Abstract
Organophosphate ester (OPE) flame retardants and plasticizers, consumer product additives with widespread human exposure, were evaluated for their effect on the activity of purified human liver carboxylesterase (hCE1). Four of the 15 OPEs tested had IC50 values lower than 100 nM, including triphenyl phosphate (TPHP), 2-ethylhexyl diphenyl phosphate (EHDPHP), 4-isopropylphenyl diphenyl phosphate (4IPPDPP), and 4-tert-butylphenyl diphenyl phosphate (4tBPDPP), as did 4 of the commercial flame retardant mixtures tested. Because hCE1 is critical for the activation of imidapril, an angiotensin-converting enzyme-inhibitor prodrug prescribed to treat hypertension, the most potent inhibitors, TPHP and 4tBPDPP, and an environmentally relevant mixture (house dust) were further evaluated for their effect on imidapril bioactivation in vitro. TPHP and 4tBPDPP were potent inhibitors of hCE1-mediated imidapril activation (Ki = 49.0 and 17.9 nM, respectively). House dust extracts (100 µg/ml) also caused significant reductions (up to 33%) in imidapril activation. Combined, these data suggest that exposure to OPEs may affect pharmacotherapy.
Keywords: organophosphate ester, flame retardant, carboxylesterase, house dust, enzyme inhibition
Current evidence suggests that human exposure to isopropylated and tert-butylated triarylphosphate esters (ITPs and TBPPs) is common; however, there are very limited data available on their fate and effects in the human body (Behl et al., 2015; Hammel et al., 2016). ITP and TBPP isomers are used as plasticizers in polymers and as flame retardants in polyurethane foam commonly applied to residential furniture (McGee et al., 2013; Phillips et al., 2017). Toxicology and epidemiological studies have demonstrated potential hazards associated with ITP exposure, ranging from developmental neurotoxicity in zebrafish assays to the reduced probability of successful fertilization, implantation, clinical pregnancy, and live birth in humans (Carignan et al., 2017; Jarema et al., 2015). Indoor house dust is a known conduit of exposure for flame retardants, and organophosphate esters (OPEs), like ITPs and TBPPs, are commonly detected at high levels (µg/g) in this matrix (Hoffman et al., 2015; Phillips et al., 2018). Due to their widespread exposure and potentially toxic properties, the U.S. EPA has prioritized their risk assessment under the Frank R. Lautenberg Chemical Safety for the 21st Century Act, with a statutory deadline for proposed action by June 22, 2019 (U.S. EPA, 2016).
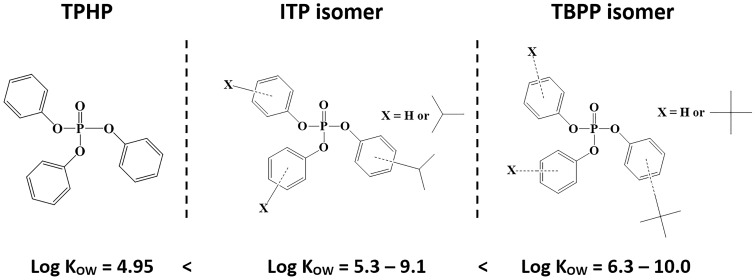
Although ITPs and TBPPs are structurally similar to organophosphate pesticides, OPEs have not been found to act as potent acetylcholinesterase inhibitors (Eldefrawi et al., 1977). However, multiple studies have shown that triphenyl phosphate (TPHP), an organophosphate chemical with an analogous structure to ITPs and TBPPs, inhibits mammalian liver carboxylesterase enzymes (Ces) (Brandt et al., 1980; Morris et al., 2014). For instance, a recent study found that mice treated with an intraperitoneal injection of 100 mg/kg TPHP had diminished hepatic Ces activity (43% decrease) compared to untreated controls 4 h following administration (Morris et al., 2014). Another study conducted in our laboratory found that rats dosed orally with 1000 µg/day Firemaster 550, an ITP and TPHP-containing flame retardant mixture, had significantly reduced hepatic carboxylesterase activity (62% decrease) compared to controls (Patisaul et al., 2013). Previous studies have indicated that increasing hydrophobicity is positively correlated with Ces inhibitor potency, and yet ITPs and TBPPs, which are more hydrophobic than TPHP, have not been evaluated for potential Ces inhibition (Figure 1) (Wadkins et al., 2007).
Figure 1.
Structures and associated log KOW ranges of triphenyl phosphate (TPHP), isopropylated triaryl phosphate isomers (ITPs), and tert-butylated triaryl phosphate isomers (TBPPs). ITPs and TBPPs are not well-studied, although previous studies have indicated that increasing hydrophobicity is positively correlated with carboxylesterase inhibitor potency (Wadkins et al., 2007).
Carboxylesterases are a class of enzymes belonging to the serine hydrolase superfamily and are known to be involved in the detoxification of pyrethroid pesticides, activation of prodrugs, and processing of endogenous lipids (Brandt et al., 1980; Morris et al., 2014). The major Ces isoforms in the human body are human liver carboxylesterase (hCE1), which is predominantly found in the liver, and hCE2, which is predominantly found in the small intestine (Schwer et al., 1997). Because an anti-hCE1 antibody was shown to inhibit 80%–95% of hepatic hydrolysis, hCE1 is thought to drive the majority of hydrolysis in the liver (Imai et al., 2006). Interestingly, more than an 8-fold range variance has been reported for hCE1 protein levels among human liver microsomes, suggesting significant interindividual variation in hCE1 expression (Hosokawa et al., 1995).
Because hCE1 activity is known to be critical for the bioactivation of many widely-used prodrugs, inhibition of hCE1 by OPE plasticizers and flame retardants could profoundly impact the efficacy of certain pharmacotherapies. Drugs that are activated by hCE1 include Tamiflu, antihyperlipidemic agents including simvastatin and lovastatin, mycophenolate, an immunosuppressant drug used to prevent rejection during organ transplantation, and capecitabine, a chemotherapy agent (Laizure et al., 2013). Our study focused on imidapril, an angiotensin-converting enzyme (ACE) inhibitor that is commonly prescribed to treat hypertension and congestive heart failure and more recently, diabetic nephropathy (Hosoya and Ishimitsu, 2002; Ren et al., 2015). Imidapril is a prodrug that is metabolized by hCE1 to imidaprilat, its bioactive form (Laizure et al., 2013). Because imidapril is well-tolerated and patients taking the drug experience a lower incidence of dry cough compared to enalapril or benazepril, imidapril is often a “first choice” ACE inhibitor (Hosoya and Ishimitsu, 2002). In fact, in Japan, imidapril was found to have the highest distribution of sales among various ACE inhibitors, accounting for 2.13 defined daily doses/1000 inhabitants per year (Imai et al., 2018). Responsiveness to imidapril treatment varies among patients, with a responder rate of approximately 50% (Huang et al., 2001). Although polymorphisms of the CES1A2 gene have been reported to affect imidapril metabolism, genetic factors alone do not account for all nonresponders even when differences in baseline blood pressure are considered (Geshi et al., 2005; Huang et al., 2001). It is possible that relatively high systemic concentrations of hCE1 inhibitors in combination with genetic polymorphisms which reduce hCE1 activity may explain this limited responsiveness.
