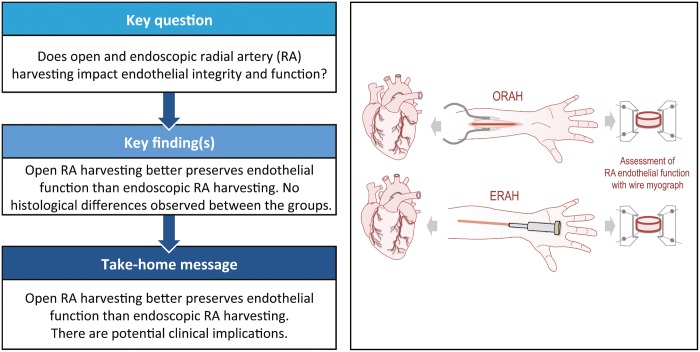
Abstract
OBJECTIVES
Both the open and endovascular techniques are commonly used for harvesting the radial artery (ORAH and ERAH, respectively), and yet, very little is known about the effects of these 2 techniques on endothelial integrity and function of the radial artery (RA). The aim of this study was to assess the endothelial integrity and function of RA harvested using the 2 approaches.
METHODS
Two independent surgical teams working in the same institution routinely use the RA for coronary artery bypass grafting exclusively employing either ORAH or ERAH. Thirty-nine consecutive patients were enrolled in this comparative study. Endothelial function after ORAH or ERAH was assessed by using the wire myograph system. The integrity of the RA endothelium was evaluated by immunohistochemical staining for erythroblast transformation specific-related gene.
RESULTS
The vasodilation in response to acetylcholine was significantly higher in RA harvested with ORAH (P ≤ 0.001 versus ERAH). Endothelial integrity was not different between the 2 groups.
CONCLUSIONS
ORAH is associated with a significantly higher endothelium-dependent vasodilation. Further investigation on the potential implications of these findings in terms of graft spasm and patency as well as clinical outcomes are needed.
Keywords: Radial artery, Coronary artery bypass grafting, Open harvesting, Endoscopic harvesting, Endothelium
INTRODUCTION
Coronary artery bypass grafting (CABG) is the gold standard approach for the treatment of multivessel coronary artery diseases. Traditionally, the internal mammary artery and saphenous veins have been used for CABG [1]. In the 1970s, the radial artery (RA) was introduced and soon abandoned because of high failure rate and traumatic harvesting procedure [2]. In the early 1990s, the RA was reconsidered after Acar et al. [3] introduced a more refined method of harvesting, which was associated with improved RA patency rate. Currently, the RA is often used as complementary arterial conduit for CABG. Data from the Society of Thoracic Surgery Adult Cardiac Surgery Database indicate that the RA is preferred to the right internal thoracic artery as the second arterial graft in the USA [4]. Recently, we published a randomized-based study demonstrating the clinical benefits of using RA versus the saphenous vein in CABG [5]. In light of these findings, it seems likely that the interest towards this conduit will increase in the near future.
Traditionally, the RA has been harvested using the open technique (ORAH) [6]. In the early 2000s, the endoscopic technique (ERAH), less invasive and cosmetically more acceptable, was developed [7]. However, the endoscopic dissection of the RA occurs in a narrow space, raising concerns regarding potential mechanical injuries to the conduit, particularly on the endothelium. Preservation of the functional integrity of the endothelium is of critical importance for maintaining the endothelial cell functions, including vascular tone regulation, anti-inflammatory and antithrombotic functions and inhibition of smooth muscle cell proliferation and migration [8]. Endothelium-derived vasoactive substances including nitric oxide, prostaglandin I2 and the endothelium-derived hyperpolarizing factor control RA vasoactive tone and flow. Hence, even skilled surgical manoeuvers during ERAH could potentially impact the ‘health’ of the endothelium and lead to graft spasm and thrombosis, ultimately compromising the clinical outcomes.
Initial clinical studies assessing the short-term patency of RAs harvested using the 2 techniques reported similar outcomes [9, 10]. However, due to the rarity of clinical events after CABG with the RA, it is likely that all the published series and meta-analyses are underpowered to detect even moderate differences.
