Abstract
CEPs (C-TERMINALLY ENCODED PEPTIDEs) inhibit Arabidopsis primary root growth by unknown mechanisms. We investigated how CEP3 levels control primary root growth. CEP3 peptide application decreased cell division, S-phase cell number, root meristematic cell number, and meristem zone (MZ) size in a dose- and CEP RECEPTOR1-dependent manner. Grafting showed that CEP3-dependent growth inhibition requires root and shoot CEPR1. CEP3 induced mitotic quiescence in MZ cells significantly faster than that induced by nutrient limitation alone. CEP3 also inhibited the restoration of S-phase to mitotically quiescence cells by nutrient resupply without quantitatively reducing TARGET OF RAPAMYCIN (TOR) kinase activity. In contrast, cep3-1 had an increased meristem size and S-phase cell number under nitrogen (N)-limited conditions, but not under N-sufficient conditions. Furthermore, cep3-1 meristematic cells remained in S-phase longer than wild-type cells during a sustained carbon (C) and N limitation. RNA sequencing showed that CEP3 peptide down-regulated genes involved in S-phase entry, cell wall and ribosome biogenesis, DNA replication, and meristem expansion, and up-regulated genes involved in catabolic processes and proteins and peptides that negatively control meristem expansion and root growth. Many of these genes were reciprocally regulated in cep3-1. The results suggest that raising CEP3 induces starvation-related responses that curtail primary root growth under severe nutrient limitation.
Keywords: Amino acid catabolism, carbon limitation, cell cycle, CEP, CEPR1, nitrogen limitation, peptide hormone, primary root growth, TOR
Nutrient availability influences root growth in a trade-off between seedling establishment and survival. CEP3 accelerates starvation-related primary root growth inhibition under nitrogen or combined carbon and nitrogen limitation.
Introduction
Upon seed germination, the root apical meristem (RAM) grows into a heterogeneous soil milieu. Positively and negatively acting plant phytohormones and peptide hormones carefully orchestrate RAM growth to deal with variations in nutrient availability and environmental conditions (Delay et al., 2013; Pacifici et al., 2015). The extent of RAM growth influences the effectiveness of water and nutrient procurement in the seedling and determines the success of seedling establishment and survival. Strong and early seedling establishment, especially in rain-fed crops, is a strong predictor of future yield as it reduces evaporative losses from the soil surface and reduces weed competition, thus reducing herbicide use (Richards et al., 2002). Therefore, understanding the mechanisms influencing RAM growth is important.
The interaction of the C-TERMINALLY ENCODED PEPTIDE (CEP) family with its major receptor, CEPR1, negatively controls RAM growth in Arabidopsis (Ohyama et al., 2008; Delay et al., 2013; Tabata et al., 2014), but the underlying mechanism is undefined. Arabidopsis encodes 15 CEP genes (Delay et al., 2013; Ogilvie et al., 2014) and, due to gene redundancy and a paucity of CEP mutants, there is a poor understanding of the function of individual CEP peptide-encoding genes. Low nitrogen (N) induces several CEP genes predominantly in roots (Delay et al., 2013; Imin et al., 2013; Tabata et al., 2014; Mohd-Radzman et al., 2015). Root-produced CEP peptides enter the xylem stream (Okamoto et al., 2009; Tabata et al., 2014; Patel et al., 2018) enabling root to shoot CEP translocation. In the shoot, CEPs interact with the receptor CEPR1/XIP1 (CEP RECEPTOR1/XYLEM INTERMIXED WITH PHLOEM1; hereafter called CEPR1) to influence systemic N demand signalling (Tabata et al., 2014; Ohkubo et al., 2017; Taleski et al., 2018). In Arabidopsis and Medicago, evidence also exists for root-localized CEP–CEPR1-dependent circuits that inhibit root system growth (Huault et al., 2014; Mohd-Radzman et al., 2015, 2016; Roberts et al., 2016; Taleski et al., 2018), but this pathway is undefined. It is not known if CEP-dependent inhibition of main root growth proceeds by fast, local effects on the main root or slower, indirect effects mediated by root to shoot to root signalling, or both.
CEP3 is an in vivo validated 15 amino acid peptide hydroxylated at proline residues at positions 4 and 11 (Tabata et al., 2014) that negatively controls primary root growth (Delay et al., 2013; Tabata et al., 2014). Consistently, the root tips of cep3-1 plants grow faster than those of wild type (WT) plants under various growth and stress conditions, including low N (Delay et al., 2013). These results suggest that CEP3 imparts a non-redundant, condition-dependent, and root growth-inhibiting phenotype that affects primary root growth (Delay et al., 2013). A lack of additional knockout mutant alleles of CEP3, however, hampers the validation of CEP3 function.
Primary root growth results from cell cycle progression in the meristematic zone (MZ) and cell elongation (Dolan et al., 1993). The stem cell initials divide to generate defined MZ cell files, giving a clear longitudinal root zonation demarked by the transition zone (TZ) and the elongation zone (EZ) (Baluska et al., 2010). The transcription factors WOX5 (WUSCHEL-RELATED HOMEOBOX 5) (Tian et al., 2014) and SCR (SCARECROW) (Wysocka-Diller et al., 2000) regulate the identity and patterning of specific root tip cell types. It is possible that CEP3 inhibits root tip growth by directly affecting root patterning, longitudinal root zonation, cell elongation, the quiescent centre, or MZ cell cycle activity. Alternatively, CEP3 could inhibit root tip growth by slower (systemic) routes, so it is important to determine the kinetics of the inhibition of primary root growth by CEP3.
Since CEP expression is altered by changes in carbon (C) (Imin et al., 2013; Chapman et al., 2019) and N levels, it is possible that CEPs affect TARGET OF RAPAMYCIN (TOR), a kinase that links growth and development to nutrient and energy status (Xiong et al., 2013). TOR regulates the RAM by phosphorylating downstream effectors including S6K1 and S6K2 (Xiong et al., 2013; Dobrenel et al., 2016; Li et al., 2017). There is a well-established link between C limitation and cell cycle progression in the RAM (Xiong et al., 2013). C limitation of young seedlings progressively induces RAM cells into mitotic quiescence, typified by a lack of TOR signalling (Xiong et al., 2013; Xiong and Sheen, 2014). The resupply of C restores S-phase (within 2 h) and TOR signalling within minutes to mitotically quiescent cells. These responses are readily visualized in S-phase cells using the thymidine analogue 5-ethynyl-2'-deoxyuridine (EdU), the incorporation of which can be detected using fluorescence, or by using antibodies to detect TOR-specific phosphorylation of target proteins (Xiong et al., 2013; Li et al., 2017). As well as low C inhibiting RAM growth, low N also negatively affects RAM growth (Martin et al., 2002), but how N limitation and imbalances in C and N affect primary root growth is unknown. Given that low N and high CO2 up-regulate CEP levels (Delay et al, 2013; Imin et al., 2013) and that CEP levels affect RAM growth, it is important to determine whether CEPs interfere with TOR signalling to exert their inhibitory effects on primary root growth.