The current study has 2 goals: (1) to investigate the potential in vitro inhibition of purified human hepatic carboxylesterase (hCE1) by ITP and TBPP isomers and related flame retardant mixtures and (2) to assess the potential for these chemicals to inhibit imidapril activation. Additionally, recently collected house dust samples were tested for their effect on hCE1-mediated imidapril activation at environmentally relevant doses.
MATERIALS AND METHODS
Materials
Bis(4-nitrophenyl) phosphate (BNPP), TPHP, tributyl phosphate (TBP), tris(4-tert-butylphenyl) phosphate (T4tBPP), diphenyl phosphate (DPHP), 2-ethylhexyl diphenyl phosphate (EHDPHP), and p-nitrophenyl acetate (PNPA) were purchased from Sigma Aldrich. Authentic standards (> 98% pure) of 2-isopropylphenyl diphenyl phosphate (2IPPDPP) and 4-isopropylphenyl diphenyl phosphate (4IPPDPP) were purchased from Wellington Laboratories. An authentic standard of 4-tert-butylphenyl diphenyl phosphate (4tBPDPP) and d3-imidapril hydrochloride were purchased from Toronto Research Chemicals. Firemaster 550 (FM 550) and Firemaster BZ-54 (BZ-54) were provided by Chemtura, the ITP commercial mixture (ITP Mix) was purchased from Jinan Great Chemical Industry Co, the TBPP commercial mixture (TBPP Mix) was produced by Ubichem and obtained from the National Toxicology Program (lot No. M062011NS) for research purposes as part of a materials transfer agreement. Kronitex 50 (K 50) was purchased from Chem Service. Isopropylated phenyl phenylphosphate (ip-PPP) and tert-butylated phenyl phenylphosphate (tb-PPP) were synthesized by the Duke Small Molecule Laboratory. Imidapril hydrochloride, imidaprilat, and d5-imidaprilat were purchased from TLC Pharmaceutical Standards Ltd. Recombinant, purified hCE1b (the major wild-type isoform in the human liver) was purchased from Corning Life Sciences (Corning, New York).
Dosing stock preparation and confirmation
Dosing stocks were prepared for the following chemicals and commercial mixtures: TPHP, EHDPHP, 2IPPDPP, 4IPPDPP, 4tBPDPP, T4tBPP, TBP, DPHP, ip-PPP, tb-PPP, FM 550, BZ-54, K 50, ITP Mix, and TBPP Mix. Chemical masses were recorded using a microbalance and then dissolved in methanol to prepare each stock solution. The final concentration of methanol used in all incubations was kept below 1%. The nominal concentration of each dosing stock was quantified via gas chromatography mass spectrometry (GC/MS) using previously published methods (Phillips et al., 2017; Stapleton et al., 2009).
Screening for hCE1 inhibition
Prior to screening experiments, the specific activity of hCE1 (16 µg/ml) for PNPA (1 mM) was evaluated. The observed specific activity agreed well with the value reported by the manufacturer and appeared to be linear for a 25-min incubation period (Supplementary Figure 1). Each chemical and commercial mixture was then individually evaluated for their ability to inhibit hCE1 (16 µg/ml) at a range of concentrations (0.1 nM–100 µM). Total hCE1 activity was determined by measuring the formation of p-nitrophenol (PNP) at 405 nm from the hydrolysis of p-nitrophenyl acetate (1 mM) (Ross and Borazjani, 2007). Following 5-min incubations in phosphate buffer (100 mM, pH 7.4) at 37°C, an equal volume of ice-cold acetonitrile was used to stop the reaction and total carboxylesterase activity was measured. Bis-p-nitrophenyl phosphate (BNPP), a known Ces inhibitor, was included as a positive control. Experiments included 3 replicates per plate and were run in triplicate. Results were corrected for spontaneous hydrolysis using controls containing PNPA but no hCE1, and inhibition curves were generated.
Effect on hCE1-mediated imidapril activation
Due to their widespread exposure and potency in screening assays, TPHP and 4tBPDPP were also tested for their effect on the bioactivation of imidapril. An LC-MS/MS method to quantify imidapril and imidaprilat was created based on the one described by Mabuchi et al. (1999). More information on this method can be found in the Supplementary Table 1. Initial range-finding experiments were performed to determine incubation duration and which concentrations of imidapril and hCE1 should be used to produce detectable amounts of imidaprilat (Supplementary Figure 2). Preliminary experiments were also conducted to establish a mass balance between imidapril and imidaprilat after the incubation of imidapril with hCE1 (Supplementary Figure 3). Following 150-min hCE1 (160 µg/ml) incubations with 10 µM imidapril and either TPHP or 4tBPDPP (0.1 nM–100 µM), an equal volume of ice-cold acetonitrile containing d3-imidapril and d5-imidaprilat was used to terminate the reaction. The incubation mixture was then cleaned using Costar Spin-X centrifuge filters (nylon membrane, pore size 0.22 µM, Corning). Extracts were transferred to autosampler vials, imidaprilat formation was monitored via LC-MS/MS, and inhibition curves were generated. Spike/recovery experiments were performed to determine the recovery of imidapril and imidaprilat following clean up (see Supplementary Material). In addition to TPHP and 4tBPDPP, house dust sample extracts (n = 20; collected in 2014–2016) were tested for their effect on imidapril activation at 10 and 100 µg/ml doses. TPHP and 4tBPDPP concentrations in dust extracts were previously quantified and their associations with the inhibition of imidapril activation were assessed (Phillips et al., 2018). All imidapril activation experiments were run in duplicate.
Kinetic and mode of inhibition experiments
Michaelis-Menten kinetic parameters were also determined using a range of imidapril concentrations (1 µM–2.5 mM) in 60-min incubations with 160 µg/ml hCE1. Mode of inhibition experiments were carried out for TPHP (30, 60, and 200 nM) and 4tBPDPP (10, 20, and 75 nM) at a range of imidapril concentrations (10–500 µM). After the addition of d3-imidapril and d5-imidaprilat in an equal volume of acetonitrile, samples were cleaned using the centrifuge filter method described above. Imidaprilat formation was quantified via LC-MS/MS.
Statistical analyses
All IC50 values were calculated using a 3-parameter nonlinear model in JMP Pro (version 11; SAS Institute Inc, Cary, North Carolina) (Roberts et al., 2015). IC50 values presented represent mean ± standard error. All analyses involving house dust extracts were conducted using SAS statistical software (version 9.4; SAS Institute Inc). One-factor ANOVA indicated a significant effect of dose (p < .01). Significant effects were further tested using Tukey’s post hoc test. Imidapril activation was log-transformed before statistical analysis. Preliminary analyses indicated that OPE concentrations in dust, as well as the percent control imidapril activation were normally-distributed. Accordingly, Pearson correlations were used to assess relationships between these 2 parameters. The Km and Vmax values and inhibition types were determined by fitting data to a competitive, noncompetitive, uncompetitive, or mixed inhibition model by nonlinear regression analysis using GraphPad Prism (version 7; GraphPad Software, Inc, San Diego, California) (Fukami et al., 2010). The Km and Vmax values depict mean ± standard error. Statistical significance was set to α = .05. More information regarding model equations used for data analysis can be found in the Supplementary Material.
