Abstract
Context
Underlying mechanisms leading to gestational diabetes mellitus (GDM) are still under investigation, and it is unclear whether the placenta plays a role in triggering glucose intolerance or if its functions are modified in response to the hyperglycemia. Circulating miRNAs are involved in placental development and function and are encapsulated in extracellular vesicles (EVs).
Objective
To compare differential expression of miRNAs in circulating EVs in pregnancies complicated by GDM vs controls.
Methods
This was a case-control study nested in a prospective pregnancy cohort including 23 women with GDM and 46 matched controls. The presence of serum EVs in early pregnancy was validated by transmission electron microscopy. Placental dimensions were assessed at 11 to 13 weeks of gestation. Differential expression of 17 miRNAs encapsulated in EVs (miR‒122-5p, miR‒132-3p, miR-1323, miR‒182-3p, miR‒210-3p, miR‒29a-3p, miR‒29b-3p, miR‒342-3p, miR‒517-5p, miR‒517a-3p, miR‒518b, miR-520h, miR‒525-5p, miR‒136-5p, miR‒342-3p, miR‒376c-5p, and miR‒494-3p) was assessed using quantitative reverse transcription PCR.
Results
EVs were present in the early phase of placentation (6 to 15 weeks of gestation) in both cases and controls. No differences were observed for placental dimensions and estimated placental volume between GDM and control groups. Ten miRNAs (miR‒122-5p; miR‒132-3p; miR‒1323; miR‒136-5p; miR‒182-3p; miR‒210-3p; miR‒29a-3p; miR‒29b-3p; miR‒342-3p, and miR-520h) showed significantly higher levels in GDM cases than in controls (P ≤ 0.05). Bioinformatics analysis showed that these miRNAs are involved in trophoblast proliferation/differentiation as well as in insulin secretion/regulation and glucose transport in pregnant women.
Conclusion
The miRNA content of blood EVs may be a promising avenue for studying the early effect of impaired glucose metabolism on placental development.
Gestational diabetes mellitus (GDM) is defined as glucose intolerance resulting in maternal hyperinsulinemia and hyperglycemia, with onset or first diagnosis during pregnancy and in the absence of existing type 1 or type 2 diabetes mellitus (1, 2). According to Diabetes Canada, 3% to 20% of pregnant women across Canada develop GDM depending on their risks factors (i.e., ethnic background, advanced maternal age, or obesity), and its worldwide prevalence is still increasing mainly as a result of the obesity pandemic (1–4). Underlying mechanisms leading to GDM are still under investigation, and it is unclear whether the placenta plays a role in triggering glucose intolerance or if its functions are modified in response to the hyperglycemic milieu. It has been proposed that the placenta may play a crucial role in protecting the fetus from adverse effects of the maternal diabetic milieu. This milieu may result in changes in placental function, particularly with respect to the uptake, transfer, and/or utilization of glucose (5, 6).
Screening for GDM is usually done between 24 and 28 weeks of pregnancy, when placentation is already established, meaning that the opportunity to reverse adaptive placental dysfunction and fetal complications is limited (7–9). Indeed, Hinkle et al. (10) reported significantly higher levels of glycated hemoglobin during the first trimester, which reflects glucose levels over the previous 4 to 8 weeks, in women who later developed GDM than in women with normal pregnancies. This supports the hypothesis that women who develop GDM may have impaired glucose homeostasis early in pregnancy, which could affect placental development. Recently, several studies reported placental epigenetic changes associated with GDM (11), including DNA methylation in term placentas (12, 13), altered expression of miRNAs in the second and third trimesters (14), and placental pathophysiological changes, such as inflammation and endocrine perturbation (15–17). The placenta produces hundreds of miRNAs, which are released into the maternal circulation encapsulated in extracellular vesicles (EVs) (18, 19). Several of these miRNAs are unique to the placenta and are coregulated in clusters (e.g., cluster chromosome 14 and cluster chromosome 19) in a time-dependent manner (14).
In this study, we used miRNAs encapsulated in EVs (EV-miRNAs) to characterize early changes in placental physiology. EVs are an important mode of cell-cell communication, capable of modifying the activity of the targeted cells; their content reflects the cytoplasmic composition of their parent cells, thus representing an intracellular “proxy” for specific organs. Their content in miRNAs, which regulate gene expression, is particularly interesting in obstetrics given the implication of EV-miRNAs in normal placenta formation and function (i.e., proliferation, migration, and invasion of extravillous trophoblasts) (20), as well as in inflammation, regulation of glucose levels, dyslipidemia, and insulin resistance, all of which are relevant for GDM (14, 15, 21–23). In this study, we investigated the ability of miRNAs to indicate specific pathophysiological placental signatures. Specifically, we measured the levels of 17 miRNAs found to be associated with adverse outcomes in pregnancy, including six miRNAs that are known to be of placental origin (miR‒517-5p, miR‒517a-3p, miR-518b, miR-520h, miR‒525-5p, and miR-1323), four miRNAs specific to the pregnancy state (miR‒136-5p, miR‒342-3p, miR‒376c-5p, and miR‒494-3p), and seven miRNAs involved in the pathophysiological process of diabetes (miR‒29a-3p, miR‒29b-3p, miR‒122-5p, miR‒132-3p, miR‒182-3p, miR‒210-3p, and miR‒342-3p) in serum EVs from pregnant women who later developed GDM (n = 23) compared with women with normal pregnancies (n = 46).
Materials and Methods
Selection of participants
Pregnant women were enrolled between 6 and 15 weeks of gestation at the Centre Hospitalier Universitaire in Sherbrooke (CHUS), QC, Canada. GDM pregnancies (n = 23) were diagnosed as women in whom the 50-g oral glucose tolerance test (OGTT) revealed a 1-hour posttest venous plasma glucose level of >10.3 mmol/L. If the 1-hour posttest venous plasma glucose level was ≥7.2 mmol/L and ≤10.3 mmol/L, a 2-hour 75-g OGTT was done. GDM was confirmed for this 2-hour 75-g OGTT if one of the following values was met or exceeded: fasting venous plasma glucose level ≥5.3 mmol/L, 1-hour plasma glucose level ≥10.6 mmol/L, or 2-hour plasma glucose level ≥9.0 mmol/L according to the guidelines of the Society of Obstetricians and Gynaecologists of Canada (8).
