Abstract
N6-Methyladenosine (m6A) is the most common and abundant mRNA modification that involves regulating the RNA metabolism. However, the role of m6A in regulating the β-cell function is unclear. Methyltransferase-like 14 (METTL14) is a key component of the m6A methyltransferase complex. To define the role of m6A in regulating the β-cell function, we generated β-cell METTL14-specific knockout (βKO) mice by tamoxifen administration. Acute deletion of Mettl14 in β-cells results in glucose intolerance as a result of a reduction in insulin secretion in β-cells even though β-cell mass is increased, which is related to increased β-cell proliferation. To define the molecular mechanism, we performed RNA sequencing to detect the gene expression in βKO islets. The genes responsible for endoplasmic reticulum stress, such as Ire1α, were among the top upregulated genes. Both mRNA and protein levels of IRE1α and spliced X-box protein binding 1 (sXBP-1) were increased in βKO islets. The protein levels of proinsulin and insulin were decreased in βKO islets. These results suggest that acute METTL14 deficiency in β-cells induces glucose intolerance by increasing the IRE1α/sXBP-1 pathway.
N6-Methyladenosine (m6A) is the most common and abundant mRNA modification, modulated by “writers” proteins, such as methyltransferase-like 14 (METTL14), methyltransferase-like 3 (METTL3), and Wilms tumor 1-associating protein (WTAP). In mammals, m6A sites in mRNA are dominated by the conserved Pu [G > A] m6AC [A/C/U] motif that localizes near stop codons in 3′-untranslated region (UTR) within long internal exons and at 5′-UTR (1). METTL3 was known as associated with mRNA methylation for a long time; in 2014, METTL14 was discovered to catalyze m6A methylation in mRNA together with METTL3 (2). These two proteins form a stable heterodimer core complex of METTL3–METTL14 that mediates m6A deposition on nuclear RNA in mammalian cells. WTAP can interact with the METTL3–METTL14 complex to affect cellular m6A deposition markedly. Furthermore, m6A modification is reversible by “erasers” proteins, such as fat mass and obesity-associated protein (FTO) and ALKBH5. Besides writers and erasers proteins, the “readers” proteins, such as YTH domain family members YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2, are also involved in m6A modification and function (3).
In vitro and in vivo data have shown that m6A influences mRNA metabolism, such as mRNA stability and translation, which affects stem-cell fates (4), sex determination (5), spermatogenesis (6), tumorigenesis (7), and naïve T cell homeostasis (8). Especially, aberrant m6A levels upon Mettl14 deficiency markedly decrease neuron stem-cell proliferation and prematurely (9), promotes terminal myeloid differentiation of normal hematopoietic stem and progenitor cells and acute myeloid leukemia cells, inhibits acute myeloid leukemia cell survival/proliferation (10), and disrupts the proper lineage commitment and differentiation of functional stem cells (11). Given the strong correlation of m6A with health and diseases, we focus on exploring the potential role of m6A in β-cells.
In humans, patients with type 2 diabetes (T2D) show lower m6A levels in mRNA in blood, which is related to higher FTO expression (12). Moreover, glucose is involved in the dynamic regulation of m6A in T2D (13). Interestingly, the m6A content was negatively associated with mRNA expression levels of METTL3, METTL14, and FTO. In addition, FTO was positively correlated with serum glucose (13). However, the role of METTL14 in β-cells is unclear. To determine its role in β-cells, we generated β-cell Mettl14-specific knockout (βKO) mice by using mouse insulin promoter (MIP)-CreERT (14). The current study was undertaken to determine whether METTL14 plays a role in regulating β-cell function.
Materials and Methods
MIN6 cell culture
Mouse MIN6 insulinoma cells were cultured in DMEM, as previously reported (15). DMEM was supplemented with 15% fetal bovine serum, antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin), 1 mM sodium pyruvate, 10 mM HEPES, and 50 μM β-mercaptoethanol. The cells were maintained at 37°C in an atmosphere of 5% CO2 and 100% humidity.
Western blot
For Western blot, the blots were probed with antibodies against IRE1α (3294; rabbit; 1:1000; Cell Signaling Technology; RRID: AB_823545) (16), spliced X-box protein binding 1 (sXBP-1; sc-7160; rabbit; 1:500; Santa Cruz Biotechnology; RRID: AB_794171) (17), METTL14 (ARP50652; rabbit; 1:1000; Aviva Systems Biology; RRID: AB_AB_10567380) (18), proinsulin (8138; rabbit; 1:1000; Cell Signaling Technology; RRID: AB_10949314) (19), and actin (A-3853; mouse; 1:5000; Sigma-Aldrich; RRID: AB_262137) (20). Antibody detection was accomplished using enhanced chemiluminescence (PerkinElmer) and the LAS-3000 imaging system (Fuji Film, Stamford, CT). The integrated density of each band was measured using National Institutes of Health ImageJ software (Bethesda, MD).
