Abstract
Context
Limited natural history data are available in patients with non-HIV–related lipodystrophy syndromes who never received disease-specific therapies, making interpretation of benefits of therapies in lipodystrophy syndromes challenging.
Objective
We assessed the natural history of non-HIV–related generalized lipodystrophy (GL) and partial lipodystrophy (PL) in patients who have never received leptin or other lipodystrophy-specific therapies.
Design/Setting/Patients
We conducted an international chart review of 230 patients with confirmed GL or PL at five treatment centers who never received leptin or other lipodystrophy-specific therapies. Patients were observed from birth to loss to follow-up, death, or date of chart abstraction.
Outcome Measures
Lifetime prevalence of diabetes/insulin resistance and select organ abnormalities, time to diabetes/insulin resistance, first organ abnormality, disease progression, and mortality were described.
Results
Diabetes/insulin resistance was identified in 58.3% of patients. Liver abnormalities were the most common organ abnormality (71.7%), followed by kidney (40.4%), heart (30.4%), and pancreatitis (13.0%). Kaplan-Meier estimates of mean (SE) time to first organ abnormality were 7.7 years (0.9) in GL and 16.1 years (1.5) in PL (P < 0.001). Mean time to diabetes/insulin resistance was 12.7 years (1.2) in GL and 19.1 years (1.7) in PL (P = 0.131). Mean time to disease progression was 7.6 years (0.8) and comparable between GL and PL subgroups (P = 0.393). Mean time to death was 51.2 years (3.5) in GL and 66.6 years (1.0) in PL (P < 0.001).
Conclusions
This large-scale study provides comprehensive, long-term data across multiple countries on the natural history of non-HIV–related lipodystrophy.
This international chart review of patients with lipodystrophy syndromes describes the disease natural history and highlights differences between generalized and partial forms.
Lipodystrophy syndromes are a heterogeneous group of rare, potentially life-threatening disorders characterized by the paucity of adipose tissue, which leads to insufficient storage capacity for excess nutrients and spillover into ectopic sites (1, 2). These pathophysiological adaptations are thought to cause much of the metabolic manifestations of lipodystrophy syndromes based on studies in animal models targeting adipocyte differentiation or lipid droplet formation (2). The estimated prevalence of lipodystrophy syndromes is 1.3 to 4.7 cases per million worldwide (based on confirmed mostly monogenic cases; inclusion of suspected cases or polygenic forms may result in higher estimates) when excluding cases associated with HIV, which are more prevalent but managed in a distinct manner and beyond the scope of the current study (3–5).
Lipodystrophy syndromes are categorized into two major forms based on the extent of adipose tissue loss across the body (generalized or partial) (1, 6). Generalized lipodystrophy (GL) is characterized by the absence or progressive loss of adipose tissue across the whole body, whereas in partial lipodystrophy (PL), adipose tissue loss is more limited, typically affecting select regions such as the limbs or the upper body (1). Lipodystrophy syndromes are also categorized by etiology, with inherited and acquired forms. The two types of inherited lipodystrophy syndromes are congenital GL (CGL) and familial PL (FPLD), and the two types of acquired lipodystrophy syndromes are acquired GL (AGL) and acquired PL (APL). The most common subtypes of CGL are caused by mutations in AGPAT2 (CGL1) or BSCL2 (CGL2) and follow an autosomal-recessive inheritance pattern, whereas the most common subtype of FPLD is caused by mutations in LMNA (FPLD2 or Dunnigan variety) and follows an autosomal-dominant inheritance pattern (2, 6). Acquired lipodystrophy syndromes can occur in association with autoimmune mechanisms, inflammatory conditions (e.g., panniculitis), previous whole-body irradiation, and medication use (4, 7). Another subgroup of acquired lipodystrophy was considered idiopathic in which the mechanism was historically not well understood, although recent discoveries suggest some of these cases may be due to inherited or de novo genetic mechanisms (4, 7).
Patients with GL and PL present with a broad range of symptoms that include organ abnormalities (e.g., hepatic steatosis, nephropathy, and pancreatitis) and metabolic abnormalities (e.g., diabetes, insulin resistance, and hypertriglyceridemia). The loss of adipose tissue and resulting ectopic accumulation of lipids throughout the body can cause severe insulin resistance and other metabolic abnormalities, which can lead to organ damage and higher rates of mortality, particularly in GL (8, 9). Other less well-documented comorbidities, such as reproductive dysfunction, psychological distress, and pain, are also gaining recognition as notable components of disease symptomology (1, 4, 10). In addition, there may be multisystem involvement driven by mechanisms distinct from adipose tissue loss and ectopic lipid accumulation.