Our current understanding of the impact of ERAH versus ORAH on the integrity of the endothelial structure and function is based on older series with negative results [11, 12]. The intrinsic limitations of the methods used in those series, and the difficulties to assess the vasoreactivity of RA due to the peculiar intense vasomotions prompted us to further investigate the effects of the ORAH versus ERAH on the structural integrity and functions of the endothelium of RA. In this study, endothelial-dependent vasorelaxation to acetylcholine (ACh) as well as quantitative structural analysis of the endothelial integrity was performed in open and endoscopic harvested RAs using a more contemporary approach.
MATERIALS AND METHODS
Study design
Two independent and experienced surgical teams working in the same institution routinely use the RA for CABG, with each individual team performing either ORAH or ERAH exclusively. From January to October 2017 all consecutive patients undergoing primary CABG with the RA were screened and included if the laboratory personnel was available for the evaluation. The study was approved by the institutional review board and patient consent was obtained prior to enrolment.
Radial artery harvesting
In the endoscopic group, the RA was harvested according to a method described by Connolly et al. [13]. Briefly, a small 2–3 cm incision was made on the distal volar aspect of the forearm just proximal to the radial styloid prominence. A 30-degree 5-mm endoscope aided by subcutaneous retractors and harmonic shears were used to harvest the RA with its surrounding pedicle. A 2–3 cm counter incision was made at the proximal end of the dissection to aid in vessel transection and ligation.
In the open group, the RA was harvested according to the method described by Lau and Gaudino [14]. Briefly, a linear incision was made from the lateral edge of the biceps tendon and carried distally following the round curvature of the brachio-radialis muscle and terminated just proximal to the radial styloid prominence. Dissection was aided with the use of a harmonic ultrasonic device and the RA was harvested as a pedicle according to the ‘no touch’ technique [15].
All harvestings were performed by highly experienced operators who routinely perform their respective techniques (namely, ERAH and ORAH); none of the RAs included in our study was harvested by trainees or rotating residents. Before harvesting, no patient received vasodilators. No tourniquet was employed irrespective of the harvesting technique. Heparin was administered in both groups.
After dissection, a 1-cm segment was cut from the proximal end of the RA and immediately placed in cold (4°C) Krebs buffered solution and transferred to the lab for functional studies. Prior to placing in Krebs solution, an RA ring of ∼2 mm in length was cut from the RA and placed in 4% formalin for histological analysis.
Assessment of radial artery vascular reactivity in organ bath
Following the harvesting by open or endoscopic approach, the RA specimens were placed in a cold (4°C) Krebs solution at pH 7.2 ± 0.2 composed of (mmol/l) NaCl 118, KCl 4.7, MgCl2 1.2, KH2PO4 1.2, CaCl2×H2O 2.5, NaHCO3 25 and glucose 11, and carboxygenated with a gas mixture of 95% O2 and 5% CO2. By using a dissecting scope (Zeiss Discovery.V8 Stereo) at low magnification, the RA was carefully dissected and cleaned of all the surrounding fat and loose connective tissue and then cut into ∼2 mm rings. From each patient, 1–4 RA rings (about 2 mm length) were mounted in the wire myograph system (Danish MyoTechnology, Denmark). Each chamber was filled with 5 ml of Krebs solution maintained at 37°C and continuously carboxygenated. Ring length (L) was measured before each experiment for pressure calculations. The rings were then allowed to equilibrate upstretched for about 60 min before passive tension was increased stepwise until they reached a transmural pressure of 60 mmHg, calculated as previously reported for large vessels [16]. A known intrinsic characteristic of the RA is the vasospasm. In in vitro experiments, when the RA were stretched beyond 60 mmHg, the vasomotions overpowered the contraction and relaxation effects in response to pharmacological agents (both, vasoconstrictors and vasodilators), creating a confounding factor of the interpretation of the data. Thus, to overcome this confounding factor in the assessment of vascular reactivity, we assessed lower transmural pressures within physiological range (90, 80, 70 and 60 mmHg), until frequency and magnitude of the vasomotions did not interfere with the pharmacological studies. Thus, we found that the optimal transmural pressure to avoid the interference of vasomotion was 60 mmHg.
Vessel stabilization and force calculations
After equilibration, the brackets were slowly moved apart and the rings were then sequentially stretched to apply a tension equivalent to 60 mmHg. The internal diameter, the length of the RA ring and the passive tension were used to calculate the pressure applied as previously reported [17].
