. This is particularly true for FP-based MHC-peptide binding analysis, as introduction of a fluorescent probe is likely to alter MHC-peptide binding affinity due to extensive and somewhat unpredictable MHC interactions all along the length of a bound peptide. To evaluate the utility of the ELBM FP assay in competitive binding assays, we selected a set of competitor peptides derived from vaccinia virus for which IC50 values and/or half-life measurements had been previously reported [49–52] (Table 2). As the full set of peptides had not been previously compared in the same assay, we evaluated the thermal stability of HLA-A2 complexes of the set of test peptides (Supplementary Fig. 2, Table 2) using a differential scanning fluorimetry assay [53]. The competition binding assay was then performed with competitor peptides titrated in 2-fold dilutions starting from 2 μM (Fig. 5). IC50 values were determined by fitting using nonlinear regression analysis with the equation:
| 5 |
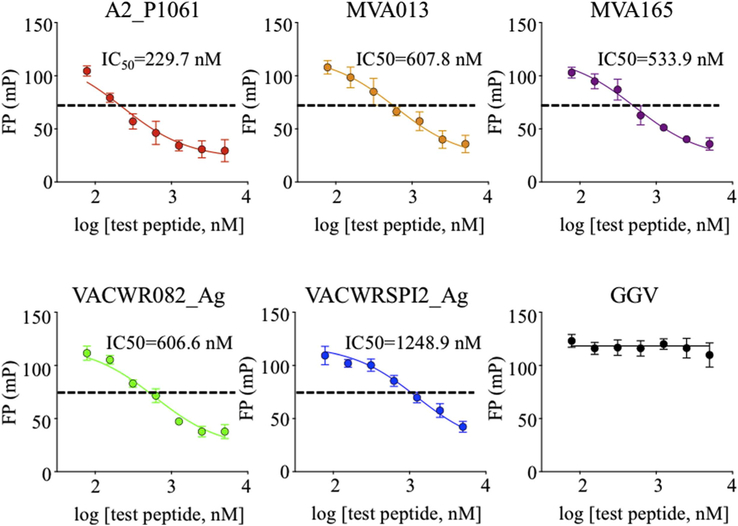
where mPmin is measured in the absence of MHC (i.e. mPfree), mPmax is measured in a control binding reaction with MHC and probe peptide but in the absence of competitor peptide, Comptot is the variable concentration of the (unlabeled) competitor peptide, and IC50 is Comptot at half-maximal competition. As expected, increasing concentration of each of the vaccinia test peptides decreased the binding of labeled probe peptide (Fig. 5). Best-fit IC50 values for these peptides ranged from ~230 nM to ~1250 nM.
Table 2.
Binding and stability parameters for test peptides.
Fig. 5. Measurement of IC50 for a set of vaccinia peptides.
An inhibition assay was performed for determination of IC50 using 500 nM MHC and 25 nM Alexa488-RT(cys), with 2-fold dilutions (beginning at 2 μM) of the vaccinia competitor peptides A2_P1061, MVA013, MVA165, VACWR082_Ag, and VACWRSPI2_Ag. The GGV peptide was similarly diluted and used as a negative control. Curves were fit using Eq. (5).