RESULTS
Dosing Stock Confirmation
Test chemicals were dissolved in methanol, and the final organic concentration was kept below 1% in all assays. All nominal dosing stock concentrations were within 10% of measured concentrations, as measured by GC/MS. Nominal concentrations were adjusted according to measured concentrations for data analysis (Supplementary Table 2).
Screening for hCE1 Inhibition
The inhibitory effects of various OPE flame retardants and related commercial mixtures on total hCE1 activity were screened in an absorbance-based assay that measured p-nitrophenol formation from p-nitrophenyl acetate hydrolysis (Ross and Borazjani, 2007). BNPP, a known carboxylesterase inhibitor, was included as a positive control for hCE1 inhibition. The measured IC50 value for BNPP in our assay, 69.3 ± 9.4 nM, agreed well with the IC50 value reported by Corning (24 nM), the enzyme’s manufacturer (Wang et al., 2011). IC50 values for the test chemicals and commercial mixtures ranged from 22.3 nM to > 90 400 nM. The TBPP commercial flame retardant mixture had the lowest IC50 value (17.6 ± 6.9 nM), and FM 550, K 50, the ITP Mix, TPHP, EHDPHP, 4IPPDPP, 4tBPDPP all had IC50 values lower than 100 nM (Table 1).
Table 1.
hCE1b IC50 Values of ITP and TBPP Isomers and Related FR Mixtures
| Test Chemical | Structure or Description | Presence in FR Mixturea | IC50 Value (nM)b | R 2 of Model |
|---|---|---|---|---|
| BNPP |
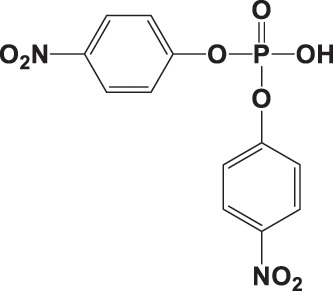
|
NA | 69.3 ± 9.4c | 0.998 |
| TPHP |
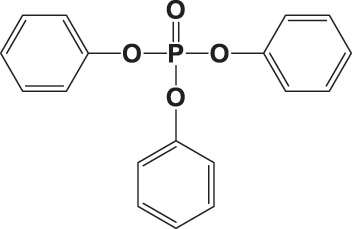
|
|
65.4 ± 8.3 | 0.993 |
| EHDPHP |
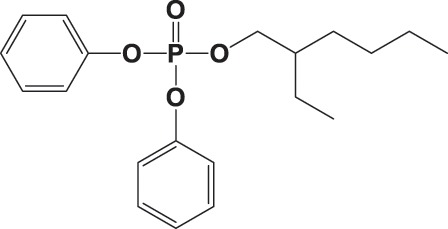
|
NA | 99.4 ± 65.6 | 0.975 |
| FM 550 | EH-TBB, BEH-TEBP and ITP mixture | NA | 31.2 ± 2.9 | 0.999 |
| BZ-54 | EH-TBB and BEH-TEBP only | NA | > 35 000 | NA |
| ITP Mix | ITP mixture | NA | 28.0 ± 15.4 | 0.979 |
| K 50 | ITP mixture | NA | 27.6 ± 16.9 | 0.978 |
| 2IPPDPP |
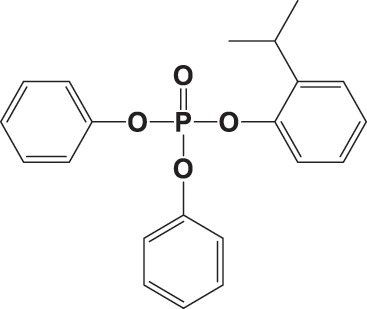
|
|
574 ± 127 | 0.996 |
| 4IPPDPP |
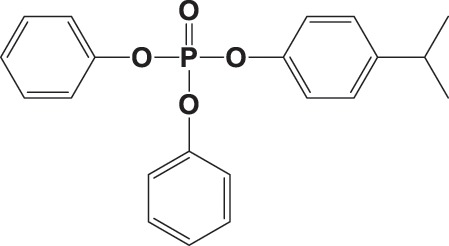
|
|
49.9 ± 21.9 | 0.984 |
| TBPP Mix | TBPP mixture | NA | 17.9 ± 6.9 | 0.988 |
| 4tBPDPP |
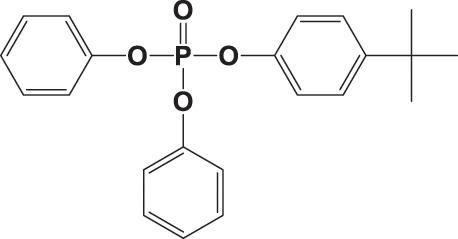
|
|
22.3 ± 6.9 | 0.977 |
| T4tBPP |
|
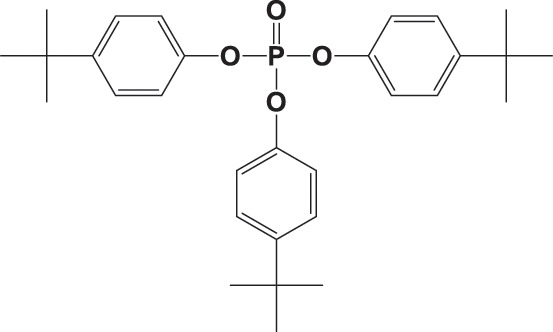
|
1740 ± 1010 | 0.979 |
| TBP |
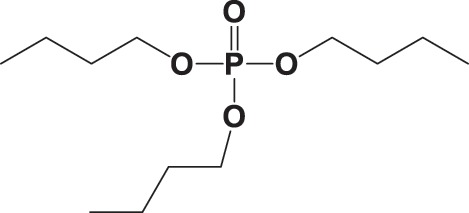
|
NA | > 19 000 | NA |
| DPHP |
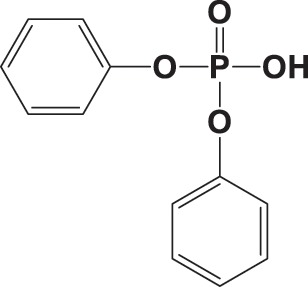
|
NA | > 90 400 | NA |
| ip-PPP |
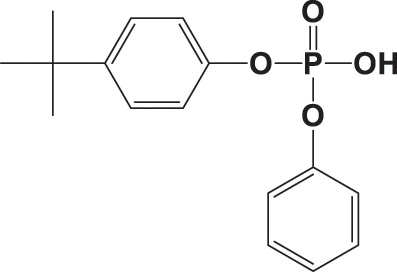
|
NA |
3420 ± 1620 |
0.985 |
| tb-PPP |

|
NA | 4820 ± 2690 | 0.979 |
Abbreviations: hCE1b, human hepatic carboxylesterase; ITP and TBPP, isopropylated and tert-butylated triarylphosphate esters; FR, flame retardant; BNPP, bis(4-nitrophenyl) phosphate; TPHP, triphenyl phosphate; EHDPHP, 2-ethylhexyl diphenyl phosphate; FM 550, Firemaster 550; BZ-54, Firemaster BZ-54; ITP, isopropylated triaryl phosphate commercial mixture; 2IPPDPP, 2-isopropylphenyl diphenyl phosphate; 4IPPDPP, 4-isopropylphenyl diphenyl phosphate; TBPP, tert-butylated triarylphosphate commercial mixture; 4tBPDPP, 4-tert-butylphenyl diphenyl phosphate; T4tBPP, tris(4-tert-butylphenyl) phosphate; TBP, tributyl phosphate; DPHP, diphenyl phosphate; ip-PPP, isopropylated phenyl phenylphosphate; tb-PPP, tert-butylated phenyl phenylphosphate; NA, not applicable.
