Abstract
Stress-induced mitochondrial calcium (Ca2+) overload is a key cellular toxic effectors and a trigger of cardiomyocyte death during cardiac ischemic injury through the opening of mitochondrial permeability transition pore (mPTP). We previously found that the valosin-containing protein (VCP), an ATPase-associated protein, protects cardiomyocytes against stress-induced death and also inhibits mPTP opening in vitro. However, the underlying molecular mechanisms are not fully understood. Here, we tested our hypothesis that VCP acts as a novel regulator of mitochondrial Ca2+ uptake proteins and resists cardiac mitochondrial Ca2+ overload by modulating mitochondrial Ca2+ homeostasis. By using a cardiac-specific transgenic (TG) mouse model in which VCP is overexpressed by 3.5 folds in the heart compared to the wild type (WT) mouse, we found that, under the pathological extra-mitochondrial Ca2+ overload, Ca2+ entry into cardiac mitochondria was reduced in VCP TG mice compared to their little-matched WT mice, subsequently preventing mPTP opening and ATP depletion under the Ca2+ challenge. Mechanistically, overexpression of VCP in the heart resulted in post-translational protein degradation of the mitochondrial Ca2+ uptake protein 1, an activator of the mitochondria Ca2+ uniporter that is responsible for mitochondrial calcium uptake. Together, our results reveal a new regulatory role of VCP in cardiac mitochondrial Ca2+ homeostasis and unlock the potential mechanism by which VCP confers its cardioprotection.
Keywords: VCP, heart, calcium uptake, mPTP, MICU1
Acute myocardial infarction (AMI) continues to be one of the leading causes of morbidity, disability, and mortality worldwide. While the ischemic episode poses numerous challenges to the heart, recent developments have shown that reperfusion may also lead to cell damage (Hausenloy and Yellon, 2013). Pathological cardiac calcium overload in the mitochondria, which began during ischemia and further exacerbated during reperfusion, is found to be a key cellular toxic effector and a trigger of cardiomyocyte death through the opening of the mitochondrial permeability transition pore (mPTP) (Baines, 2009; Halestrap and Pasdois, 2009; Ong et al., 2015; Perez and Quintanilla, 2017). Thus, preventing excessive Ca2+ entering mitochondria serves as a promising target in pursuing protection against cardiomyocyte death caused by ischemia/reperfusion (I/R) damage.
One of the major conduits of Ca2+ uptake into the mitochondria is the mitochondria Ca2+ uniporter (MCU) (Santo-Domingo and Demaurex, 2010). However, the MCU does not function on its own but being controlled by MCU-associated regulators, among which mitochondrial Ca2+ uptake proteins (MICUs) have been raised most attentions; for example, increasing evidence indicates that MICU1 activates the MCU at high Ca2+ concentrations, while MICU2 acts as an inhibitor of the MCU at lower Ca2+ concentrations (Matesanz-Isabel et al., 2016). These findings highlight the possibility of modulating mitochondrial Ca2+-handling proteins to enhance cell resistance against Ca2+ overload during stress, and subsequently inhibit mPTP opening and cell death. Although several MCU-associated regulators have recently been identified (De Stefani et al., 2015), the underlying regulatory mechanisms of mitochondrial Ca2+ homeostasis in the heart remain largely unknown.
The valosin-containing protein (VCP), also known as Cdc48 in yeast and p97 plants, belongs to the type II AAA (ATPases-Associated with diverse cellular Activities) protein family, known for their involvement in key cellular activities (Asai et al., 2002; Dai et al., 1998; Egerton et al., 1992; Frohlich et al., 1991; Patel and Latterich, 1998; Pleasure et al., 1993; Rabinovich et al., 2002; Schulte et al., 1994). Increased expression of VCP is correlated to growth and viability of cancer cells, highlighting the importance of VCP for cell survival (Tsujimoto et al., 2004; Yamamoto et al., 2003, 2005). We previously demonstrated that overexpressing VCP confers protection on cardiomyocytes against stress-induced apoptosis in vitro (Lizano et al., 2013), as well as dramatically reduces infarct size of I/R in vivo in cardiac-specific VCP transgenic (TG) mice when compared to wild type (WT) controls (Lizano et al., 2017). We also found that VCP overexpression inhibits mPTP opening both in intact cardiomyocytes and in mitochondria isolated from heart tissues of VCP TG mice (Lizano et al., 2017). However, the underlying mechanisms have not yet been resolved.
Based on our previous studies and other’s findings, our present study hypothesized that VCP presents a novel regulator of mitochondrial Ca2+ uptake proteins and resists cardiac mitochondrial Ca2+ overload by modulating mitochondrial Ca2+ homeostasis. By using a cardiac-specific VCP TG mouse model which has shown cardiac protection against I/R injury, we revealed that VCP decreases Ca2+ entry cardiac mitochondria under the pathological Ca2+ overload, protects against ATP depletion, and modulates the expression of MCU regulators post-translationally.
MATERIALS AND METHODS
Animal model
A TG mouse (FVB) with cardiac-specific overexpression of VCP was generated and characterized as previous described (Zhou et al., 2017). At 2–4 months of age, male and female VCP TG mice were studied alongside litter-matched WT mice as controls. All animal procedures were performed in accordance with the NIH guidance (Guide for the Care and Use of Laboratory Animals, revised 2011) and the protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University.
Mitochondrial isolation from heart tissues
In order to compare our findings with our previous studies, mitochondria were isolated from mouse left ventricular tissue as previously described (Koentges et al., 2015; Lizano et al., 2017; Qiu et al., 2011). Briefly, left ventricular tissue was homogenized in a buffer (220 nmol/L mannitol 220, 70 nmol/L sucrose, 10 nmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 nmol/L ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), and pH 7.4 at 4°C) supplemented with 0.25% bovine serum albumin and protease inhibitors (cOmplete™, Roche), using a Potter-Elvehjem glass homogenizer. After an initial spin at 100 × g (5 min) to discard the cellular debris and unbroken cells, the nuclear fraction was pelleted at low-speed centrifugation (1000 × g, 5 min). The supernatant was further centrifuged (10 000 × g, 10 min) to pellet the mitochondrial fraction. The resulting supernatant was ultra-centrifuged (100 000 × g, 90 min) to obtain the cytosolic fraction (supernatant) and a microsomal fraction (pellet). The mitochondrial pellet was resuspended in storage buffer (220 nmol/L mannitol 220, 70 nmol/L sucrose, 10 nmol/L HEPES, pH 7.4 at 4°C). All other pellets were washed 3 times with 1× phosphate-buffered saline and resuspended in radioimmunoprecipitation assay buffer (RIPA buffer) [150 mM NaCl, 1% NP40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris (pH 8.0)] (Lizano et al., 2017). The purification of the isolated mitochondria from heart tissue was verified by Western blotting, using glyceraldehyde 3-phosphate dehydrogenase (GAPDH, cytosol), Lamin C (nucleus), and voltage-dependent anion channel (VDAC, mitochondria) as loading controls.
Mitochondrial calcium homeostasis assays under Ca2+ overloading
Isolated mitochondria (37.5 μg) were resuspended in buffer (120 mM KCl, 10 mM Tris-HCl, 5 mM 3-(N-morpholino)propanesulfonic acid (MOPS), 5 mM Na2HPO4, 10 mM glutamate, and 2 mM malate) in a total volume of 150 μl. The isolated mitochondria were then stimulated with higher doses of Ca2+ at 50 mM in the buffer in the absence or presence of cyclosporin A (CSA) and the Ca2+ retention capacity (CRC) assay was used to measure mPTP opening by using a membrane permeant Ca2+ indicator, Rhod-2 AM. Another membrane impermeant Ca2+ Green-5N (5 µM; Molecular Probes, Eugene, Oregon) was used to measure extra-mitochondrial Ca2+ when the isolated mitochondria were treated with a series of doses of Ca2+ pulses (from 2.5 μM to 175 μM) in the absence or presence of CSA (Nguyen et al., 2011). Uptake rates were defined as the change Ca-5N fluorescence in mitochondrial medium compared to the blank buffer which contained no mitochondria ((Fb − F)/Fb), where F refers to fluorescence and b refers to blank control.