3.7. Concentration dependence of IC50
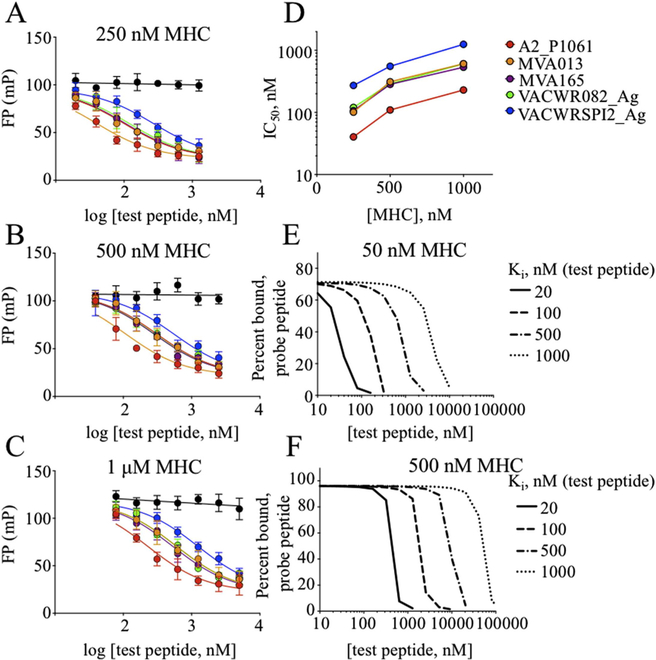
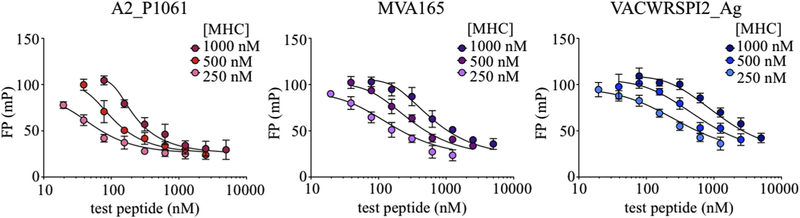
IC50 measurements are suitable for relative assessments of binding affinity, but often the absolute binding affinity of the inhibitor peptide, Ki, is desired. Under certain conditions, the IC50 value obtained in a competition binding assay approximates the Ki, in particular when the concentration of peptide is below its Kd and the concentration of MHC is low relative to the concentration of test and probe peptides. For FP assays with MHC proteins, these conditions are difficult to achieve in practice. In our assay, the concentration of labeled peptide was necessarily > 10 nM to obtain reliable FP measurements, but this is near the Kd, and the MHC concentration needed to be several fold greater in order to bring the bound fraction into a region where competition could be observed. We evaluated the concentration dependence of IC50 for three of the test peptides that spanned the range of observed IC50 values. Experiments at different MHC concentrations yielded different IC50 values, although the test peptide rank order and relative IC50 values were similar under the different conditions (Fig. 6A–D, Table 2).
Fig. 6. Dependence of IC50 on MHC concentration.
(A–D) An inhibition assay to determine IC50 values was performed with different MHC concentrations and 25 nM Alexa488-RT(cys), with 2-fold dilutions of competitor peptides beginning at 5 μM (1 μM MHC), 2.5 μM (500 nM MHC), and 1.25 μM (250 nM MHC). As MHC concentration doubles from (A) 250 nM to (B) 500 nM to (C) 1 μM, IC50 values approximately double (D) for the vaccinia peptides tested (shown in Table 2.) Curves were fit using Eq. (5). (E–F) Simulated competition binding curves, calculated using Eq. (8), for 25 nM test peptide with Kd = 20 nM and other parameters as shown.
3.8. Estimation of Ki using Cheng-Prusoff and Munson-Rodbard approaches
Several approaches have been suggested in the literature to correct for these concentration effects and convert IC50 values to Ki [54–59]. In the Cheng-Prusoff approach, IC50 values can be converted to Ki using the known concentration of the labeled test peptide and its binding constant:
| 6 |
Under our experimental conditions, the probe peptide concentration was similar to its binding affinity, with Peptot/Kd ~1.5, so the correction factor was substantial (Table 3). However, this equation does not consider the effect of high MHC concentration on depleting peptide, so that the free and total peptide concentrations are substantially different. Thus, even after application of the Cheng-Prusoff correction, we observed Ki,corr to vary with [MHC] (Table 3).
Table 3.