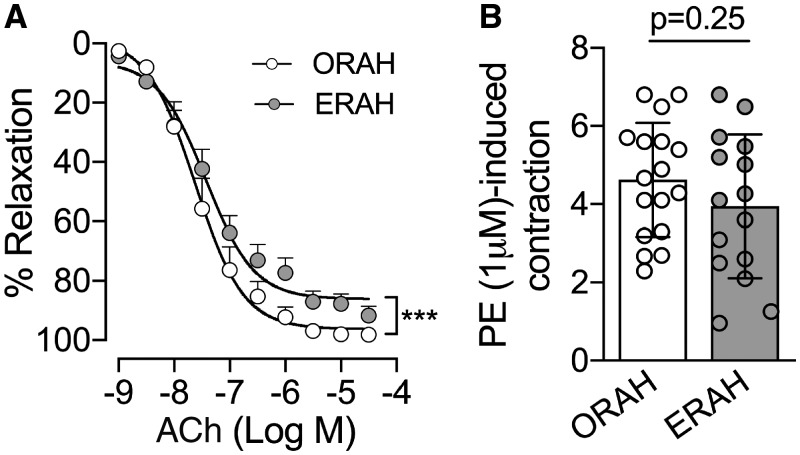
The vasoreactivity to the pharmacological agents was assessed once the tension of RA rings was stable (the tension equivalent to the one the ring was exposed at 60 mmHg). RA rings were constricted with phenylephrine (PE, 1 × 10−6 mol/l) until the tension evoked was consistent between 2 consecutive PE stimulations. Next, the RA rings were preconstricted with PE (1 × 10−6 mol/l) followed by a cumulative concentration–response curve of ACh (1 × 10−9–3 × 10−5 mol/l) to evaluate the integrity and the function on the RA endothelium (Fig. 1). Of note, ACh induces an endothelium-dependent vasorelaxation. Vessels were washed between experiments and allowed to re-stabilize, before repeating the concentration–response curve of ACh.
Figure 1:
Ach-mediated vasodilation was preserved in the radial artery (RA) harvested via open technique. Two to three RA rings from each patient were mounted in the wire myograph system (620M, DMT). (A) After stabilization, RA rings were assessed for vasodilation in response to increasing concentrations of ACh (1 × 10−9–3 × 10−6 M) as indicated. Two-way analysis of variance was used for the statistical analysis. ***P < 0.001 for the group effect, specifically ORAH compared to ERAH. ORAH = 23 patients; ERAH = 16 patients. (B) Vasoconstriction in response to PE (1 × 10−6 M) was also assessed in both groups, ORAH (n = 8) and ERAH (n = 9). Data are presented in (A) as mean with standard error of the mean and (B) as mean with standard deviation. ACh: acetylcholine; ERAH: endoscopic radial artery harvesting; ORAH: open radial artery harvesting; PE: phenylephrine.
Histological analysis
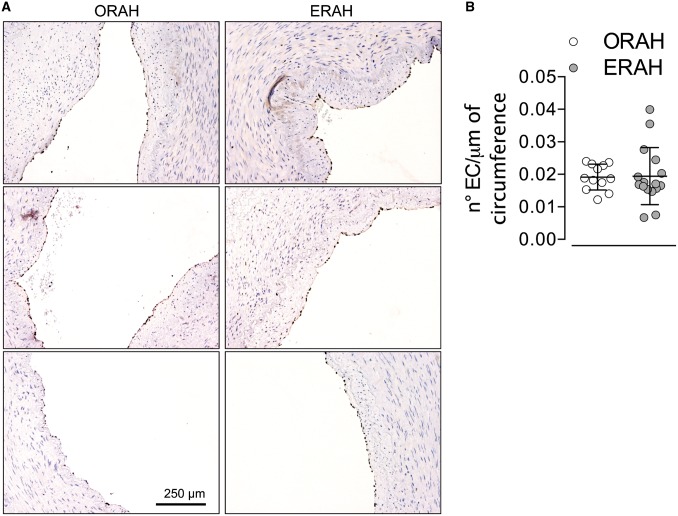
Following harvesting, RA specimens were immediately fixed in a 10% formalin solution and embedded in paraffin. Immunohistochemistry was performed on 4-μm-thick sections. The unstained slides were deparaffinized and rinsed in deionized water, followed by antigen retrieval, by using the sodium citrate buffer (pH = 6). The endothelial cells were detected by performing immunohistochemical staining for erythroblast transformation specific-related gene [18] (ERG, Abcam, Cambridge, MA, USA. Cat. N. ab92513). The sections were incubated with anti-ERG antibody (1:100 dilution) for 25 min at room temperature. ERG was detected using an horseradish peroxidase conjugated compact polymer system and 3,3′-diaminobenzidine as the chromogen. Each section was counterstained with haematoxylin and mounted with Leica Micromount.