ATP production
Mitochondrial ATP production was measured using the Molecular Probes’ ATP determination kit (A22066) which uses a convenient bioluminescence assay for quantitative determination of ATP with recombinant firefly luciferase and its substrate D-luciferin. A reaction solution was made according to manufacturer’s instructions. Mitochondria were isolated as described previously and energized in the prepared reaction solution with 10 mM glutamate/5 mM malate. A standard curve was established with known concentrations of ATP (0 µM–500 µM), then the reaction was started in the mitochondrial preparations by the addition of adenosine diphosphate (ADP). The fluorescence was measured with a fluorimeter (i3max, Molecular Devices).
RNA extraction and real-time PCR
RNA was extracted from isolated left ventricular tissue by using the Quick-RNA MiniPrep kit (Genesee Scientific, San Diego, California) according to the manufacturer’s instruction. RNA concentration was determined through photometric measurement on the Nanodrop 2000 (Peqlab, Erlangen, Germany). Quantitative real-time PCR was performed on a CFX96 Touch™ Real-Time PCR Detection System by using iTaq™ Universal SYBR® Green Supermix (Bio-Rad, Hercules, California) according to the manufacturer’s manual. All real-time PCR experiments were performed in triplicate (Zhou et al., 2017).
Protein extraction and subcellular fraction
Following collection and homogenization of left ventricular tissue, total protein was extracted using Tissue Extraction Reagent I (Cat No. FNN0071, Life Technologies Corporation, Grand Island, New York). Subcellular fractionation was achieved by differential centrifugation. After an initial spin at 100 × g (5 min) to discard the cellular debris and unbroken tissue, the nuclear fraction was pelleted at low-speed centrifugation (500 × g, 10 min). The supernatant was further centrifuged (10 000 × g, 10 min) to pellet the mitochondrial fraction. The resulting supernatant was ultra-centrifuged (100 000 × g, 90 min) to obtain the cytosolic fraction (supernatant) and a microsomal fraction (pellet). All pellets were washed 3 times with 1× phosphate-buffered saline and resuspended in RIPA buffer (150 mM NaCl, 1% NP40, 0.5% deoxycholate, 0.1% SDS, and 50 mM Tris (pH 8.0)) (Lizano et al., 2017).
Co-immunoprecipitation (Co-IP) and Western blot
Total protein was extracted then measured by Western blotting and detected using a LI-COR Odyssey® Infrared Imaging System (Lincoln, Nebraska) as previously described (Rashed et al., 2015; Zhou et al., 2017). IP was performed by using the Dynabeads protein G kit (Life Technologies Corporation). Briefly, the primary antibody (Ab) was resuspended with total protein (Ag) and incubated overnight at 4°. The dynabeads were then incubated with the samples overnight to form the Dynabeads-Ab-Ag complex. The tubes were then placed on the magnets and the supernatant was removed. The Dynabeads-Ab-Ag complexes were washed three times using washing buffer (Life Technologies Corporation). Finally, the dynabeads-Ab-Ag complexes were resuspended in elution buffer (Life Technologies Corporation) and 20 μL of eluate was added to the loading dye for Western blot experiments. Western blots were quantitated using the ImageJ software version 1.51, available April, 2018.
Proteasome activity
The chymotrypsin-like activity of the proteasome was measured with the fluorogenic substrate Suc-LLVY-AMC (Boston Biochem, Cambridge, Massachusetts) and fluorescence detected with a Turner TD-700 fluorometer (Turner Designs, Sunnyvale, California).
Cell culture, transfection, and treatments
H9C2 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen), supplemented with 10% fetal bovine serum (FBS) (Invitrogen). We generated an adenovirus harboring a hairpin targeting the VCP sequence for silencing VCP (Si-VCP-Ad). VCP knockdown was performed by transfecting Si-VCP-Ad in H9C2 cell for 48 h with a standard procedure as previously described (Qiu et al., 2011) and a non-relevant short hairpin against luciferase was used for control experiments, as before (Qiu et al., 2011). These cells were further treated with cycloheximide (CHX, 100 μg/ml), which blocks mRNA translation, for different time periods as indicated, with either the absence or the presence of 10 μM MG132 for 2 h before cell lysis. Dimethylsulfoxide (DMSO) was used as a vehicle control for the drug treatment. Cells harvested at the starting point have been considered for the basal level of the protein.
Statistical analysis
Results are presented as the mean ± SEM for the number of samples indicated in the figure legends, where n = 5 means that 5 mice were used per group. One-way ANOVA or two-way ANOVA was used to test for significance between groups. Student-Newman-Keuls post hoc correction was applied for multiple pairwise comparisons. A value of p < .05 was considered statistically significant.
RESULTS
Overexpression of VCP in the Mouse Heart Prevents Calcium Overload-Induced mPTP Opening
We previously showed that overexpression of VCP in the heart reduced mPTP opening in isolated mitochondria by using an indirect measurement based on the mitochondrial morphological changes under Ca2+ stimulation (Lizano et al., 2017). To further reveal the effects of VCP on mitochondrial Ca2+ dynamics during Ca2+ stress, here we measured the Ca2+ levels retained inside the mitochondria upon stimulating mPTP opening using a membrane permeant Ca2+ indicator.
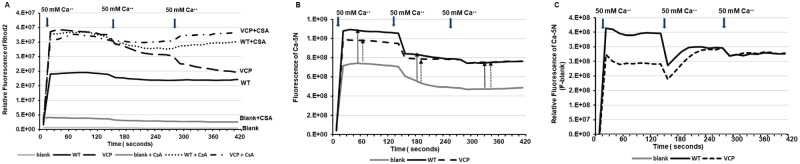
To maximally induce mPTP opening, mitochondria isolated from both VCP TG and their litter-matched WT mice were exposed to a high concentration of CaCl2 at 50 mM to exaggerate extramitochondrial Ca2+ overload. We then examined the intramitochondrial Ca2+ levels by using a known membrane permeant dye, Rhod-2 AM. As shown in Figure 1A, despite adding Ca2+, no Rhod-2 AM fluorescence was detected in our blank control where mitochondria were absent, confirming the specificity of Rhod-2 AM in measuring intramitochondrial Ca2+. We found that, under exposure to 50 mM Ca2+, mitochondria from VCP TG mice exhibited higher Ca2+ retention in the mitochondria compared with their litter-matched WTs, reflected by a higher fluorescence of Rhod-2 AM in the mitochondria from VCP TG mice compared with WTs (Figure 1A). We further determined whether the mPTP were fully opened in the mitochondrial of WT mice by repeating the stimulation with two additions of CaCl2 at 50 mM. There were no more changes in Rhod-2 AM fluorescence in WT mice upon the additional Ca2+ stimulation, while the Rhod-2 AM fluorescence in VCP TG mice showed a delayed decrease with these additional stimulation, indicating that the mitochondria isolated from VCP TG mice exhibited higher tolerance to high Ca2+ challenge.
Figure 1.
Valosin-containing protein (VCP) inhibits mitochondrial permeability transition pore (mPTP) under extensive calcium-overloading. A, The relative change of the fluorescence level of Rhod2 (indicator of Ca2+ inside mitochondria) in samples during the additions of 50 mM Ca2+ in the mitochondria isolated from wild type (WT) and VCP transgenic (TG) mice, compared to the blank buffer without mitochondria, upon the absence or presence of mPTP inhibitor [cyclosporin A, (CSA)]. Blank is the control buffer with the same amount of Ca2+ loading as the measured samples but without mitochondria. B, The increase of fluorescence of Ca-green-5N (indicator of Ca2+ outside mitochondria) in the samples compared to the blank control. C, The relative fluorescence of Ca-green-5N of the mitochondrial samples (F) from the blank buffer (Blank).
In addition, pharmacological blockage of mPTP opening using CSA, a known inhibitor of mPTP (Halestrap and Pasdois, 2009; Nguyen et al., 2011), resulted in similar levels of mitochondrial calcium retention between WT and VCP, indicating inhibiting effects of CSA on mPTP opening in both groups (Figure 1A). Importantly, the effect of CSA on WT mitochondria was similar to the results obtained from VCP TG mitochondria without CSA at the first 50 mM Ca2+, highlighting that overexpression of VCP in the heart inhibits Ca2+ overloading stimulated by mPTP opening. Furthermore, CSA treatments showed a further inhibition on VCP TG mice at the two additional prolonged stimulations (Figure 1A). These data may also suggest that the mechanism whereby VCP confers its effects was different to those induced by CSA.