Patients with lipodystrophy syndromes often have a deficiency in leptin, a hormone produced in adipose tissue (1, 10). Recombinant human methionyl leptin (metreleptin) is approved in the United States and European Union as a therapy for treating the complications of leptin deficiency in patients with CGL or AGL. The European Medicines Agency has also recently approved the therapy to treat such complications in patients with FPLD and APL. Although there are ongoing studies evaluating leptin therapy (LT) or other investigational therapies in patients with lipodystrophy syndromes (11), little is known about the natural history of patients with GL or PL and how such therapies could alter the course of disease. Published studies are often limited to small samples and individual countries, which can limit external validity (9, 12–16). Only three studies included longitudinal assessments of the natural history, yet these studies did not include data on patients with PL (9, 12, 16). Other studies that assessed the burden of illness had cross-sectional designs, which provide limited insights into disease progression (13, 14). The lack of such data makes efforts to interpret the benefits of therapies for lipodystrophy syndromes particularly challenging.
In light of this knowledge gap, the objective of the current study was to assess the natural history of patients with GL and PL who have never been treated with LT or any other lipodystrophy-specific therapies using longitudinal, retrospective data in patient medical charts sourced from five treatment centers in three countries. These data provide a reference point for future studies to estimate the treatment effect of lipodystrophy-specific therapies on long-term clinical outcomes, such as mortality.
Materials and Methods
Study population
Retrospective data for this study were obtained from patient medical charts at five treatment centers across three countries: Brazil (University of São Paulo and the Federal University of Ceará), Turkey (Dokuz Eylül University), and the United States (National Institutes of Health and the University of Michigan). Medical charts of patients with a diagnosis of non-HIV–related GL or PL, prior to 1 January 2015, were eligible for inclusion. The year 2015 was selected to enable sufficient observation time following the index date. Medical charts of patients who received lipodystrophy-specific therapies such as LT at any time were not eligible for this study.
Each treatment center received local institutional review board approvals prior to initiation of data collection.
Study design
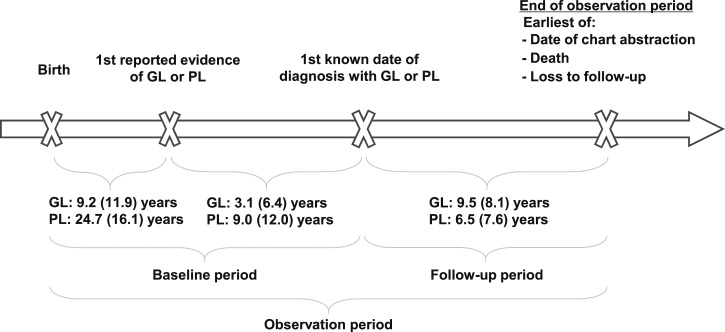
To capture data across the entire time period for which data were available within each patient medical chart, the study observation period was defined as the time period that spanned from birth until loss to follow-up, death, or date of chart abstraction, whichever occurred first. The date when the first signs of lipodystrophy appeared (e.g., visible lipodystrophy, diagnosis of diabetes and/or insulin resistance, and elevated triglycerides or liver enzymes) was denoted as the “first reported evidence of GL or PL.” Any time prior to the initial diagnosis of GL or PL was defined as the “baseline period,” and any time on or following this diagnosis was defined as the “follow-up period.” The date of last available data in each medical chart, at which a patient may be lost to follow-up, deceased, or still alive and being followed at their respective treatment centers, marked the end of the observation period for all patients. The study design is presented in Fig. 1.
Figure 1.
Study design. The study observation period spanned from the patients' birth until loss to follow-up, death, or date of chart abstraction, whichever occurred first, and includes the baseline and follow-up periods. The baseline period spanned the time from birth until the date of diagnosis with GL or PL. The follow-up period spanned the time from date of diagnosis with GL or PL to the end of the observation period. Durations of the various study periods in years are reported as means (SD).