Here, we explored the function of CEP3 in Arabidopsis. We examine how altering CEP3 levels affects RAM growth and whether nutrient-limited or nutrient-imbalanced conditions affect CEP3 level-dependent RAM growth. We examined if CEP3 inhibition of primary root growth was dose and CEPR1 dependent, and if post-transcriptional silencing of CEP3 recapitulated the enhanced root growth phenotype of cep3-1. Grafting studies assessed whether CEP3 interacts with CEPR1 in the root or shoot to inhibit primary root growth. We assessed whether CEP3 levels alter tissue zonation in the root tip using WT, cep3-1, WT overexpressing CEP3 (oe), or WT roots treated with CEP3 peptide. Reporter lines, flow cytometry, and an EdU-based S-phase assay were used to determine if altering CEP3 levels affects RAM cell patterning and zonation, the stem cell niche, or cell cycle progression. We assessed whether altering CEP3 levels affects the speed at which RAM cells enter mitotic quiescence under nutrient-limited or nutrient-imbalanced conditions. To determine if CEP3 affects RAM growth directly or indirectly, we measured the kinetics of CEP3 inhibition of RAM growth. We also determined whether CEP3 counteracted the ability of nutrient resupply to restore S-phase to mitotically quiescent RAM cells by affecting TOR kinase activity. To identify transcripts affected by CEP3 levels, we undertook RNA sequencing (RNA-Seq) of the roots of cep3-1 and CEP3-treated and untreated WT plants.
Materials and methods
Plant lines
CEP3oe, cep3-1 (Delay et al., 2013), and 35S::S6K1-HA (Xiong and Sheen, 2012) lines were described previously. Philip Benfey (Duke University) provided the pSCR2.0:SCR:GFP (Wysocka-Diller et al., 2000), pWOX5:YFP (Sarkar et al., 2007), and pCYCB1;1:CYCB1;1:GFP (Colon-Carmona et al., 1999) lines. The homozygous Col-0 cepr1-3 mutant was isolated from the T-DNA line 467C01 generated by the GABI-Kat program and provided by Bernd Weisshaar (Chapman et al., 2019; Kleinboelting et al., 2012). The CEP3-knockdown line (amiR-CEP3) in the Col-0 background was generated using artificial miRNA-mediated gene silencing using the vector pMDC32B-AtMIR390a-B/c (Carbonell et al., 2014) containing an insert targeting CEP3 mRNA (Table S1 at JXB online) designed using the P-SAMS tool (Fahlgren et al., 2016).
Synthetic peptides
CEP3 peptide (TFRhyPTEPGHShyPGIGH; hyP is hydroxyproline) and the scrambled CEP peptide (TPSGFhyPGDHHhyPTRGI) (Delay et al., 2013) were synthesized by GL Biochem (Shanghai) or the Biomolecular Resource Facility at the Australian National University to >95% purity and used at 1 µM unless specified. HPLC and MS confirmed peptide mass and purity.
Growth conditions and analysis
Arabidopsis seed were sterilized and stratified (Delay et al., 2013). For plate-based assays measuring root meristem size, cell cycle progression, stem cell niche, and radial patterning, half-strength Murashige and Skoog medium (no sucrose; referred to as standard 1/2 MS) and modified N-free 1/2 MS medium were used (Delay et al., 2013). Seedlings were grown vertically on 90 mm square plates at 22 °C (16 h photoperiod and 100 μmol m–2 s–1 of photosynthetically active radiation). Ambient CO2 levels were used for all assays. For plate assay root system measurements, images were analysed using the SmartRoot plugin (Lobet et al., 2011) in ImageJ.
For liquid assays, three seeds were sown, germinated, and grown per well with 1 ml of 1/2 MS medium (Corning Costar 12-well plates; Sigma Aldrich). For C limitation, plants were grown with standard 1/2 MS medium under controlled conditions with a 12 h photoperiod at 21 °C at ≤50 μmol m–2 s–1 (Xiong et al., 2013). For N starvation, plants were grown in N-free 1/2 MS medium with 15 mM sucrose (16 h photoperiod and 100 μmol m–2 s–1 of photosynthetically active radiation). Glucose (Glc), CEP3 peptide, or mock treatments were added and mixed evenly.
Quantitative reverse transcription–PCR (qRT–PCR) analysis
To validate the CEP3-silenced line, total RNA was extracted from snap-frozen tissue using a modified Trizol (Life Technologies) extraction with columns from the RNeasy plant mini kit (QIAGEN) (Delay et al., 2013). cDNA synthesis was carried out using oligo(dT)12–18 primers and Superscript III reverse transcriptase (Invitrogen). Quantitative RT–PCR was carried out using Fast SYBR Green fluorescent dye (Applied Biosystems) with samples run on a ViiA 7 Real-Time PCR System (Applied Biosystems) following the manufacturer’s specifications. Data were analysed using the ΔΔCT method (Livak and Schmittgen, 2001). The reference gene At4g24550 (ADAPTOR PROTEIN-4 MU-ADAPTIN) was used for normalization (Czechowski et al., 2005). Primers used are listed in Supplementary Table S1.
Hypocotyl grafting
Seedlings were grown for 6 d on 1/2 MS with 0.5% sucrose prior to hypocotyl grafting (Branco and Masle, 2019). Five days after grafting, plants were transferred to 1/2 MS medium with 1% sucrose with or without added 1 µM CEP3.
Microscopy and imaging
Whole seedlings were submerged in 100 μg ml–1 propidium iodide (PI; Sigma Aldrich) for 150 s. Seedlings were mounted on slides in water under a coverslip for imaging on a Zeiss LSM780 UV-NLO confocal microscope (Carl Zeiss MicroImaging, Jena, Germany). Images were acquired using Zen2012 software (Zeiss), and cell number and root zones were determined manually (Verbelen et al., 2006). The TZ is defined as the zone encompassing the boundaries between dividing and expanding cells in the different files, whereas the MZ is defined as the zone where cells are dividing at the root tip.
EdU-based S-phase assay
EdU incorporation and visualization was carried out using described methods (Kotogany et al., 2010). Briefly, 1 μM EdU was added to wells for 30 min. Samples were fixed with 4% (w/v) formaldehyde solution in phosphate-buffered saline (PBS; pH 7.2) with 0.1% Triton X-100 for 30 min and washed three times with PBS. Alexa-Fluor-488 azide was coupled to the EdU alkyne using the Click-It EdU Alexa Fluor 488 Imaging Kit (ThermoFisher Scientific). Samples were mounted in water and observed with a Leica DM5500 microscope.
Flow cytometry
Samples were prepared using described methods (Galbraith et al., 2001; Dolezel et al., 2007). Root tips (5 mm) from at least 300 seedlings were excised using a razor blade and chopped in 1 ml of ice-cold Galbraith’s buffer for no more than 2 min. Samples were mixed by pipetting, passed through a 50 µm nylon mesh, and centrifuged for 10–15 min at 150 g. The supernatant was removed, leaving ~200 μl of remaining liquid. Samples were stored on ice for up to 4 h. Approximately 20 min before analysis, 100 μg ml–1 PI was added and mixed. Samples were vortexed again prior to and during flow cytometry. Nuclei were analysed with a BD LSRFortessa flow cytometer (BD Biosciences, San Jose, CA, USA) at the Cytometry and Imaging Facility, Australian National University. Cell-cycle analysis was performed with ModFitLT software (Verity Software House).