We also used Ca2+ Green™-5N (Ca-5N), a known membrane-impermeant probe, to detect the change of extra-mitochondrial Ca2. Upon the addition of 50 mM Ca2+, there was a dramatic increase in the Ca-5N fluorescence in the mitochondria from both VCP TG and WT mice compared with the blank buffer control (ie buffer containing no mitochondria), indicating that there was Ca2+ efflux from the mitochondria into the buffer upon the high Ca2+ stimulation (Figs. 1B and 1C). Notably, this increase was greater in WT compared to VCP TG mice during the first Ca2+ stimulation (Figs. 1B and 1C). These data are consistent with less Ca2+ inside the mitochondria in WT mice and further confirmed the less mPTP opening in VCP TG versus WT mice.
VCP Resists Ca2+ Uptake into Mitochondria Under the Ca2+ Overloading
Next, we investigated the potential mechanism by which VCP inhibits mPTP opening. Considering that Ca2+ overload due to excessive Ca2+ entry into mitochondria is a primary factor triggering mPTP opening, we investigated whether there was a difference in Ca2+ uptake between the groups before the fully opening of mPTP. We exposed mitochondria isolated from VCP TG and WT mice to a lower concentration of Ca2+ challenge and then measured the changes of Ca2+ in the buffer inside and outside the mitochondria.
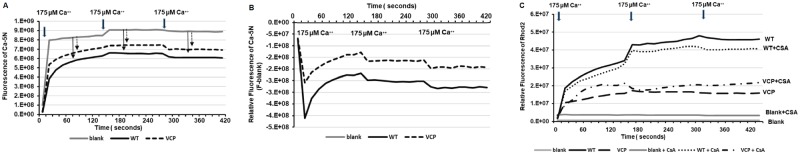
We first investigated the mitochondrial Ca2+ uptake with the addition of 175 μM Ca2+ boluses. Under the stimulation of this concentration, extra-mitochondrial Ca2+ fluorescence (by Ca-5N) at both WT and VCP TG mice were less than blank buffer, indicating no additional Ca2+ out from the mitochondria into the buffer upon this Ca2+ stimulation (Figure 2A). Mitochondria from VCP TG mouse hearts showed higher extra-mitochondrial Ca2+ fluorescence compared with WT (Figure 2A). After the first bolus, there was a dramatic reduction of Ca-5N fluorescence in both groups, indicating an influx of Ca2+ into mitochondria. Compared with WT, mitochondria from VCP TG mouse hearts showed less reduction of extra-mitochondrial Ca2+ fluorescence (Figure 2B), indicating less mitochondrial Ca2+ entry. This difference between the two groups remained similar after two additional 175 μM Ca2+ boluses (Figure 2B).
Figure 2.
Valosin-containing protein (VCP) resists mitochondrial Ca2+ overload via inhibiting Ca2+ uptake. The changes of fluorescence of Ca-green-5N (indicator of Ca2+ outside mitochondria) in the samples compared to the blank control, during the additions of 175 µM Ca2+ in the mitochondria isolated from wild type (WT) and VCP transgenic (TG) mice. B, The decrease of fluorescence of Ca-green-5N of the mitochondrial samples (F) from the blank buffer (Blank). C, The relative change of the fluorescence level of Rhod2 (indicator of Ca2+ inside mitochondria) in samples during the additions of 175 µM Ca2+ in the mitochondria isolated from wild type (WT) and VCP transgenic (TG) mice, compared to the blank buffer without mitochondria, upon the absence or presence of mPTP inhibitor [cyclosporin A, (CSA)].
We then used Rhod-2 AM to measure the intramitochondrial Ca2+ levels. As showed in Figure 2C, mitochondria from VCP TG mouse hearts showed lower mitochondrial Ca2+ fluorescence compared with WT (Figure 2C), indicating less Ca2+ uptake in the mitochondria isolated from VCP TG mice compared with WT mice. Different from the observation in the high Ca2+ stimulation in which the mPTP was opened, at this concentration, CSA treatment did not change the intramitochondrial Ca2+ fluorescence in both groups (Figure 2C), suggesting that lower concentration of Ca2+ inside the mitochondria of VCP TG mice was not due to more mPTP opening, but rather due to the less Ca2+ moving into mitochondria. These data indicated that VCP TG resisted Ca2+ uptake into mitochondria under the Ca2+ overload before the mPTP opening. Together, our data showed a dual effect of VCP on mitochondrial calcium homeostasis under the pathological Ca2+ stimulation, eg, preventing mitochondrial Ca2+ uptake and inhibiting the mPTP opening.
VCP Regulates Mitochondrial Ca2+ Uptake Based on a Range of Ca2+ Challenge
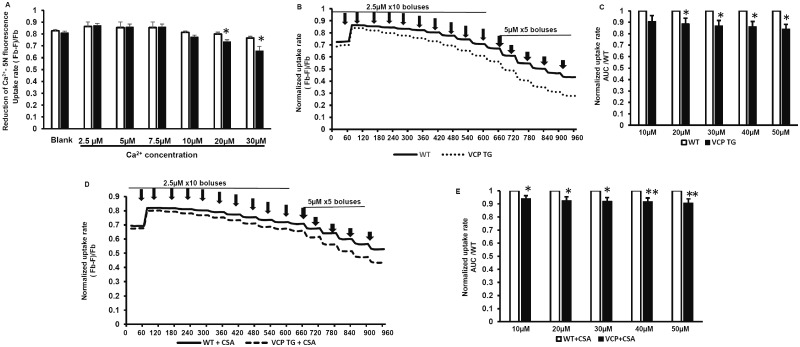
Considering the different effects of VCP on mitochondrial calcium homeostasis under the different Ca2+ concentrations, we then explored the sensitivity and extent of VCP on resistance to Ca2+ loading using a series of doses of Ca2+ overload in the isolated cardiac mitochondria. As showed in Figure 3A, there was no difference in Ca2+ uptake between VCP TG and WT mice at low concentrations of Ca2+ (at 2.5 µM, 5 µM, 7.5 µM, and 10 µM). However, significant inhibition of Ca2+ uptake rates were found in mitochondria isolated from VCP TG mice compared with WT mice when the concentration of Ca2+ was over 20 µM, reflected by decreased reduction in Ca2+-5N fluorescence outside of the mitochondria (Figure 3A).
Figure 3.
Valosin-containing protein regulates Ca2+ uptake into mitochondria varying with a range of Ca2+ challenge. A, The relative changes of fluorescence of Ca2+-5N at different doses of Ca2+ compared to reading at corresponding blank control. B, The normalized mitochondrial uptake rate, as measured by the relative changes in fluorescence of Ca2+-5N compared to blank control under chronic increase of extra-mitochondrial Ca2+. C, The quantitative average of the area under curves (AUC) of panel B. n = 5/group. *p < .05, **p < .01 versus corresponding wild type (WT). D, The normalized mitochondrial uptake rate in each group under chronic increase of extra-mitochondrial Ca2+ in the presence of cyclosporin A (CSA). E, The quantitative average of the AUC of panel D. n = 4–5/group. *p < .05, **p < .01 versus corresponding WT. Abbreviations: F, fluorescence detected in each sample under each bolus of CaCl2; Fb, fluorescence of blank buffer without mitochondria; TG, transgenic, VCP, valosin-containing protein.
To further explore the sensitivity of VCP on chronic Ca2+ overload, we exposed the isolated mitochondria to Ca2+ stimulation using cumulative Ca2+ boluses (2.5 μM for 10 times and 5 µM for 5 times (Figure 3B). Mitochondria isolated from VCP TG mouse hearts showed a trend of less mitochondrial uptake than WT mitochondria under this lower Ca2+ loading (Figure 3B). Although there is no significant difference in the Ca-5N fluorescence at each bolus of 2.5 μM Ca2+ between VCP TG and WT samples, the normalized quantitative Ca2+ uptake rate during these repeated additions, as reflected by the area under the curve (AUC), was significantly lower in VCP TG when the cumulative Ca2+ was 20 μM or higher (Figure 3D). These data indicate that the mitochondria from VCP TG mice exhibited a progressive resistance to Ca2+ overload by reducing the excessive Ca2+ entry into mitochondria under Ca2+ challenge. In addition, these differences in Ca2+ uptake rates between VCP TG and WT mice were not eliminated by the CSA treatment, supporting that the differences in Ca2+ fluorescence does not result from changes in mPTP opening, but may be due to the difference of mitochondrial Ca2+ uptake between VCP TG versus WT (Figs. 3D and 3E).