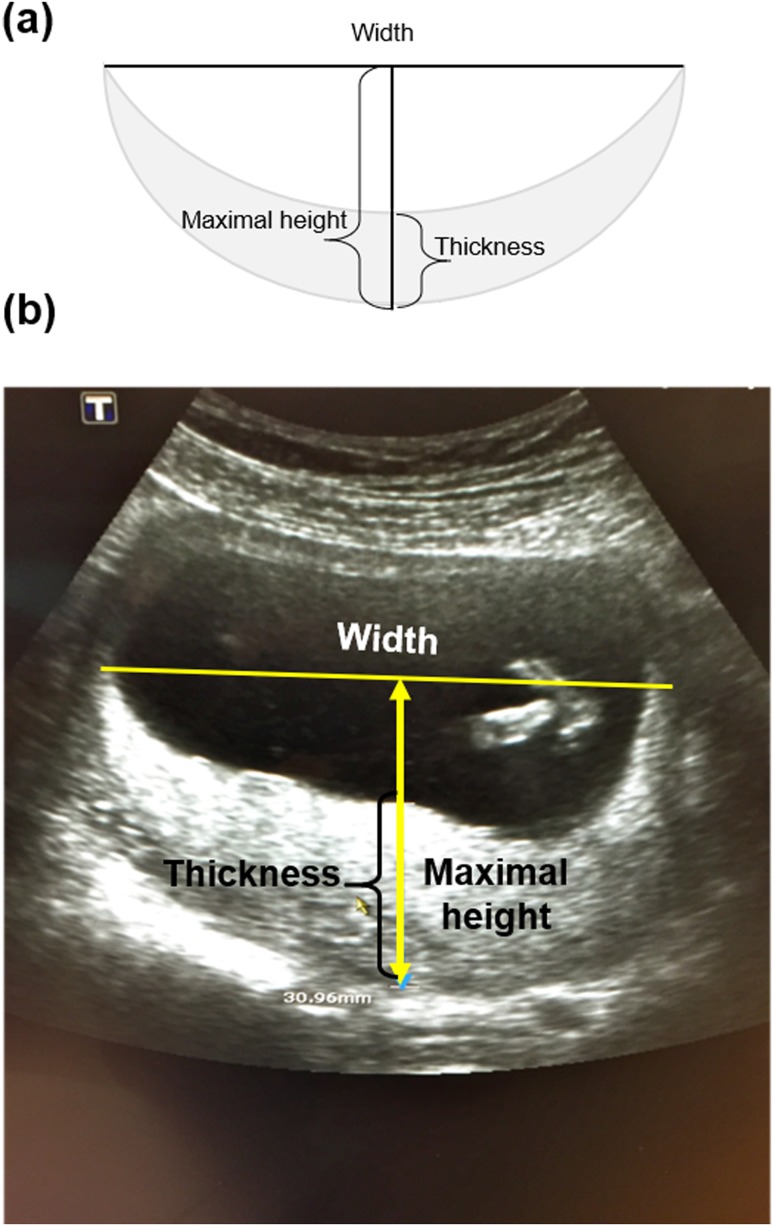
Control pregnancies (n = 46) were defined as those without any complications, resulting in healthy infants and birth weights of ≥2700 g after 37 weeks of gestation with vaginal or cesarean section delivery. Two controls per case were selected to obtain two groups balanced for gestational age (±6 days) at blood sampling. Gestational age was determined using crown-rump length between 11 and 13 weeks of pregnancy. Placental location and shape were assessed using standard two-dimensional ultrasound techniques at 11 to 13 weeks of gestation. When possible, placental thickness was measured at the level of the cord insertion, thus maintaining close proximity to the perpendicular of the placental surface (24). Maximal width and height were acquired from an image of the placenta as illustrated in Fig. 1. Measurements to estimate placental volume used the following convex-concave shell formula: , where V = volume, W = maximal width, H = maximal height, and T = thickness at maximal height. We collected the length measurements only when we were able to visualize both edges of the placenta. Placental measurements were available for 35 pregnancies (14 GDM and 21 controls).
Figure 1.
(a) Schema of the placenta and (b) representative grayscale two-dimensional ultrasonographic image of the placenta in the first trimester of gestation demonstrating the thickness, width, and maximal height measurements used to calculate an EPV. The height is always greater than the thickness, and when the placenta is flat, the thickness and height have the same measurement. EPV, estimated placental volume.
The study was approved by the ethics committee of the CHUS (IRB no. 05-057-S1). All participants provided written informed consent.
Isolation and morphology of EVs
To confirm the presence of EVs in our samples, we isolated EVs from serum samples of women with GDM (n = 2) and controls (n = 2). Briefly, 500 μL of serum samples were thawed on ice and then diluted with an equal volume of filtered (0.2 μm) 1× PBS. To isolate intact EVs for subsequent characterization by transmission electron microscopy (TEM), we used the differential centrifugation method of reference for EV isolation as described (25). Briefly, the serum was centrifuged at 16,500g for 20 minutes at 4°C; the supernatant was filtered through a 0.45-μm filter (Cat No. 16555; Sartorius, Göttingen, Germany) to remove apoptotic bodies and large aggregates and then centrifuged at 120,000g [Ultracentrifuge Sorvall Discovery 90 with the rotor T-8100 (K Factor 17.7; Thermo Fischer Scientific, Waltham, MA)] for 90 minutes at 4°C. The final EV pellet was resuspended in filtered (0.1 µm) PBS 1×. For the characterization of EVs by TEM, purified EVs were applied to a formvar carbon-coated nickel grid for 60 minutes at room temperature. After three washing steps with filtered (0.1 µm) PBS, the grid containing the EVs was placed in 2% paraformaldehyde for 10 minutes to allow fixation and then placed in a solution of primary antibody, a mouse monoclonal IgG2a placental alkaline phosphatase (PLAP) antibody diluted 1:50 (to working concentration at 4 µg/mL) in filtered (0.1 µm) PBS [PLAP antibody (8B6): sc-47691; Santa Cruz Biotechnology Inc., Dallas, TX]. Then, 12 nm Colloidal Gold-AffiniPure goat anti-mouse IgG secondary antibody (Cat No. 115-205-166; Jackson ImmunoResearch Laboratories Inc., Baltimore, PA) diluted 1:20 in filtered (0.1 µm) PBS was applied for 60 minutes at room temperature, and samples were fixed with 2.5% glutaraldehyde. Contrast was created with 2% uranyl acetate. Samples were embedded in 0.4% (w/v) uranyl acetate and 1.8% (w/v) methylcellulose. Finally, excess liquid was removed, the grid was allowed to dry at room temperature, and the sample was then viewed in a TEM (Hitachi H-7500; Tokyo, Japan).
Blood collection and total RNA extraction from EVs
Blood samples were obtained at enrollment, between 6 and 15 weeks of gestation. Four milliliters of maternal peripheral blood was collected in serum separation tubes (Cat. No 367989; Becton Dickinson, Franklin Lakes, NJ) and centrifuged at 2000g for 20 minutes at 4°C within 1 hour after collection to separate the plasma fraction. Aliquots of cell-free serum were immediately stored at −20°C for downstream analysis. For the isolation of EVs and total RNA extraction, serum samples (1 mL) were thawed on ice and filtered through a 0.8-μm membrane unit (MiniSart sterile syringe filters, Cat No. 16592; Sartorius). After filtering, total RNA was extracted from EVs using the exoRNeasy Serum/Plasma Maxi kit (Cat No. 77064; Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Quantification of miRNA was done using the Qubit® microRNA Assay Kit (Cat No. Q32880; Life Technologies, Carlsbad, CA) and the Qubit® 2.0 Fluorometer (Thermo Fisher Scientific).
Quantitative real-time PCR
We conducted a literature review and identified 17 miRNA candidates that were most frequently associated with GDM disorders or involved in placental function. Details of these miRNAs are shown in Table 1. Primer design for these miRNAs was conducted using the miRbase v21 database, as described by Griffiths-Jones et al. (26), and validation was done as described by Brosseau et al. (27). In every quantitative PCR (qPCR) run, a no-template control was performed for each primer pair, and these were consistently negative. Primer sequences are provided in Table 2. Reverse transcription (RT) was performed using 10 ng of RNA with Maxima Enzyme Mix (Maxima First Strand cDNA Synthesis Kit for RT-qPCR, Cat No. K1671; Thermo Fisher Scientific), stem-loop RT specific primer (shared-mir_rev; see Table 2), deoxyribonucleotide triphosphates (Cat No. R0181; Roche Diagnostics, Bale, Switzerland), and 10 units of RNAseOUT (Cat No. 10777019; Invitrogen, Carlsbad, CA) in a total volume of 20 µL according to manufacturer’s protocol. Forward and reverse primers were individually resuspended to 20 to 100 μM stock solution in Tris-EDTA buffer and diluted as a primer pair to 1 μM in RNase DNase-free water (Cat No. AM9935; Invitrogen). All qPCR reactions were performed in 384-well plates on a CFX-384 thermocycler (Bio-Rad; Hercules, CA) iTaq Universal SYBR Green Supermix (Cat No. 17225122; Bio-Rad). Relative expression quantification was calculated using the qBase method (41) with standard error propagation; the spike-in control synthetic Caenorhabditis elegans miR-39-5p (Cat No. 217184; Qiagen) was used as a reference gene because it shows no sequence homology to any human miRNAs. Calculations were performed from the triplicate cycle threshold readings (CFX384 Real-Time System; Bio-Rad) using single well regression analysis for cycle threshold attribution. Assay layouts were designed to avoid any interrun bias by loading all samples in a single run for each miRNA target.