Images of stained RA were acquired by using ×20 objective of Aperio AT2 whole slide scanner (Leica Biosystems, San Diego, CA, USA). The scanned images were evaluated for quality and loaded onto the HALO™ Digital Image Analysis (DIA) platform (Indica Labs, Corrales, NM, USA) for the quantification of the endothelial cell ERG-positive and the circumference of the RA, as previously described [19]. The endothelial integrity of each RA specimen was expressed as the number of cells per unit circumference (mm). To increase the robustness of the analysis, the same RA specimens were quantified manually and blindly for the number of endothelial cells per length of circumference of RA. The outcome was consistent between the 2 methods of quantification. The data represented in Fig. 2 are the quantification performed with the manual approach.
Figure 2:
Histological analysis did not evidence endothelial disruption in ERAH versus ORAH. (A) Immunohistochemistry for erythroblast transformation specific-related gene, transcriptional factor expressed in the endothelial cells, was performed in 16 ERAH and 12 ORAH formalin-fixed specimens. Representative images erythroblast transformation specific-related gene-staining of the endothelium in both groups. (B) Quantitative analysis of the number of the endothelial cells lining the circumference of the radial artery. The data were expressed as ratio of the number of the endothelial cells and the circumference (μm). Data are presented as mean with standard deviation. ERAH: endoscopic radial artery harvesting; ORAH: open radial artery harvesting.
Statistical analysis
Data are expressed as mean ± standard error of the mean in Fig. 1A, and as mean ± standard deviation in Figs 1B and 2B. The concentration–response curves for the 2–3 RA rings per patient have been averaged. Thus, for each patient only one concentration–response curve to ACh was considered. A two-way analysis of variance was employed for statistical analyses in Fig. 1A. Two-tailed unpaired Student’s t-test was used for statistical analysis in Figs 1B and 2B. Differences were considered statistically significant at P-value <0.05. All tests were 2-sided. The GraphPad Prism software (version 8.0, GraphPad Software) was used for all statistical analyses.
Based on preliminary data from 7 patients we estimated that the mean of endothelial cells/micron in the open group was 0.03 with a standard deviation of 0.01. In order to detect a 33% difference (from 0.03 to 0.02) with a power of 0.80 at alpha of 0.05, 32 patients (16 from each group) were required. The same sample size give >80% power to detect a difference ≥10% in the mean of the maximal relaxation response to ACh.
RESULTS
Patient population
From a total of 138-screened patients, 39 were enrolled in the study (23 in the ORAH group and 16 in the ERAH). The baseline patient profile was similar between the 2 groups (see Table 1).
Table 1:
Baseline patients profile
| ORAH (n = 23) | ERAH (n = 16) | P-value | |
|---|---|---|---|
| Age (years), mean ± SD | 61.35 ± 9.72 | 59.19 ± 9.88 | 0.50 |
| Male gender, n (%) | 20 (87) | 13 (81) | 0.67 |
| Height (m), mean ± SD | 1.71 ± 0.10 | 1.7 ± 0.07 | 0.73 |
| Weight (kg), mean ± SD | 86.78 ± 14.27 | 84.88 ± 29.45 | 0.79 |
| BMI (kg/m2), mean ± SD | 29.74 ± 4.27 | 29.87 ± 8.89 | 0.95 |
| Hypertension, n (%) | 18 (78) | 16 (100) | 0.06 |
| Hyperlipidaemia, n (%) | 17 (74) | 14 (87) | 0.43 |
| Congestive heart failure, n (%) | 5 (22) | 0 (0) | 0.06 |
| Obesity, n (%) | 8 (34) | 9 (56) | 0.32 |
| Smoking history, n (%) | 13 (56) | 8 (50) | 0.94 |
| Diabetes, n (%) | 9 (39) | 10 (62) | 0.27 |
Hypertension was defined as either a systolic blood pressure >140/90 mmHg. Obesity was defined as a body mass index ≥30 kg/m2. Congestive heart failure was defined by laboratory findings of elevated natriuretic peptides in patients with concomitant signs and symptoms consistent with this syndrome. Dyslipidaemia was defined as LDL-cholesterol above recommended levels as a function of patients total cardiovascular risk. Diabetes was defined as fasting plasma glucose ≥7.0 mmol/l (126 mg/dl) or 2 h plasma glucose ≥11.1 mmol/l (200 mg/dl).