IC50 as a function of MHC and estimated Ki using Cheng-Prusoff or Munson-Rodbard approaches.
| Peptide | [MHC] (nM) | IC50 (nM) | Ki Cheng-Prusoff (nM) | Ki Munson-Rodbard (nM) | Ki cubic fita(nM) |
|---|---|---|---|---|---|
| A2_P1061 | 250 | 40 | 24 | 30 | |
| 50 | 110 | 65 | 47 | 5 (3–9) | |
| 1000 | 230 | 137 | 59 | ||
| MVA165 | 250 | 106 | 64 | 51 | |
| 500 | 284 | 169 | 85 | 23 (17–42) | |
| 1000 | 534 | 319 | 100 | ||
| VACWRSPI2_Ag | 250 | 272 | 162 | 103 | |
| 500 | 553 | 323 | 145 | 60 (49–138) | |
| 1000 | 1249 | 745 | 196 |
Single value representing global fit to inhibition curves obtained at three MHC concentrations. Values in parentheses show range of Ki values obtained from fits, allowing for observed experimental uncertainty in mPfree, mPbound, Kd, and MHC active fraction parameters.
In the Munson-Rodbard approach, ligand depletion is considered explicitly using a term (y0) corresponding to the ratio of bound and free test peptide in the absence of inhibitor:
| 7 |
Although in principle this correction is exact, in practice y0 can have substantial experimental uncertainty, leading to large deviations of the observed Ki,corr from expected values. Under our assay conditions, at low [MHC] the y0 factor was 1.9, with a reasonable 95% confidence interval of 1.4–2.8 based on the experimental uncertainly in mPmin and mPbound, but at high [MHC], the y0 factor was 8.8, with a very large 95% confidence interval of 4.7–36. Thus, even after Munson-Rodbard correction, we observed substantial Ki,corr divergence (Table 3).
3.9. Estimation of Ki using a cubic equation
In order to determine more directly how MHC concentration (MHCtot), probe peptide concentration (Peptot), and probe peptide binding affinity (Kd) would affect IC50 curves obtained, we modeled IC50 curves as a function of concentration of competitor peptide. Because of the effects of ligand depletion on both probe and test peptides, this requires a cubic equation [60,61]. We derived an exact equation for the free MHC concentration in terms of the other experimental parameters (see Appendix B):
| 8 |
and used this to simulate competition binding curves under different conditions, using the test conservation equations [MHCpep] = MHCtot-[MHC] and Peptot = [Pepfree]+[MHCpep] to relate the free MHC concentration to the percentage of test peptide bound observed experimentally. Calculated competition curves shift as each of the experimental variables (MHCtot, Ki, Kd) are changed (Fig. 6E–F), but as we observed experimentally, differences between the IC50 curves are distinguishable regardless of the changes in these parameters.
We next simultaneously fit a family of experimental IC50 curves for a single test peptide obtained under different [MHC], with the idea that this might provide a reliable consensus Ki value, similarly to how simultaneous fits of MHC and peptide titrations provided a robust Kd determination as described above in Section 3.3. We encountered difficulty implementing a non-linear least squares curve-fitting approach using the equation shown as Eq. (8), but we were able to simultaneously fit a single Ki value to sets of experimental binding inhibition curves obtained at different MHC concentrations (Fig. 7). For this analysis we used an implicit equation version of the cubic equation (Appendix C). This fit included values for mPfree, mPbound, [MHC], active fraction, Kd and Peptot in addition to Ki. Known or estimated values for these parameters could be included in the fit procedure, or values could be determined simultaneously with Ki. In practice, we found that the fit had a relatively narrow radius of convergence, so that initial values had to be chosen thoughtfully, and that Ki values were somewhat coupled to the probe peptide Kd and MHC active fraction, although they were relatively independent of mPfree and mPbound. Best fit values for Ki for the test peptides, along with ranges consistent with experimental uncertainties in Kd and MHC active fraction, are shown in Table 3.
Fig. 7. Fitting competitive binding inhibition curves using a cubic equation.
To determine Ki values for the A2_P1061, MVA165, and VACWRSPI2_Ag peptides, three curves obtained at different [MHC] were simultaneously fit using an implicit cubic equation (Appendix C). Best fit Ki values obtained with Kd = 16 nM and fract = 0.2 shown in Table 3, along with a range of Ki values obtained with reasonable parameter values (mPfree = 22 to 28, mPbound = 115 to 150, Kd = 10–20 nM, and fract = 0.08 to 0.2).