Table 1.
Genomic Location, Sequence, and Biological Relevance of Selected miRNAs
| miRNAs (miRBase ID) | Genomic Location | miRNA Sequence | Related Roles/Functions |
|---|---|---|---|
| hsa-miR‒517-5pa | 19: 53721076-53721142 [+] | 5′-CCUCUAGAUGGAAGCACUGUCU-3′ | Trophoblast cell proliferation, conferment of viral resistance to nonplacental maternal cell (28, 29) |
| hsa-miR‒517a-3pa | 19: 53712268-53712354 [+] | 5′-AUCGUGCAUCCCUUUAGAGUGU-3′ | |
| +hsa-miR-518ba | 19: 53702737-53702819 [+] | 5′-CAAAGCGCUCCCCUUUAGAGGU-3′ | |
| hsa-miR-520ha | 19: 53742512-53742599 [+] | 5′-ACAAAGUGCUUCCCUUUAGAGU-3′ | |
| hsa-miR‒525-5pa | 19: 53697533-53697617 [+] | 5′-CUCCAGAGGGAUGCACUUUCU-3′ | |
| hsa-miR-1323a | 19: 53671968-53672040 [+] | 5′-UCAAAACUGAGGGGCAUUUUCU-3′ | |
| hsa-miR‒136-5pb | 14: 100884702-100884783 [+] | 5′-ACUCCAUUUGUUUUGAUGAUGGA-3′ | Cellular proliferation, migration, and invasion (29, 30) |
| hsa-miR‒342-3pb | 14: 100109655-100109753 [+] | 5′-UCUCACACAGAAAUCGCACCCGU-3′ | |
| hsa-miR‒376c-5pb | 14: 101039690-101039755 [+] | 5′-GGUGGAUAUUCCUUCUAUGUU-3′ | |
| hsa-miR‒494-3pb | 14: 101029634-101029714 [+] | 5′-AGGUUGUCCGUGUUGUCUUCUCU-3′ | |
| hsa-miR‒29a-3p | 7: 130212046 - 130212109 [−] | 5′-UAGCACCAUCUGAAAUCGGUUA-3′ | Modulation of angiogenic properties of human endothelial cells (31), upregulated in β cells by glucose, and decreased glucose-stimulated insulin secretion (22, 34) |
| hsa-miR‒29b-3p | 7: 130212758 - 130212838 [−] | 5′-UAGCACCAUUUGAAAUCAGUGUU-3′ | Apoptosis, invasion and angiogenesis of trophoblast cells (32), and insulin-stimulated glucose uptake (33) |
| hsa-miR‒122-5p | 18: 54269286 - 54269370 [+] | 5′-UGGAGUGUGACAAUGGUGUUUG-3′ | Vascular inflammation and diabetes (34) |
| hsa-miR‒132-3p | 17: 2049908-2050008 [−] | 5′-UAACAGUCUACAGCCAUGGUCG-3′ | Dyslipidemia and diabetes (23) |
| hsa-miR‒182-3p | 7: 129770383-129770492 [−] | 5′-UGGUUCUAGACUUGCCAACUA-3′ | Type 2 diabetes pathogenesis (35), insulin resistance, and coronary artery disease (36, 37) |
| hsa-miR‒210-3p | 11: 568089-568198 [−] | 5′-CUGUGCGUGUGACAGCGGCUGA-3′ | Hypoxia, insulin resistance (38), and antiangiogenesis (29, 39) |
| hsa-miR‒483-3p | 11: 2134134-2134209 [−] | 5′-UCACUCCUCUCCUCCCGUCUU-3′ | Insulin resistance, onset diabetes mellitus, and cardiovascular disease (40) |
miRNAs belonging to the chromosome 19 miRNA cluster, which are placenta-specific miRNAs.
miRNAs belonging to the chromosome 14 miRNA cluster, which are pregnancy-related miRNAs highly expressed in the placenta.
Table 2.
Sequences of the Primers Used for miRNA Analysis by Quantitative Real-Time PCR
| qPCR Forward Primer | Specific RT Primer | |
|---|---|---|
| cel-miR‒39-5p.SpFwd2 | GCGCTCACCGGGTGTAAATC | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAAGCT |
| hsa-miR‒122-5p.SpFwd2 | GCGCTGGAGTGTGACAATGG | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAAACA |
| hsa-miR‒132-3p.SpFwd2 | CGCTGTTAACAGTCTACAGCCA | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCGACCA |
| hsa-miR‒1323.SpFwd2 | GCGTCAAAACTGAGGGGCA | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGAAAA |
| hsa-miR‒136-5p.SpFwd2 | TGGTCGGACTCCATTTGTTTTGAT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCCATC |
| hsa-miR‒182-3p.SpFwd2 | GCTGTCGTGGTTCTAGACTTGC | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTAGTTG |
| hsa-miR‒210-3p.SpFwd2 | GCGCTGTGCGTGTGACAGC | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAGCC |
| hsa-miR‒29a-3p.SpFwd2 | GCTGTCGTAGCACCATCTGAAAT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTAACCG |
| hsa-miR‒29b-3p.SpFwd2 | GCTGTCGTAGCACCATTTGAAATC | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACACT |
| hsa-miR‒342-3p.SpFwd2 | GCGCTCTCACACAGAAATCGC | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACGGGT |
| hsa-miR‒376c-5p.SpFwd2 | GCTGTCGGGTGGATATTCCTTC | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACATA |
| hsa-miR‒483-3p.SpFwd2 | GCGCTCACTCCTCTCCTCC | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAGACG |
| hsa-miR‒494-3p.SpFwd2 | GCGCTGAAACATACACGGGA | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGAGGTT |
| hsa-miR‒517-5p.SpFwd2 | GCGCCCTCTAGATGGAAGCA | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGACAG |
| hsa-miR‒517a-3p.SpFwd2 | GCGCATCGTGCATCCCTTTA | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACACTC |
| hsa-miR‒518b.SpFwd2 | GCGCAAAGCGCTCCCCTTT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACCTCT |
| hsa-miR‒520h.SpFwd2 | GCGCACAAAGTGCTTCCCTT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACTCTA |
| hsa-miR‒525-5p.SpFwd2 | GCGCTCCAGAGGGATGCA | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGAAAG |
| qPCR reverse primer | GTGCAGGGTCCGAGGT | Shared-mir_reva |
Primer that targets the added stem-loop sequence in reverse transcription.
Statistical analysis
We used standard descriptive statistics to compare participant characteristics reported as mean (SD) or number of observations (%). The Student t test or χ2 test was used to compare characteristics between cases and controls (Table 3). Placental ultrasonography measurements were normally distributed. Thus, to compare placental characteristics between study groups, we used the Student t test. Then, to examine the relationship between placental characteristics and miRNAs, which did not always follow a normal distribution, we used the Spearman correlation test. Analysis of covariance (SAS PROC GLM) was used to assess differences in miRNA expression between cases and controls [adjustment for body mass index (BMI) was done]. In addition, to avoid selection bias when controls were selected and matched to the cases, we compared patient characteristics of the 46 control pregnancies in this study with the characteristics of all the women who had a normal pregnancy in the initial source cohort of 400 pregnancies using the Student t test or χ2 test, and no differences were found (data not shown). All statistical analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC).
Table 3.