BMI: body mass index; ERAH: endoscopic radial artery harvesting; LDL: low density lipoprotein ORAH: open radial artery harvesting; SD: standard deviation.
Endothelial-dependent vasodilation was preserved by open radial artery harvesting approach
As shown in Fig. 1A, ACh-induced vasodilation was significantly reduced (P < 0.001) in the ERAH rings compared to ORAH suggesting that the endothelium was better preserved in conduits harvested with the open compared to the endoscopic approach (Fig. 1A). Maximal relaxation response was significantly higher in the ORAH versus ERAH group (98.2 ± 0.7% and 89.8 ± 3.7%, respectively, with P = 0.03). The EC50 values for ACh were not statistically significant between the 2 groups (P = 0.51). The tension induced by PE was comparable between both groups (Fig. 1B, P = 0.25), suggesting that different precontractions by PE were not accountable for the greater ACh-induced endothelial-dependent vasodilation in the ORAH group.
Endothelial integrity of radial artery assessed by immunohistochemistry
The number of endothelial cells per section were quantified and expressed as a ratio to the length of the internal circumference. The endothelial cells/internal circumference ratio was not statistically different between the ERAH and ORAH groups (Fig. 2).
DISCUSSION
This study shows for the first time that ORAH harvesting is associated with better preservation of the endothelial function compared to the endoscopic harvesting.
Notably, the proximal RA segments were investigated in light of the demonstrated higher vasospastic tendency, greater incidence of string sign and lower midterm perfect patency rate of the distal RA segments; for these characteristics the proximal RA is indeed considered the segment of choice when performing CABG [20].
In the past, several studies have compared the clinical, angiographic or biological results of the open and endoscopic techniques for RA harvesting. In a small randomized trial, Burns et al. [21] reported similar mid-term patency rate for RA grafts harvested using the open and endoscopic techniques. Bisleri and Muneretto [22] in a propensity score analysis including 470 patients found significantly lower incidence of wound infection, significantly lower pain and better wound healing with the endoscopic technique, in the absence of any difference in cardiac-related mortality.
In a meta-analysis of 6 randomized controlled and propensity matched studies including 743 patients, Rahouma et al. [10] found that the use of the endoscopic technique was associated with a significantly reduced incidence of wound complications [odds ratio (OR) 0.33, 95 confidence interval (CI) 0.14–0.77] in the absence of significant differences in graft patency and 5-year clinical outcomes.
However, due to a very low event rate in patients with RA grafts, all these studies, including the meta-analysis, are probably underpowered to detect even moderate differences in outcomes.
A number of studies have previously compared endoscopic versus open harvesting, mostly with regard to saphenous vein graft [23]. A post hoc analysis from the PREVENT IV trial showed higher risk of graft failure following endoscopic versus open harvesting of the saphenous vein (OR 1.41, 95% CI 1.16–1.71), although the trial itself was not adequately powered to detect significant differences between the 2 techniques [24]. In the recently published REGROUP trial, 1150 patients were randomized to undergo open versus endoscopic saphenous vein harvesting. Over a median follow-up of 2.78 years, no differences were shown between the 2 groups in terms of primary composite end point of major adverse cardiac events, including death from any cause, non-fatal myocardial infarction and repeat revascularization (hazard ratio 1.12, 95% CI 0.83–1.51; P = 0.47) [23].
Different studies investigated the endothelial mechanical damage of the endoscopic versus open RA harvesting techniques, by using the histological analysis. In agreement with the previous studies [12, 25], histological analysis showed no difference in the endothelial coverage of the RA between ORAH and ERAH. However, it needs to be considered that the histological analysis can only detect mechanical damage to significant degrees, such as loss of endothelial cells or disruption of the endothelial layer. The histological approach might not evidence ‘micro-mechanical damages’ that may not physically break the endothelial layer of the RA, but compromise the endothelial function, as shown by the endothelial-dependent vasodilation (Fig. 1A). Our study has demonstrated for the first time not only that ORAH better preserves the functional integrity of the endothelium compared to ERAH, but also that assessing the endothelial-dependent vasodilation of RA should be the gold standard approach to evaluate the preservation of the RA endothelium between the 2 surgical approaches.