RNA-Seq analysis
Three treatments were used for RNA-Seq: WT, WT plus CEP3 (12 h treatment with 1 μM CEP3 peptide), and cep3-1. Samples were grown in liquid N-free medium with suboptimal light conditions (≤50 μmol m–2 s–1) for 6 d. On day 6, half of the WT roots were treated with CEP3 for 12 h, and the remaining WT and cep3-1 samples received a mock treatment. RNA was extracted (Delay et al., 2013) using Trizol (Life Technologies) and purified using spin columns and buffers from the RNeasy plant mini kit (QIAGEN). RNA extracted from three pooled biological replicates per treatment (nine samples in total) was sent to the Australian Genome Research Facility (Melbourne, Australia) for library preparation and RNA-Seq. RNA was prepared, run, and analysed at the AGRF using standard procedures and pipelines for differential gene expression. RNA quality was assessed using a Bioanaylzer (Agilent Technologies). Nine samples were pooled and run in a single lane on a HiSeq2000 with single-end, 50 bp reads. Illumina sequencing adaptor reads were removed. The sequence reads were aligned against the Arabidopsis genome (Build version TAIR10) using the Tophat aligner v2.0.13 to align the 20–25 million mapped reads per sample to the genome. The read count mapping to each known gene in the latest Araport annotation (Araport11) was summarized using the featureCounts v1.4.6-p5 utility of the subread package (Liao et al., 2014) and used for expression analysis. The RUVseq package (Risso et al., 2014) was used to remove noise in the data before testing for expression changes between the groups. The differential gene expression analysis was performed using the bioconductor package edgeR (McCarthy et al., 2012). Gene Ontology (GO) analysis was performed using API scripts from the ThaleMine application and Araport project resources. GO term enrichment analysis was performed on differentially expressed genes changing at least 1.5-fold with a false discovery rate (FDR) cut-off of ≤0.05. Statistically significant GO terms from the ‘biological process’ category were selected using the Benjamini–Hochberg correction with a P-value cut-off of ≤0.05.
TOR kinase assays
The roots from >400 seedlings were excised using a razor blade and then ground in liquid nitrogen before adding 40 µl of 2× SDS for the western blot. Phospho-p70 S6 kinase (p-Thr449) polyclonal antibody (Agrisera) was used to detect TOR kinase phosphorylation of T449 in Arabidopsis S6K1. HA-tagged protein was detected by anti-HA (Roche) monoclonal antibodies using standard techniques (Li et al., 2017).
Results
CEP3 inhibits root apical meristem cell number via CEPR1 activity in roots and shoots
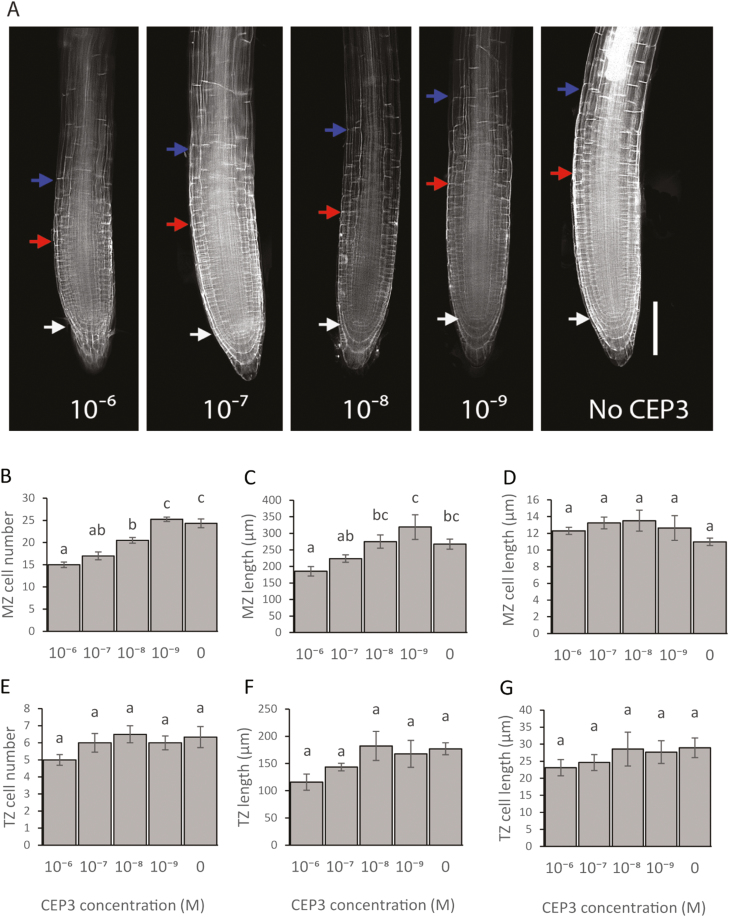
We first determined the physiological basis for CEP3 peptide inhibition of primary root growth and whether this was dose and CEP receptor dependent. CEP peptide addition inhibited RAM growth by reducing the number of MZ cells (Fig. 1). CEP3 reduced MZ cell number at 10–6–10–8 M concentrations in a dose-dependent manner, but did not affect cell elongation in the MZ or TZ (Fig. 1). CEP3 significantly reduced MZ length at 10–6 M (Fig. 1C).
Fig. 1.
CEP3 affects MZ size by reducing MZ cell number and MZ length. (A) Representative images of root meristems stained with propidium iodide after 6 d growth on standard 1/2 MS medium with CEP3 peptide added at concentrations ranging from 0 to 10–6 M. The white arrow indicates the quiescent centre. The red arrow indicates the boundary of the meristematic zone. The blue arrow indicates the boundary of the transition zone. Scale bar=100 µm. (B–G) Measurements of cortical cell number, zone length, and cell length for the MZ and TZ of plants grown as described in (A). (B) MZ cell number. (C) MZ length. (D) MZ cell length. (E) TZ cell number. (F) TZ length. (G) TZ cell length. Error bars show the SE. Letters indicate significant differences (P<0.05, ANOVA using Bonferroni multiple comparisons test). n≥4.
We then confirmed that the decrease in MZ cell number by CEP3 peptide addition depended on its receptor, CEPR1. As expected, the MZ of the Columbia cepr1-3 mutant was insensitive to CEP3 peptide (Supplementary Fig. S1A). CEP3 addition did not affect mature cell length in either the WT or cepr1-3 (Supplementary Fig. S1B). An assessment of the effect of CEP3 peptide on the length of the primary root on reciprocally grafted plants showed that CEP3 peptide inhibition of primary root growth required both root and shoot CEPR1 (Supplementary Fig. S1C), suggesting a role, in part, for systemic CEP3–CEPR1 interactions in controlling primary root growth.