Clinical Characteristics and Demographic Information of the Study Participants
| Characteristics | All (n = 69) | GDM (n = 23) | Controls (n = 46) | P Value |
|---|---|---|---|---|
| Age, ya | 28.5 (4.8) | 29.8 (5.3) | 27.9 (4.4) | 0.12c |
| Body mass index, kg/m2a | 25.7 (5.9) | 28.2 (7.2) | 24.5 (4.7) | 0.01c |
| Multiparousb | 43 (62.3) | 19 (82.6) | 24 (52.2) | 0.002d |
| Systolic blood pressure, mm Hga | 120.9 (11.8) | 120.6 (15.2) | 121.1 (9.9) | 0.86c |
| Diastolic blood pressure, mm Hga | 76.2 (8.1) | 76.6 (9.1) | 76.0 (7.7) | 0.77c |
| Smoking statusb | 0.58d | |||
| Never | 48 (69.6) | 17 (73.9) | 31 (67.4) | |
| Former | 21 (30.4) | 6 (26.1) | 15 (32.6) | |
| Current | — | — | — | |
| Country of birthb | 0.51d | |||
| Canada | 64 (92.8) | 22 (95.6) | 42 (91.3) | |
| Other | 5 (7.2) | 1(4.4) | 4 (8.7) | |
| Educationb | 0.70d | |||
| High school | 22 (31.9) | 9 (39.1) | 13 (28.3) | |
| College | 22 (31.9) | 6 (34.8) | 16 (34.8) | |
| University | 24 (34.8) | 8 (26.1) | 16 (34.8) | |
| Other | 1 (1.4) | — | 1 (2.1) | |
| Placental characteristics (first trimester), cma | ||||
| Width | 5.7 (1.3) | 5.5 (1.3) | 5.8 (1.4) | 0.45c |
| Height | 3.1 (1.3) | 3.6 (1.5) | 2.9 (1.1) | 0.15c |
| Thickness | 1.9 (0.4) | 2.1 (0.5) | 1.8 (0.3) | 0.06c |
| Estimated placental volume | 51.7 (29.3) | 55.8 (33.6) | 49.5 (27.4) | 0.57c |
| Gestational age, wa | ||||
| At sampling | 10.6 (2.4) | 10.5 (2.5) | 10.6 (2.4) | 0.90c |
| At delivery | 39.2 (1.6) | 38.4 (2.1) | 39.6 (1.2) | 0.003c |
| Birth weight, ga | 3458 (411) | 3424 (458) | 3475 (390) | 0.64c |
| Cesarean sectionb | 8 (11.6) | 3 (13.0) | 5 (10.9) | 1.00d |
| Baby’s sexb | 0.61d | |||
| Male | 39 (56.5) | 14 (60.9) | 25 (54.3) | |
| Female | 30 (43.5) | 9 (39.1) | 21 (45.7) |
Data are mean (SD).
Data are number of observations (%).
Student t test.
χ 2 test.
Analysis of miRNA targets
The following miRNA accession IDs and their respective expression fold-changes were analyzed through the use of Ingenuity pathway analysis (Qiagen; www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis/): miR‒122-5p, miR‒132-3p, miR‒136-5p, miR‒182-3p, miR‒210-3p, miR‒29b-3p, miR‒342-3p, miR‒520h-3p, and miR-1323 (42). The molecular activity predictor application is a function of the Ingenuity pathway analysis software. Using this feature, one can interrogate subnetworks and canonical pathways and generate hypotheses by selecting a molecule of interest, indicating upregulation or downregulation, and simulating directional consequences of downstream molecules and the inferred activity upstream in the network or pathway. It contains content from TarBase, TargetScan, miRecords, and micro-RNA‒related findings from the published literature. A total of 4335 initial targets were identified and then filtered down to 313 by selecting only molecules that are transmembrane receptors, kinases, and G protein‒coupled receptors, in search of viable biomarkers. The targets were further narrowed down to 58 by selecting only highly predicted or experimentally observed confident levels. These targets were found to be involved in 257 different canonical pathways. After the pathways were gone through individually, three were selected: the type 2 diabetes mellitus signaling pathway, the insulin receptor signaling pathway, and the AMP-activated protein kinase (AMPK) signaling pathway. The miRNAs were then connected to the selected pathways by limiting the grow application uniquely to our data set. A molecular activity predictor application was then used to determine the effects of each involved miRNA on the pathways.
Results
Clinical characteristics of patients
Table 3 shows the clinical characteristics of the pregnant women and their newborns. Compared with their matched controls, women with GDM were more likely to have a higher BMI at enrollment (28.2 ± 7.2 kg/m2 vs 24.5 ± 4.7 kg/m2; P = 0.01), be multiparous (83% vs 52% controls; P < 0.01), and deliver earlier (38.4 ± 2 weeks vs 39.6 ± 1 weeks of gestation; P < 0.01). Regarding placental dimensions assessed by ultrasonography at 11 to 13 weeks of gestation, placental estimated volume as well as measured width, height, and thickness were weakly correlated with gestational age (Pearson r = 0.21, 0.18, 0.14, and 0.19, respectively, with P > 0.20). BMI at the first trimester was weakly correlated with placental width, height, and thickness (Pearson r = 0.13, 0.16, and 0.16, respectively, with P > 0.35) and marginally correlated with placental estimated volume (Pearson r = 0.27; P = 0.14). No difference was observed for width (P = 0.45), height (P = 0.15), or estimated placental volume (P = 0.57) between GDM and control groups. Placental thickness was slightly higher in the GDM group than in controls (2.1 cm vs 1.8 cm, respectively; P = 0.06).
EVs and PLAP-positive EVs were present in the maternal circulation as early as the eighth week of gestation
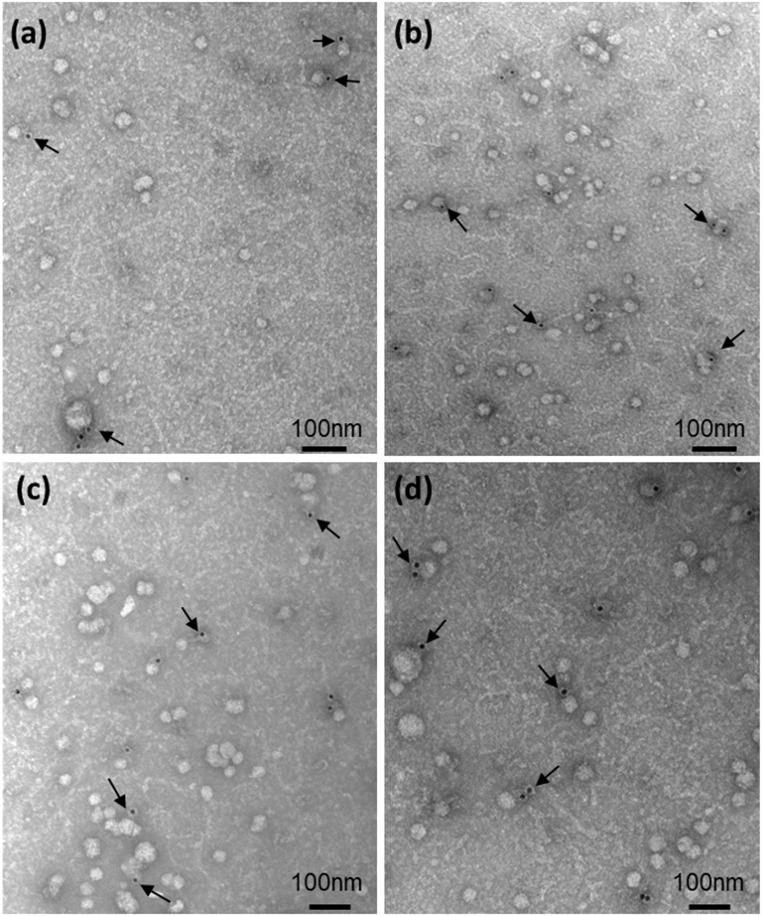
The presence of EVs in four serum samples was confirmed by TEM (Fig. 2). Nanovesicles with a diameter of 30 to 150 nm and a cup-shaped form, characteristic of EVs, were present in samples from both women with GDM and women with a normal pregnancy. Using the specific surface marker of the placental-derived EVs, PLAP, we confirmed their presence in the maternal blood as early as the eighth week of gestation (Fig. 2).