Percent composition is expressed on a wt/wt basis (Phillips et al., 2017).
IC50 values were calculated using a 3 parameter nonlinear model in JMP Pro 11 (Roberts et al., 2015). More information regarding the model equation can be found in the Supplementary Material.
This value is comparable to the IC50 value determined by the manufacturer (24 nM) (Wang et al., 2011).
Imidaprilat Formation Kinetics
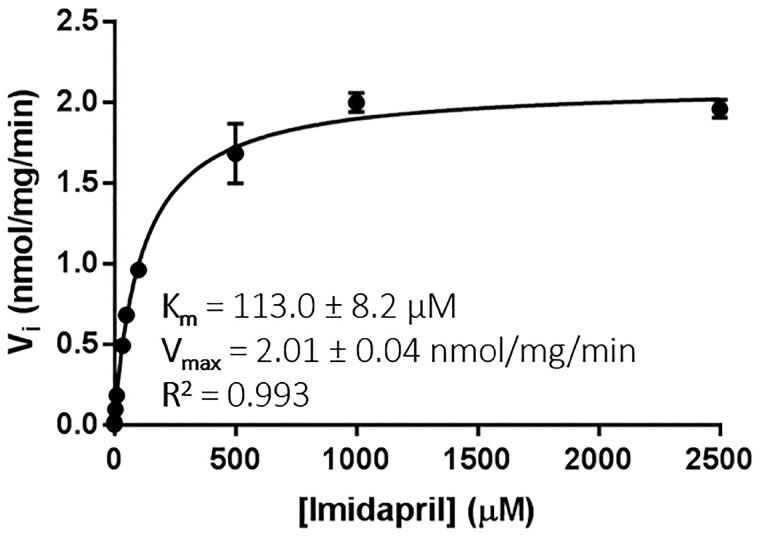
The kinetics of imidaprilat formation was investigated using purified hCE1. Imidaprilat formation showed typical Michaelis-Menten enzyme kinetics, and the model fit was excellent (r2 = .993). The apparent Km for the formation of imidaprilat from imidapril was determined to be 113.0 ± 8.2 µM, and the Vmax was estimated to be 2.01 nmol/min/mg hCE1 (Figure 2). Although these values were not identical to previously-reported values obtained using recombinant enzyme expressed in Sf21 cells (Km = 3.2 ± 8.2 mM, Vmax = 24.5 nmol/mg/min), their semblance was deemed acceptable due to the difference in enzyme source (Fukami et al., 2010). The interday variation was assessed by performing the assay on 2 separate days (n = 2 per day) using optimized conditions (100 µM imidapril and 160 µg/ml hCE1 for a 60-min incubation). The results showed no statistical difference among the 2 days (ANOVA; p > .05).
Figure 2.
The rate of formation of imidaprilat (nmol/mg/min) resulting from the incubation of imidapril with 160 µg/ml human hepatic carboxylesterase (hCE1b) for 60 min. The Michaelis constant (Km) and the maximal reaction velocity (Vmax) were obtained from nonlinear regression analysis in GraphPad Prism 7 (see Supplementary Material for model equation). The experiment was run in duplicate and each point depicts mean ± SEM.
Effect of TPHP and 4tPDPP on hCE1-mediated Imidapril Activation
The inhibitory effects on imidapril hydrolase activity by hCE1 were investigated using TPHP and 4tBPDPP. TPHP was chosen due to its ubiquity in the indoor environment and 4tBPDPP was chosen due to its potency in the screening assay. TPHP and 4tBPDPP were dissolved in methanol (< 1% vol/vol), and inhibition was calculated as percent inhibition of the control activity (ie, imidaprilat formed without presence of inhibitors).
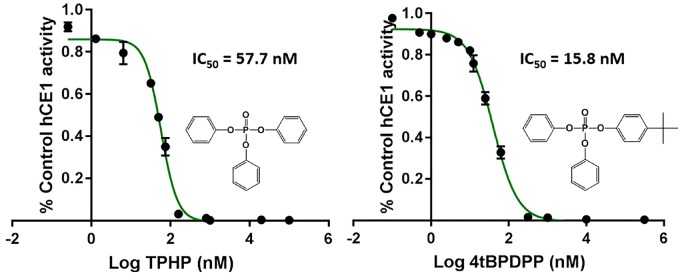
TPHP and 4tBPDPP were found to be potent inhibitors of imidapril activation (IC50 = 57.7 and 15.8 nM, respectively) (Figure 3).
Figure 3.
Inhibition curves for triphenyl phosphate (TPHP) and 4-tert-butylphenyl diphenyl phosphate (4tBPDPP) using 10 µM imidapril and 160 µg/ml human hepatic carboxylesterase (hCE1b). Activity was normalized to control and was determined by measuring imidaprilat formation following 150-min incubations. Experiments were run in duplicate and each point depicts mean ± SEM. IC50 values were calculated using a 3 parameter linear model in JMP Pro 11 (Roberts et al., 2015). More information regarding the model equation can be found in the Supplementary Material.
Mode of Inhibition of TPHP and 4tBPDPP on Imidapril Activation
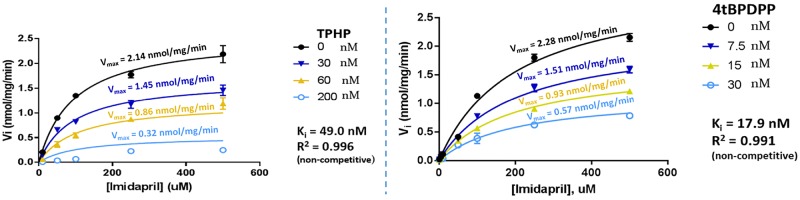
The type of inhibition (competitive, noncompetitive, uncompetitive, or mixed inhibition) was investigated by calculating the Michaelis-Menten parameters (Vmax and Km values) using various concentrations of the OPE inhibitor (either TPHP or 4tBPDPP) (Figure 4). The Vmax rate was statistically lower with both increasing TPHP and 4tBPDPP concentrations but the apparent Km did not significantly vary with inhibitor concentration. For example, the maximal rate of imidaprilat formation decreased from 2.14 to 0.32 nmol/mg/min upon addition of 200 nM TPHP, although the Km did not change significantly (98.9 vs 115.1 µM, p > .05). Similarly, the Km did not vary significantly upon the addition of 30 nM 4tBPDPP (85.8 vs 109.3 µM, p > .05) but the maximal rate of imidaprilat formation decreased from 2.28 to 0.57. These observations in enzymatic constants are indicative of noncompetitive inhibition.
Figure 4.