4. Discussion
In this work, we describe a method whereby MHC-I peptide affinities can be measured by direct binding or competition assays. While fluorescence polarization has been employed previously to measure the rate of peptide exchange and affinity in MHC-I and MHC-II binding assays [34,42,43], here we have used a novel recombinant HLA-A2 protein with a proteolytically cleavable linker tethered to the β2m subunit, which allows for efficient peptide release and measurement of probe peptide Kd. We show that relative binding affinities of competitor peptides can be measured by determination of IC50 values using this assay. Lastly, to estimate absolute Ki from IC50 values, we propose use of a cubic equation and demonstrate its application to determination of the Ki of competitor peptides.
A potential limitation of the ELBM method is the difficulty that may be encountered in designing a linker peptide with the appropriate features. For efficient exchange to occur, this peptide must exhibit sufficient affinity for binding but also must be weak enough to be released after enzymatic cleavage. A different linker peptide for each MHC allele of interest must also be designed due to distinct allelespecific binding requirements. Suitable peptides similarly have been difficult to design for dipeptide and photocleavable peptide exchange assays, given that following cleavage, peptide fragments may inefficiently dissociate from the MHC protein [27,62]. The primary advantage of the ELBM approach over other available methods for producing MHC-I for peptide interaction measurements [11,25,27,28,43] is that covalent linkage of a replaceable peptide ligand allows for expression in mammalian cells, while at the same time allowing for high efficiency of peptide exchange in vitro. We used the ELBM constructs to characterize binding behavior of a known HLA-A2 ligand derived from HIV reverse-transcriptase.
To help understand how observed competition binding curves depend on experimental conditions and relate to intrinsic binding affinities for probe and test peptides, we developed a cubic equation that relates observable fluorescence polarization changes to intrinsic assay parameters. Conventional approaches for analyzing competition binding data and extracting intrinsic binding parameters [54,55] were developed for cases where the receptor concentration is very low and the sensitivity of detection high, such as for radiolabeled ligand binding to receptors on the surface of intact cells. These approaches were not applicable to our FP-based assay of peptides binding to recombinant soluble MHC-I proteins, and the derived Ki,app values were highly dependent on experimental conditions. Fluorescence polarization assays in general measure the fraction of probe that is bound to receptor, and thus require sufficient receptor concentration to achieve substantial fractional occupancy. Moreover, sensitivity of conventional microplate fluorescence readers in polarization mode is limited to probe concentrations of ~25 nM or greater, which is in the vicinity of many MHC-I-peptide binding constants. Thus, key assumptions used in conventional competition binding analysis to relate observed IC50 values to intrinsic Ki do not hold for our experimental conditions. Despite these caveats, relative IC50 values reliably reported relative binding affinity. In order to explore estimation of absolute Ki values from our competition binding data, we derived from first principles a cubic equation relating concentrations of all bound and free species present in the reactions and their binding constants. We also derived an intrinsic equation relating observed fluorescence polarization changes to these parameters, which proved more useful for routine curve fitting. Similar development of cubic equations for fitting other types of competition binding assays have been presented previously [60,61]. Using this approach, we were able to estimate intrinsic binding constants for a set of vaccinia-derived HLA-A2-binding peptides, although robust curve fitting required experiments at a range of MHC concentrations as well as a previously-determined value for the Kd of the probe peptide.
The ELBM approach may also represent a useful tool in additional applications such as generation of MHC-I tetramers with different peptide ligands to characterize antigen-specific T cell responses. ELBM potentially additionally could be employed to produce MHC-I-peptide complexes for biophysical studies. In sum, this work describes a novel method for producing specific MHC-I-peptide complexes by peptide exchange, with particular application to assaying peptide binding affinity. This method may have applications in epitope discovery and assessment of peptide-MHC interactions and may prove useful in vaccine and immunotherapy development.
Supplementary Material
Acknowledgments
We thank Guoqi Li for refolding of HLA-A2 complexes and Liusong Yin for assistance with Alexa488-RT(cys) labeling.
Funding
This work was supported by the National Institutes of Health: R01-AI038996 (LJS) and T32-AI07349 (MMJ), HHSN272201300006C (JDA).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ab.2019.05.017.