Figure 2.
Transmission electron microscopy images of EVs isolated from the serum of (a and b) two controls and (c and d) two women with GDM. The presence of EVs of placental origin was confirmed by immunostaining with mouse monoclonal antihuman-PLAP IgG followed by 12 nm Colloidal Gold AffiniPure goat anti-mouse IgG (Cat No. 115-205-166; Jackson ImmunoResearch Laboratories Inc). Samples were stained with uranyl acetate. Arrows point to gold particles linked to the antibody anti-PLAP. Scale bar = 100 nm.
miRNAs in circulating EVs were differentially expressed early in complicated vs control pregnancies
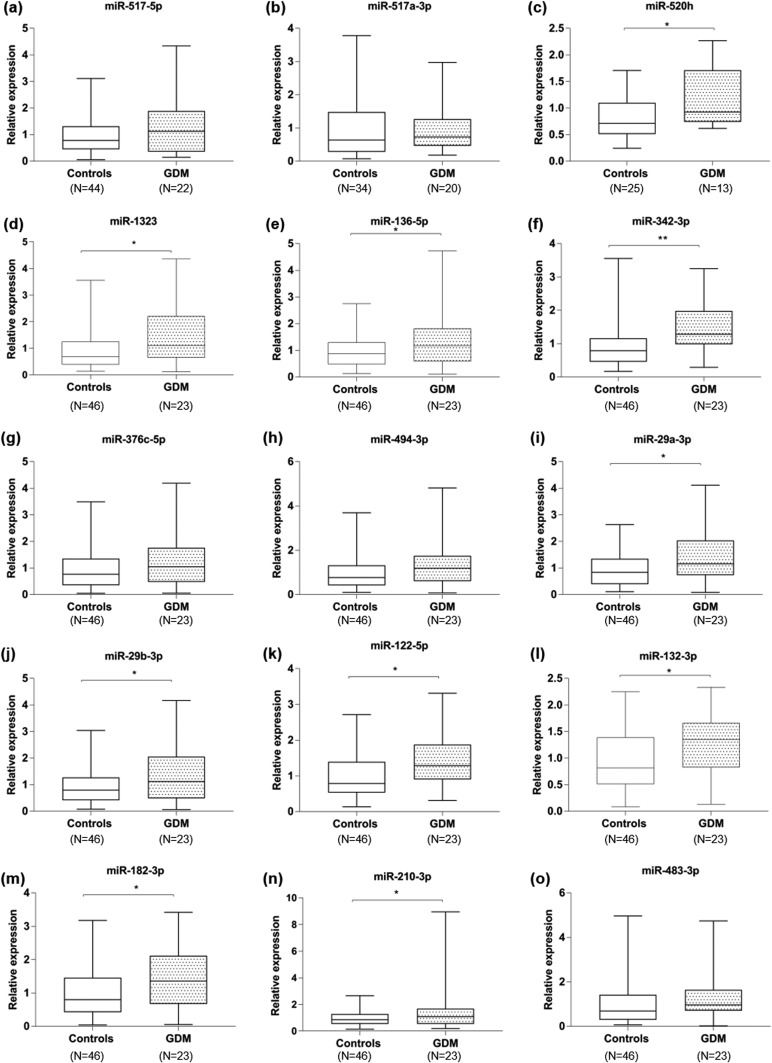
Among the 17 miRNAs selected, 15 miRNAs were quantifiable in serum EVs among women with GDM and controls (miR-518b and miR‒525-5p were not detected). We measured placenta-specific miRNAs from both the C19 miRNA cluster (miR‒517-5p, miR‒517a-3p, miR-520h, and miR-1323) and the C14 miRNA cluster specific to pregnancy (miR‒136-5p, miR‒342-3p, miR‒376c-5p, and miR‒494-3p) in circulating EVs as well as the miRNAs previously shown to exhibit differential expression in placental-related pregnancy complications (Fig. 3). Overall, increased levels of 12 miRNAs were observed in the GDM group compared with their matched controls, with significantly higher levels for 10 miRNAs: miR-520h (mean, 1.2 ± 0.2 vs 0.80 ± 0.07; P = 0.03); miR-1323 (mean, 1.54 ± 0.26 vs 0.97 ± 0.13; P = 0.03); miR‒136-5p (mean, 1.48 ± 0.24 vs 0.99 ± 0.10; P = 0.03); miR‒342-3p (mean, 1.50 ± 0.18 vs 0.95 ± 0.11; P = 0.008); miR‒29a-3p (mean, 1.43 ± 0.22 vs 0.97 ± 0.09; P = 0.03); miR‒29b-3p (mean, 1.40 ± 0.22 vs 0.95 ± 0.10; P = 0.04); miR‒122-5p (mean, 1.42 ± 0.17 vs 0.98 ± 0.09; P = 0.01); miR‒132-3p (mean, 1.30 ± 0.12 vs 0.94 ± 0.08; P = 0.03); miR‒182-3p (mean, 1.51 ± 0.20 vs 0.99 ± 0.11; P = 0.01), and miR‒210-3p (mean, 1.67 ± 0.39 vs 0.97 ± 0.08; P = 0.02) (Fig. 3). Simultaneously, a trend toward increased levels of miR‒494-3p (mean, 1.40 ± 0.23 vs 1.00 ± 0.12; P = 0.10) and miR‒517-5p (mean, 1.30 ± 0.22 vs 0.96 ± 0.10; P = 0.12) was identified in women who developed GDM. The levels of miR‒517a-3p, miR‒376c-5p, and miR‒483-3p were similar between GDM and control pregnancies (P = 0.84, P = 0.26, and P = 0.47, respectively).
Figure 3.
Boxplots of different levels of EV-miRNAs in pregnancies with GDM compared with normal pregnancies. (a‒o) Relative quantification for each miRNA is indicated for both normal pregnancies (controls) and pregnancies complicated by GDM. (a‒d) miRNAs from C19MC, (e‒h) miRNAs of C14MC, and (i‒o) miRNAs not specific to the placenta. The upper and lower limits of the boxes represent the 75th and 25th percentiles, respectively. The upper and lower whiskers represent the maximum and minimum values. *P ≤ 0.05; **P ≤ 0.01.
In a subgroup of 35 pregnancies, we correlated placental ultrasonography measurements at 11 to 13 weeks of gestation with the expression levels of miRNAs. In general, there were no statistically significant correlations between placental measurements and miRNA expression levels, with the exception of marginally correlated miR‒517-5p with placental height (ρ = −0.37; P = 0.08) and miR‒136-5p with placental height and estimated placental volume (ρ = −0.32; P = 0.08 and ρ = −0.30; P = 0.09, respectively) (Table 4).
Table 4.