Variables and data collection
Data were extracted by local staff at centers of excellence for the diagnosis and management of lipodystrophy syndromes between 6 March 2017 and 20 March 2018 and all patients with GL and PL seen at centers if they had never received lipodystrophy-specific treatment were potentially eligible. Records were available from as early as 1977 for patients in the United States, 1979 for patients in Brazil, and 1992 for patients in Turkey. The majority of the cases were followed after 2000 (84.4%). Data were entered into internet-based case report forms, either from electronic or paper charts available at the study sites, which formed a database of de-identified patient records. Diagnoses recorded in original patient medical charts were assumed to conform to prevailing clinical definitions at the time of diagnosis in the patient’s country of residence.
Patient demographics and clinical characteristics included age (as of initial diagnosis), sex, race, country of residence, years between the first reported evidence of GL or PL and diagnosis, and lipodystrophy by type. Physical characteristics associated with lipodystrophy included acanthosis nigricans, acromegaloid features, lack of fat in face, muscular appearance, prominent veins, and splenomegaly. These were reported as of the last visit of the follow-up period (or at the last visit when such characteristics were recorded).
The study focus was on documentation of abnormalities in organ systems affected by the pathophysiological adaptation mechanisms associated with metabolic abnormalities. Thus, data collection tools and protocols were developed in a manner that focused on capturing abnormalities related to insufficient storage capacity for excess nutrients or spillover to ectopic sites rather than global defects that can be caused by other heterogeneous molecular mechanisms affecting multiple systems. The following abnormalities recorded during the observation period were specifically investigated: diabetes and/or insulin resistance, elevated laboratory values [triglyceride levels, HbA1c, alanine aminotransferase (ALT), and aspartate aminotransferase (AST)], organ abnormalities (liver abnormalities, kidney abnormalities, heart abnormalities, and pancreatitis), and death. The definitions of organ abnormalities were based on existing literature, clinical relevance, and author discussions and are described in Table 1. Abnormality rates were reported as lifetime prevalence, defined as the percentage of patient medical records in which a specific abnormality has been recorded at least once over the observation period. In addition, information on causes and contributing factors to death (if available) were recorded (e.g., myocardial infarction, cardiac arrest, infection, and liver disease). Data on background medication use in the baseline period were also recorded. As this was a medical chart review study, data were abstracted as found in each patient record. Abnormalities and medication use were abstracted when specific terminology was identified by data abstractors and were not inferred.
Table 1.
Definition of Observed Variables
| Physical Characteristics | Metabolic Abnormalities | Elevated Laboratory Values | Organ Abnormalities |
|---|---|---|---|
| Acanthosis nigricans | Diabetes and/or IRa | Tg >150 mg/dL | Liver |
| Acromegaloid features | Tg >500 mg/dL | Hepatic steatosisb | |
| Lack of fat in face | HbA1c >5.7% | Hepatomegalyc | |
| Muscular appearance | HbA1c >6.5% | Cirrhosis | |
| Prominent veins | HbA1c >8.5% | Kidney | |
| Splenomegaly | ALT >35 U/L | Nephropathyd | |
| ALT >55 U/L | Chronic renal failuree | ||
| AST >35 U/L | ESRD | ||
| AST >48 U/L | Transplant | ||
| Otherf | |||
| Heart | |||
| Coronary artery diseaseg | |||
| Cardiac arrhythmiah | |||
| Cardiomyopathyi | |||
| Heart failure | |||
| Transplant | |||
| Otherj | |||
| Pancreas | |||
| Pancreatitis |
Abbreviations: ESRD, end-stage renal disease; IR, insulin resistance; Tg, triglycerides.
Based on documentation in patient medical records; includes diabetes (recorded in drop-down lists), insulin resistance (recorded in open-text fields only), and diabetes/insulin resistance (recorded in open-text fields only).
Includes nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and steatohepatitis.
Hepatic steatosis is the principle driver of hepatomegaly in lipodystrophy. However, a low percentage of patients may have hepatomegaly driven by other conditions.
Includes albuminuria, microalbuminuria, and proteinuria.
Includes mentions of chronic renal failure (recorded in open-text fields), kidney dysfunction (recorded in open-text fields), decreased kidney function (recorded in open-text fields), and ESRD (including kidney transplant).
Includes hematuria, kidney stones, nephromegaly, and renal hypoplasia.
Includes atherosclerosis, bypass surgery, ischemia, myocardial infarction, and probable anteroseptal infarct.
Includes atrial fibrillation, atrial flutter, bradycardia, and tachycardia.