References
- [1].Irvine K, Bennink J, Factors influencing immunodominance hierarchies in TCD8+ -mediated antiviral responses, Expert Rev. Clin. Immunol 2 (2006) 135–147. [DOI] [PubMed] [Google Scholar]
- [2].van der Burg SH, Visseren MJ, Brandt RM, Kast WM, Melief CJ, Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability, J. Immunol 156 (1996) 3308–3314. [PubMed] [Google Scholar]
- [3].Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB, Kranz DM, Meredith SC, Rowley DA, Schreiber H, Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity, Cancer Cell 23 (2013) 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Regner M, Mullbacher A, Blanden RV, Lobigs M, Immunogenicity of two peptide determinants in the cytolytic T-cell response to flavivirus infection: inverse correlation between peptide affinity for MHC class I and T-cell precursor frequency, Viral Immunol 14 (2001) 135–149. [DOI] [PubMed] [Google Scholar]
- [5].Fairchild PJ, Wraith DC, Peptide-MHC interaction in autoimmunity, Curr. Opin. Immunol 4 (1992) 748–753. [DOI] [PubMed] [Google Scholar]
- [6].Busch DH, Pamer EG, T cell affinity maturation by selective expansion during infection, J. Exp. Med 189 (1999) 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dockree T, Holland CJ, Clement M, Ladell K, McLaren JE, van den Berg HA, Gostick E, L.M.K., Llewellyn-Lacey S, Bridgeman JS, Man S, Bailey M, Burrows SR, Price DA, Wooldridge L, CD8(+) T-cell specificity is compromised at a defined MHCI/CD8 affinity threshold, Immunol. Cell Biol 95 (2017) 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sette A, Sidney J, del Guercio MF, Southwood S, Ruppert J, Dahlberg C, Grey HM, Kubo RT, Peptide binding to the most frequent HLA-A class I alleles measured by quantitative molecular binding assays, Mol. Immunol 31 (1994) 813–822. [DOI] [PubMed] [Google Scholar]
- [9].Chen BP, Parham P, Direct binding of influenza peptides to class I HLA molecules, Nature 337 (1989) 743–745. [DOI] [PubMed] [Google Scholar]
- [10].Olsen AC, Pedersen LO, Hansen AS, Nissen MH, Olsen M, Hansen PR, Holm A, Buus S, A quantitative assay to measure the interaction between immunogenic peptides and purified class I major histocompatibility complex molecules, Eur. J. Immunol 24 (1994) 385–392. [DOI] [PubMed] [Google Scholar]
- [11].Sidney J, Southwood S, Moore C, Oseroff C, Pinilla C, Grey HM, Sette A, Measurement of MHC/peptide Interactions by Gel Filtration or Monoclonal Antibody Capture, Curr Protoc Immunol, Chapter 18, (2013) Unit 18 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parker KC, Bednarek MA, Coligan JE, Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains, J. Immunol 152 (1994) 163–175. [PubMed] [Google Scholar]
- [13].Harndahl M, Rasmussen M, Roder G, Buus S, Real-time, high-throughput measurements of peptide-MHC-I dissociation using a scintillation proximity assay, J. Immunol. Methods 374 (2011) 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schumacher TN, Heemels MT, Neefjes JJ, Kast WM, Melief CJ, Ploegh HL, Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro, Cell 62 (1990) 563–567. [DOI] [PubMed] [Google Scholar]
- [15].Hosken NA, Bevan MJ, Defective presentation of endogenous antigen by a cell line expressing class I molecules, Science 248 (1990) 367–370. [DOI] [PubMed] [Google Scholar]
- [16].Kessler JH, Benckhuijsen WE, Mutis T, Melief CJ, van der Burg SH, Drijfhout JW, Competition-based cellular peptide binding assay for HLA class I (Chapter 18), Curr. Protoc. Im 61 (1) (2004) Unit 18 12. [DOI] [PubMed] [Google Scholar]