Imidaprilat formation rate resulting from the incubation of imidapril (10–500 µM) with varying concentrations of triphenyl phosphate (TPHP) (0, 30, 60, and 200 nM) or 4-tert-butylphenyl diphenyl phosphate (4tBPDPP) (0, 7.5, 15, or 30 nM) with 160 µg/ml human hepatic carboxylesterase (hCE1b) for 60 min. Kinetic data were fit to competitive, noncompetitive, uncompetitive, and mixed inhibition models by nonlinear regression in GraphPad Prism and were best modeled by noncompetitive inhibition (Ki = 49.0 nM, R2 = .996 for TPHP; Ki = 17.9 nM, R2 = .991 for 4tBPDPP; see Supplementary Material for model equations). Km values ranged from 98.9 to 115.1 µM for TPHP and from 85.8 to 109.3 µM for 4tBPDPP. The experiment was run in duplicate and each point depicts mean ± SEM.
Effect of House Dust Extracts on hCE1-mediated Imidapril Activation
To evaluate potential inhibition with environmentally relevant mixtures, imidapril was incubated with hCE1 in the presence of 2 doses (10 and 100 µg/ml) of individual house dust extracts (n = 20 per dose). Although limited hCE1b inhibition was observed by dust extracts at 10 µg/ml, significant inhibition (reductions of up to 33%) was observed for 10 of the 20 extracts tested at 100 µg/ml. Both doses tested were environmentally relevant concentrations (Figure 5). The interday variation was assessed by performing the assay on 2 separate days (n = 2 per day) using optimized conditions (10 µM imidapril and 160 µg/ml hCE1 for a 150-min incubation). The results showed no statistical difference among the 2 days (ANOVA; p > .05). Percent inhibition was negatively correlated with both log10TPHP and log104tBPDPP concentrations measured in the dust using mass spectrometry (rP = −.65 and rP = −.49, p < .05), suggesting that a large proportion of the observed hCE1 inhibition from house dust was driven by these chemicals (Figure 6).
Figure 5.
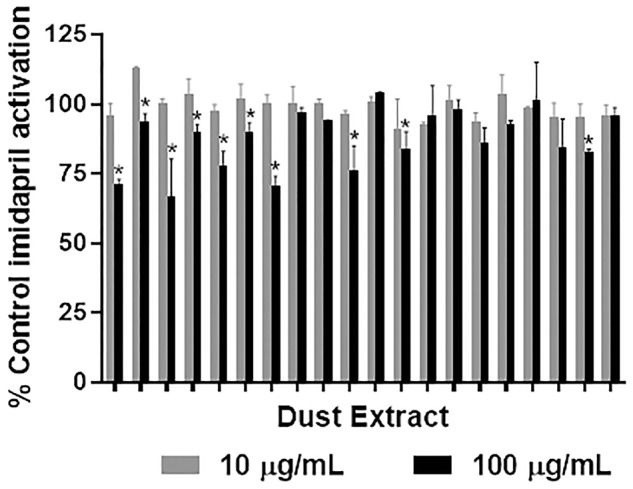
Following the incubation of dust extracts (10 or 100 µg/ml, n = 20 per dose) with 160 µg/ml human hepatic carboxylesterase (hCE1b) and 10 µM imidapril for 150 min, imidaprilat formation was monitored via LC-MS/MS. Samples were run in duplicate on separate days and bars depict mean ± SEM. *p < .05 indicates a significant difference from control.
Figure 6.
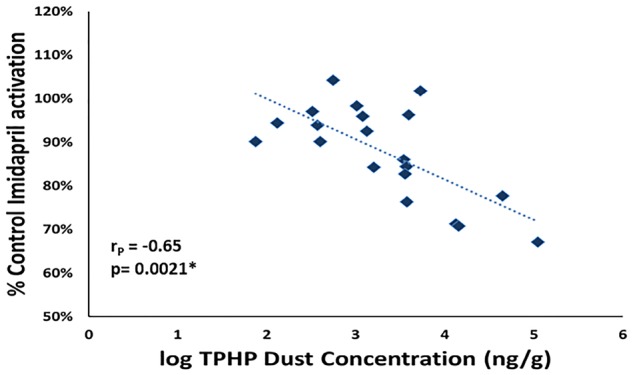
Following the incubation of dust extracts (100 µg/ml, n = 20) with 160 µg/ml hCE1b and 10 µM imidapril for 150 min, imidaprilat formation was monitored via LC-MS/MS. Triphenyl phosphate (TPHP) concentration in dust extracts were previously quantified (Phillips et al., 2018). Pearson analysis showed that percent control imidapril activation was negatively correlated (rP = −.65, p = .0021, n = 20) with log10TPHP dust concentration.
DISCUSSION
Screening for hCE1 Inhibition
Of 2IPPDPP and 4IPPDPP, the 2 ITP isomers tested, 4IPPDPP had a much lower IC50 value compared to 2IPPDPP, suggesting that steric effects (ie, alkyl substitution position on the ring) may contribute to inhibitor potency. Of 4tBPDPP and T4tBPP, the mono- and tri-substituted TBPP isomers, the measured IC50 value for 4tBPDPP was multiple orders of magnitude lower than IC50 for T4tBPP, suggesting that 4tBPDPP and possibly B4tBPPPP (not tested in this study) were the main contributors to the observed potency of the TBPP mixture (Table 1). Consistent with the previously observed positive trend between inhibitor potency and hydrophobicity, 4tBPDPP (para-substituted, tert-butylated isomer, log KOW = 6.3) had an IC50 value that was approximately half of the IC50 value found for 4IPPDPP (para-substituted, isopropylated isomer, log KOW = 5.7) (Wadkins et al., 2006). Because the active site of hCE1 is lined with mostly hydrophobic amino acids, it is unsurprising that an inhibitor’s potency is related to its log KOW value, the octanol-water partitioning coefficient that is correlated with hydrophobicity (Wang et al., 2018). Interestingly, hCE1 activity was unaffected even at the highest test concentration for TBP (log KOW = 2.9). The diester metabolites that were tested (DPHP, ip-PPP, and tb-PPP; log KOW = 1.4–3.1) were also less potent hCE1 inhibitors compared to their corresponding triester parent compounds (TPHP, 4IPPDPP, and 4tBPDPP; log KOW = 4.6–6.3), potentially reflecting the previously noted positive correlation between hCE1 inhibitor potency and hydrophobicity. Although most of the chemicals tested in this study are additives in construction materials and consumer products, EHDPHP has long been approved by the Food and Drug Administration for use in food packaging and has been detected in food on numerous occasions (Castle et al., 1988; Poma et al., 2017). This is particularly concerning due the high potential for human exposure and its potency as an hCE1 inhibitor in our assay (IC50 = 99.4 nM).
Notably, the FM 550 and BZ-54 mixtures, which both contain the brominated compounds EH-TBB and BEH-TEBP, had different effects on hCE1 activity. BZ-54, which only contains EH-TBB and BEH-TEBP had no effect on hCE1 activity even at the highest test concentration, whereas FM 550, which contains TPHP and ITP isomers in addition to EH-TBB and BEH-TEBP was a potent inhibitor of hCE1 activity. This indicates that organophosphate components of the FM 550 drove the reduction of liver microsomal carboxylesterase activity observed in previous in vivo experiments (Patisaul et al., 2013). Both of the ITP mixtures that were tested, ITP Mix and K 50, had IC50 values just under 30 nM, reflecting their nearly identical ITP isomer composition profiles. Because the main components of the TBPP mixture are 4tBPDPP and TPHP (24% and 36% wt/wt, respectively) and T4tBPP is relatively minor component (3% wt/wt), it is unsurprising that the IC50 for the TBPP mixture more closely resembles the IC50 value for 4tBPDPP and TPHP than the IC50 value for T4tBPP (Phillips et al., 2017).