VCP Attenuated Ca2+ Overload-Induced Mitochondrial Impairment of ATP Production
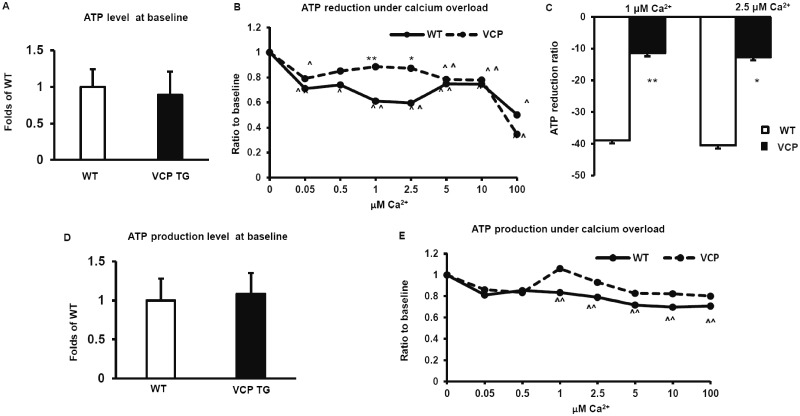
We next tested whether VCP overexpression altered ATP consumption in isolated mitochondria under Ca2+ overload. We used a commercially available luciferase/luciferin kit which utilizes luciferin and ATP produced by the mitochondria to emit light, which can then be read in a luminometer. As shown in Figure 4A, there was no significant difference in ATP levels at baseline with no Ca2+ stimulation, suggesting that basal ATP consumption in WT and VCP mitochondria are similar. We then subjected the mitochondria to various concentrations of Ca2+ (ranging from 0.05 µM to 100 µM), to determine whether ATP consumption changed during Ca2+ uptake. When exposed to Ca2+, ATP levels were significantly decreased in WT cardiac mitochondria. However, this loss of ATP was lower in VCP cardiac mitochondria compared with WT controls (Figure 4B). Significantly less reduction in ATP levels was found in VCP TG samples versus WT samples when mitochondria are placed in 1 µM and 2.5 µM Ca2+ (Figure 4C), although there was no difference at the higher concentrations of Ca2+.
Figure 4.
Valosin-containing protein (VCP) attenuates Ca2+ overload-induced mitochondrial impairment of ATP production. A, The relative ATP levels at baseline without Ca2+ overload in cardiac mitochondria from wild type (WT) and VCP transgenic (TG) mice. p > .05 versus WT. n = 5–6/group. B, The dose response of ATP levels upon extra-mitochondrial Ca2+ stimulation in the absence of ADP. n = 5–6/group. ^p < .05, ^^p < .01 versus baseline without calcium loading. C, The quantitative average of reduction of ATP levels in mitochondria from VCP TG versus WT under 1 μM and 2.5 μM Ca2+ stimulation. *p < .05, **p < .01 versus WT. D, ATP production upon the addition of ADP with the absence of calcium. E, The dose response of ATP production to extra-mitochondrial calcium overload upon the addition of ADP. ^^p < .01 versus baseline without calcium loading. N = 6/group and each sample with triplication.
We also measured the impairment of ATP production under Ca2+ overloading stimulations. When ADP was added to the buffer, mitochondria were pushed towards producing ATP and the resulting luminescence was read. We found that there were no significant differences in the ATP produced by isolated mitochondria from WT and VCP mouse hearts in the absence of Ca2+ (Figure 4D). At Ca2+ concentrations above 1 µM, ATP production in mitochondria isolated from WT mouse hearts was impaired, compared with baseline. However, this impairment was not observed in VCP TG mitochondria, indicating that the ability to produce ATP was preserved in mitochondria of VCP TG mice under Ca2+ overload (Figure 4E).
VCP Regulates Mitochondrial Ca2+ Uptake Proteins in the Mouse Hearts
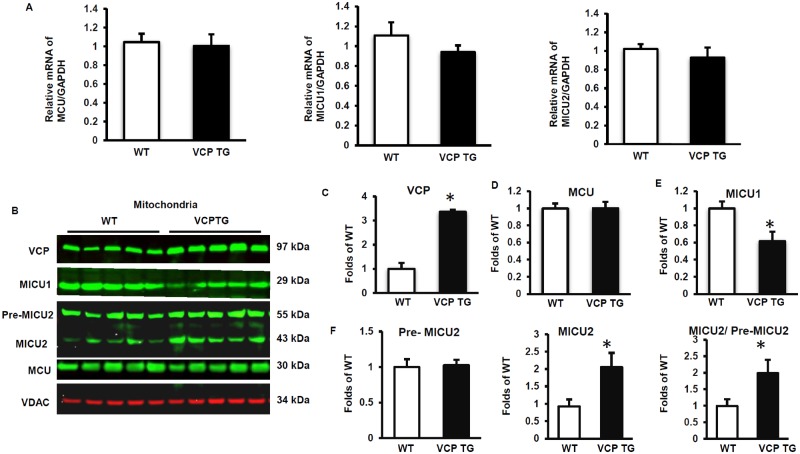
We then determined the molecular mechanisms that were responsible for these alterations of Ca2+ handling in the mitochondria in VCP TG mouse hearts. The expression of the mitochondrial Ca2+ uniporter (MCU) and its regulators, MICU1 and MICU2, were measured at mRNA levels by qPCR, using freshly extracted heart tissues of both VCP TG and their litter-matched WT mice. As shown in Figure 5A, there were no significant differences in the mRNA levels of MCU, MICU1, and MICU2 between VCP TG and WT mouse hearts, indicating that VCP does not affect the transcription of these genes. We then measured the effect of VCP on these Ca2+ handling molecules at the protein level. Mitochondrial fractions of fresh heart tissues were obtained, and as seen in Figures 5B and 5C, overexpression of VCP leads to a ∼3.5 fold increase of VCP protein in the mitochondrial fraction from VCP TG mouse hearts versus WT mice. There was no significant difference between VCP TG and WT mice in the protein expression of MCU in the mitochondrial fraction of the hearts (Figs. 5B and 5D). However, we found that the protein level of MICU1, the activator of MCU, was significantly reduced in the mitochondrial fraction of VCP TG mouse heart compared with WT (Figs. 5B and 5E). In addition, we detected two predominant bands of MICU2 in the cardiac mitochondrial fraction, one at about 50 kDa corresponding to the unprocessed precursor (pre-MICU2, upper band), and another faster migrating band at 42 kDa corresponding to the fully processed mature or active form of MICU2 (active MICU2, lower band) (Figure 5B). Compared with WT, VCP TG mice exhibited a significant increase in the mature or active isoform of MICU2 in the mitochondrial fraction while there was no significant difference in the pre-MICU2 expression between the two groups (Figure 5F), resulting in an increase in the ratio of active MICU2/pre-MICU2 (Figure 5F).
Figure 5.
Valosin-containing protein (VCP) regulates mitochondria Ca2+ uniporter (MCU) complex in the mouse heart tissue. A, qPCR data show mRNA levels of MCU, MICU1, and MICU2, indicating that overexpression of VCP does not affect transcription of these genes. B–F, Western blot showing protein expression of VCP (C), MCU (D), MICU1 (E), and MICU2 (F) in mitochondrial fractions of heart tissues. *p < .05 versus wild type (WT). N = 5/group. Abbreviations: GADPH, glyceraldehyde 3-phosphate dehydrogenase; TG, transgenic; VDAC, voltage-dependent anion channel;
VCP Decreases MICU1 by Post-Translational Degradation via Proteasome
Since it has been shown that MICU1 activates the MCU at high Ca2+ concentrations while MICU2 inhibits the MCU at lower Ca2+ concentrations (Matesanz-Isabel et al., 2016), we focused our attention on investigating the mechanism of the reduction of mitochondrial MICU1 mediated by VCP as most of our data were obtained at concentrations beyond those regulated by MICU2.