Shapira et al. [25] in a small randomized cohort found no differences between the open versus conventional harvesting of RA in the maximal relaxation in response to ACh and nitroglycerine, in RA precontracted with U46619, a thromboxane A2 mimetic. It is difficult to compare their findings with ours as the experimental conditions used were different. For instance, RA rings were stored in papaverine before pharmacological studies, which were conducted in the presence of indomethacin, cyclooxygenase inhibitor. The resting tension applied was the one necessary to induce the maximal response to 80 mmol/l KCl [25], and the RA rings were precontracted with U46619 whereas we used PE.
Shapira et al. also assessed the expression of adhesion molecules by immunohistochemistry between the 2 groups and found no differences. Adhesion molecules such as intercellular adhesion molecule-1, vascular cadherin adhesion molecule and P-selectin are considered as markers of endothelial activation following proinflammatory cytokine stimulation, such as tumour necrosis factor-α, or mechanical stimuli (disturbed shear stress, injury) [26]. Although no images of adhesion molecule staining were included in the article, the expression of adhesion molecules as means to discriminate the mechanical damage or stress on the endothelium imposed by the open versus the endoscopic procedure might not be a reliable method. Indeed, the narrow time frame between the harvesting and the fixation of the RA specimens, may not be sufficient to allow the expression of adhesion molecules. Most likely, what the authors reported reflected an underline vascular inflammatory condition of the patients undergoing CABG.
Nowicki et al. [27] used immunohistochemistry specifically for CD31 and endothelial nitric oxide synthase (eNOS), to evaluate endothelial integrity of RA grafts after open and endoscopic harvesting. They reported significantly higher endothelium preservation using the endoscopic approach. This fairly unique finding is in sharp contrast with almost all the published literature on the comparison of endoscopic versus open conduit harvesting (including the saphenous vein) and is mainly explained by the surprising 42% endothelial preservation in the open group (a finding that was never reproduced in the other published series). One puzzling aspect of their data is the expression of CD31 and eNOS in all the cells of the intima, media and adventitia. This finding suggests a poor specificity of the immunohistochemical approach employed. It is also important to consider that RA are from patients affected by cardiovascular diseases who have risk factors and therefore there is a generalized disease level of the vasculature, including inflammation, neointima proliferation and some lipid depositions. In our study, we stained the endothelium with ERG which is a transcriptional factor expressed in the endothelial cells in some pathological conditions and has been reported as a better marker than CD31 [18]. Indeed, the ERG staining is highly specific and restricted to the endothelium (Fig. 2A).
Finally, Medalion et al. [12] used organ bath studies to evaluate the differences between the 2 harvesting techniques and found similar results between the 2 groups. Unfortunately, in this study the concentration–response to ACh was not performed but it was assessed only with vasodilation induced by 1 × 10−6 M of ACh, which limits data interpretation. It is noteworthy that although not statistically significant, the vasodilation induced by ACh 1 × 10−6 M had a tendency towards higher values in the open compared to the endoscopic RA, suggesting that the concentration–response curve of ACh could have evidenced differences between the 2 groups while a single dose of ACh might have overlooked. In this study, haematoxylin and eosin, Masson’s trichrome and von Gieson staining revealed that all 3 arterial layers were preserved, indicating that no major mechanical damages were caused by the surgical procedure. However, there was no specific staining for the endothelial cells and quantification of the endothelial integrity was not performed, and the histology-based conclusions were rather qualitative.
Of note, it has been suggested that endoscopic saphenous vein graft harvesting is associated with lower patency rates [28], which closely correlates with higher endothelial damage during the endoscopic compared to open procedure. Considering that arteries have spastic characteristics compared to veins, and that RA tends to be more spastic than other arterial graft, it is intuitive that even a small endothelial damage may potentially have a greater impact on the patency of the RA compared to the saphenous vein.
The RA is the most used complementary arterial graft for CABG [8], and it is a class IB recommendation in the 2018 ESC/EACTS Guidelines in the cases of target vessels with >90% stenosis [29]. Due to its muscular wall, the RA is more prone to spasm than any other conduit used for CABG. For this reason, preservation of the functional integrity of the endothelium during harvesting is of paramount importance. It must be reminded that the suboptimal results reported in initial experiences with RA grafts were mainly attributed to the traumatic preparation technique, and leading to high degree of vessel wall damage during harvesting [30].