Spearman Correlation Coefficients (ρ) for Placental Dimensions and miRNA Expression
| Width | Height | Thickness | EPV | |
|---|---|---|---|---|
| miR‒122-5p | ||||
| ρ | −0.15 | −0.17 | 0.01 | −0.24 |
| P value | 0.40 | 0.35 | 0.98 | 0.19 |
| miR‒132-3p | ||||
| ρ | 0.03 | −0.04 | 0.20 | −0.11 |
| P value | 0.86 | 0.81 | 0.24 | 0.54 |
| miR-1323 | ||||
| ρ | −0.03 | −0.28 | −0.08 | −0.23 |
| P value | 0.84 | 0.12 | 0.63 | 0.20 |
| miR‒136-5p | ||||
| ρ | −0.02 | −0.32 | −0.04 | −0.30 |
| P value | 0.92 | 0.08 | 0.81 | 0.10 |
| miR‒182-3p | ||||
| ρ | −0.13 | −0.21 | −0.09 | −0.29 |
| P value | 0.47 | 0.25 | 0.63 | 0.11 |
| miR‒210-3p | ||||
| ρ | −0.10 | −0.04 | −0.01 | −0.16 |
| P value | 0.57 | 0.83 | 0.94 | 0.41 |
| miR‒29a-3p | ||||
| ρ | −0.04 | −0.17 | −0.02 | −0.19 |
| P value | 0.84 | 0.34 | 0.92 | 0.29 |
| miR‒29b-3p | ||||
| ρ | −0.18 | −0.23 | −0.12 | −0.32 |
| P value | 0.29 | 0.21 | 0.48 | 0.08 |
| miR‒342-3p | ||||
| ρ | 0.13 | 0.01 | 0.14 | −0.05 |
| P value | 0.45 | 0.95 | 0.41 | 0.80 |
| miR‒376c-5p | ||||
| ρ | −0.04 | −0.21 | −0.10 | −0.18 |
| P value | 0.83 | 0.25 | 0.58 | 0.34 |
| miR‒483-3p | ||||
| ρ | 0.01 | −0.23 | −0.06 | −0.20 |
| P value | 0.97 | 0.20 | 0.75 | 0.28 |
| miR‒494-3p | ||||
| ρ | −0.09 | −0.29 | −0.12 | −0.27 |
| P value | 0.59 | 0.11 | 0.49 | 0.14 |
| miR‒517-5p | ||||
| ρ | −0.08 | −0.21 | −0.12 | −0.29 |
| P value | 0.64 | 0.24 | 0.50 | 0.11 |
| miR‒517a-3p | ||||
| ρ | 0.13 | −0.37 | −0.05 | −0.25 |
| P value | 0.54 | 0.09 | 0.83 | 0.26 |
| miR-520h | ||||
| ρ | −0.20 | −0.15 | −0.05 | −0.34 |
| P value | 0.41 | 0.55 | 0.85 | 0.17 |
Analysis was performed for all patients (n = 35) with GDM and controls combined. Negative value indicates a negative correlation; positive value indicates a positive correlation. Placental dimensions were measured between 11 and 13 wk of pregnancy. ρ: Spearman correlation coefficient; all P values are >0.05.
Abbreviation: EPV, estimated placental volume.
Pathway analysis revealed that miRNAs upregulated in GDM are involved in insulin/glucose regulation
Target analysis revealed that the miRNAs upregulated in GDM are involved in several relevant pathways for GDM—the insulin receptor signaling pathway, the AMPK signaling pathway, and the epidermal growth factor receptor-phosphatidylinositol 3-kinase-Akt pathway—which play important roles in placental development, fetal growth, and insulin and glucose regulation. In the insulin receptor signaling pathway, increased expression of miR‒122-5p, miR‒132-3p, miR‒182-3p, miR‒210-3p, and miR‒29b-3p is predicted to inhibit insulin binding to the insulin receptor protein. In the type 2 diabetes mellitus signaling pathway, miR‒122-5p, miR‒132-3p, miR‒182-3p, miR‒29b-3p, miR‒342-3p, and miR‒520h-3p are predicted to inhibit GLUT 2 and trigger impaired insulin secretion in pancreatic β islet cells. In AMPK signaling, the increased 122-5p, 132-3p, 210-3p, 29b-3p, 342-3p, 520g-3p, and 1323 miRNAs observed in GDM are predicted to inhibit AMPK and ultimately β-oxidation and glucose transport.
Discussion
Our study shows a significant difference early in pregnancy in the expression of EV-miRNAs and placenta-specific miRNAs in EVs between GDM and normal pregnancies. Our study has certain limitations (despite the use of quantitative RT-PCR, which is currently the most sensitive, reproducible gold standard method to quantify miRNAs). We acknowledge the possibility that other EV-miRNAs, which were not screened in our analysis, may be present in the maternal blood circulation and may be associated with GDM (43). As expected, women who developed GDM had a higher BMI in the first trimester and delivered earlier, as these are risk factors for GDM and a common outcome, respectively (44). The strengths of our study include the nested case-control design within a prospective cohort study, with careful matching by gestational age at sampling. In addition, we very carefully evaluated several potential confounders in the association between EV-miRNA levels and GDM, and we adjusted for BMI. We also provide ultrasonography estimations of placental volume and show that the increase in certain miRNAs is not simply due to the increased volume of placental tissue. Actually, the majority of correlations between miRNAs and placental measurements were either close to zero or negative; however, all were statistically nonsignificant. Although ultrasonography estimation is an indirect measure of placental volume, it has been accurate in predicting actual placental weight (24). Moreover, the homogeneity of the study population (white women) reduces the bias of unmeasured confounding factors that may be attributed to ethnic background (a well-known risk-factor for GDM) but limits the external validation of our findings in larger and more diverse epidemiological studies.
We investigated the specific miRNAs that are involved in the development of GDM, as well as C19MC and C14MC miRNAs that are specifically expressed by placental cells, trophoblasts, and syncytiotrophoblasts (45). We confirmed that placenta-specific miRNAs—C19MC miRNAs and C14MC miRNAs as well as miRNAs previously reported in GDM—are expressed in EVs in the maternal circulation early in pregnancy (6 to 15 weeks of gestation). This is consistent with a study by Luo et al. (18), who reported that C19MC miRNAs were released into the maternal circulation encapsulated in placental EVs in normal pregnancies.
Our findings show a consistent pattern of increased miRNA expression in pregnant women with GDM vs pregnant controls. Because no similar studies have been published, we compared our results with studies done on placentas or in maternal blood (without EV isolation), often sampled in the second or third trimester. This can explain why we found differences between our results and previous data. Indeed, Morales-Pietro et al. (45, 46) characterized levels of C19MC and C14MC in maternal circulation during the first, second, and third trimesters and reported changes throughout pregnancy: The expression of C19MC miRNAs was higher in the second and third trimesters, whereas C14MC miRNAs were expressed mostly during the first trimester. This can also explain why we were unable to significantly detect miR-518b and miR‒525-5p (miRNAs belonging to C19MC) in our samples. Contrary to our results, miR‒29a-3p and miR‒132-3p were reported to be downregulated in the plasma during the second trimester in GDM pregnancies (47). However, this comparison is limited, as we carried out analysis on serum, not plasma; we isolated miRNAs from EVs not directly from blood; and samples were collected at an earlier gestational age.
Pathway analysis showed that five upregulated miRNAs (miR‒122-5p, miR‒132-3p, miR‒29b-3p, miR‒182-3p, and miR‒29a-3p) modulated key target genes for regulation of glucose homeostasis and insulin secretion. Indeed, in agreement with bioinformatics analysis, in vitro and human studies indicate that miR‒29b-3p, miR‒122-5p, and miR‒132-3p are related to insulin resistance (48, 49), obesity (48), and glucose level regulation (50, 51), which are all hallmarks of GDM. Overexpression of miR-29 represses insulin-stimulated glucose uptake and may result in insulin resistance (34). miR-122 has been reported to be overexpressed in patients with type 2 diabetes mellitus and patients with insulin resistance (48, 52) and targets AMPK, a sensor of intracellular energy status that acts as a key regulator in glucose and fatty acid metabolism (53). More recently, circulating miR‒122-5p, which is localized in hepatic-derived EVs, was associated with the risk of new-onset metabolic syndrome, suggesting that it can be used as an early biomarker (54). Moreover, miR‒29a-3p was involved in insulin resistance by targeting peroxisome proliferator-activated receptor δ in skeletal muscle cells (55), and miR‒182-3p (also upregulated) was reported to be involved in the pathogenic process of dyslipidemia and type 2 diabetes mellitus (36).