RNA isolation and real-time quantitative PCR analysis
Total RNA was isolated using the Trizol reagent (Invitrogen), and reverse transcription was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The probe of Ire1α (Mm00470233_m1) and Mettl14 (Mm01318173_m1) was from Invitrogen. Primers used were as follows: Itpr1, forward 5′-CGTTTTGAGTTTGAAGGCGTTT-3′, reverse 5′-CATCTTGCGCCAATTCCCG-3′; Thbs1, forward 5′-GGGGAGATAACGGTGTGTTTG-3′, reverse 5′-CGGGGATCAGGTTGGCATT-3′; Pdia4, forward 5′-TCCCATTGCTGTAGCGAAGAT-3′, reverse 5′-GGGGTAGCCACTCACATCAAAT-3′; Tmem27, forward 5′-TTCCTGGTGACTACTATTCACGC-3′, reverse 5′-AGAGGTATTCTTGATCCGTGTCC-3′; Igfbp5, forward 5′-CCCTGCGACGAGAAAG CTC-3′, reverse 5′-GCTCTTTTCGTTGAGGCAAACC-3′; Ins1, forward 5′-CCTGTTGGTGCACTTCCTA-3′, reverse 5′-TCTGAAGGTCCCCGGGGCT-3′; premature ins2, forward 5′-GGGGAGCGTGGCTTCTTCTA-3′, reverse 5′-GGGGACAGAATTCAGTGGCA-3′; mature ins2, forward 5′-TGGCTTCTTCTACACACCCAAG-3′, reverse 5′-ACAATGCCACGCTTCTGCC-3′; and actin, forward 5′-TCATCACTATTGGCAACGAGCG-3′, reverse 5′- CGGATGTCAACGTCACACTTCA-3′. Relative expression levels of mRNA were determined using the comparative CT method and were normalized to actin levels.
RNA-sequencing, m6A-sequencing, and gene-expression analysis
Polyadenylated RNA was further enriched from total RNA by using the Dynabeads® mRNA Purification Kit (Invitrogen). All samples were sequenced using Illumina Hiseq 4000 at The University of Chicago Genomics Facility. Sequence reads were aligned to mouse transcriptome (GRCm38.vM17 downloaded from genecode) and quantified using Kallisto (21) at pair-end mode with 30 times the bootstrap. Transcript level differential expression analysis was performed using sleuth (22). Inference of differential expression between wild-type (WT) and βKO mice was made by a likelihood ratio test, and log fold change was obtained using the Wald test. The inference leveraged the bootstrap to take the uncertainty of quantification into account and leveraged biological replicates to take into account the biological variability. For m6A-sequencing (m6A-seq), total RNA was extracted from MIN6 cells. After mRNA purification and fragment, m6A-specific antibodies (202 003; rabbit; Synaptic Systems, Goettingen, Germany; RRID: AB_2279214) (23) were used to immunoprecipitate RNA. RNA is reverse transcribed to cDNA and sequenced. Deep sequencing provides high-resolution reads of m6A-methylated RNA. Sequencing reads were aligned to the mouse genome (mm9) by Tophat with Bowtie1 incorporated. Only unique mapping reads were retained for the following analysis. The exome Peak software package was introduced to identify m6A peaks. In brief, readings from immunoprecipitated samples were compared with reads from input samples. The enriched regions were identified and concatenated as a candidate m6A peak. A false-discovery rate < 0.05 was set to filter the low-confidence peaks. Peaks were then annotated to transcriptome, based on University of California Santa Cruz RefSeq database.
Isolation of primary mouse pancreatic islets
Mouse islets were isolated by using collagenase and filtration, as previously described (24). After isolation, islets were cultured for 4 hours in 10 mM glucose RPMI media (containing 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum; pH was adjusted to 7.4 with NaOH) before being handpicked and cultured overnight at 37°C with 5% CO2 and saturated humidity.
Generation of floxed Mettl14 mice
With the use of a traditional recombineering approach, we generated a targeting construct with a single loxP site in intron 6 and a flippase recognition target (FRT)–flanked neomycin-resistant gene, coupled with a loxP site in intron 9 [Fig. 1(A)]. We transfected the targeting construct into 129 mouse embryonic stem cells, selected for neomycin resistance, screened for homologous recombination by Southern blotting, and used selected clones to generate chimeric mice by injection into Black-6 mouse blastocysts. Chimeric mice were bred to WT Black-6 mice to test for germline transmission of the mutant allele, which was identified by PCR. The PCR-positive lines were crossed with commercially available mice containing either a β-actin (Actb), promoter-driven Flp recombinase (to remove the neomycin-resistant gene via FRT site recombination) or an Mx2 promoter-driven Cre recombinase (to remove Mettl14 exons 7–9, as well as the included neomycin gene and FRT sites, via loxP site recombination). Offspring from the Actb-Flp cross harbor a Mettl14 gene whose sixth through ninth exons are loxP flanked (floxed), allowing for subsequent Cre-mediated deletion of the methyltransferase active site encoded within exon 7. Mice with the loxP site were crossed to a transgenic mouse line with MIP-CreERT (14).