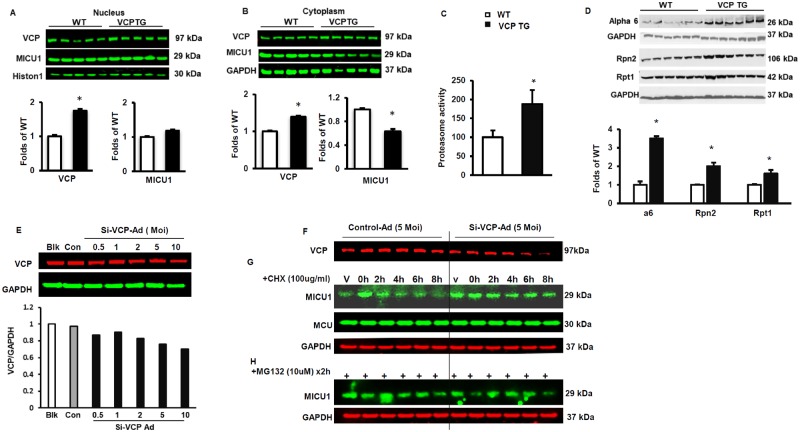
Giving the finding that the mRNA of MICU1 are similar between VCP TG and WT mice, we first determined whether the decrease in MICU1 protein in the mitochondria of VCP TG mouse hearts is due to the translocation of MICU1 to other subcellular fractions. Our results showed that, while there was no significant difference in the nuclear fraction between the groups (Figure 6A), MICU1 was also significantly decreased in the cytoplasmic fraction of VCP TG mouse hearts compared with WT (Figure 6B). Together, these data suggest that the decrease in MICU1 expression was not due to the downregulation of synthesis or subcellular translocation but may be due to increased degradation of this protein.
Figure 6.
Valosin-containing protein (VCP) increases MICU1 degradation via proteasome. A and B, Subcellular distribution of MICU1 in the mouse heart tissue. Western blot showing protein expression of VCP and MICU1 in nuclear fractions (A) and cytoplasmic fractions (B) *p < .05 versus wild type (WT). N = 5/group. C, Overexpression of VCP increases proteasome activity in heart tissues of VCP transgenic (TG) mice versus WT mice. N = 4/group with triplication in each samples *p < .05 versus WT. D, Western blot showing protein expression of key components of the Proteasome: a6, Rpn2, and Rpt 1. N = 6/group. *p < .05 versus WT. E, Western blotting showing a dose response of the reduction of VCP in H9ce cell upon the knock down of VCP by a Si-VCP adenovirus versus control. F, VCP protein levels in H9C2 cells upon the addition of 5 MOI Si-VCP Ad. G, A cycloheximide (CHX)-chase assay showing half-life of MICU1 and MCU in H9C2 cells upon 5 MOI Si-VCP Ad versus controls. H, Representative Western blots showing MICU1 levels with the treatment of proteasome inhibitor (MG132) in CHX-treated cells for 2 h. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control of the total proteins from the cells.
To explore this potential mechanism, we measured the chymotrypsin-like activity of the proteasome, and found proteasome activity was significantly increased in VCP TG samples versus WT mice (Figure 6C). In addition, we measured the abundance of representative components of the proteasome by Western blotting, namely Rpt1 and Rpn2 proteins for the 19S subunit, and α6 proteins for the 20S subunit, and found that these proteasome components were significantly increased in the VCP TG samples compared with WT (Figure 6D).
We further investigated whether VCP was necessary for the degradation of MICU1 by the proteasome by using an alternative cardiomyocyte model. To test this aim, we knocked down VCP in H9C2 cells with a Si-VCP Adenovirus (Si-VCP Ad). As showed in Figure 6E, addition of this adenovirus to H9c2 cells dose-dependently reduced VCP abundance. Since our preliminary results showed that more than 30% decrease of VCP dramatically increased cell death, we used the dose of adenovirus (5MOI) which resulted in 25% decrease of VCP level to treat H9c2 cells and compared it with its control (luciferase) (Figure 6F). A CHX-chase assay was performed in these H9C2 cells to determine the half-life of the MICU1. The Western blotting showed that, while the MCU protein remained unchanged, the treatment of CHX resulted in a rapid degradation of MICU1 through the time course, intracellular levels of MICU1 drastically dropped by more than 50% within 2 h of treatment (Figure 6G). However, knockdown of VCP dramatically attenuated the degradation of MICU1, which was comparable to the effect of the proteasome inhibitor MG132 in CHX-treated cells (Figure 6H). These results indicate that MICU1 was rapidly and selectively degraded by proteasome in a VCP-dependent manner.
VCP Selectively Facilitates the Degradation of the Ubiquitinated MICU1
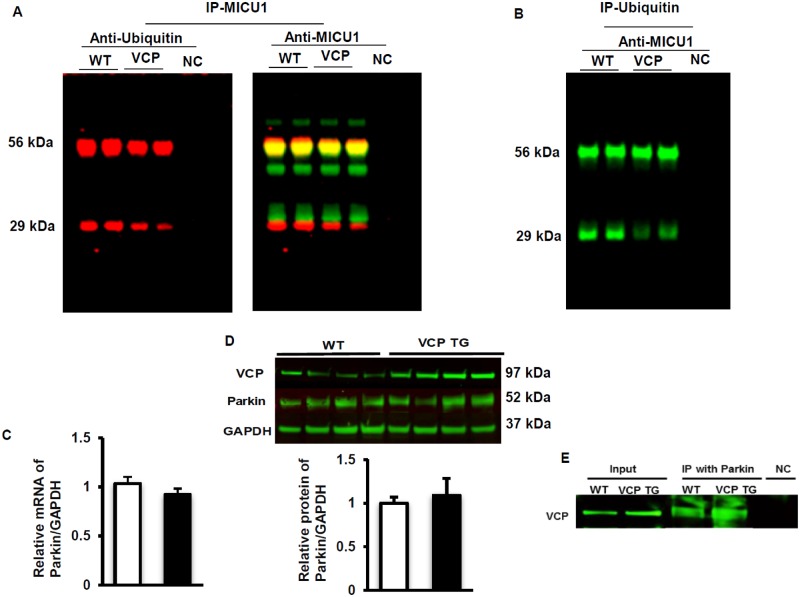
Considering the results described above, we further investigated the mechanisms by which VCP specifically modulates MICU1 degradation. Co-IP was performed with the total lysate of heart tissues from both VCP TG and WT mice by using specific anti-MICU1 antibody, and the resulting Western blot was probed with an anti-ubiquitin antibody to detect the ubiquitinated MICU1. The membrane was also probed with an anti-MICU1 antibody as control. As showed in Figure 7A, the ubiquitinated MICU1proteins was remarkably decreased in hearts of VCP TG mice versus WT, which was likely due to the rapid degradation by the increased proteasome activity in VCP TG mouse heart. In another direction, ubiquitin was also immunoprecipitated from whole cell lysate and was probed with an anti-MCU1 antibody to detect the co-immunoprecipitated MICU1. Our results showed a similar reduction in the ubiquinated MICU1 (Figure 7B).
Figure 7.
Valosin-containing protein (VCP) selectively mediates ubiquitinated MICU1 degradation. A, Co-immunoprecipitation (co-IP) of the total lysate of heart tissues from both VCP transgenic (TG) and wild type (WT) mice by using specific anti-MICU1 antibody, and probed with an anti-ubiquitin antibody and anti-MICU1 antibody. B, Co-immunoprecipitation (co-IP) of the total lysate of heart tissues from both VCP TG and WT mice using specific anti-ubiquitin antibody, and probed with an anti-MICU1 antibody. C, mRNA of Parkin in mouse heart tissue measured by qPCR. D, Protein expression of Parkin measured by Western blotting. E, Western blotting showing the protein interaction between VCP and Parkin after IP with Parkin antibody. Abbreviations: GADPH, glyceraldehyde 3-phosphate dehydrogenase; NC, negative control.
In addition, we measured VCP’s effect on Parkin, a known specific regulator of MICU1 degradation (Matteucci et al., 2018). There was no difference in Parkin expression at both mRNA and protein levels between VCP TG and WT mice (Figs. 7C and 7D), indicating that overexpression of VCP does not affect the expression of Parkin. We then tested whether there is any protein-protein interactions among VCP and Parkin. We found that, although there is no direct interaction between VCP and MICU1, Parkin interacted with VCP (Figure 7E). These data together, indicate that VCP may mediate degradation of ubiquitinated MICU1 via interaction with Parkin.