CONCLUSIONS
Our data show that the functional integrity of the endothelium of RA is better preserved with ORAH compared to ERAH. We cannot speculate on the effect of the observed differences in the endothelial layer on short and long-term clinical outcomes. It is possible that the compromised endothelial function imposed by the ERAH is a self-limiting effect without any detrimental consequence in terms of graft spasm or failure.
However, confirmation on our results in a larger cohort, including mid and long-term comparison of the patency rate and clinical outcomes of RA grafts obtained using the 2 harvesting techniques are needed to clarify this important question.
Funding
This work was supported by a grant from the Weill Cornell Clinical and Translational Science Centre to M.F.G. and National Institutes of Health [grant R01HL126913 to A.D.L.].
Conflict of interest: none declared.
Presented at the 32nd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 18–20 October 2018.
REFERENCES
- 1. Diodato M, Chedrawy EG.. Coronary artery bypass graft surgery: the past, present, and future of myocardial revascularisation. Surg Res Pract 2014;2014:726158.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curtis JJ, Stoney WS, Alford WC Jr, Burrus GR, Thomas CS Jr. Intimal hyperplasia. A cause of radial artery aortocoronary bypass graft failure. Ann Thorac Surg 1975;20:628–35. [DOI] [PubMed] [Google Scholar]
- 3. Acar C, Jebara VA, Portoghese M, Beyssen B, Pagny JY, Grare P et al. . Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg 1992;54:652–9; discussion 59–60. [DOI] [PubMed] [Google Scholar]
- 4. Schwann TA, Ramia PS, Habib JR, Engoren MC, Bonnell MR, Habib RH.. Effectiveness of radial artery-based multiarterial coronary artery bypass grafting: role of body habitus. J Thorac Cardiovasc Surg 2018;156:43–51.e2. [DOI] [PubMed] [Google Scholar]
- 5. Gaudino M, Benedetto U, Taggart DP.. Radial-artery grafts for coronary-artery bypass surgery. N Engl J Med 2018;379:1967–8. [DOI] [PubMed] [Google Scholar]
- 6. Bisleri G, Giroletti L, Hrapkowicz T, Bertuletti M, Zembala M, Arieti M et al. . Five-year clinical outcome of endoscopic versus open radial artery harvesting: a propensity score analysis. Ann Thorac Surg 2016;102:1253–9. [DOI] [PubMed] [Google Scholar]
- 7. Genovesi MH, Torrillo L, Fonger J, Patel N, McCabe JC, Subramanian VA.. Endoscopic radial artery harvest: a new approach. Heart Surg Forum 2001;4:223–4; discussion 24–5. [PubMed] [Google Scholar]
- 8. Kinlay S, Libby P, Ganz P.. Endothelial function and coronary artery disease. Curr Opin Lipidol 2001;12:383–9. [DOI] [PubMed] [Google Scholar]
- 9. Kim G, Jeong Y, Cho Y, Lee J, Cho J.. Endoscopic radial artery harvesting may be the procedure of choice for coronary artery bypass grafting. Circ J 2007;71:1511–15. [DOI] [PubMed] [Google Scholar]
- 10. Rahouma M, Kamel M, Benedetto U, Ohmes LB, Di Franco A, Lau C et al. . Endoscopic versus open radial artery harvesting: a meta-analysis of randomized controlled and propensity matched studies. J Card Surg 2017;32:334–41. [DOI] [PubMed] [Google Scholar]
- 11. Shapira OM, Eskenazi BR, Hunter CT, Anter E, Bao Y, Murphy R et al. . Endoscopic versus conventional radial artery harvest—is smaller better? J Cardiac Surgery 2006;21:329–35. [DOI] [PubMed] [Google Scholar]
- 12. Medalion B, Tobar A, Yosibash Z, Stamler A, Sharoni E, Snir E et al. . Vasoreactivity and histology of the radial artery: comparison of open versus endoscopic approaches. Eur J Cardiothorac Surg 2008;34:845–9. [DOI] [PubMed] [Google Scholar]
- 13. Connolly MW, Torrillo LD, Stauder MJ, Patel NU, McCabe JC, Loulmet DF et al. . Endoscopic radial artery harvesting: results of first 300 patients. Ann Thorac Surg 2002;74:502–5; discussion 6. [DOI] [PubMed] [Google Scholar]