Includes ventricular hypertrophy.
Includes aortic insufficiency, aortic outflow murmur, aortic regurgitation, aortic stenosis, ascending aorta dilated, asymmetric septal hypertrophy with a sigmoid septum, atrial-level shunt and ventricular dilation, arteriovenous (AV) malformation, AV shunt, cardiomegaly, dilated left atrium, effusion pericardial, grade II/VI midsystolic murmur at the base of the left sternal border, heart murmurs, left ventricular relaxation deficit, mild mitral insufficiency, mild mitral valve regurgitation, mild tricuspid insufficiency, mild tricuspid valve regurgitation, mitral insufficiency, mitral valve insufficiency, mitral valve prolapse, mitral valve regurgitation, moderate mitral insufficiency, pulmonary AV malformation, pulmonic valve regurgitation, subaortic stenosis, subaortic ventricular septal defect, tricuspid insufficiency, tricuspid valve regurgitation, valvular heart disease, and ventricle diastolic-systolic dysfunction.
Data abstractors recorded the presence or absence of prespecified physical characteristics into the case report forms through a series of multiple-choice questions, with “unknown” being a possible third choice for cases that could not be determined from available information within the patient record. Data abstractors used drop-down lists in the case report forms to indicate the presence of diabetes and/or insulin resistance, prespecified organ abnormalities associated with lipodystrophy, and baseline use of prespecified medications for managing diabetes, dyslipidemia, cardiovascular disease, kidney disease, reproductive health, and depression. These drop-down lists also included an option to select “other,” which then prompted the data abstractor to specify the condition or specific medication in an open-text field. Laboratory results during the observation period were also recorded in the case report forms. No inference of clinical diagnoses was attempted after data abstraction based on laboratory values recorded in case report forms.
Analyses
Means and frequencies of demographic and clinical characteristics as of the date of data abstraction and the frequency and proportion of patients with physical characteristics associated with lipodystrophy were reported. In addition, lifetime prevalence of diabetes and/or insulin resistance, elevated laboratory values, and organ abnormalities were calculated for the entire sample and separately for the GL and PL subgroups. Age-adjusted lifetime prevalence was also calculated, as patients with PL were generally older than patients with GL. Time to a diagnosis of diabetes and/or insulin resistance was plotted using separate Kaplan-Meier curves for the GL and PL subgroups and defined as the length of time (in years) between first evidence of GL or PL and a diagnosis of diabetes and/or insulin resistance. Time to first organ abnormality and time to disease progression were also plotted using separate Kaplan-Meier curves for the GL and PL subgroups. Time to first organ abnormality was defined as the length of time (in years) between the first reported evidence of GL or PL and the first organ abnormality thereafter. The length of time (in years) between the first organ abnormality and the diagnosis of another abnormality in a different organ thereafter (i.e., on a different date) was assessed as a proxy for disease progression (time to disease progression). A canonical clinical definition of progression for lipodystrophy syndromes is not yet available. Log-rank tests were conducted to compare time to diagnosis of diabetes and/or insulin resistance, time to first organ abnormality, and time to disease progression between the GL and the PL subgroups.
Overall survival was also described using a separate Kaplan-Meier curve for the GL and PL subgroups. A log-rank test was conducted to compare survival between patients with GL and PL.
Statistical analyses were performed using SAS 9.3 or SAS 9.4 (SAS Institute, Cary, NC).
Results
Data availability
Medical charts of 230 patients with a diagnosis of GL or PL meeting eligibility criteria were included, and available data over the observation period (i.e., baseline plus follow up) for each chart were extracted. The mean (SD) duration of the baseline period was 26.2 years (18.4) in the overall study cohort, 12.3 years (13.7) in the GL subgroup, and 33.7 years (16.1) in the PL subgroup. The mean duration of the follow up period was 7.6 years (7.9) in the overall cohort, 9.5 years (8.1) in the GL subgroup, and 6.5 years (7.6) in the PL subgroup. By country, the mean duration of the baseline period was 32.8 years (18.5) for patients in the US, 24.5 years (16.4) for patients in Turkey, and 16.2 years (16.1) for patients in Brazil, whereas the mean duration of the follow-up period was 8.0 years (9.9) for patients in the United States, 5.0 years (4.9) for patients in Turkey, and 10.5 years (6.0) for patients in Brazil. The start of the observation period ranged from 1977 to 2017 for patients in the United States, from 1992 to 2016 for patients in Turkey, and from 1979 to 2014 for patients in Brazil.