Imidaprilat Formation Kinetics
The measured Vmax value for imidaprilat formation using purified hCE1(2.01 nmol/min/mg) was an order of magnitude lower than the Vmax value reported using human liver microsomes (39.1 nmol/min/mg) by Fukami et al. (2010), which is in line with values reported by the enzyme’s manufacturer for fluorescein diacetate (purified hCE1 = 0.31 µmol/min/mg; HLM = 18.5 µmol/min/mg) (Wang et al., 2011). However, Takahashi et al. (2009) reported a Vmax of 2.4 nmol/min/mg for imidaprilat formation using human liver microsomes, which closely matches the observed value in this study. Different Vmax values could reflect the substantial interindividual variability (31-fold) in imidaprilat hydrolysis by human liver microsomes that has been reported previously (Takahashi et al., 2009). It is also possible that the recombinant enzyme purchased from Corning had lower activity than the carboxylesterases found in human liver microsomes, or that other enzymes present in human liver microsomes also participate in imidapril activation.
Effect of TPHP and 4tPDPP on hCE1-mediated Imidapril Activation
TPHP and 4tBPDPP were found to inhibit hCE1-mediated imidapril activation at environmentally-relevant levels, highlighting the potential for these chemicals to interfere with certain pharmacotherapies. TPHP is used as both a flame retardant and plasticizer and is one the primary components of multiple currently-used commercial flame retardant mixtures including FM 550, ITP mixtures, and TBPP mixtures (Phillips et al., 2017). TPHP is also found in some nail polishes, hydraulic fluids, lubricating oils, electrical equipment, and lacquers (Carlsson et al., 2000; Mendelsohn et al., 2016). TPHP has been detected in indoor and outdoor air, house dust, surface water, fish, and human hair, nails, and breastmilk (Liu et al., 2016; Phillips et al., 2018; Sundkvist et al., 2010; Wilford et al., 2004; Xu et al., 2016). Similarly, 4tBDPP has been detected in the indoor environment, and has been detected frequently in indoor house dust (Phillips et al., 2018). Geometric mean and maximum levels of ip-PPP, a urinary metabolite of ITPs, have been measured to range from 6 to 200 nM, concentrations which are well within IC50 values found for TPHP and 4tBPDPP in our imidapril assay (Butt et al., 2016; Phillips et al., 2018).
Mode of Inhibition of TPHP and 4tBPDPP on Imidapril Activation
Our kinetic experiments suggest that TPHP and 4tBPDPP are noncompetitive inhibitors of hCE1b (Ki = 49.0 and 17.9 nM, respectively), further raising concern about their ability to compromise certain drug treatments. Although competitive inhibition in the context of xenobiotic-drug interactions likely has little relevance due to the stark differences in substrate concentration (ie, intentionally-administered drugs are found at much higher levels in the human body than xenobiotics), noncompetitive enzyme inhibition by a pervasive xenobiotic, like TPHP, could potentially affect a pharmaceutical’s efficacy. In the case of noncompetitive inhibition of hCE1 by TPHP or 4tBPDPP, the formation of imidaprilat from imidapril in the liver would be reduced, resulting in a lower concentration of imidaprilat in circulation than the intended dose and potentially, the attenuation of the therapeutic effect (reduction in blood pressure). As TPHP and 4tBPDPP are likely noncompetitive inhibitors of hCE1, increasing the dose of imidapril will not necessarily lead to increased imidaprilat formation. This is because the liver of each individual has a finite amount of hCE1 content, and a fixed percentage of hCE1 will always be inactivated by a noncompetitive inhibitor, such as a TPHP or 4tBPDPP (Bisswanger, 2011).
Effect of House Dust Extracts on hCE1-mediated Imidapril Activation
Because inadvertent ingestion of contaminated house dust is a primary route of exposure for these OPEs, testing house dust extracts for their effects on imidapril bioactivation is especially relevant. The U.S. EPA recommended values for the central tendency of the general population for dust ingestion range from 30 to 60 mg/day, depending on age (U.S. EPA, 2011). Recent exposure assessments estimate a total daily intake for TPHP attributed to house dust to be 37–54 ng/kg bw/day for the 95th percentile of toddlers and measured urinary levels of DPHP, a metabolite of TPHP, range from 27 to 153 nM (geometric mean-maximum concentrations) (Phillips et al., 2018; Tajima et al., 2014). Importantly, imidapril activation takes place in the liver where the levels of parent compound are likely to be the highest prior to metabolism. Although 4tBPDPP was a more potent inhibitor than TPHP, a stronger correlation was observed for TPHP perhaps because TPHP is present in house dust at much higher levels compared to 4tBPDPP. To the authors’ knowledge, other known carboxylesterase inhibitors such as benzil, BNPP, and trifluoromethyl ketones have not been detected at substantial levels in house dust.
Limitations and Future Directions
Because OPEs are known to be rapidly metabolized and the diester metabolites were less potent hCE1 inhibitors than the triester parents, IC50 values measured in our experiments with purified hCE1 may be lower than they would have been had experiments been performed with human liver microsomes or in cell culture (Cooper et al., 2011). Although multiple in vivo experiments have noted hepatic carboxylesterase activity reductions resulting from OPE administration, the doses used often exceed environmental relevance and significant interspecies variation in microsomal carboxylesterase activity may limit the translation of these findings to humans (Hosokawa et al., 1995). Although human and rodent carboxylesterases share a high degree of sequence homology (approximately 70%), different species express distinct carboxylesterase isoforms which have been shown to exhibit different inhibitor sensitivities, making comparison across species difficult (Xie et al., 2002). Future studies should focus on using human-derived cell culture lines or other metabolically active model systems to evaluate hCE1 inhibition by OPE flame retardants and plasticizers. Additional studies should also be conducted to evaluate the potential reversibility of carboxylesterase inhibition by OPEs. For example, uncharged oximes reactivate carboxylesterases inactivated by some organophosphate compounds, but certain organophosphate compounds cause inhibition that is refractory to oxime reactivation (Maxwell et al., 1994). The data presented herein highlight the potential for drug-xenobiotic interactions via hCE1 inhibition, but future studies should be conducted to confirm that the inhibition observed in vitro is biologically meaningful in vivo.