CEP3-dependent inhibition of MZ size is influenced by N availability
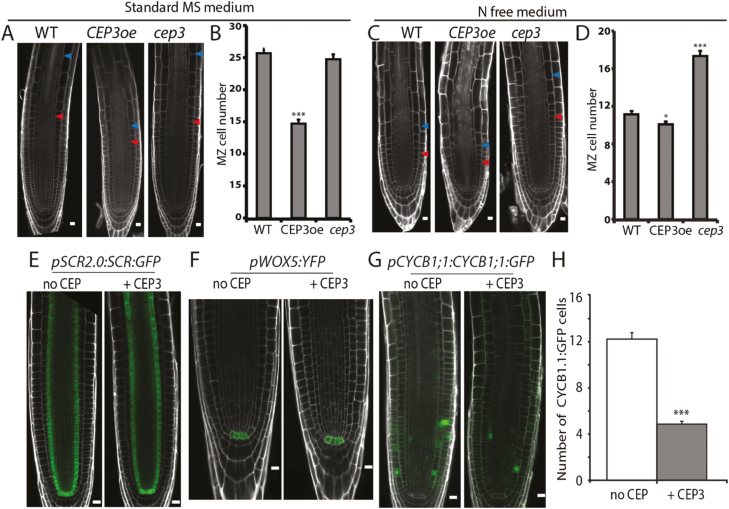
We next determined whether N availability in the growth medium influenced CEP3-dependent primary root growth inhibition (Fig. 2A–D). The cep3-1 root tips had a high number of MZ cells when grown in an N-free medium (Fig. 2C, D), but not when grown in sucrose-free MS medium (‘standard 1/2 MS’) (Fig. 2A, B). In contrast, CEP3oe had a diminished number of MZ cells and size of the TZ irrespective of the growth medium (Fig. 2A, B).
Fig. 2.
CEP3 levels affect root meristem size and cell cycle progression. (A, C) Confocal images of propidium iodide-stained 6-day-old root meristems. (B, D) Enumerated MZ cell number (n≥14). Red and blue arrowheads in (A) and (C) indicate the MZ and TZ boundary, respectively. (A, B) Plants were grown on standard 1/2 MS medium. (C, D) Plants were grown on modified N-free 1/2 MS medium. (E–G) Confocal images of CEP3 peptide-treated and non-treated reporter lines grown on standard 1/2 MS medium without and with 1 µM CEP3 peptide: (E) pSCR2.0:SCR:GFP; (F) pWOX5:YFP, and (G) pCYCB1;1:CYCB1;1:GFP. (H) Number of pCYCB1;1:CYCB1;1:GFP-positive cells per primary root tip. Focal planes right through the root were examined in (H). n=30 root tips. *P<0.05; **P<0.01; ***P<0.001 (two-sample t-test, Genstat). Scale bars=10 μm in (A), (C), (E–G).
To independently assess the negative effect of CEP3 on root growth, we silenced CEP3 using artificial miRNA-mediated gene silencing (Carbonell et al., 2014). This confirmed that lowering CEP3 expression increased primary root length, consistent with previous reports for cep3-1 (Delay et al., 2013) (Supplementary Fig. S2).
Increased CEP3 levels reduce the number of cells in the cell cycle
We determined whether raising CEP3 levels reduced MZ cell number by affecting radial cell patterning, quiescent cell identity, or cell cycle progression in the RAM (Fig. 2E–H). CEP3 did not affect the expression of the radial patterning marker, SCR, or the quiescent centre marker, WOX5 (Fig. 2E, F), but did reduce CYCB1;1 expression, which marks the G2–M transition. CEP3 treatment reduced the number of fluorescent cells expressing pCYCB1;1:CYCB1;1:GFP (Colon-Carmona et al., 1999) down to 40% (Fig. 2G, H).
CEP3 inhibits S phase entry
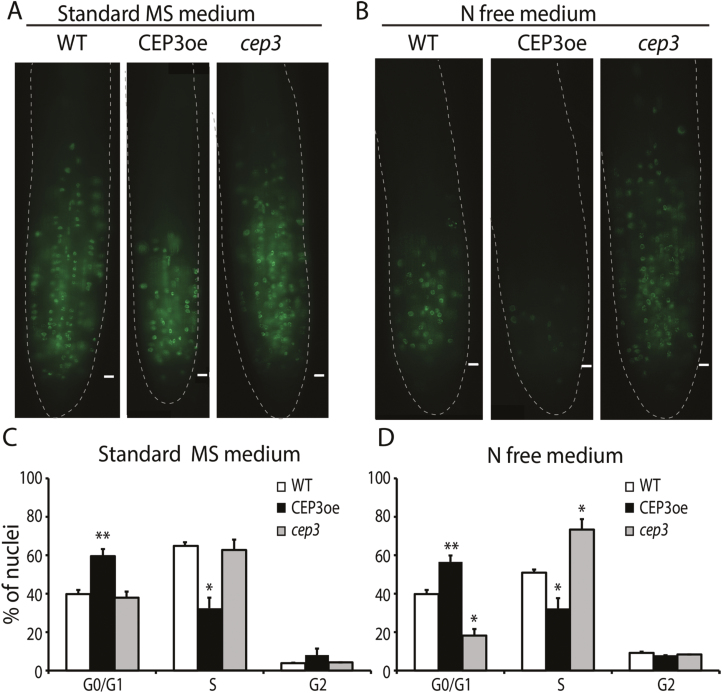
The effects of CEP3 levels on the MZ were evident under N-free conditions. Therefore, to determine if CEP3 levels affected cell cycle progression prior to the G2–M transition, we used EdU incorporation (Xiong et al., 2013) to enumerate S-phase cells in plants grown in the presence and absence of N (Fig. 3A, B). CEP3oe plants showed an ~50% reduction in S-phase cell number under sufficient N and an almost complete absence of S-phase cells under N depletion (Fig. 3A, B). Consistent with Fig. 2, there was no detectable difference between WT and cep3-1 S-phase cell number under sufficient N conditions; however, there were ~50% more S-phase cells in the cep3-1 MZ grown under no N conditions, compared with the WT (Fig. 3A, B).
Fig. 3.
CEP3 regulates S-phase entry in response to N limitation. An EdU assay was used to visualize S-phase cells in the meristems of plants grown on standard medium (A) or N-free medium (B). Flow cytometry analysis of nuclei extracted from root tips of 6-day-old WT, CEP3oe, and cep3-1 plants grown on standard medium (C) or N-free medium (D). Meristem-enriched cells were isolated by harvesting tissue within 5 mm of the root tip. There were 30% more nuclei in G0/G1 phase in samples from CEP3oe plants compared with the WT and 30% less in the S-phase (C). In N-free medium (D), there was <50% the number of nuclei from cep3-1 plants in G0/G1 phase and 40% more in S-phase (two-sample t-test, Genstat). Scale bars=10 μm.
To determine the cell cycle stage affected by CEP3, we conducted flow cytometry on nuclei isolated from PI-treated root tips from WT, cep3-1, and CEP3oe grown under sufficient (Fig. 3C) or no N (Fig. 3D) conditions. Under sufficient N, there were no measurable differences between cep3-1 and the WT; however, elevated levels of CEP3 diminished S-phase entry (i.e. there were ~30% more nuclei in G0/G1 phase in CEP3oe samples compared with the WT and ~48% less in S-phase; Fig. 3C). In contrast, under a no N condition, cep3-1 plants had ~50% fewer nuclei in G0/G1 phase and ~40% more nuclei in S-phase, whereas CEP3oe gave nearly opposite results (Fig. 3D). These results suggest that CEP3 levels affect root growth by directly or indirectly regulating S-phase entry in the RAM.
CEP3 peptide inhibits S-phase entry under C limitation
We next tested if CEP3 levels influence C starvation-dependent exit of RAM MZ cells from the cell cycle into mitotic quiescence by using EdU incorporation to assess the number of cells in S-phase.