DISCUSSION
Increasing evidence demonstrates that excessive mPTP opening plays a central role in mediating both the necrotic and apoptotic components of I/R injury, particularly at the onset of reperfusion (Garcia-Dorado et al., 2009; Heusch et al., 2010). The present study provides direct evidences highlighting that VCP protects mouse cardiac mitochondria against Ca2+ overload-induced mPTP opening by measuring the calcium retained within the mitochondria, which further supported our previous studies in vitro in cardiomyocytes and confirmed our previous measurements of the swelling assay, which depends on the morphological change of mitochondria to indirectly reflect mPTP opening. In addition, the results from the present study further revealed a new role of VCP on resisting mitochondrial Ca2+ overload through preventing excessive Ca2+ entry into mitochondria. Furthermore, our study also established a molecular mechanistic link between the VCP and the regulation of Ca2+ uptake proteins. Together, our findings in this study not only revealed a novel regulator of Ca2+ homeostasis in the cardiac mitochondria against Ca2+ overload but also offered a potential explanation for the mechanism for the VCP-mediated inhibition of mPTP opening and cardiac protection against cell death that we previously observed in isolated cardiomyocytes and in the VCP TG mouse (Lizano et al., 2013, 2017).
Although calcium (Ca2+) is critical to the cardiac mitochondrial function under physiological conditions, excessive mitochondrial Ca2+ induced by stress has been founded to be toxic (Konstantinidis et al., 2012; Santulli et al., 2015). It has been shown that excessive mitochondrial Ca2+ entry is a primary factor triggering mPTP opening during IR, resulting in the loss of the ability of mitochondria to generate ATP (Wong et al., 2012), eventually leading to I/R-induced cell death (Finkel et al., 2015). Therefore, resisting excessive Ca2+ entering mitochondria is essential for cellular adaptation in response to cardiac stress, such as the conditions of AMI or I/R injury. To determine the mechanisms by which VCP inhibits mPTP opening, we then measured Ca2+ uptake into isolated mitochondria before the mPTP fully opened. We found that mitochondria from VCP TG mouse hearts exhibited a less mitochondrial Ca2+ and a much higher extra-mitochondrial Ca2+ levels compared with WT control. Since higher levels of extra-mitochondrial calcium can be due to both decreased mitochondrial Ca2+ influx and increased mitochondrial Ca2+ efflux, to resolve the potential contribution of mPTP opening to this increase in extra-mitochondrial Ca2+ levels, we used CSA to inhibit mPTP opening and found that CSA did not attenuate the difference in Ca2+ fluorescence in VCP mitochondria compared with WT under low Ca2+ overload (175 µM), suggesting that mPTP opening plays a negligible role in this increase of extra-mitochondrial Ca2+ and the change is due to lowered mitochondrial uptake. We also noticed that VCP has little effect on Ca2+ uptake at low Ca2+ stimulation (below 20 µM), but inhibits Ca2+ uptake when Ca2+ concentrations are 20 µM or higher. In addition to the mPTP opening, the mitochondrial Na+/Ca2+ exchanger may also play a role in Ca2+ efflux from mitochondria. However, this Ca2+ release is largely dependent on extra-mitochondrial Na+ or Li+ stimulation. Since our experimental buffer did not contain Na+ or Li+, it is unlikely that increase of extra-mitochondria Ca2+ is due to the additional Ca2+ efflux through the activation of mitochondrial Na+/Ca2+ exchanger. Therefore, our data indicate that cardiac-specific VCP overexpression resists Ca2+ entry into mitochondria, preventing mitochondrial Ca2+ overload under Ca2+ stress.
Since mitochondrial Ca2+ uptake may also affect ATP production in both physiological and pathological conditions (Tarasov et al., 2012), we further investigated whether VCP altered Ca2+-induced ATP consumption and generation in isolated mitochondria. Our results showed that under pathological Ca2+ stimulation, mitochondria from VCP TG hearts were protected against the reduction in ATP levels observed in WT mouse cardiac mitochondria, compared with no Ca2+. In addition, the ATP production was significantly impaired under Ca2+ overload in WT compared with mitochondria with no Ca2+, as is characteristic of mitochondria experiencing Ca2+ overload (Tarasov et al., 2012). However, this Ca2+ overload-induced impairment of ATP production was not observed in VCP TG mouse hearts. Instead, the ability to produce ATP was preserved in VCP TG mitochondria under the calcium challenge. The ability to resist ATP depletion and impaired production is vital to mechanisms that protect cardiomyocytes during I/R stress. It is also notable that despite the protective effects observed under Ca2+ overload, overexpression of VCP did not affect ATP production in the absence of Ca2+, indicating that VCP’s effect is stress-dependent.
Our previous studies have shown that upon VCP overexpression, there is a subsequent increase of VCP in the nucleus of cardiac cells where VCP plays important roles in regulating other gene expressions via signaling pathway involving AKT/NF-κβ/Stat3/iNOS (Lizano et al., 2013). We believed that the increased nuclear expression of VCP was also linked to the translocation of VCP to the mitochondria where it conferred its effects on mitochondrial function as we observed in our previous studies (Lizano et al., 2017) and in this study. There is a general consensus that mitochondrial Ca2+ uptake is mediated by the MCU complex (Santo-Domingo and Demaurex, 2010), a multi-subunit Ca2+ channel complex whose expression may vary depending on the cell type, subtype, and even Ca2+ stimulation (Markus et al., 2016). Although the precise molecular composition of the cardiac MCU complex is still being studied, a few proteins have been identified, particularly: the pore-forming protein MCU, its regulatory heterodimer composed by the stimulatory component MICU1, the inhibitory component, MICU2 (Matesanz-Isabel et al., 2016), and essential MCU regulator (EMRE), a single-pass membrane protein that links MCU and MICU1 together (Nemani et al., 2018). Since the MCU complex is a major mode of mitochondrial Ca2+ influx, inhibiting its activity poses as an attractive means to prevent Ca2+ overload, mPTP opening, and subsequent cardiomyocyte death (Kwong, 2017). Our results from this study demonstrated that VCP participates in the regulation of the MCU complex via a comprehensive mechanism. First, we noticed that there is no significant difference of MCU level at both mRNA and protein levels between the VCP TG and WT mice, indicating that the effect of VCP on the mitochondrial Ca2+ uptake was not due to the reduction of mitochondria number or the formation of MCU pores. Second, overexpression of VCP in the heart significantly decreases the protein expression of MICU1 in mitochondrial fraction compared with WT. Third, VCP TG mice exhibited a significant increase in the active MICU2 (42 kDa) with no difference at pre-MICU2 levels when compared with WT, and no difference at mRNA levels of MICU2 between the two groups. These data indicate that VCP may modify the MICU2 at the post-translational level in the heart without altering the transcription level.
Considering that MICU1 plays a major role on the regulation of MCU at high Ca2+ levels while MICU2 regulates MCU at the lower Ca2+ level (<7 µm) (Matesanz-Isabel et al., 2016), it is reasonable to assume that the VCP-mediated modulation of mitochondrial Ca2+ homeostasis observed in our experiments were more likely due to the changes in MICU1 since the Ca2+ levels was out of the range for triggering MICU2 function. As such, we further investigated the potential mechanisms by which VCP decreased MICU1 in the mitochondria. There are some possibilities that VCP may reduce MICU1 in the mitochondrial fraction, including the decrease of MICU1 synthase, intracellular redistribution and degradation of the proteins. Our data showed that there is no difference of MICU1 mRNA level between the two groups, no increase of MICU1 in other cellular fractions. Instead, MICU1 was significantly decreased in the cytoplasm while no significant difference was seen in the nuclear fraction. These data together suggested that the decrease of MICU1 in the mitochondrial fraction in VCP TG mouse hearts is not the result from the decrease of protein synthesis or from intracellular redistribution of the protein, but rather may result from increased degradation of MICU1. Our data further confirmed that overexpression of VCP dramatically increased both proteasome abundance and activity compared with WT. Although a universal mechanism underlying the regulation of proteasome expression remains elusive, recent studies have identified a transcription factor nuclear factor erythroid-derived 2-related factor 1 (NFE2L1, also known as Nrf1) was identified to be a pivotal regulator promoting the expression of all proteasome subunits in mammals. Interestingly, studies in other cells demonstrated that the activation of Nrf1 depends on VCP, by which VCP not only helps Nrf1 extracted from the endoplasmic reticulum (ER), but also contributes to Nrf1 proteolytic processing to release Nrf1’s active region and allow it to be translocated to the nucleus, where it becomes transcriptionally active (Motosugi and Murata, 2019; Radhakrishnan et al., 2010, 2014; Sha and Goldberg, 2014). In addition, our data showed that MICU1 is short-lived protein and is degraded in a VCP-dependent manner since impaired VCP remarkably delayed the degradation of MICU1. Since the ubiquitin-proteasome system (UPS) is the most important mechanism of proteolysis in the heart (Lilienbaum, 2013; Zheng and Wang, 2010), these data support that VCP acts as an integral component of the ubiquitin/proteasome pathway and play a role in the regulation of MCU complex function through the protein degradation via promoting UPS activity.