- [17].Kessler JH, Mommaas B, Mutis T, Huijbers I, Vissers D, Benckhuijsen WE, Schreuder GM, Offringa R, Goulmy E, Melief CJ, van der Burg SH, Drijfhout JW, Competition-based cellular peptide binding assays for 13 prevalent HLA class I alleles using fluorescein-labeled synthetic peptides, Hum. Immunol 64 (2003) 245–255. [DOI] [PubMed] [Google Scholar]
- [18].Storkus WJ, Zeh HJ 3rd, Salter RD, Lotze MT, Identification of T-cell epitopes: rapid isolation of class I-presented peptides from viable cells by mild acid elution, J. Immunother. Emphas. Tumor Immunol 14 (1993) 94–103. [PubMed] [Google Scholar]
- [19].Townsend A, Ohlen C, Bastin J, Ljunggren HG, Foster L, Karre K, Association of class I major histocompatibility heavy and light chains induced by viral peptides, Nature 340 (1989) 443–448. [DOI] [PubMed] [Google Scholar]
- [20].Miles KM, Miles JJ, Madura F, Sewell AK, Cole DK, Real time detection of peptide-MHC dissociation reveals that improvement of primary MHC-binding residues can have a minimal, or no, effect on stability, Mol. Immunol 48 (2011) 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khilko SN, Jelonek MT, Corr M, Boyd LF, Bothwell AL, Margulies DH, Measuring interactions of MHC class I molecules using surface plasmon resonance, J. Immunol. Methods 183 (1995) 77–94. [DOI] [PubMed] [Google Scholar]
- [22].Sylvester-Hvid C, Kristensen N, Blicher T, Ferre H, Lauemoller SL, Wolf XA, Lamberth K, Nissen MH, Pedersen LO, Buus S, Establishment of a quantitative ELISA capable of determining peptide - MHC class I interaction, Tissue Antigens 59 (2002) 251–258. [DOI] [PubMed] [Google Scholar]
- [23].Wulf M, Hoehn P, Trinder P, Identification of human MHC class I binding peptides using the iTOPIA-epitope discovery system, Methods Mol. Biol 524 (2009) 361–367. [DOI] [PubMed] [Google Scholar]
- [24].Silver ML, Parker KC, Wiley DC, Reconstitution by MHC-restricted peptides of HLA-A2 heavy chain with beta 2-microglobulin, in vitro, Nature 350 (1991) 619–622. [DOI] [PubMed] [Google Scholar]
- [25].Garboczi DN, Hung DT, Wiley DC, HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides, Proc. Natl. Acad. Sci. U. S. A 89 (1992) 3429–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ferre H, Ruffet E, Blicher T, Sylvester-Hvid C, Nielsen LL, Hobley TJ, Thomas OR, Buus S, Purification of correctly oxidized MHC class I heavy-chain molecules under denaturing conditions: a novel strategy exploiting disulfide assisted protein folding, Protein Sci 12 (2003) 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Saini SK, Ostermeir K, Ramnarayan VR, Schuster H, Zacharias M, Springer S, Dipeptides promote folding and peptide binding of MHC class I molecules, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 15383–15388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Toebes M, Coccoris M, Bins A, Rodenko B, Gomez R, Nieuwkoop NJ, van de Kasteele W, Rimmelzwaan GF, Haanen JB, Ovaa H, Schumacher TN, Design and use of conditional MHC class I ligands, Nat. Med 12 (2006) 246–251. [DOI] [PubMed] [Google Scholar]
- [29].Rodenko B, Toebes M, Celie PH, Perrakis A, Schumacher TN, Ovaa H, Class I major histocompatibility complexes loaded by a periodate trigger, J. Am. Chem. Soc 131 (2009) 12305–12313. [DOI] [PubMed] [Google Scholar]
- [30].Buus S, Colon S, Smith C, Freed JH, Miles C, Grey HM, Interaction between a “processed” ovalbumin peptide and Ia molecules, Proc. Natl. Acad. Sci. U. S. A 83 (1986) 3968–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim A, Ishizuka I, Hartman I, Poluektov Y, Narayan K, Sadegh-Nasseri S, Studying MHC class II peptide loading and editing in vitro, Methods Mol. Biol 960 (2013) 447–459. [DOI] [PubMed] [Google Scholar]
- [32].Roche PA, Cresswell P, High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR, J. Immunol 144 (1990) 1849–1856. [PubMed] [Google Scholar]