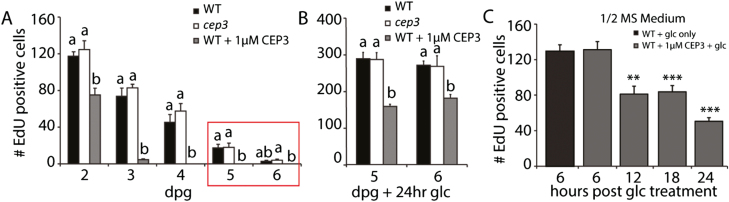
First, we induced C depletion by incubating WT, cep3-1, and CEP3-treated WT plants under suboptimal light and no added sugars. Under these conditions, C depletes gradually over a 6 d period, which induces mitotic quiescence (Xiong et al., 2013). We found that CEP3 peptide accelerated the reduction in S-phase cell number in WT plants by 2–3 d compared with non-treated WT plants, whereas there was no detectable difference in the reduction in S-phase cell number in cep3-1 and WT plants (Fig. 4A).
Fig. 4.
CEP3 peptide accelerates entry into mitotic quiescence under C limitation and inhibits Glc-dependent recovery into S-phase. (A) Time course showing the effect of C limitation of S-phase cell number. Plants were grown with restricted light (≤50 µmol m–2 s–1) to prevent photoautotrophic growth. Treated WT plants were continually exposed to the CEP3 peptide from imbibition. The red box indicates the age of plants used in (B). (B) Number of EdU-positive cells after 24 h of incubation with Glc. dpg=days post-germination. (C) After 5 d of C-limited growth, WT plants were treated with Glc (1 mM) or Glc (1 mM) plus CEP3 peptide. CEP3 treatment reduces the number of root meristem cells re-entering S-phase of the cell cycle within 12 h after Glc addition. Plants were grown in suboptimal light to day 5 to synchronize meristematic cells into mitotic quiescence. Glc (1 mM) or CEP3 (1 µM)+Glc was added at time 0 after synchronization and plants were assayed at the specified times. n≥5 for each time point in (A–C). Plants in (A–C) were grown in standard 1/2 MS medium. Lower case letters in (A) and (B) indicate statistically significant differences within each time point (P<0.05, ANOVA with Bonferroni correction for multiple comparison; Genstat). In (C), **P<0.01; ***P<0.001 (t-test, compared with no CEP treatment at the same time point).
Mitotically quiescent RAM cells under C limitation can be rapidly restored to S-phase by Glc addition (Xiong et al., 2013). To confirm that the RAM cells with diminished or no S-phase cells (i.e. akin to the cells in the red box in Fig. 4A) were indeed mitotically quiescent, we added Glc to 5 d and 6 d C-limited plants. Glc raised S-phase cell number in all treatments by a minimum of 8-fold (Fig. 4B). This confirmed that the RAMs lacking S-phase cells were mitotically quiescent.
Although Glc treatment significantly increased S-phase cells in all samples, we noted that significantly fewer RAM cells recovered into S-phase after CEP3 treatment compared with untreated WT and cep3-1 RAMs (Fig. 4B). This suggests that CEP3 peptide prevents the full recovery of mitotically quiescent cells into S-phase. To investigate this, we induced RAM cells into mitotic quiescence by exposing WT plants to suboptimal light for 5 d, before dividing the sample and adding Glc with or without CEP peptide (Fig. 4C). Glc addition restored S-phase in mitotically quiescent cells, but CEP3 peptide significantly inhibited the extent of S-phase recovery within 12 h. The magnitude of the CEP3 peptide-dependent inhibition of Glc-induced recovery of S-phase increased with increasing CEP3 exposure time (Fig. 4C). We then tested if the pre-treatment of CEP3 peptide inhibited Glc-induced recovery of mitotically quiescent cells into S-phase. This showed that inhibition of Glc-induced recovery of S-phase required between 6 h and 12 h of CEP3 pre-treatment (Supplementary Fig. S3).
CEP3-dependent reduction of S-phase recovery after nutrient resupply is independent of TOR signalling
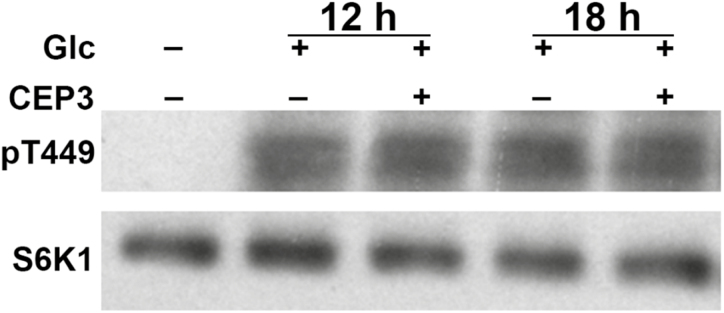
TOR kinase activates cell cycle machinery by phosphorylating downstream effectors (Xiong et al., 2013). Therefore, we tested if the CEP3-dependent inhibitory effects upon the recovery of mitotically quiescent cells into S-phase by nutrient supply depended on TOR kinase activity. We measured T449 phosphorylation of S6K1 by TOR, which is conserved in plants and animals (Xiong et al., 2013). CEP3 pre-treatment of mitotically quiescent cells did not inhibit S6K1 phosphorylation in response to Glc resupply (Fig. 5) but still reduced S-phase cell number from the 12 h time point onwards (Supplementary Fig. S3).
Fig. 5.
CEP3 does not affect TOR kinase activity in roots following nutrient resupply to C-starved seedlings. 35S::S6K1-HA seedlings were grown in liquid 1/2 MS for 4 d then underwent a mock or 1 µM CEP3 pre-treatment for 12 or 18 h before being treated with 15 mM Glc for 15 mins. Phosphorylation of T449 in S6K1 was detected. Levels of S6K1-HA were determined as a loading control (Xiong et al., 2013).
CEP3 peptide inhibits N-dependent recovery into S-phase
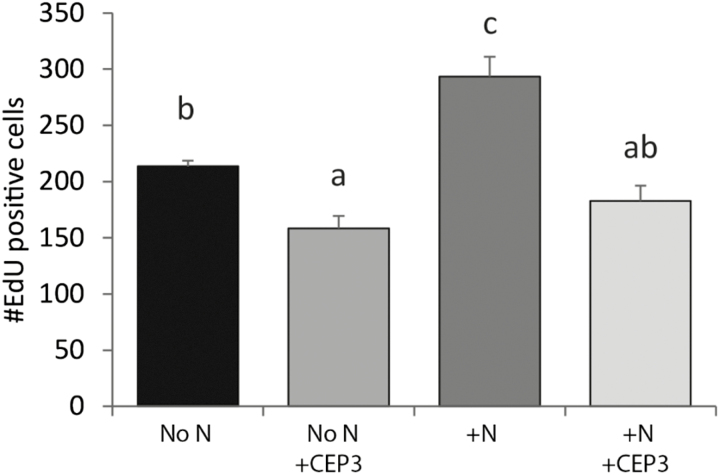
We also assessed the effect of CEP3 on the RAM under N-limited but C-sufficient conditions in liquid medium. Nitrate supply increased S-phase cell number after N limitation (Fig. 6). The addition of CEP3 reduced S-phase cell number under N limitation and also inhibited the increase in S-phase cell number by N resupply (Fig. 6).
Fig. 6.