- 14. Lau C, Gaudino M.. Open radial artery harvesting. Multimed Man Cardiothorac Surg 2018;2018, doi:10.1510/mmcts.2018.021. [DOI] [PubMed] [Google Scholar]
- 15. Blitz A, Osterday RM, Brodman RF.. Harvesting the radial artery. Ann Cardiothorac Surg 2013;2:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He GW, Yang CQ.. Characteristics of adrenoceptors in the human radial artery: clinical implications. J Thorac Cardiovasc Surg 1998;115:1136–41. [DOI] [PubMed] [Google Scholar]
- 17. He GW, Angus JA, Rosenfeldt FL.. Reactivity of the canine isolated internal mammary artery, saphenous vein, and coronary artery to constrictor and dilator substances: relevance to coronary bypass graft surgery. J Cardiovasc Pharmacol 1988;12:12–22. [DOI] [PubMed] [Google Scholar]
- 18. Sullivan HC, Edgar MA, Cohen C, Kovach CK, HooKim K, Reid MD.. The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: an analysis of 25 cases. J Clin Pathol 2015;68:44–50. [DOI] [PubMed] [Google Scholar]
- 19. McIntire PJ, Zhong E, Patel A, Khani F, D'Alfonso T, Chen Z et al. . Hotspot enumeration of CD8+ tumor infiltrating lymphocyte using digital image analysis in triple negative breast cancer yields consistent results. Hum Pathol 2019;85:27–32. [DOI] [PubMed] [Google Scholar]
- 20. Gaudino M, Nasso G, Canosa C, Glieca F, Salica A, Alessandrini F et al. . Midterm angiographic patency and vasoreactive profile of proximal versus distal radial artery grafts. Ann Thorac Surg 2005;79:1987–9. [DOI] [PubMed] [Google Scholar]
- 21. Burns DJ, Swinamer SA, Fox SA, Romsa J, Vezina W, Akincioglu C et al. . Long-term patency of endoscopically harvested radial arteries: from a randomized controlled trial. Innovations (Phila) 2015;10:77–84. [DOI] [PubMed] [Google Scholar]
- 22. Bisleri G, Muneretto C.. Endoscopic saphenous vein and radial harvest: state-of-the-art. Curr Opin Cardiol 2015;30:624–8. [DOI] [PubMed] [Google Scholar]
- 23. Zenati MA, Bhatt DL, Bakaeen FG, Stock EM, Biswas K, Gaziano JM et al. . Randomized trial of endoscopic or open vein-graft harvesting for coronary-artery bypass. N Engl J Med 2019;380:132–41. [DOI] [PubMed] [Google Scholar]
- 24. Hess CN, Lopes RD, Gibson CM, Hager R, Wojdyla DM, Englum BR et al. . Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation 2014;130:1445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shapira OM, Eskenazi BR, Anter E, Joseph L, Christensen TG, Hunter CT et al. . Endoscopic versus conventional radial artery harvest for coronary artery bypass grafting: functional and histologic assessment of the conduit. J Thorac Cardiovasc Surg 2006;131:388–94. [DOI] [PubMed] [Google Scholar]
- 26. Galkina E, Ley K.. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 2007;27:2292–301. [DOI] [PubMed] [Google Scholar]
- 27. Nowicki M, Misterski M, Malinska A, Perek B, Ostalska-Nowicka D, Jemielity M et al. . Endothelial integrity of radial artery grafts harvested by minimally invasive surgery—immunohistochemical studies of CD31 and endothelial nitric oxide synthase expressions: a randomized controlled trial. Eur J Cardiothorac Surg 2011;39:471–7. [DOI] [PubMed] [Google Scholar]
- 28. Lopes RD, Hafley GE, Allen KB, Ferguson TB, Peterson ED, Harrington RA et al. . Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med 2009;361:235–44. [DOI] [PubMed] [Google Scholar]
- 29. Sousa-Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al. . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4–90. [DOI] [PubMed] [Google Scholar]
- 30. Gaudino M, Crea F, Cammertoni F, Massetti M.. The radial artery: a forgotten conduit. Ann Thorac Surg 2015;99:1479–85. [DOI] [PubMed] [Google Scholar]