A tabular summary of the data availability metrics, including the number of medical evaluations across patient charts and the proportion with data on mortality status, physical characteristics, laboratory values, and medication use, is presented in Table 2. The proportion of patients with fewer than three medical evaluations over the observation period was 11.1% in the GL subgroup (n = 9 out of 81), 15.4% in the PL subgroup (n = 23 out of 149), and 13.9% overall (n = 32 out of 230). Data on medication use at baseline were only available from 38.7% (n = 89 out of 230) of patient records. The extent of missing data on the prevalence of diabetes and/or insulin resistance and organ abnormalities could not be assessed.
Table 2.
Data Availability Metrics
| Parameter | Overall (N = 230) | GL (n = 81) | PL (n = 149) |
|---|---|---|---|
| Number of medical evaluations, n (%) | |||
| ≥3 | 198 (86.1) | 72 (88.9) | 126 (84.6) |
| <3 | 32 (13.9) | 9 (11.1) | 23 (15.4) |
| Survival status available at last observation date, n (%) | 198 (86.1) | 64 (79.0) | 134 (89.9) |
| Physical characteristics data available at last observation date, n (%) | |||
| Acanthosis nigricans | 186 (80.9) | 72 (88.9) | 114 (76.5) |
| Acromegaloid features | 149 (64.8) | 64 (79.0) | 85 (57.0) |
| Lack of fat in face | 190 (82.6) | 70 (86.4) | 120 (80.5) |
| Muscular appearance | 205 (89.1) | 77 (95.1) | 128 (85.9) |
| Prominent veins | 149 (64.8) | 59 (72.8) | 90 (60.4) |
| Splenomegaly | 168 (73.0) | 62 (76.5) | 106 (71.1) |
| One or more laboratory value measurements available over entire observation period, n (%) | |||
| HbA1c | 200 (87.0) | 67 (82.7) | 133 (89.3) |
| Tg | 225 (97.8) | 79 (97.5) | 146 (98.0) |
| ALT | 221 (96.1) | 77 (95.1) | 144 (96.6) |
| AST | 211 (91.7) | 76 (93.8) | 135 (90.6) |
| Medication use data at baseline available, n (%) | 89 (38.7) | 30 (37.0) | 59 (39.6) |
Abbreviation: Tg, triglycerides.
Patient demographics
The overall study cohort included 81 patients (35.2%) with GL and 149 patients (64.8%) with PL. Ninety-eight patients (42.6%) were from the two US treatment centers, 80 patients (34.8%) were from Turkey, and 52 patients (22.6%) were from the two treatment centers in Brazil. The mean (SD) age at initial diagnosis was 26.2 years (18.4) in the overall cohort, 12.3 years (13.7) for patients with GL, and 33.7 years (16.1) for patients with PL. Males represented 40.7% (n = 33) of the GL subgroup, 24.8% (n = 37) of the PL subgroup, and 30.4% (n = 70) overall. First symptoms of lipodystrophy were typically identified during childhood among patients with GL [mean (SD) age: 9.2 years (11.9)], whereas such symptoms were typically identified during adulthood among patients with PL [mean age: 24.7 years (16.1)]. The mean (SD) number of years between the first reported evidence of GL or PL and diagnosis was 3.1 years (6.4) for patients with GL and 9.0 years (12.0) for patients with PL. The majority of patients in the GL subgroup had CGL (n = 72; 88.9%), whereas the majority of patients in the PL subgroup had FPLD (n = 121; 81.2%). Patient demographic and clinical characteristics are reported by type of lipodystrophy in Table 3 and by country in Table 4.
Table 3.