CEP3 peptide inhibits N-dependent recovery into S-phase. The number of meristematic cells undergoing DNA synthesis in 7-day-old seedlings. Plants were grown in modified N-free liquid medium (No N) with 15 mM sucrose and 100 µmol m–2 s–1 light for 6 d then treated for 24 h with 5 mM KNO3 (+N) and/or 1 µM CEP3. Error bars show the SE. Letters indicate significant differences (P<0.05, ANOVA using Bonferroni multiple comparisons test). n≥4.
CEP3 levels affect the mitotic activity of the RAM cells under C and N limitation
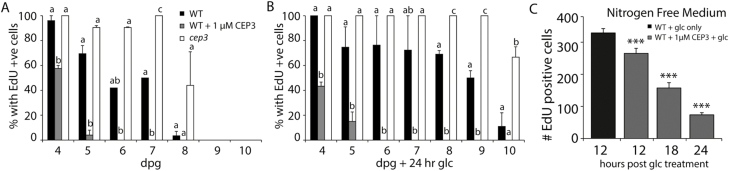
We measured the effect of raising CEP3 levels on primary root growth under a combined C and N limitation by determining the percentage of roots with mitotically active MZ cells in WT, CEP3-treated WT, and cep3-1 plants. Elevating CEP3 peptide levels significantly reduced the percentage of roots with S-phase cells after 4 d exposure and, by day 6, no cells were in S-phase (Fig. 7A). Consistently, there were higher levels of S-phase cells in cep3-1 RAMs than in the RAMs of WT plants after 7 d and 8 d of N and C limitation (Fig. 7A).
Fig. 7.
The cep3-1 mutant sustains mitotic activity for longer under C and N limitation, and CEP3 peptide impedes the recovery of mitotic cells into S-phase. (A) Time course showing the percentage of roots with EdU-florescent cells relative to levels in day four samples. Plants were grown in modified N-free liquid medium with suboptimal light to prevent photoautotrophic growth. (B) Percentage of roots with EdU-positive cells after 24 h of incubation with 1 mM Glc. n≥10 roots. Letters indicate statistically significant differences within each time point (P<0.05, ANOVA with Bonferroni multiple comparison; Genstat). (C) After growth under N and C limitation, CEP3 peptide treatment reduces the number of root meristem cells in the S-phase of the cell cycle within 12 h. Plants were grown in modified N-free liquid medium under suboptimal light for 6 d to synchronize meristematic cells. Glc or CEP3+Glc was added at time 0 (after synchronization) and plants were assayed at the specified times. ***P<0.001 (t-test, compared with no peptide control). n=8 roots per time point.
We confirmed that cep3-1 and untreated WT RAM cells were able to withstand prolonged C- and N-limited conditions as Glc resupply restored the S-phase to WT and cep3-1 RAMs deprived for up to 10 d of C and N (Fig. 7B). The RAMs of cep3-1 remained Glc responsive for a significantly longer time than those of WT roots. At day 10, for instance, Glc restored 75% of cep3-1 RAMs into S-phase compared with only 14% for WT RAMs (Fig. 7B). In contrast, Glc failed to rescue CEP3-treated WT RAM cells into S-phase if the treatment time exceeded 6 d (Fig. 7B).
We determined how quickly CEP3 treatment affects the reactivation of S-phase in mitotically quiescence WT RAMs grown for 5 d under C and N limitation (Fig. 7C). Glc restored S-phase, whereas a combined Glc plus CEP3 treatment significantly reduced the extent of S-phase recovery within 12 h of CEP addition, and the magnitude of the reduction increased with longer CEP exposure time (Fig. 7C).
RNA-Seq shows that CEP3 levels affect the transcription of cell cycle genes involved in G1–S phase transition and cell wall and ribosome synthesis
We conducted RNA-Seq to identify differentially expressed genes that depended on the levels of CEP3. Since the greatest difference in the mitotic response of plants to CEP3 levels occurred under C and N limitation (Fig. 7), WT and cep3-1 were grown in liquid N-free medium under suboptimal light for 6 d and half the WT plants were treated with CEP peptide or a mock treatment for 12 h to allow for transcriptional reprogramming. There were 1250 differentially expressed genes (minimum ±2-fold change, P<0.05; FDR <0.05) in the CEP3-treated samples, with 257 and 993 genes up- and down-regulated, respectively, compared with the WT (Supplementary Table S2; Supplementary Fig. S4). There were 996 differentially expressed genes in the cep3-1 mutant, with 450 and 546 genes up- and down-regulated, respectively, compared with the WT (Supplementary Table S3; Supplementary Fig. S4). Of these, there was an inverse regulation of 217 genes in CEP3-treated WT compared with cep3-1 (Supplementary Table S4).
GO term enrichment and pathway analysis of the differentially displayed genes identified biological processes affected by altering CEP levels (Supplementary Fig. S5). Congruent with its growth-retarding effects, CEP3 peptide down-regulated multiple genes involved in cell wall organization and biosynthesis (e.g. extensin, polygalacturonase/pectinase, proline-rich protein, expansin, xyloglucan transferase/hydrolysase, cellulose synthase, and arabinogalactan biosynthesis genes) (Supplementary Table S2). CEP3 also down-regulated 30 small and large ribosomal subunit protein-coding genes (Supplementary Table S2). Many of these genes were up-regulated in cep3-1 (Supplementary Table S4).
We determined whether CEP3 down-regulated genes required for DNA replication, which would be congruent with its ability to inhibit Glc-dependent S-phase entry. CEP3 down-regulated 34 core genes required for G1–S phase transition and DNA replication (Vandepoele et al., 2002, 2005; Menges et al., 2006) including five CYCLIN Ds, two E2F transcription factors, several E2F-dependent replication enzymes and origin factors, as well as CYCLIN A, CYCLIN B (including CYCLIN B1; congruent with the pCYCB1;1:CYCB1;1:GFP data in Fig. 1G, H), and CYCLIN DEPENDENT KINASE B (Supplementary Table S5), and a further eight genes known to be involved in G1–S phase transition (Vandepoele et al., 2005).
CEP3 peptide affects genes controlling meristem expansion and root growth
Consistent with its activity on primary root growth, CEP3 peptide down-regulated transcripts for signalling molecules known to increase MZ size and up-regulated transcripts of proteins known to restrict root tip growth. CEP peptide down-regulated two ROOT GROWTH FACTOR genes (RGF2 and RGF8) by ~2-fold and up-regulated the plant-specific FANTASTIC FOUR2 (FAF2) and FAF4 genes (Wahl et al., 2010) by 3- to 5-fold. RGFs promote MZ growth through PLETHORA-dependent pathways (Matsuzaki et al., 2010), whereas FAF genes negatively influence meristem maintenance at both growing poles of Arabidopsis (Wahl et al., 2010). CEP3 also up-regulated CLE3, CLE4, and CLE7 (Araya et al., 2014) by 3.6- to 13.5-fold. CLE3, CLE4, and CLE7 are expressed predominantly in pericycle cells of N-deficient roots. These CLEs play a somewhat similar role to CEPs in reducing lateral root primordium growth and lateral root emergence (Araya et al., 2014).