Finally, we observed by TEM that EVs of placental origin are present in the maternal circulation as early as the eighth week of pregnancy. Because specific placental miRNAs were measured in EVs from all samples collected between 6 and 15 weeks of gestation, this suggests that placental EVs are released into the maternal circulation as early as the sixth week of gestation. This finding is consistent with a study by Salomon et al. (56), who also reported the release of placental EVs from the first trimester. Interestingly, they also showed that the secretion of EVs from all origins was slightly higher in women with GDM than in women who had a normal pregnancy and that the release of placental EVs tended to increase over the pregnancy, with a 2.2-fold higher concentration in GDM (57). Our findings of increased miRNAs in GDM thus could result from increased EV secretion by syncytiotrophoblasts. However, of the 10 increased miRNAs, only four are known to be placental miRNAs (miR-520h, miR-1323, miR‒136-5p, and miR‒342-3p). Given that we cannot be sure that the EVs are placental, this explanatory hypothesis remains speculative and needs to be tested on specific placental EVs.
Our findings of increased levels of placenta-specific miRNAs before GDM diagnosis could reflect the epigenetic response of the placenta to early insulin resistance and/or proinflammatory status, even when there are still no major ultrasonography-detectable morphological changes happening in the placenta in pregnancies destined to develop GDM. Given that GDM diagnosis is made at 24 to 28 weeks, EVs in the maternal blood circulation offer an opportunity to detect early pathophysiological responses of fetal organs, such as the placenta, to maternal metabolic phenotype. Further studies using high-throughput miRNA-sequencing technology represent the best method to identify miRNAs contained in EVs. This would enhance miRNA biomarker research of EVs and knowledge of the regulatory roles mediated by EVs in GDM (58).
Acknowledgments
The authors thank Dr. Darel Hunting for his valuable feedback throughout the study and the preparation of the manuscript. We also thank all study participants and personnel of the Obstetric Service of the CHUS, Quebec, Canada.
Financial Support: This work was supported in part by Pilot Project funding from the Harvard Chan-NIEHS Center for Environmental Health (ES000002) (to A.B.), by institutional funds from the department of Obstetrics and Gyneacology of the CHUS (to A.O.), and by Internal funding PAFI of the Foundation of the CHUS (to A.O.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. V.G. was supported by a Quebec Training Network in Perinatal Research Scholarship.
Current Affiliation: R. S. Rodosthenous’ current affiliation is Cardiovascular Research Center, Massachusetts General Hospital, Boston, Massachusetts 02114. K. Brennan’s and A. Baccarelli’s current affiliation is Environmental Health Sciences Department, Mailman School of Public Health, Columbia University, New York, New York 10032.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AMPK
AMP-activated protein kinase
- BMI
body mass index
- CHUS
Centre Hospitalier Universitaire in Sherbrooke
- EV
extracellular vesicle
- EV-miRNA
miRNA encapsulated in extracellular vesicle
- GDM
gestational diabetes mellitus
- OGTT
oral glucose tolerance test
- PLAP
placental alkaline phosphatase
- qPCR
quantitative PCR
- RT
reverse transcription
- TEM
transmission electron microscopy
References and Notes
- 1. Feig DS, Berger H, Donovan L, Godbout A, Kader T, Keely E, Sanghera R. Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes and pregnancy. Can J Diabetes. 2018;42(Suppl 1):S255–S282. [DOI] [PubMed] [Google Scholar]
- 2. Schiavone M, Putoto G, Laterza F, Pizzol D. Gestational diabetes: an overview with attention for developing countries. Endocr Regul. 2016;50(2):62–71. [DOI] [PubMed] [Google Scholar]
- 3. Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59(7):1396–1399. [DOI] [PubMed] [Google Scholar]
- 4. Amaral LM, Cunningham MW Jr, Cornelius DC, LaMarca B. Preeclampsia: long-term consequences for vascular health. Vasc Health Risk Manag. 2015;11:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Osmond DT, Nolan CJ, King RG, Brennecke SP, Gude NM. Effects of gestational diabetes on human placental glucose uptake, transfer, and utilisation. Diabetologia. 2000;43(5):576–582. [DOI] [PubMed] [Google Scholar]
- 6. Jarmuzek P, Wielgos M, Bomba-Opon D. Placental pathologic changes in gestational diabetes mellitus. Neuroendocrinol Lett. 2015;36(2):101–105. [PubMed] [Google Scholar]
- 7. Mack LR, Tomich PG. Gestational diabetes: diagnosis, classification, and clinical care. Obstet Gynecol Clin North Am. 2017;44(2):207–217. [DOI] [PubMed] [Google Scholar]
- 8. Berger H, Gagnon R, Sermer M, Basso M, Bos H, Brown RN, Bujold E, Cooper SL, Gagnon R, Gouin K, McLeod NL, Menticoglou SM, Mundle WR, Roggensack A, Sanderson FL, Walsh JD. Diabetes in pregnancy. J Obstet Gynaecol Can. 2016;38(7):667–679.e1. [DOI] [PubMed] [Google Scholar]
- 9. McIntyre HD, Sacks DA, Barbour LA, Feig DS, Catalano PM, Damm P, McElduff A. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39(1):53–54. [DOI] [PubMed] [Google Scholar]
- 10. Hinkle SN, Tsai MY, Rawal S, Albert PS, Zhang C. HbA1c measured in the first trimester of pregnancy and the association with gestational diabetes. Sci Rep. 2018;8(1):12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moen G-H, Sommer C, Prasad RB, Sletner L, Groop L, Qvigstad E, Birkeland KI. Mechanisms in endocrinology: epigenetic modifications and gestational diabetes: a systematic review of published literature. Eur J Endocrinol. 2017;176(5):R247–R267. [DOI] [PubMed] [Google Scholar]
- 12. Cardenas A, Gagné-Ouellet V, Allard C, Brisson D, Perron P, Bouchard L, Hivert M-F. Placental DNA methylation adaptation to maternal glycemic response in pregnancy. Diabetes. 2018;67(8):1673–1683. [DOI] [PubMed] [Google Scholar]
- 13. Desgagné V, Hivert M-F, St-Pierre J, Guay S-P, Baillargeon J-P, Perron P, Gaudet D, Brisson D, Bouchard L. Epigenetic dysregulation of the IGF system in placenta of newborns exposed to maternal impaired glucose tolerance. Epigenomics. 2014;6(2):193–207. [DOI] [PubMed] [Google Scholar]
- 14. Poirier C, Desgagné V, Guérin R, Bouchard L. MicroRNAs in pregnancy and gestational diabetes mellitus: emerging role in maternal metabolic regulation. Curr Diab Rep. 2017;17(5):35. [DOI] [PubMed] [Google Scholar]
- 15. Sáez T, de Vos P, Sobrevia L, Faas MM. Is there a role for exosomes in foetoplacental endothelial dysfunction in gestational diabetes mellitus? Placenta. 2018;61:48–54. [DOI] [PubMed] [Google Scholar]
- 16. Corrêa-Silva S, Alencar AP, Moreli JB, Borbely AU, de S Lima L, Scavone C, Damasceno DC, Rudge MVC, Bevilacqua E, Calderon IMP. Hyperglycemia induces inflammatory mediators in the human chorionic villous. Cytokine. 2018;111:41–48. [DOI] [PubMed] [Google Scholar]
- 17. Buschur E, Stetson B, Barbour LA. Diabetes in pregnancy. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000. Available at:www.ncbi.nlm.nih.gov/books/NBK279010/. Accessed 11 September 2018.