- [33].Tompkins SM, Rota PA, Moore JC, Jensen PE, A europium fluoroimmunoassay for measuring binding of antigen to class II MHC glycoproteins, J. Immunol. Methods 163 (1993) 209–216. [DOI] [PubMed] [Google Scholar]
- [34].Yin L, Stern LJ, Measurement of peptide binding to MHC class II molecules by fluorescence polarization, Curr. Protoc. Im 106 (2014) 5 10 11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stern LJ, Wiley DC, The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide, Cell 68 (1992) 465–477. [DOI] [PubMed] [Google Scholar]
- [36].Frayser M, Sato AK, Xu L, Stern LJ, Empty and peptide-loaded class II major histocompatibility complex proteins produced by expression in Escherichia coli and folding in vitro, Protein Expr. Purif 15 (1999) 105–114. [DOI] [PubMed] [Google Scholar]
- [37].Hansen BE, Andersson EC, Madsen LS, Engberg J, Sondergaard L, Svejgaard A, Fugger L, Functional characterization of HLA-DRA1*0101/DRB1*0401 molecules expressed in Drosophila melanogaster cells, Tissue Antigens 51 (1998) 119–128. [PubMed] [Google Scholar]
- [38].Moro M, Cecconi V, Martinoli C, Dallegno E, Giabbai B, Degano M, Glaichenhaus N, Protti MP, Dellabona P, Casorati G, Generation of functional HLA-DR*1101 tetramers receptive for loading with pathogen- or tumour-derived synthetic peptides, BMC Immunol 6 (2005) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hansen T, Yu YY, Fremont DH, Preparation of stable single-chain trimers engineered with peptide, beta2 microglobulin, and MHC heavy chain (Chapter 17), Curr. Protoc. Im 87 (1) (2009) Unit17 15. [DOI] [PubMed] [Google Scholar]
- [40].Kozono H, White J, Clements J, Marrack P, Kappler J, Production of soluble MHC class II proteins with covalently bound single peptides, Nature 369 (1994) 151–154. [DOI] [PubMed] [Google Scholar]
- [41].Wooster AL, Anderson TS, Lowe DB, Expression and characterization of soluble epitope-defined major histocompatibility complex (MHC) from stable eukaryotic cell lines, J. Immunol. Methods 464 (2019) 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dedier S, Reinelt S, Rion S, Folkers G, Rognan D, Use of fluorescence polarization to monitor MHC-peptide interactions in solution, J. Immunol. Methods 255 (2001) 57–66. [DOI] [PubMed] [Google Scholar]
- [43].Buchli R, VanGundy RS, Hickman-Miller HD, Giberson CF, Bardet W, Hildebrand WH, Development and validation of a fluorescence polarization-based competitive peptide-binding assay for HLA-A*0201–a new tool for epitope discovery, Biochemistry 44 (2005) 12491–12507. [DOI] [PubMed] [Google Scholar]
- [44].Chang HC, Bao Z, Yao Y, Tse AG, Goyarts EC, Madsen M, Kawasaki E, Brauer PP, Sacchettini JC, Nathenson SG, et al. , A general method for facilitating heterodimeric pairing between two proteins: application to expression of alpha and beta T-cell receptor extracellular segments, Proc. Natl. Acad. Sci. U. S. A 91 (1994) 11408–11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Beckett D, Kovaleva E, Schatz PJ, A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation, Protein Sci 8 (1999) 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Khan F, He M, Taussig MJ, Double-hexahistidine tag with high-affinity binding for protein immobilization, purification, and detection on ni-nitrilotriacetic acid surfaces, Anal. Chem 78 (2006) 3072–3079. [DOI] [PubMed] [Google Scholar]
- [47].Reeves PJ, Callewaert N, Contreras R, Khorana HG, Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line, Proc. Natl. Acad. Sci. U. S. A 99 (2002) 13419–13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Joshi RV, Zarutskie JA, Stern LJ, A three-step kinetic mechanism for peptide binding to MHC class II proteins, Biochemistry 39 (2000) 3751–3762. [DOI] [PubMed] [Google Scholar]