CEP3 peptide up-regulates genes controlling catabolic processes and low energy responses
An analysis of GO terms up-regulated by CEP3 revealed a multitude of genes associated with the catabolism of organic acids and amino acids and low energy responses (Supplementary Table S2) most notably including genes such as ASN1 (GLUTAMINE-DEPENDENT ASPARAGINE SYNTHETASE 1), BCAT-2 (BRANCHED-CHAIN-AMINO-ACID TRANSAMINASE2), ERD5/PRODH (EARLY RESPONSIVE TO DEHYDRATION 5/PROLINE DEHYDROGENASE), 3-METHYLCROTONYL-COA CARBOXYLASE genes MCCA and MCCB, and bZIP1 (BASIC LEUCINE-ZIPPER 1) (Dröge-Laser and Weiste, 2018).
Discussion
The results conclusively demonstrate that CEP3 inhibits primary root growth in a dose- and CEPR1-dependent manner by reducing the number of MZ cells and MZ size. This is consistent with the inhibitory effects of CEPR1 orthologues on root system expansion in Medicago (Imin et al., 2013; Mohd-Radzman et al., 2015). This suggests that CEP–CEPR1 pathways inhibit root growth across species. Grafting experiments confirm that CEP3–CEPR1 interactions in the root and shoot underpin this root growth inhibition.
We show that CEP3 inhibited primary root growth by reducing MZ cell number and MZ size. The results indicate a role for CEP3 in the plant’s response to N starvation since cep3-1 had more MZ cells than the WT when grown on medium lacking N. The increased root growth of the cep3-1 and CEP3 knockdown lines under various growth and stress conditions other than low N (Delay et al., 2013) also suggests that CEP3 plays a more expansive role in negatively controlling growth that goes beyond low-N conditions. It is possible that CEPs may be induced under nutrient-imbalanced conditions to curtail primary root growth.
Reporter gene and EdU incorporation assays show that increased CEP3 levels reduce the number of MZ cells engaged in the cell cycle. CEP3 fails to influence the expression of gene markers implicated in root patterning or the maintenance of the stem cell niche, but reporter gene and flow cytometry measurements support CEP3 reducing the number of MZ cells in the cell cycle and particularly those transitioning from G1 into S-phase.
Although CEP3 slows the growth rate under most conditions, under severe nutrient limitation or imbalanced conditions (either low C, low N, or combined low C and N), CEP3 greatly accelerates the speed at which MZ cells enter mitotic quiescence, eventually resulting in fewer detectable cells in S-phase. CEP3 also reduces the extent to which nutrient resupply restores mitotically quiescent cells into S-phase without quantitatively reducing the strength of TOR signalling. Under either a low N or combined low N and C limitation, the cep3-1 mutant has a distinctive phenotype; it is able to maintain MZ cells in S-phase longer than either the WT or, in particular, the WT with elevated CEP3 levels. In addition, after a prolonged N and C starvation, more cep3-1 cells recover into S-phase upon nutrient resupply. This suggests that CEP3 has a definitive role in responses to nutrient starvation, with the addition of CEP3 triggering a premature nutrient starvation response. Therefore, root growth in cep3-1 plants persists longer under nutrient limitation compared with WT plants and in particular WT plants with elevated CEP3 levels.
Roots require a 6–12 h period of CEP3 exposure to show a significant reduction in the number of S-phase cells. This suggests that CEP3 does not directly target MZ cells but rather curtails growth in part by systemic mechanisms. This is consistent with the grafting results showing that CEP3 requires CEPR1 in both roots and shoots to inhibit root growth.
RNA-Seq studies confirm that CEP3 peptide causes the widespread reduction of the transcription of genes involved in the cell cycle, G1–S phase transition, cell wall and ribosome synthesis, as well as genes required for meristem expansion, and up-regulates genes which restrict meristem size. As CEP3 addition did not alter the length of mature cells in the primary root, this suggests that the differences in the expression of the cell wall-related genes may be due to the reduction of the number of cells in the cell cycle after CEP3 treatment, which leads to a lower number of elongating cells and, hence, slower growth.
CEP addition also induced the expression of low N-induced CLE genes (CLE3, CLE4, and CLE7). This may suggest a synergistic interplay between CEP–CEPR1 and low N-related CLE–CLV1 pathways to curtail overall root growth. It is postulated that the up-regulation of these CLE genes involves systemic low N signalling (Araya et al., 2014, 2016). Given the role of CEP–CEPR1 in systemic N-demand signalling (Tabata et al., 2014; Ohkubo et al., 2017), it would be of interest to determine if up-regulation of CLE3, CLE4, and CLE7 under low N is dependent on CEP–CEPR1. Finally, the induction by CEP3 of key genes involved in catabolic and low energy processes supports a role for CEP3–CEPR1 interactions in curtailing root growth under severe nutrient stress, potentially to promote seedling survival. Therefore, manipulating CEP levels in crops may be an avenue to fine-tune root growth to ensure optimal seedling establishment.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. The effect of CEP3 peptide on the main root growth is dependent on CEPR1 in both shoot and root.
Fig. S2. Artificial miRNA-mediated knockdown of CEP3 results in increased main root growth.
Fig. S3. CEP3 pre-treatment interferes with Glc-dependent recovery of S phase in mitotically quiescent cells.
Fig. S4. Number of genes differentially regulated by raising or lowering CEP3 levels as determined by RNA-Seq analysis.
Fig. S5. Biological processes that were significantly over-represented in the differentially displayed gene sets.
Table S1. List of primers used.
Table S2. RNA-sequencing data of CEP3 peptide treatment versus the WT.
Table S3. RNA-sequencing data of the cep3-1 mutant versus the WT.
Table S4. Genes reciprocally regulated after comparing CEP3 peptide treatment with cep3-1.
Table S5. Key cell cycle-related genes significantly down-regulated by CEP3 peptide addition.
Acknowledgements
Philip Benfey and Jamie Van Norman are thanked for providing Arabidopsis constructs and mutants. CD and MT received Australian postgraduate awards; CD was supported by a GRDC Scholarship; KC was awarded an ANU PhD scholarship. YW, KC, and MT contributed equally to this work. An Australian Research Council grant to MAD (DP150104250) supported this work.