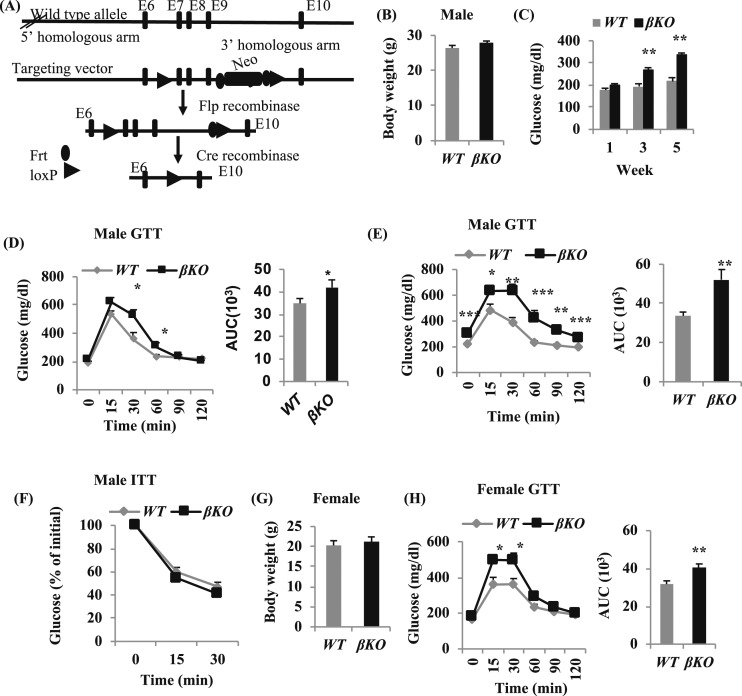
Figure 1.
Specific deletion of Mettl14 in β-cells induces glucose intolerance. (A) Generation of Mettl14 conditional KO mouse. (B) Body weight of 19-wk-old male mice, 8 wk after tamoxifen treatment. (C) Feeding glucose levels of male mice, 1, 3, and 5 wk after tamoxifen treatment. (D and E) Blood glucose levels after intraperitoneal injection of dextrose (2 g/kg) in male mice, (D) 2 wk or (E) 4 wk after tamoxifen treatment. Area under the blood glucose curves (AUC), using the (left) data in the mice. (F) Glucose levels in response to 0.75 U/kg body weight insulin in 16-wk-old male mice, 5 wk after tamoxifen treatment. (G) Body weight of 17-wk-old female mice. (H) Blood glucose levels after intraperitoneal injection of dextrose (2 g/kg) in 17-week-old female mice, 6 wk after tamoxifen treatment. AUC, using the (left) data in the mice. All of the mice are fed a normal chow diet. *P < 0.05, **P < 0.01, ***P < 0.001 compared with WT mice (n = 12 to 15). E, exon; Flp, flippase; Frt, flippase recognition target; GTT, glucose tolerance test; ITT, insulin tolerance test; Neo, neomycin.
In vivo characterization of mice
Mettl14 flox/flox mice in a mixed background (C57BL/6-129/SV) were used for experiments. The mice were fed a normal chow diet (Harlan Teklad) and maintained in a standard 12-hour light/12-hour dark cycle. At the age of 10 weeks, both male and female mice were intraperitoneally injected with 250 µL (20 mg/mL) tamoxifen every other day for three times to delete Mettl14 in β-cells. Intraperitoneal glucose tolerance tests were performed on mice at the age of 15 weeks after a 5-hour fast (2 g/kg dextrose). Insulin levels were measured at 0, 15, and 30 minutes after glucose challenge by using the Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem, Chicago, IL; RRID: AB_2783626) (25). Insulin tolerance tests were performed after a 5-hour fast by administering human recombinant insulin (0.75 U/kg). We quantified the β-cell area from anti-insulin-stained pancreas sections counterstained with hematoxylin using the intensity-thresholding function of the integrated morphometry package in ImageJ. The relative β-cell area was measured from anti-insulin-stained pancreas sections, counterstained with hematoxylin, using ImageJ software. At least 10 pancreatic serial sections (6 μm), spaced 50 μm apart per block, were stained for each animal (n = 3 mice of each group). The ratio of stained islet to the pancreas was calculated, and these same measurements, on at least 10 sections, were performed. At last, the average β-cell mass was calculated by multiplying the β-cell area: pancreas area ratio × pancreatic weight. Ki67 staining was performed, as previously described (15, 26). For Ki67 staining, at least 10,000 β-cells were counted per mouse, and the Ki67 antibody (AB9260; rabbit; 1:300; Millipore; RRID: AB_2142366) (27) was used. All animal experiments in this study were performed under protocols approved by The University of Chicago Animal Studies Committee.
Statistical analysis
The two-tailed unpaired Student’s t test was used to assess the statistical significance of differences between two sets of data. In all experiments, the number of asterisks was used to designate the following levels of statistical significance: *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the Mettl14+/+CreERT+ (WT) group. Results were presented as means ± SEM. Each experiment was repeated three times.