Our results indicate a selective degradation of MICU1 in VCP TG mice. While the underlying mechanisms need to be further explored, there are several possibilities for this effect of VCP: first, in addition to the increase of proteomic activity, VCP also facilitates the extraction of ubiquitinated proteins in various subcellular location and chaperons them to the proteasome, which is necessary for these proteins’ degradation (Xu et al., 2011). For example, it has been shown that Parkin, a 465-residue E3 ubiquitin ligase, was recruited to mitochondria and ubiquitinated proteins at the OMM. A recent study has demonstrated that Parkin directly participates in the selective regulation of MICU1 by acting as a scaffold for the proteasome-mediated degradation of MICU1 (Matteucci et al., 2018). Interestingly, VCP was also found to be recruited at mitochondria by parkin (Narendra et al., 2008; Pickrell and Youle, 2015). Our data in the VCP TG mouse model further showed that parkin interacted with both MICU1 and VCP, an interaction may result in the selective degradation of ubiquitinated MICU1 in mitochondria. Secondly, VCP can also remodel proteins in OMM which may contribute to its selective regulatory effect in protein quality control (Heidelberger et al., 2018). Thirdly, it was also shown that VCP can act as an alternate subunit of proteasomes by interacting with the 20S Peptidase to form an ATP-powered proteasome (Meyer and Weihl, 2014; Tran and Brodsky, 2014), which may provide an additional explanation to the increased proteasome activity in TG mice and may also result in a selective effect on the degradation on the proteins that were bound to it.
It has been known that the majority mitochondrial proteins imported from the nuclei and cytosol undergo a proteolytic cleavage by the mitochondrial processing peptidase (MPP), which is necessary for the maturation of the pre-proteins to their functional forms in mitochondria. Although MICU2 maturation in mitochondria was observed in a recent report (Matteucci et al., 2018) and in our present study, the mechanism underlying the processing is yet unclear. We noticed that there was no physical direct interaction between VCP and MICU2 proteins (data not shown), we speculate that VCP likely functions through binding other proteins (its cofactors) that can specifically bind and recognize the targeting signal of MICU2, resulting in a proteolytic cleavage on MICU2 by MPP, thus lead to its maturation in cardiac mitochondria. It might be also hypothetical that VCP-mediated decrease of MICU1 interrupts the formation of the MICU1/MICU2 heterodimers, which may stimulate the adaptive recruitment of MICU2 to mitochondria and lead to a secondary MICU2 maturation.
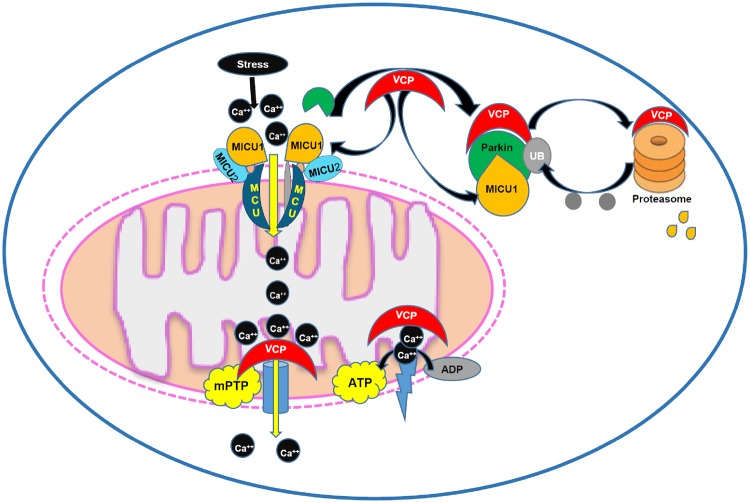
In summary, as also illustrated in the Figure 8, our results from this study revealed a new role of VCP on mitochondrial Ca2+ homeostasis through inhibiting Ca2+ uptake, preventing mitochondrial Ca2+ overload, and subsequently resisting Ca2+ challenge. Mechanistically, VCP regulates mitochondrial Ca2+-handling by decreasing the MCU activator, MICU1, through the increase of degradation of this protein. These results, together with our previous studies, indicate that VCP presents a novel regulator of Ca2+ homeostasis in mitochondria that leads to the prevention of mPTP opening and cardiac protection against I/R injury.
Figure 8.
Summary of the findings. The results from this study indicate a new role of valosin-containing protein (VCP) on mitochondrial Ca2+ homeostasis through inhibiting Ca2+ uptake, preventing mitochondrial permeability transition pore (mPTP), and subsequently resisting Ca2+ challenge. Mechanistically, VCP regulates mitochondrial Ca2+-handling by decreasing the MCU activator, MICU1, through the increase of degradation of this protein.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The National Institutes of Health (NIH) (HL115195-01 to H.Q., HL137962 to H.Q., and HL142291 H.Q. and G.Q.).
Author Contributions
H.Q., S.S., and G.Q. made substantial contributions to conceptual design, acquisition of data, analysis, and interpretation of data, and have been involved in drafting and revising the manuscript critically for important intellectual content. S.S. performed all the measurement of mitochondrial Ca2+ regulation and Western blotting and qPCR. J.X. performed IP and Western blotting as well as data analysis. B.M. performed in vitro experiments on cells and Co-IP on heart tissues. C.L. contributed to animal and experimental support, data analysis, and interpretation. E.J.B. contributed to Ca2+ uptake experiments, data analysis, and interpretation.