References
- Araya T, Miyamoto M, Wibowo J, Suzuki A, Kojima S, Tsuchiya YN, Sawa S, Fukuda H, von Wirén N, Takahashi H. 2014. CLE–CLAVATA1 peptide–receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proceedings of the National Academy of Sciences, USA 111, 2029–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya T, von Wirén N, Takahashi H. 2016. CLE peptide signaling and nitrogen interactions in plant root development. Plant Molecular Biology 91, 607–615. [DOI] [PubMed] [Google Scholar]
- Baluska F, Mancuso S, Volkmann D, Barlow PW. 2010. Root apex transition zone: a signalling–response nexus in the root. Trends in Plant Science 15, 402–408. [DOI] [PubMed] [Google Scholar]
- Branco R, Masle J. 2019. Systemic signalling through TCTP1 controls lateral root formation in Arabidopsis. Journal of Experimental Botany. doi:10.1093/jxb/erz204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell A, Takeda A, Fahlgren N, Johnson SC, Cuperus JT, Carrington JC. 2014. New generation of artificial microRNA and synthetic trans-acting small interfering RNA vectors for efficient gene silencing in Arabidopsis. Plant Physiology 165, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K, Taleski M, Ogilvie HA, Imin N, Djordjevic MA. 2019. CEP–CEPR1 signalling inhibits the sucrose-dependent enhancement of lateral root growth. Journal of Experimental Botany. doi:10.1093/jxb/erz207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P. 1999. Technical advance: spatio-temporal analysis of mitotic activity with a labile cyclin–GUS fusion protein. The Plant Journal 20, 503–508. [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. 2005. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology 139, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay C, Imin N, Djordjevic MA. 2013. CEP genes regulate root and shoot development in response to environmental cues and are specific to seed plants. Journal of Experimental Botany 64, 5383–5394. [DOI] [PubMed] [Google Scholar]
- Dobrenel T, Caldana C, Hanson J, Robaglia C, Vincentz M, Veit B, Meyer C. 2016. TOR signaling and nutrient sensing. Annual Review of Plant Biology 67, 261–285. [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. 1993. Cellular organisation of the Arabidopsis thaliana root. Development 119, 71–84. [DOI] [PubMed] [Google Scholar]
- Dolezel J, Greilhuber J, Suda J. 2007. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols 2, 2233–2244. [DOI] [PubMed] [Google Scholar]
- Dröge-Laser W, Weiste C. 2018. The C/S1 bZIP network: a regulatory hub orchestrating plant energy homeostasis. Trends in Plant Science 23, 422–433. [DOI] [PubMed] [Google Scholar]
- Fahlgren N, Hill ST, Carrington JC, Carbonell A. 2016. P-SAMS: a web site for plant artificial microRNA and synthetic trans-acting small interfering RNA design. Bioinformatics 32, 157–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith DW, Lambert GM, Macas J, Dolezel J. 2001. Analysis of nuclear DNA content and ploidy in higher plants. Current Protocols in Cytometry 2, 7.6.1–7.6.22. [DOI] [PubMed] [Google Scholar]
- Huault E, Laffont C, Wen J, Mysore KS, Ratet P, Duc G, Frugier F. 2014. Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLoS Genetics 10, e1004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imin N, Mohd-Radzman NA, Ogilvie HA, Djordjevic MA. 2013. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. Journal of Experimental Botany 64, 5395–5409. [DOI] [PubMed] [Google Scholar]
- Kleinboelting N, Huep G, Kloetgen A, Viehoever P, Weisshaar B. 2012. GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Research 40, D1211–D1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotogány E, Dudits D, Horváth GV, Ayaydin F. 2010. A rapid and robust assay for detection of S-phase cell cycle progression in plant cells and tissues by using ethynyl deoxyuridine. Plant Methods 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cai W, Liu Y, Li H, Fu L, Liu Z, Xu L, Liu H, Xu T, Xiong Y. 2017. Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proceedings of the National Academy of Sciences, USA 114, 2765–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lobet G, Pagès L, Draye X. 2011. A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiology 157, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Oswald O, Graham IA. 2002. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon:nitrogen availability. Plant Physiology 128, 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y, Ogawa-Ohnishi M, Mori A, Matsubayashi Y. 2010. Secreted peptide signals required for maintenance of root stem cell niche in Arabidopsis. Science 329, 1065–1067. [DOI] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK. 2012. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Research 40, 4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges M, Samland AK, Planchais S, Murray JA. 2006. The D-type cyclin CYCD3;1 is limiting for the G1-to-S-phase transition in Arabidopsis. The Plant Cell 18, 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Radzman NA, Binos S, Truong TT, Imin N, Mariani M, Djordjevic MA. 2015. Novel MtCEP1 peptides produced in vivo differentially regulate root development in Medicago truncatula. Journal of Experimental Botany 66, 5289–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Radzman NA, Laffont C, Ivanovici A, Patel N, Reid D, Stougaard J, Frugier F, Imin N, Djordjevic MA. 2016. Different pathways act downstream of the CEP peptide receptor CRA2 to regulate lateral root and nodule development. Plant Physiology 171, 2536–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie HA, Imin N, Djordjevic MA. 2014. Diversification of the C-TERMINALLY ENCODED PEPTIDE (CEP) gene family in angiosperms, and evolution of plant-family specific CEP genes. BMC Genomics 15, 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo Y, Tanaka M, Tabata R, Ogawa-Ohnishi M, Matsubayashi Y. 2017. Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nature Plants 3, 17029. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Ogawa M, Matsubayashi Y. 2008. Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis. The Plant Journal 55, 152–160. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Ohnishi E, Sato S, Takahashi H, Nakazono M, Tabata S, Kawaguchi M. 2009. Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant & Cell Physiology 50, 67–77. [DOI] [PubMed] [Google Scholar]
- Pacifici E, Polverari L, Sabatini S. 2015. Plant hormone cross-talk: the pivot of root growth. Journal of Experimental Botany 66, 1113–1121. [DOI] [PubMed] [Google Scholar]
- Patel N, Mohd-Radzman NA, Corcilius L, et al. 2018. Diverse peptide hormones affecting root growth identified in the Medicago truncatula secreted peptidome. Molecular & Cellular Proteomics 17, 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RA, Rebetzke GJ, Condon AG, van Herwaarden AF. 2002. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals presented at the 1999 CSSA Symposium on water use efficiency. Crop Science 42, 111–121. [DOI] [PubMed] [Google Scholar]
- Risso D, Ngai J, Speed TP, Dudoit S. 2014. Normalization of RNA-seq data using factor analysis of control genes or samples. Nature Biotechnology 32, 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I, Smith S, Stes E, et al. 2016. CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. Journal of Experimental Botany 67, 4889–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T. 2007. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446, 811–814. [DOI] [PubMed] [Google Scholar]
- Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y. 2014. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346, 343–346. [DOI] [PubMed] [Google Scholar]
- Taleski M, Imin N, Djordjevic MA. 2018. CEP peptide hormones: key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. Journal of Experimental Botany 69, 1829–1836. [DOI] [PubMed] [Google Scholar]
- Tian H, Wabnik K, Niu T, et al. 2014. WOX5–IAA17 feedback circuit-mediated cellular auxin response is crucial for the patterning of root stem cell niches in Arabidopsis. Molecular Plant 7, 277–289. [DOI] [PubMed] [Google Scholar]
- Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombauts S, Inzé D. 2002. Genome-wide analysis of core cell cycle genes in Arabidopsis. The Plant Cell 14, 903–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepoele K, Vlieghe K, Florquin K, Hennig L, Beemster GT, Gruissem W, Van de Peer Y, Inzé D, De Veylder L. 2005. Genome-wide identification of potential plant E2F target genes. Plant Physiology 139, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbelen JP, De Cnodder T, Le J, Vissenberg K, Baluska F. 2006. The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities: meristematic zone, transition zone, fast elongation zone and growth terminating zone. Plant Signaling & Behavior 1, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl V, Brand LH, Guo YL, Schmid M. 2010. The FANTASTIC FOUR proteins influence shoot meristem size in Arabidopsis thaliana. BMC Plant Biology 10, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, Benfey PN. 2000. Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development 127, 595–603. [DOI] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J. 2013. Glucose–TOR signalling reprograms the transcriptome and activates meristems. Nature 496, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Sheen J. 2012. Rapamycin and glucose-target of rapamycin (TOR) protein signaling in plants. Journal of Biological Chemistry 287, 2836–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Sheen J. 2014. The role of target of rapamycin signaling networks in plant growth and metabolism. Plant Physiology 164, 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.