Results
Mettl14-deficient mice develop glucose intolerance
To define the role of METTL14 in vivo, we set up the crosses of global heterozygous mice for Mettl14 and obtained 73 live-born pups. Of these mice, 39 were WT, 34 were heterozygous, and 0 were homozygous for Mettl14, indicating that complete loss of Mettl14 is embryonic lethal. Thus, to study the in vivo-specific function of METTL14 in β-cells, we generated βKO mice using MIP-CreERT+ [exons 7 to 9 would be removed after tamoxifen treatment; Fig. 1(A)]. For male mice after tamoxifen treatment, there was no significant difference in the body weight between Mettl14F/FMIP-CreERT+ (βKO) mice and WT mice [Fig. 1(B)], but glucose levels were changed, 3 and 5 weeks after tamoxifen treatment, and the feeding glucose levels of βKO mice were significantly higher compared with WT mice [Fig. 1(C)]—55.1% higher in βKO mice than those in WT mice, 5 weeks after tamoxifen treatment [Fig. 1(C)]. Even 2 weeks after tamoxifen treatment, βKO mice showed glucose intolerance during glucose tolerance test. The area under the curve (AUC) was 20.3% higher in βKO mice than that in WT mice [Fig. 1(E)]. Four weeks after tamoxifen treatment, βKO mice develop glucose intolerance [Fig. 1(E)]. The AUC was increased by 54.7% in βKO mice [P < 0.01; Fig. 1(E)]. The fasting glucose levels (fasting 5 to 6 hours) were 307.5 ± 24.3 mg/dL in βKO mice and 219.0 ± 7.8 mg/dL in WT mice [P < 0.001; Fig. 1(E)]. However, the response to insulin was similar between βKO and WT mice [Fig. 1(F)].
Like male mice, the female βKO mice also develop glucose intolerance after tamoxifen treatment. The body weight between female WT and βKO mice was not significantly different [Fig. 1(G)], whereas the glucose levels were significantly higher in female βKO mice than in WT mice [Fig. 1(H)], and the AUC was increased by 27.4% in female βKO mice compared with WT mice [P < 0.01; Fig. 1(H)].
Mettl14 deficiency in mice induces a decrease in insulin secretion
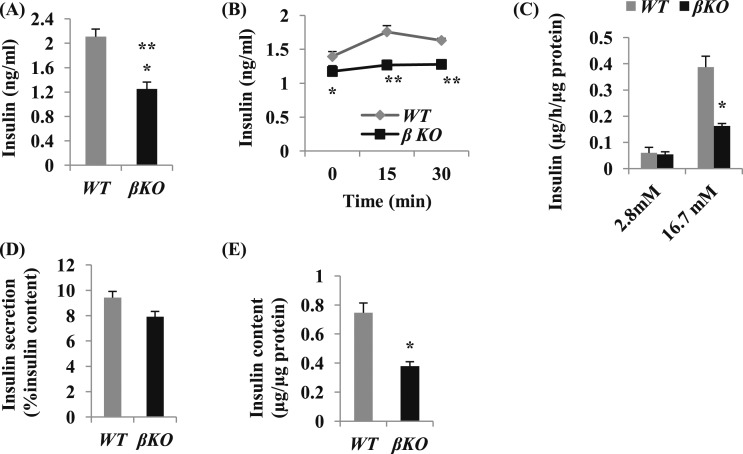
To explore the cellular mechanisms of glucose intolerance in βKO mice, we analyzed the insulin levels. Eight weeks after tamoxifen treatment, the feeding insulin levels in βKO mice were significantly decreased by 58.9% compared with WT mice [P < 0.01; Fig. 2(A)]. The serum insulin levels from βKO mice were significantly lower at 0, 15, and 30 minutes after glucose challenge [Fig. 2(B)]. To confirm further the effect of Mettl14 deficiency on insulin secretion, we isolated islets from βKO and WT mice. The insulin secretion from islets after incubation at 2.8 mM glucose was similar. However, the insulin secretion from βKO islets was less than one-half of that from WT islets after incubation at 16.7 mM glucose [Fig. 2(C)]. Interestingly, the fractional insulin secretion rate, calculated as the percent of insulin content in islets, was similar between these two groups [Fig. 2(D)]. The insulin content in βKO islets was significantly decreased compared with WT islets [P < 0.05; Fig. 2(E)]. These results indicated that METTL14 deficiency in β-cells induces a decrease in insulin secretion related to the reduction of insulin content in βKO islets.
Figure 2.