References
- Asai T., Tomita Y., Nakatsuka S., Hoshida Y., Myoui A., Yoshikawa H., Aozasa K. (2002). VCP (p97) regulates NFkappaB signaling pathway, which is important for metastasis of osteosarcoma cell line. Jpn. J. Cancer Res. 93, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines C. P. (2009). The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res. Cardiol. 104, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai R. M., Chen E., Longo D. L., Gorbea C. M., Li C. C. (1998). Involvement of valosin-containing protein, an ATPase Co-purified with IkappaBalpha and 26 S proteasome, in ubiquitin-proteasome-mediated degradation of IkappaBalpha. J. Biol. Chem. 273, 3562–3573. [DOI] [PubMed] [Google Scholar]
- De Stefani D., Patron M., Rizzuto R. (2015). Structure and function of the mitochondrial calcium uniporter complex. Biochim. Biophys. Acta 1853, 2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton M., Ashe O. R., Chen D., Druker B. J., Burgess W. H., Samelson L. E. (1992). VCP, the mammalian homolog of cdc48, is tyrosine phosphorylated in response to T cell antigen receptor activation. EMBO J. 11, 3533–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Menazza S., Holmström K. M., Parks R. J., Liu J., Sun J., Liu J., Pan X., Murphy E. (2015). The ins and outs of mitochondrial calcium. Circ. Res. 116, 1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich K. U., Fries H. W., Rudiger M., Erdmann R., Botstein D., Mecke D. (1991). Yeast cell cycle protein CDC48p shows full-length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation, and gene expression. J. Cell Biol. 114, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Dorado D., Ruiz-Meana M., Piper H. M. (2009). Lethal reperfusion injury in acute myocardial infarction: facts and unresolved issues. Cardiovasc. Res. 83, 165–168. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Pasdois P. (2009). The role of the mitochondrial permeability transition pore in heart disease. Biochim. Biophys. Acta 1787, 1402–1415. [DOI] [PubMed] [Google Scholar]
- Hausenloy D. J., Yellon D. M. (2013). Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger J. B., Voigt A., Borisova M. E., Petrosino G., Ruf S., Wagner S. A., Beli P. (2018). Proteomic profiling of VCP substrates links VCP to K6-linked ubiquitylation and c-Myc function. EMBO Rep. 19, e44754.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch G., Boengler K., Schulz R. (2010). Inhibition of mitochondrial permeability transition pore opening: the holy grail of cardioprotection. Basic Res. Cardiol. 105, 151–154. [DOI] [PubMed] [Google Scholar]
- Koentges C., Konig A., Pfeil K., Holscher M. E., Schnick T., Wende A. R., Schrepper A., Cimolai M. C., Kersting S., Hoffmann M. M., et al. (2015). Myocardial mitochondrial dysfunction in mice lacking adiponectin receptor 1. Basic Res. Cardiol. 110, 495.. [DOI] [PubMed] [Google Scholar]
- Konstantinidis K., Whelan R. S., Kitsis R. N. (2012). Mechanisms of cell death in heart disease. Arterioscler. Thromb. Vasc. Biol. 32, 1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong J. Q. (2017). The mitochondrial calcium uniporter in the heart: energetics and beyond. J. Physiol. 595, 3743–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienbaum A. (2013). Relationship between the proteasomal system and autophagy. Int. J. Biochem. Mol. Biol. 4, 1–26. [PMC free article] [PubMed] [Google Scholar]
- Lizano P., Rashed E., Kang H., Dai H., Sui X., Yan L., Qiu H., Depre C. (2013). The valosin-containing protein promotes cardiac survival through the inducible isoform of nitric oxide synthase. Cardiovasc. Res. 99, 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizano P., Rashed E., Stoll S., Zhou N., Wen H., Hays T. T., Qin G., Xie L. H., Depre C., Qiu H. (2017). The valosin-containing protein is a novel mediator of mitochondrial respiration and cell survival in the heart in vivo. Sci. Rep. 7, 46324.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus N. M., Hasel P., Qiu J., Bell K. F., Heron S., Kind P. C., Dando O., Simpson T. I., Hardingham G. E. (2016). Expression of mRNA encoding MCU and other mitochondrial calcium regulatory genes depends on cell type, neuronal subtype, and Ca2+ signaling. PLoS One 11, e0148164.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matesanz-Isabel J., Arias-del-Val J., Alvarez-Illera P., Fonteriz R. I., Montero M., Alvarez J. (2016). Functional roles of MICU1 and MICU2 in mitochondrial Ca(2+) uptake. Biochim. Biophys. Acta 1858, 1110–1117. [DOI] [PubMed] [Google Scholar]
- Matteucci A., Patron M., Reane D. V., Gastaldello S., Amoroso S., Rizzuto R., Brini M., Raffaello A., Cali T. (2018). Parkin-dependent regulation of the MCU complex component MICU1. Sci. Rep. 8, 14199.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Weihl C. C. (2014). The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J. Cell Sci. 127, 3877–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motosugi R., Murata S. (2019). Dynamic regulation of proteasome expression. Front. Mol. Biosci. 6, 30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani N., Shanmughapriya S., Madesh M. (2018). Molecular regulation of MCU: implications in physiology and disease. Cell Calcium 74, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T., Stevens M. V., Kohr M., Steenbergen C., Sack M. N., Murphy E. (2011). Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J. Biol. Chem. 286, 40184–40192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S. B., Samangouei P., Kalkhoran S. B., Hausenloy D. J. (2015). The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell Cardiol. 78, 23–34. [DOI] [PubMed] [Google Scholar]
- Patel S., Latterich M. (1998). The AAA team: related ATPases with diverse functions. Trends Cell Biol. 8, 65–71. [PubMed] [Google Scholar]
- Perez M. J., Quintanilla R. A. (2017). Development or disease: duality of the mitochondrial permeability transition pore. Dev. Biol. 426, 1–7. [DOI] [PubMed] [Google Scholar]
- Pickrell A. M., Youle R. J. (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure I. T., Black M. M., Keen J. H. (1993). Valosin-containing protein, VCP, is a ubiquitous clathrin-binding protein. Nature 365, 459–462. [DOI] [PubMed] [Google Scholar]
- Qiu H., Lizano P., Laure L., Sui X., Rashed E., Park J. Y., Hong C., Gao S., Holle E., Morin D., et al. (2011). H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of STAT3 and accelerates the transition into heart failure on cardiac overload. Circulation 124, 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich E., Kerem A., Frohlich K. U., Diamant N., Bar-Nun S. (2002). AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol. Cell Biol. 22, 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S. K., den Besten W., Deshaies R. J. (2014). p97-dependent retrotranslocation and proteolytic processing govern formation of active Nrf1 upon proteasome inhibition. eLife 3, e01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S. K., Lee C. S., Young P., Beskow A., Chan J. Y., Deshaies R. J. (2010). Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell 38, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed E., Lizano P., Dai H., Thomas A., Suzuki C. K., Depre C., Qiu H. (2015). Heat shock protein 22 (Hsp22) regulates oxidative phosphorylation upon its mitochondrial translocation with the inducible nitric oxide synthase in mammalian heart. PLoS One 10, e0119537.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo-Domingo J., Demaurex N. (2010). Calcium uptake mechanisms of mitochondria. Biochim. Biophys. Acta 1797, 907–912. [DOI] [PubMed] [Google Scholar]
- Santulli G., Xie W., Reiken S. R., Marks A. R. (2015). Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 112, 11389–11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte R. J., Campbell M. A., Fischer W. H., Sefton B. M. (1994). Tyrosine phosphorylation of VCP, the mammalian homologue of the Saccharomyces cerevisiae CDC48 protein, is unusually sensitive to stimulation by sodium vanadate and hydrogen peroxide. J. Immunol. 153, 5465–5472. [PubMed] [Google Scholar]
- Sha Z., Goldberg A. L. (2014). Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr. Biol. 24, 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov A. I., Griffiths E. J., Rutter G. A. (2012). Regulation of ATP production by mitochondrial Ca(2+). Cell Calcium 52, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran J. R., Brodsky J. L. (2014). The Cdc48-Vms1 complex maintains 26S proteasome architecture. Biochem. J. 458, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Tomita Y., Hoshida Y., Kono T., Oka T., Yamamoto S., Nonomura N., Okuyama A., Aozasa K. (2004). Elevated expression of valosin-containing protein (p97) is associated with poor prognosis of prostate cancer. Clin. Cancer Res. 10, 3007–3012. [DOI] [PubMed] [Google Scholar]
- Wong R., Steenbergen C., Murphy E. (2012). Mitochondrial permeability transition pore and calcium handling. Methods Mol. Biol. 810, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Peng G., Wang Y., Fang S., Karbowski M. (2011). The AAA-ATPase p97 is essential for outer mitochondrial membrane protein turnover. Mol. Biol. Cell 22, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Tomita Y., Nakamori S., Hoshida Y., Nagano H., Dono K., Umeshita K., Sakon M., Monden M., Aozasa K. (2003). Elevated expression of valosin-containing protein (p97) in hepatocellular carcinoma is correlated with increased incidence of tumor recurrence. J. Clin. Oncol. 21, 447–452. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Tomita Y., Uruno T., Hoshida Y., Qiu Y., Iizuka N., Nakamichi I., Miyauchi A., Aozasa K. (2005). Increased expression of valosin-containing protein (p97) is correlated with disease recurrence in follicular thyroid cancer. Ann. Surg. Oncol. 12, 925–934. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Wang X. (2010). Autophagy and the ubiquitin-proteasome system in cardiac dysfunction. Panminerva Med. 52, 9–25. [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Lee J. J., Stoll S., Ma B., Costa K. D., Qiu H. (2017). Rho kinase regulates aortic vascular smooth muscle cell stiffness via actin/SRF/myocardin in hypertension. Cell Physiol. Biochem. 44, 701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Lee J. J., Stoll S., Ma B., Wiener R., Wang C., Costa K. D., Qiu H. (2017). Inhibition of SRF/myocardin reduces aortic stiffness by targeting vascular smooth muscle cell stiffening in hypertension. Cardiovasc. Res. 113, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Ma B., Stoll S., Hays T. T., Qiu H. (2017). The valosin-containing protein is a novel repressor of cardiomyocyte hypertrophy induced by pressure overload. Aging Cell 16, 1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]