Implications
Taken together, these results highlight the possibility for Ces inhibition by ITP and TBPP isomers at environmentally relevant concentrations. Although TPHP and other organophosphate have been shown to inhibit Ces previously, these data add to our current knowledge of hCE1 inhibitors. Unfortunately, ITPs and TBPPs are rapidly metabolized and direct measurement of ITP and TBPP concentrations at the target (ie, human liver tissue) is not feasible. To approximate what a relevant concentration in the liver might be, urinary metabolite levels can be used as a surrogate. Geometric mean concentrations of isopropyl phenyl diphenyl phosphate (ip-PPP), a urinary metabolite of ITPs, range from 2 to 7 ng/ml in recent human exposure assessments, with reported maximum levels as high as 61 ng/ml (Butt et al., 2016; Phillips et al., 2018). IC50 values measured in this study for TPHP, EHDPHP, FM 550, ITP Mix, K 50, 4IPPDPP, and 4tBPDPP fall within this urinary concentration range (6–200 nM). It is unknown whether urinary metabolite concentrations under- or over-estimate the levels of ITPs and TBPPs in the liver following exposure, and the present study should be interpreted in the context of this uncertainty. For example, urinary metabolite levels may exceed corresponding liver concentrations (Bankir and Yang, 2012). However, because OPEs also undergo biliary excretion in addition to urinary elimination, urinary metabolite levels might also underestimate total exposure. Although biochemical experiments sometimes overstate actual cellular potency, our study illustrates biological plausibility for Ces inhibition by OPEs at doses that might occur in real life exposure scenarios.
Our data also demonstrate the potential of OPE flame retardants and plasticizers to interfere with pharmaceuticals that rely on bioactivation by hCE1b. Because plasticizers are often used as excipients in drug formulations, plasticizer excipients should be evaluated for hCE1 inhibition, especially when used in drugs that require hCE1-mediated activation (Bhyan et al., 2011). Other drugs that are activated by hCE1b include Tamiflu, antihyperlipidemic agents including simvastatin and lovastatin, mycophenolate, an immunosuppressant drug used to prevent rejection during organ transplantation, and capecitabine, a chemotherapy agent (Laizure et al., 2013). Similarly, xenobiotics and drugs inactivated by hCE1b include Ritalin, cocaine, meperidine (an opioid pain medication), and pyrethroid pesticides like bioresmethrin (Ross et al., 2006). As such, inhibition of hCE1b by organophosphate aryl esters at environmentally relevant levels has far-reaching public health ramifications and brings attention to xenobiotic-drug interactions, an idea that receives little attention in the current literature but could have profound implications for the effective use of pharmaceuticals.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to TESIE participants for providing the dust samples used in this study. We also thank the National Toxicology Program for providing the TBPP mixture for research purposes as part of a materials transfer agreement.
FUNDING
This work was funded by the National Institute of Environmental Health Sciences (R01 ES016099 to H.M.S., T32-ES021432 to A.L.P.). This work was also supported by an American College of Toxicology North American Graduate Fellowship and P.E.O. Scholar Award to A.L.P.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Bankir L., Yang B. (2012). New insights into urea and glucose handling by the kidney, and the urine concentrating mechanism. Kidney Int. 81, 1179–1198. [DOI] [PubMed] [Google Scholar]
- Behl M., Hsieh J.-H., Shafer T. J., Mundy W. R., Rice J. R., Boyd W. A., Freedman J. H., Hunter E. S., Jarema K. A., Padilla S. (2015). Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 52, 181–193. [DOI] [PubMed] [Google Scholar]
- Bhyan B., Jangra S., Kaur M., Singh H. (2011). Orally fast dissolving films: Innovations in formulation and technology. Int. J. Pharm. Sci. Rev. Res. 9, 50–57. [Google Scholar]
- Bisswanger H. (2011). Principles and methods. Enzyme 691, 83–96. [Google Scholar]
- Brandt E., Heymann E., Mentlein R. (1980). Selective inhibition of rat liver carboxylesterases by various organophosphorus diesters in vivo and in vitro. Biochem. Pharmacol. 29, 1927–1931. [DOI] [PubMed] [Google Scholar]
- Butt C. M., Hoffman K., Chen A., Lorenzo A., Congleton J., Stapleton H. M. (2016). Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ. Int. 94, 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan C. C., Mínguez-Alarcón L., Butt C. M., Williams P. L., Meeker J. D., Stapleton H. M., Toth T. L., Ford J. B., Hauser R. (2017). Urinary concentrations of organophosphate flame retardant metabolites and pregnancy outcomes among women undergoing in vitro fertilization. Environ. Health Perspect. 125, 087018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson H., Nilsson U., Ostman C. (2000). Video display units: An emission source of the contact allergenic flame retardant triphenyl phosphate in the indoor environment. Environ. Sci. Technol. 34, 3885–3889. [Google Scholar]
- Castle L., Mercer A. J., Startin J. R., Gilbert J. (1988). Migration from plasticized films into foods 3. Migration of phthalate, sebacate, citrate and phosphate esters from films used for retail food packaging. Food Addit. Contam. 5, 9–20. [DOI] [PubMed] [Google Scholar]
- Cooper E. M., Covaci A., van Nuijs A. L. N., Webster T. F., Stapleton H. M. (2011). Analysis of the flame retardant metabolites (1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 401, 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi A. T., Mansour N. A., Brattsten L. B., Ahrens V. D., Lisk D. J. (1977). Further toxicologic studies with commercial and candidate flame retardant chemicals. Part II. Bull. Environ. Contam. Toxicol. 17, 720–726. [DOI] [PubMed] [Google Scholar]
- Fukami T., Takahashi S., Nakagawa N., Maruichi T., Nakajima M., Yokoi T. (2010). In vitro evaluation of inhibitory effects of antidiabetic and antihyperlipidemic drugs on human carboxylesterase activities. Drug Metab. Dispos. 38, 2173–2178. [DOI] [PubMed] [Google Scholar]
- Geshi E., Kimura T., Yoshimura M., Suzuki H., Koba S., Sakai T., Saito T., Koga A., Muramatsu M., Katagiri T. (2005). A single nucleotide polymorphism in the carboxylesterase gene is associated with the responsiveness to imidapril medication and the promoter activity. Hypertens. Res. 28, 719–725. [DOI] [PubMed] [Google Scholar]
- Hammel S., Hoffman K., Webster T. F., Anderson K. A., Stapleton H. M. (2016). Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes. Environ. Sci. Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K., Garantziotis S., Birnbaum L. S., Stapleton H. M. (2015). Monitoring indoor exposure to organophosphate flame retardants: Hand wipes and house dust. Environ. Health Perspect. 123, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa M., Endo T., Fujisawa M., Hara S., Iwata N., Sato Y., Satoh T. (1995). Interindividual variation in carboxylesterase levels in human liver microsomes. Drug Metab. Dispos. 23, 1022–1027. [PubMed] [Google Scholar]
- Hosoya K., Ishimitsu T. (2002). Protection of the cardiovascular system by imidapril, a versatile angiotensin-converting enzyme inhibitor. Cardiovasc. Drug Rev. 20, 93–110. [DOI] [PubMed] [Google Scholar]
- Huang P. J., Chien K. L., Chen M. F., Lai L. P., Chiang F. T. (2001). Efficacy and safety of imidapril in patients with essential hypertension: A double-blind comparison with captopril. Cardiology 95, 146–150. [DOI] [PubMed] [Google Scholar]