Mettl14 deficiency in mice induces a decrease in insulin secretion. (A) Random feeding blood insulin levels in the male mice, 8 wk after tamoxifen treatment (n = 12 to 15). (B) Insulin levels measured at 0, 15, and 30 min after intraperitoneal dextrose in male mice, 8 wk after tamoxifen treatment (n = 12 to 15). (C) Insulin release in isolated islets from male mice, 4 wk after tamoxifen treatment. Islets were cultured in Krebs-Ringer-HEPES-bicarbonate medium containing 2.8 mM glucose for 1 h and then transferred to 16.7 mM glucose for 1 h at 37°C. Insulin concentration in the medium and islets was measured by ELISA (n = 3 to 5 mice per group). (D) Insulin secretion percentage of insulin content. Insulin release in (C) was calculated as the percent of insulin content. (E) Insulin content in βKO and WT islets (n = 3 to 5 mice per group). *P < 0.05, **P < 0.01 compared with WT mice.
Mettl14 deficiency in mice induces an increase in β-cell mass
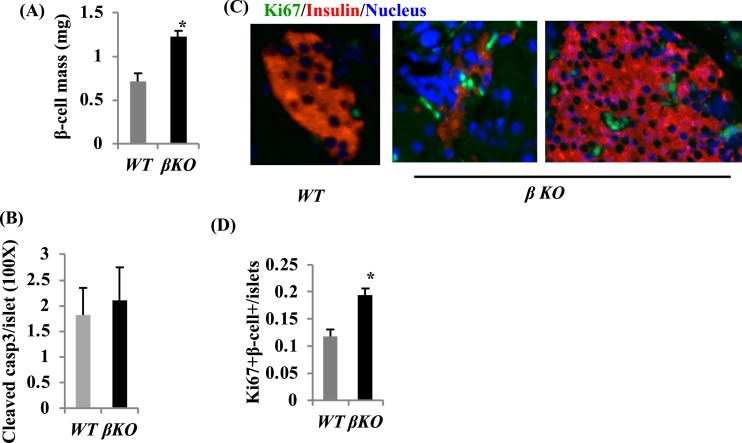
To determine whether the insufficient insulin secretion in βKO mice was also related to decreased β-cell mass, we examined β-cell mass. Surprisingly, β-cell mass in βKO mice was 55.9% higher than that in WT mice [P < 0.05; Fig. 3(A)]. To determine whether increased β-cell mass in βKO mice is related to decreased β-cell death and/or increased β-cell proliferation, we measured β-cell death by cleaved caspase3 staining and β-cell proliferation by Ki67 staining. There is no significant different in cleaved caspase3-positive β-cells between βKO and WT islets [Fig. 3(B)]. However, Ki67 staining showed that β-cell proliferation in βKO islets was increased by 1.3-fold more than that in WT islets [Fig. 3(C) and 3(D)]. These results indicate that the insufficient insulin secretion in βKO mice was not related to β-cell mass, and the increased β-cell mass in βKO mice mainly relies on β-cell proliferation.
Figure 3.
β-Cell mass is increased in Mettl14-deficient mice. (A) β-Cell mass in male βKO mice, 8 wk after tamoxifen treatment. Histological analysis of pancreatic islets and quantitation of group data for β-cell mass (n = 3 to 4 per group). (B) Cleaved caspase3 (casp3) staining in islets. (C) Ki67+ labeling of pancreatic β-cells from 18-week-old mice; original magnification, ×200. (D) Quantitative Ki67+β-cells data are shown (n = 3 to 5 per group). *P < 0.05 compared with WT mice.
Mettl14 deficiency induces upregulation of IRE1α
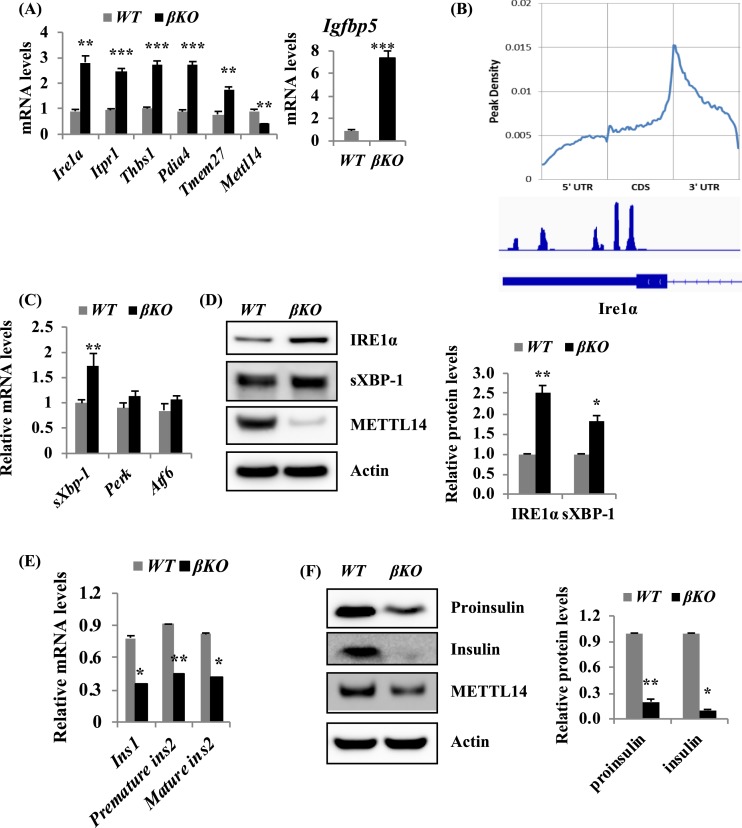
To understand the molecular mechanism of glucose intolerance and the insufficient insulin secretion, we performed RNA-sequencing to detect the gene expression in βKO and WT islets isolated from male mice, 8 weeks after tamoxifen treatment. RNA-sequencing results showed that expression levels of 106 transcripts were significantly altered in βKO islets compared with WT islets (28). Among those altered genes, 104 genes were upregulated (P < 0.001), and 2 genes were downregulated. The genes responsible for endoplasmic reticulum (ER) stress, such as Ire1α (Ern1), Itpr1, Thbs1, Pdia4, Igfbp5, and Tmem27, were among the top upregulated genes (29). Quantitative RT-PCR analysis confirmed that mRNA levels of these genes were increased at least by twofold [Fig. 4(A)]. As we cannot get enough mRNA from islets for m6A immunoprecipitation to detect directly levels of m6A methylation on each transcript in β-cells, we applied m6A-seq in MIN6 cells and confirmed that m6A modification was enriched at the 3′-UTR region, with a consensus sequence of U (A/C) GGAC in MIN6 cells [Fig. 4(B)]. These results suggest a potential role for m6A in regulating gene expression in β-cells. In addition, Ire1α, an ER stress gene with transmembrane kinase and endoribonuclease activity, was indeed modified