- [49].Gilchuk P, Spencer CT, Conant SB, Hill T, Gray JJ, Niu X, Zheng M, Erickson JJ, Boyd KL, McAfee KJ, Oseroff C, Hadrup SR, Bennink JR, Hildebrand W, Edwards KM, Crowe JE Jr., Williams JV, Buus S, Sette A, Schumacher TN, Link AJ, Joyce S, Discovering naturally processed antigenic determinants that confer protective T cell immunity, J. Clin. Investig 123 (2013) 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Assarsson E, Sidney J, Oseroff C, Pasquetto V, Bui HH, Frahm N, Brander C, Peters B, Grey H, Sette A, A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection, J. Immunol 178 (2007) 7890–7901. [DOI] [PubMed] [Google Scholar]
- [51].Oseroff C, Kos F, Bui HH, Peters B, Pasquetto V, Glenn J, Palmore T, Sidney J, Tscharke DC, Bennink JR, Southwood S, Grey HM, Yewdell JW, Sette A, HLA class I-restricted responses to vaccinia recognize a broad array of proteins mainly involved in virulence and viral gene regulation, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 13980–13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pasquetto V, Bui HH, Giannino R, Banh C, Mirza F, Sidney J, Oseroff C, Tscharke DC, Irvine K, Bennink JR, Peters B, Southwood S, Cerundolo V, Grey H, Yewdell JW, Sette A, HLA-A*0201, HLA-A*1101, and HLA-B*0702 transgenic mice recognize numerous poxvirus determinants from a wide variety of viral gene products, J. Immunol 175 (2005) 5504–5515. [DOI] [PubMed] [Google Scholar]
- [53].Hellman LM, Yin L, Wang Y, Blevins SJ, Riley TP, Belden OS, Spear TT, Nishimura MI, Stern LJ, Baker BM, Differential scanning fluorimetry based assessments of the thermal and kinetic stability of peptide-MHC complexes, J. Immunol. Methods 432 (2016) 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cheng Y, Prusoff WH, Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction, Biochem. Pharmacol 22 (1973) 3099–3108. [DOI] [PubMed] [Google Scholar]
- [55].Munson PJ, Rodbard D, An exact correction to the “Cheng-Prusoff” correction, J. Recept. Res 8 (1988) 533–546. [DOI] [PubMed] [Google Scholar]
- [56].Cer RZ, Mudunuri U, Stephens R, Lebeda FJ, IC50-to-Ki: a web-based tool for converting IC50 to Ki values for inhibitors of enzyme activity and ligand binding, Nucleic Acids Res 37 (2009) W441–W445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wyllie DJ, Chen PE, Taking the time to study competitive antagonism, Br. J. Pharmacol 150 (2007) 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nikolovska-Coleska Z, Wang R, Fang X, Pan H, Tomita Y, Li P, Roller PP, Krajewski K, Saito NG, Stuckey JA, Wang S, Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization, Anal. Biochem 332 (2004) 261–273. [DOI] [PubMed] [Google Scholar]
- [59].Zhen J, Antonio T, Dutta AK, Reith ME, Concentration of receptor and ligand revisited in a modified receptor binding protocol for high-affinity radioligands: [3H]Spiperone binding to D2 and D3 dopamine receptors, J. Neurosci. Methods 188 (2010) 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang ZX, An exact mathematical expression for describing competitive binding of two different ligands to a protein molecule, FEBS Lett 360 (1995) 111–114. [DOI] [PubMed] [Google Scholar]
- [61].Douglass EF Jr., Miller CJ, Sparer G, Shapiro H, Spiegel DA, A comprehensive mathematical model for three-body binding equilibria, J. Am. Chem. Soc 135 (2013) 6092–6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Negroni MP, Stern LJ, The N-terminal region of photocleavable peptides that bind HLA-DR1 determines the kinetics of fragment release, PLoS One 13 (2018) e0199704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.