- 18. Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A, Ohkuchi A, Matsubara S, Takeshita T, Takizawa T. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81(4):717–729. [DOI] [PubMed] [Google Scholar]
- 19. Salomon C, Yee SW, Mitchell MD, Rice GE. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions [published online ahead of print 14 September 2014] BioMed Res Int. doi: 10.1155/2014/693157. [DOI] [PMC free article] [PubMed]
- 20. Xie L, Sadovsky Y. The function of miR-519d in cell migration, invasion, and proliferation suggests a role in early placentation. Placenta. 2016;48:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, Zamudio S, Illsley NP, Myatt L, Colvis C, Costantine MM, Haas DM, Sadovsky Y, Weiner C, Rytting E, Bidwell G. Placental origins of adverse pregnancy outcomes: potential molecular targets: an executive workshop summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215(1Suppl):S1–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bagge A, Clausen TR, Larsen S, Ladefoged M, Rosenstierne MW, Larsen L, Vang O, Nielsen JH, Dalgaard LT. MicroRNA-29a is up-regulated in beta-cells by glucose and decreases glucose-stimulated insulin secretion. Biochem Biophys Res Commun. 2012;426(2):266–272. [DOI] [PubMed] [Google Scholar]
- 23. Collares CVA, Evangelista AF, Xavier DJ, Rassi DM, Arns T, Foss-Freitas MC, Foss MC, Puthier D, Sakamoto-Hojo ET, Passos GA, Donadi EA. Identifying common and specific microRNAs expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients. BMC Res Notes. 2013;6(1):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Azpurua H, Funai EF, Coraluzzi LM, Doherty LF, Sasson IE, Kliman M, Kliman HJ. Determination of placental weight using two-dimensional sonography and volumetric mathematic modeling. Am J Perinatol. 2010;27(2):151–155. [DOI] [PubMed] [Google Scholar]
- 25. Lasser C, Eldh M, Lotvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012;(59):e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(Suppl 1):D140–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brosseau J-P, Lucier J-F, Lapointe E, Durand M, Gendron D, Gervais-Bird J, Tremblay K, Perreault J-P, Elela SA. High-throughput quantification of splicing isoforms. RNA. 2010;16(2):442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, Sadovsky Y, Coyne CB. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci USA. 2013;110(29):12048–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen DB, Wang W. Human placental microRNAs and preeclampsia. Biol Reprod. 2013;88(5):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morales-Prieto DM, Ospina-Prieto S, Schmidt A, Chaiwangyen W, Markert UR. Elsevier Trophoblast Research Award Lecture: origin, evolution and future of placenta miRNAs. Placenta. 2014;35(Suppl):S39–S45. [DOI] [PubMed] [Google Scholar]
- 31. Yang Z, Wu L, Zhu X, Xu J, Jin R, Li G, Wu F. MiR-29a modulates the angiogenic properties of human endothelial cells. Biochem Biophys Res Commun. 2013;434(1):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li P, Guo W, Du L, Zhao J, Wang Y, Liu L, Hu Y, Hou Y.. microRNA-29b contributes to pre-eclampsia through its effects on apoptosis, invasion and angiogenesis of trophoblast cells. Clin Sci (Lond). 2013;124(1):27–40. [DOI] [PubMed] [Google Scholar]
- 33. He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2007;21(11):2785–2794. [DOI] [PubMed] [Google Scholar]
- 34. Hromadnikova I, Kotlabova K, Hympanova L, Krofta L. Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PLoS One. 2015;10(9):e0138383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou J, Meng Y, Tian S, Chen J, Liu M, Zhuo M, Zhang Y, Du H, Wang X. Comparative microRNA expression profiles of cynomolgus monkeys, rat, and human reveal that mir-182 is involved in T2D pathogenic processes. J Diabetes Res. 2014;2014:760397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hromadnikova I, Kotlabova K, Ondrackova M, Pirkova P, Kestlerova A, Novotna V, Hympanova L, Krofta L. Expression profile of C19MC microRNAs in placental tissue in pregnancy-related complications. DNA Cell Biol. 2015;34(6):437–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayor-Lynn K, Toloubeydokhti T, Cruz AC, Chegini N. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod Sci. 2011;18(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delic D, Eisele C, Schmid R, Luippold G, Mayoux E, Grempler R. Characterization of micro-RNA changes during the progression of type 2 diabetes in Zucker diabetic fatty rats. Int J Mol Sci. 2016;17(5):665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anton L, Olarerin-George AO, Schwartz N, Srinivas S, Bastek J, Hogenesch JB, Elovitz MA. miR-210 inhibits trophoblast invasion and is a serum biomarker for preeclampsia. Am J Pathol. 2013;183(5):1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gallo W, Esguerra JLS, Eliasson L, Melander O. miR-483-5p associates with obesity and insulin resistance and independently associates with new onset diabetes mellitus and cardiovascular disease. PLoS One. 2018;13(11):e0206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8(2):R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30(4):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33(20):e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Garrison A. Screening, diagnosis, and management of gestational diabetes mellitus. Am Fam Physician. 2015;91(7):460–467. [PubMed] [Google Scholar]
- 45. Morales-Prieto DM, Ospina-Prieto S, Chaiwangyen W, Schoenleben M, Markert UR. Pregnancy-associated miRNA-clusters. J Reprod Immunol. 2013;97(1):51–61. [DOI] [PubMed] [Google Scholar]
- 46. Morales-Prieto DM, Chaiwangyen W, Ospina-Prieto S, Schneider U, Herrmann J, Gruhn B, Markert UR. MicroRNA expression profiles of trophoblastic cells. Placenta. 2012;33(9):725–734. [DOI] [PubMed] [Google Scholar]
- 47. Zhao C, Dong J, Jiang T, Shi Z, Yu B, Zhu Y, Chen D, Xu J, Huo R, Dai J, Xia Y, Pan S, Hu Z, Sha J. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS One. 2011;6(8):e23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones A, Danielson KM, Benton MC, Ziegler O, Shah R, Stubbs RS, Das S, Macartney-Coxson D. miRNA signatures of insulin resistance in obesity. Obesity (Silver Spring). 2017;25(10):1734–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang YM, Seo SY, Kim TH, Kim SG. Decrease of microRNA-122 causes hepatic insulin resistance by inducing protein tyrosine phosphatase 1B, which is reversed by licorice flavonoid. Hepatology. 2012;56(6):2209–2220. [DOI] [PubMed] [Google Scholar]
- 50. Esguerra JLS, Bolmeson C, Cilio CM, Eliasson L. Differential glucose-regulation of microRNAs in pancreatic islets of non-obese type 2 diabetes model Goto-Kakizaki rat. PLoS One. 2011;6(4):e18613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alrob OA, Khatib S, Naser SA. MicroRNAs 33, 122, and 208: a potential novel targets in the treatment of obesity, diabetes, and heart-related diseases. J Physiol Biochem. 2017;73(2):307–314. [DOI] [PubMed] [Google Scholar]
- 52. Wang R, Hong J, Cao Y, Shi J, Gu W, Ning G, Zhang Y, Wang W. Elevated circulating microRNA-122 is associated with obesity and insulin resistance in young adults. Eur J Endocrinol. 2015;172(3):291–300. [DOI] [PubMed] [Google Scholar]
- 53. Louden E, Chi MM, Moley KH. Crosstalk between the AMP-activated kinase and insulin signaling pathways rescues murine blastocyst cells from insulin resistance. Reproduction. 2008;136(3):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Willeit P, Skroblin P, Moschen AR, Yin X, Kaudewitz D, Zampetaki A, Barwari T, Whitehead M, Ramírez CM, Goedeke L, Rotllan N, Bonora E, Hughes AD, Santer P, Fernández-Hernando C, Tilg H, Willeit J, Kiechl S, Mayr M. Circulating microRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes. 2017;66(2):347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou Y, Gu P, Shi W, Li J, Hao Q, Cao X, Lu Q, Zeng Y. MicroRNA-29a induces insulin resistance by targeting PPARδ in skeletal muscle cells. Int J Mol Med. 2016;37(4):931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, Longo S, Duncombe G, Mitchell MD, Rice GE, Illanes SE. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes. 2016;65(3):598–609. [DOI] [PubMed] [Google Scholar]
- 57. Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, Illanes SE, Mitchell MD, Rice GE. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One. 2014;9(6):e98667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu T, Zhang Q, Zhang J, Li C, Miao Y-R, Lei Q, Li Q, Guo A-Y. EVmiRNA: a database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D89–D93. [DOI] [PMC free article] [PubMed] [Google Scholar]