by m6A in its last exon and the 3′-UTR region [Fig. 4(B)]. Quantitative RT-PCR analysis further confirmed that mRNA levels of Ire1α and sXbp-1, one target gene of Ire1α, were significantly increased in βKO islets [Fig. 4(A) and 4(C)]. The mRNA levels of Perk and Atf6 were not significantly altered [Fig. 4(C)]. We further confirmed that the protein levels of IRE1α and sXBP-1 were increased in βKO islets [Fig. 4(D)]. As activated IRE1α can cleave insulin mRNA (30), we examined the mRNA and protein levels of insulin. Indeed, the mRNA levels of Ins1, premature ins2, and mature ins2 were significantly decreased in βKO islets [Fig. 4(E)]. Consistently, the protein levels of proinsulin and insulin were decreased in βKO islets compared with WT islets [Fig. 4(F)].
Figure 4.
Mettl14 deficiency induces ER stress. (A) Quantitative RT-PCR analysis of expression levels of a number of genes involved in the ER stress in islets from male mice, 4 wk after tamoxifen treatment. (B) m6A peak density in mRNA in MIN6 cells. Four days after METTL14 knockdown, m6A-seq analysis of MIN6 cells shows m6A enrichment at the 3′-UTR region and confirms the Ire1α gene is one of the m6A targets. (C) Quantitative RT-PCR analysis of expression levels of sXbp-1, Perk, and Atf6 in islets from male mice, 4 wk after tamoxifen treatment. (D) Protein levels of IRE1α and sXBP-1 in islets isolated from male mice, 8 wk after tamoxifen treatment. (E) Quantitative RT-PCR analysis of expression levels of a number of genes involved in the insulin secretory pathway in islets from male mice, 4 to 6 wk after tamoxifen treatment (n = 3). (F) Protein levels of proinsulin and insulin in islets isolated from male mice, 6 to 8 wk after tamoxifen treatment. *P < 0.05, **P < 0.01, ***P < 0.001 compared with WT mice. CDS, coding sequences.
Discussion
It has been demonstrated that m6A-RNA methylation by the m6A methyltransferase complex (METTL14, METTL3, and WTAP) has a pivotal role in promoting adipogenesis in vitro and the development of obesity in vivo (31, 32). In addition, m6A is reduced in blood mRNA of patients with T2D (13), suggesting that m6A-RNA methylation may be involved in the pathogenesis of T2D. The present experiments demonstrated that Mettl14 deficiency in β-cells induced a decrease in insulin secretion, resulting in glucose intolerance. Interestingly, β-cell mass was increased in βKO mice, which is related to an increase in β-cell proliferation. One potential explanation for the increased β-cell mass in βKO mice is the compensation for the insufficient insulin secretion.
In β-cells, the ER has an essential role for insulin synthesis, folding, and secretion of insulin. The disruption in ER homeostasis and accumulation of unfolded and misfolded protein in the ER lumen cause ER stress. ER stress is sensed by three ER transmembrane proteins: IRE1, PERK, and ATF6. IRE1 is an ER transmembrane kinase with endoribonuclease activity. In response to ER stress, IRE1 is activated and leads to activation of its endoribonuclease activity and unconventional splicing of XBP-1 mRNA, which regulates chaperone and ER-associated protein degradation protein expression (30). The current studies also demonstrate that Mettl14 deficiency in β-cells induces the increase in IRE1α and sXBP-1 mRNA and protein. In addition to cleavage of XBP-1, IRE1α plays a role in the cleavage of other ER-associated mRNAs, such as insulin mRNA (30). Thus, the increase in IRE1α and sXBP-1 in βKO islets may be the reason for the decrease in mRNA levels of insulin. However, as the IRE1α–sXBP-1 pathway is essential for the glucose response and protection of β-cells (33), whether increased IRE1α also has a protective role in Mettl14-deficient β-cells needs to be further investigated.