- Imai S., Fushimi K., Andersson Sundell K. (2018). Impact of new efficacy information on sales of antihypertensive medicines in Japan and Sweden. Health Policy Technol. 7, 194–199. [Google Scholar]
- Imai T., Taketani M., Shii M., Hosokawa M., Chiba K. (2006). Substrate specificity of carboxylesterase isozymes and their contribution to hydrolase activity in human liver and small intestine. Drug Metab. Dispos. 34, 1734–1741. [DOI] [PubMed] [Google Scholar]
- Jarema K. A., Hunter D. L., Shaffer R. M., Behl M., Padilla S. (2015). Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol. 52, 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laizure S. C., Herring V., Hu Z., Witbrodt K., Parker R. B. (2013). The role of human carboxylesterases in drug metabolism: Have we overlooked their importance? Pharmacotherapy 33, 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.-Y., He K., Hites R. A., Salamova A. (2016). Hair and nails as noninvasive biomarkers of human exposure to brominated and organophosphate flame retardants. Environ. Sci. Technol. 50, 3065–3073. [DOI] [PubMed] [Google Scholar]
- Mabuchi M., Kano Y., Fukuyama T., Kondo T. (1999). Determination of imidapril and imidaprilat in human plasma by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 734, 145–153. [DOI] [PubMed] [Google Scholar]
- Maxwell D. M., Lieske C. N., Brecht K. M. (1994). Oxime-induced reactivation of carboxylesterase inhibited by organophosphorus compounds. Chem. Res. Toxicol. 7, 428–433. [DOI] [PubMed] [Google Scholar]
- McGee S. P., Konstantinov A., Stapleton H. M., Volz D. C. (2013). Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: Potential role of the aryl hydrocarbon receptor. Toxicol. Sci. 133, 144–156. [DOI] [PubMed] [Google Scholar]
- Mendelsohn E., Hagopian A., Hoffman K., Butt C. M., Lorenzo A., Congleton J., Webster T. F., Stapleton H. M. (2016). Nail polish as a source of exposure to triphenyl phosphate. Environ. Int. 86, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris P. J., Medina-Cleghorn D., Heslin A., King S. M., Orr J., Mulvihill M. M., Krauss R. M., Nomura D. K. (2014). Organophosphorus flame retardants inhibit specific liver carboxylesterases and cause serum hypertriglyceridemia. ACS Chem. Biol. 9, 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul H. B., Roberts S. C., Mabrey N., McCaffrey K. A., Gear R. B., Braun J., Belcher S. M., Stapleton H. M. (2013). Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: An exploratory assessment. J. Biochem. Mol. Toxicol. 27, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A., Hammel S., Konstantinov A., Stapleton H. M. (2017). Characterization of individual isopropylated and tert-butylated triarylphosphate (ITP & TBPP) isomers in several commercial flame retardant mixtures and house dust standard reference material SRM 2585. Environ. Sci. Technol. 51, 13443–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. L., Hammel S. C., Hoffman K., Lorenzo A. M., Chen A., Webster T. F., Stapleton H. M. (2018). Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ. Int. 116, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poma G., Glynn A., Malarvannan G., Covaci A., Darnerud P. O. (2017). Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations. Food Chem. Toxicol. 100, 1–7. [DOI] [PubMed] [Google Scholar]
- Ren F., Tang L., Cai Y., Yuan X., Huang W., Luo L., Zhou J., Zheng Y. (2015). Meta-analysis: The efficacy and safety of combined treatment with ARB and ACEI on diabetic nephropathy. Ren. Fail. 37, 548–561.. [DOI] [PubMed] [Google Scholar]
- Roberts S. C., Bianco A. C., Stapleton H. M. (2015). Disruption of type 2 iodothyronine deiodinase activity in cultured human glial cells by polybrominated diphenyl ethers. Chem. Res. Toxicol. 28, 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M. K., Borazjani A. (2007). Enzymatic activity of human carboxylesterases In Jessica Lawler (editor). Current Protocols in Toxicology, Chapter 4, Volume: 33, pp: 4.24.1-4.24.14. United Kingdom:John Wiley & Sons. [DOI] [PubMed] [Google Scholar]
- Ross M. K., Borazjani A., Edwards C. C., Potter P. M. (2006). Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases. Biochem. Pharmacol. 71, 657–669. [DOI] [PubMed] [Google Scholar]
- Schwer H., Langmann T., Daig R., Becker A., Aslanidis C., Schmitz G. (1997). Molecular cloning and characterization of a novel putative carboxylesterase, present in human intestine and liver. Biochem. Biophys. Res. Commun. 233, 117–120. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Klosterhaus S., Eagle S., Fuh J., Meeker J. D., Blum A., Webster T. F. (2009). Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol. 43, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundkvist A. M., Olofsson U., Haglund P. (2010). Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit. 12, 943–951. [DOI] [PubMed] [Google Scholar]
- Tajima S., Araki A., Kawai T., Tsuboi T., Ait Bamai Y., Yoshioka E., Kanazawa A., Cong S., Kishi R. (2014). Detection and intake assessment of organophosphate flame retardants in house dust in Japanese dwellings. Sci. Total Environ. 478, 190–199. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Katoh M., Saitoh T., Nakajima M., Yokoi T. (2009). Different inhibitory effects in rat and human carboxylesterases. Drug Metab. Dispos. 37, 956–961. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (2011). Exposure Factors Handbook: 2011 Edition. National Center for Environmental Assessment, Washington DC; EPA/600/R-09/052F.
- U.S. EPA ( 2016). EPA acts on new chemical law to fast-track five chemicals United States Environmental Protection Agency News Release from Headquarters. U.S. EPA, Washington, DC. [Google Scholar]
- Wadkins R. M., Hyatt J. L., Edwards C. C., Tsurkan L., Redinbo M. R., Wheelock C. E., Jones P. D., Hammock B. D., Potter P. M. (2007). Analysis of mammalian carboxylesterase inhibition by trifluoromethylketone-containing compounds. Mol. Pharmacol. 71, 713–723. [DOI] [PubMed] [Google Scholar]
- Wang D., Zou L., Jin Q., Hou J., Ge G., Yang L. (2018). Human carboxylesterases: A comprehensive review. Acta Pharm. Sin. B 8, 699–712.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Williams E. T., Bourgea J., Wong Y. N., Patten C. J. (2011). Characterization of recombinant human carboxylesterases: Fluorescein diacetate as a probe substrate for human carboxylesterase 2. Drug Metab. Dispos. 39, 1329–1333. [DOI] [PubMed] [Google Scholar]
- Wilford B. H., Harner T., Zhu J., Shoeib M., Jones K. C. (2004). Passive sampling survey of polybrominated diphenyl ether flame retardants in indoor and outdoor air in Ottawa, Canada: Implications for sources and exposure. Environ. Sci. Technol 38, 5312–5318. [DOI] [PubMed]
- Xie M., Yang D., Liu L., Xue B., Yan B. (2002). Human and rodent carboxylesterases: Immunorelatedness, overlapping substrate specificity, differential sensitivity to serine enzyme inhibitors, and tumor-related expression. Drug Metab. Dispos. 30, 541–547. [DOI] [PubMed] [Google Scholar]
- Xu F., Giovanoulis G., van Waes S., Padilla-Sanchez J. A., Papadopoulou E., Magnér J., Haug L. S., Neels H., Covaci A. (2016). Comprehensive study of human external exposure to organophosphate flame retardants via air, dust, and hand wipes: The importance of sampling and assessment strategy. Environ. Sci. Technol. 50, 7752–7760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.