In this study, we found that 104 transcripts were upregulated among the altered transcripts in islets from βKO mice. Studies on cultured cell lines have established that one important function of m6A methylation was to decrease RNA stability, as a result of accelerated decay (34). Previous studies suggest that m6A-mediated mRNA decay operates through YTHDF2 as a major pathway in transcriptome switching during cell differentiation and development (35–37). Upregulation of mRNAs after Mettl14 deletion was expected, but downregulation of mRNAs, such as insulin, was surprising. Thus, the effect of METTL14 and other writer proteins on transcriptome in β-cells needs to be further investigated.
It also remains to be determined whether downregulation of mRNAs after Mettl14 deficiency in β-cells is a result of the unique function of m6A methylation in β-cells, mediated through other reader proteins, or is caused by the secondary effect following chronically altered β-cell functions, as a result of Mettl14 deficiency.
In summary, we reported that METTL14 deficiency in β-cells induces glucose intolerance and a decrease in insulin secretion. We found that the increase in β-cell mass in βKO mice was related to β-cell proliferation and also observed elevated mRNA and protein levels of Ire1α and sXBP-1 in βKO islets. As METTL14 deficiency in β-cells induces glucose intolerance, it is possible that a selective increase of METTL14 in β-cells may be beneficial in diabetes treatment.
Acknowledgments
The authors thank Dr. Kenneth Polonsky for insightful suggestions, advice, and discussions.
Financial Support: This study was supported by Grant P30-DK020595 (from the Diabetes Research and Training Center, The University of Chicago).
Author Contributions: D.R. designed research, analyzed data, and wrote the paper. L.M., J.S., G.L., and D.R. performed research.
Glossary
Abbreviations:
- AUC
area under the curve
- ER
endoplasmic reticulum
- FTO
fat mass and obesity-associated protein
- m6A
N6-methyladenosine
- m6A-seq
N6-methyladenosine-sequencing
- METTL
methyltransferase-like
- MIP
mouse insulin promoter
- sXbp-1
spliced X-box protein binding 1
- T2D
type 2 diabetes
- UTR
untranslated region
- WT
wild-type
- WTAP
Wilms tumor 1-associating protein
- YTHDF
YTH domain family member
- βKO
β-cell METTL14-specific knockout
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, Cheng T, Gao M, Shu X, Ma H, Wang F, Wang X, Shen B, Wang Y, Feng X, He C, Liu J. VIRMA mediates preferential m6A mRNA methylation in 3'UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hsu PJ, He C. Identifying the m6A methylome by affinity purification and sequencing. Methods Mol Biol. 2018;1649:49–57. [DOI] [PubMed] [Google Scholar]
- 4. Cao G, Li HB, Yin Z, Flavell RA. Recent advances in dynamic m6A RNA modification. Open Biol. 2016;6(4):160003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, Kreim N, Andrade-Navarro MA, Poeck B, Helm M, Roignant JY. m6A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540(7632):242–247. [DOI] [PubMed] [Google Scholar]
- 6. Xu K, Yang Y, Feng GH, Sun BF, Chen JQ, Li YF, Chen YS, Zhang XX, Wang CX, Jiang LY, Liu C, Zhang ZY, Wang XJ, Zhou Q, Yang YG, Li W. Mettl3-mediated m6A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27(9):1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG, Riggs AD, He C, Shi Y. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Reports. 2017;18(11):2622–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y, Wang G, Broughton JP, Chen YG, Kluger Y, Simon MD, Chang HY, Yin Z, Flavell RA. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548(7667):338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Li Y, Yue M, Wang J, Kumar S, Wechsler-Reya RJ, Zhang Z, Ogawa Y, Kellis M, Duester G, Zhao JCN. N6-Methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications [published correction appears in Nat Neurosci. 2018;21(8):1139]. Nat Neurosci. 2018;21(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, Shi H, Skibbe J, Shen C, Hu C, Sheng Y, Wang Y, Wunderlich M, Zhang B, Dore LC, Su R, Deng X, Ferchen K, Li C, Sun M, Lu Z, Jiang X, Marcucci G, Mulloy JC, Yang J, Qian Z, Wei M, He C, Chen J. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell. 2018;22(2):191–205.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng TG, Lu X, Guo L, Hou GM, Ma XS, Li QN, Huang L, Fan LH, Zhao ZH, Ou XH, OuYang YC, Schatten H, Li L, Wang ZB, Sun QY. Mettl14 is required for mouse postimplantation development by facilitating epiblast maturation. FASEB J. 2019;33(1):1179–1187. [DOI] [PubMed] [Google Scholar]
- 12. Shen F, Huang W, Huang JT, Xiong J, Yang Y, Wu K, Jia GF, Chen J, Feng YQ, Yuan BF, Liu SM. Decreased N(6)-methyladenosine in peripheral blood RNA from diabetic patients is associated with FTO expression rather than ALKBH5. J Clin Endocrinol Metab. 2015;100(1):E148–E154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Shen F, Huang W, Qin S, Huang JT, Sergi C, Yuan BF, Liu SM. Glucose is involved in the dynamic regulation of m6A in patients with type 2 diabetes. J Clin Endocrinol Metab. 2019;104(3):665–673. [DOI] [PubMed] [Google Scholar]
- 14. Wicksteed B, Brissova M, Yan W, Opland DM, Plank JL, Reinert RB, Dickson LM, Tamarina NA, Philipson LH, Shostak A, Bernal-Mizrachi E, Elghazi L, Roe MW, Labosky PA, Myers MG Jr, Gannon M, Powers AC, Dempsey PJ. Conditional gene targeting in mouse pancreatic ß-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59(12):3090–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ren D, Sun J, Wang C, Ye H, Mao L, Cheng EH, Bell GI, Polonsky KS. Role of BH3-only molecules Bim and Puma in β-cell death in Pdx1 deficiency. Diabetes. 2014;63(8):2744–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. RRID:AB_823545, https://scicrunch.org/resolver/AB_823545.
- 17. RRID:AB_794171, https://scicrunch.org/resolver/AB_794171.
- 18. RRID:AB_10567380, https://scicrunch.org/resolver/AB_10567380.
- 19. RRID:AB_10949314, https://scicrunch.org/resolver/AB_10949314.
- 20. RRID:AB_262137, https://scicrunch.org/resolver/AB_262137.
- 21. Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification [published correction appears in Nat Biotechnol. 2016;34(8):888]. Nat Biotechnol. 2016;34(5):525–527. [DOI] [PubMed] [Google Scholar]
- 22. Pimentel H, Bray NL, Puente S, Melsted P, Pachter L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat Methods. 2017;14(7):687–690. [DOI] [PubMed] [Google Scholar]
- 23. RRID:AB_2279214, https://scicrunch.org/resolver/AB_2279214.
- 24. Johnson JD, Ahmed NT, Luciani DS, Han Z, Tran H, Fujita J, Misler S, Edlund H, Polonsky KS. Increased islet apoptosis in Pdx1+/− mice. J Clin Invest. 2003;111(8):1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. RRID:AB_2783626, https://scicrunch.org/resolver/AB_2783626.
- 26. Chintinne M, Stangé G, Denys B, Ling Z, In ’t Veld P, Pipeleers D. Beta cell count instead of beta cell mass to assess and localize growth in beta cell population following pancreatic duct ligation in mice. PLoS One. 2012;7(8):e43959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. RRID:AB_2142366, https://scicrunch.org/resolver/AB_2142366.
- 28. Men L, Sun J, Luo G, Ren D. Data from: Acute deletion of METTL14 in β-cells of adult mice results in glucose intolerance. figshare 2019. Deposited 12 July 2019. https://figshare.com/articles/Acute_Deletion_of_METTL14_in_-cells_of_adult_mice_results_in_glucose_intolerance/8850860. [DOI] [PMC free article] [PubMed]
- 29. Men L, Sun J, Luo G, Ren D. Data from: Acute deletion of METTL14 in β-cells of adult mice results in glucose intolerance. figshare 2019. Deposited 12 July 2019. https://figshare.com/articles/Acute_Deletion_of_METTL14_in_-cells_of_adult_mice_results_in_glucose_intolerance/8861309. [DOI] [PMC free article] [PubMed]
- 30. Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab. 2011;22(7):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kobayashi M, Ohsugi M, Sasako T, Awazawa M, Umehara T, Iwane A, Kobayashi N, Okazaki Y, Kubota N, Suzuki R, Waki H, Horiuchi K, Hamakubo T, Kodama T, Aoe S, Tobe K, Kadowaki T, Ueki K. The RNA methyltransferase complex of WTAP, METTL3, and METTL14 regulates mitotic clonal expansion in adipogenesis. Mol Cell Biol. 2018;38(16):e00116-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loos RJ, Yeo GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hassler JR, Scheuner DL, Wang S, Han J, Kodali VK, Li P, Nguyen J, George JS, Davis C, Wu SP, Bai Y, Sartor M, Cavalcoli J, Malhi H, Baudouin G, Zhang Y, Yates JR III, Itkin-Ansari P, Volkmann N, Kaufman RJ. The IRE1α/XBP1s pathway is essential for the glucose response and protection of β cells. PLoS Biol. 2015;13(10):e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C. N6-Methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, Wu L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7(1):12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao BS, He C. “Gamete on” for m6A: YTHDF2 exerts essential functions in female fertility. Mol Cell. 2017;67(6):903–905. [DOI] [PubMed] [Google Scholar]
- 37. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-Methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.