Patient Demographic and Clinical Characteristics
| Overall (N = 230) | GL (n = 81) | PL (n = 149) | |
|---|---|---|---|
| Age at first symptoms in y, mean (SD) | 19.2 (16.5) | 9.2 (11.9) | 24.7 (16.1) |
| Age at initial diagnosis in y, mean (SD) | 26.2 (18.4) | 12.3 (13.7) | 33.7 (16.1) |
| Age at first visit to treatment center in y, mean (SD) | 28.7 (18.2) | 16.1 (13.9) | 35.6 (16.6) |
| Years from first symptoms to diagnosis, mean (SD) | 6.9 (10.8) | 3.1 (6.4) | 9.0 (12.0) |
| Duration of follow-up period in y, mean (SD) | 7.6 (7.9) | 9.5 (8.1) | 6.5 (7.6) |
| Males, n (%) | 70 (30.4) | 33 (40.7) | 37 (24.8) |
| Race/ethnicity,a n (%) | |||
| Caucasian/white | 166 (72.2) | 46 (56.8) | 120 (80.5) |
| African descent/black | 17 (7.4) | 14 (17.3) | 3 (2.0) |
| Hispanic/Latino | 11 (4.8) | 2 (2.5) | 9 (6.0) |
| Other | 21 (9.1) | 16 (19.7) | 5 (3.4) |
| Unknown | 16 (7.0) | 3 (3.7) | 13 (8.7) |
| Country of residence, n (%) | |||
| Brazil | 52 (22.6) | 25 (30.9) | 27 (18.1) |
| Turkey | 80 (34.8) | 32 (39.5) | 48 (32.2) |
| United States | 93 (40.4) | 22 (27.2) | 71 (47.7) |
| Otherb | 5 (2.2) | 2 (2.5) | 3 (2.0) |
| Treatment center, n (%) | |||
| National Institutes of Health (United States) | 66 (28.7) | 23 (28.4) | 43 (28.9) |
| University of Michigan (United States) | 32 (13.9) | 1 (1.2) | 31 (20.8) |
| Dokuz Eylül University (Turkey) | 80 (34.8) | 32 (39.5) | 48 (32.2) |
| Federal University of Ceará (Brazil) | 23 (10.0) | 19 (23.5) | 4 (2.7) |
| Universidade de São Paulo (Brazil) | 29 (12.6) | 6 (7.4) | 23 (15.4) |
| Type of lipodystrophy, n (%) | |||
| AGL | 7 (3.0) | 7 (8.6) | — |
| APL | 28 (12.2) | — | 28 (18.8) |
| CGL | 72 (31.3) | 72 (88.9) | — |
| FPLD | 121 (52.6) | — | 121 (81.2) |
| Generalized progeroid lipodystrophy | 2 (0.9) | 2 (2.5) | — |
One patient in the United States was marked as “Caucasian” and “Other.” Because of this, the sum of patient counts for the race/ethnicity categories may exceed the total number of patients.
Other countries included Argentina, Bahamas, Greece, Israel, and the United Kingdom.
Table 4.
Patient Demographic and Clinical Characteristics by Country
| United States (n = 98) | Turkey (n = 80) | Brazil (n = 52) | |
|---|---|---|---|
| Age at first symptoms in y, mean (SD) | 23.4 (17.5) | 18.3 (15.5) | 12.8 (13.8) |
| Age at initial diagnosis in y, mean (SD) | 32.8 (18.5) | 24.5 (16.4) | 16.2 (16.1) |
| Age at first visit to treatment center in y, mean (SD) | 37.3 (17.4) | 26.0 (15.9) | 16.7 (15.2) |
| Years from first symptoms to diagnosis, mean (SD) | 9.4 (12.7) | 6.2 (9.4) | 3.4 (7.2) |
| Duration of follow-up period in y, mean (SD) | 8.0 (9.9) | 5.0 (4.9) | 10.5 (6.0) |
| Males, n (%) | 30 (30.6) | 29 (36.3) | 11 (21.2) |
| Race/ethnicity,a n (%) | |||
| Caucasian/white | 74 (75.5) | 80 (100.0) | 12 (23.1) |
| African descent/black | 13 (13.3) | — | 4 (7.7) |
| Hispanic/Latino | 6 (6.1) | — | 5 (9.6) |
| Other | 6 (6.1) | — | 15 (28.8) |
| Unknown | — | — | 16 (30.8) |
| Type of lipodystrophy, n (%) | |||
| AGL | 4 (4.1) | 1 (1.3) | 2 (3.8) |
| APL | 11 (11.2) | 15 (18.8) | 2 (3.8) |
| CGL | 18 (18.4) | 31 (38.8) | 23 (44.2) |
| FPLD | 63 (64.3) | 33 (41.3) | 25 (48.1) |
| Generalized progeroid lipodystrophy | 2 (0.9) | — | — |
One patient in the United States was marked as “Caucasian” and “Other.” Because of this, the sum of patient counts for the race/ethnicity categories may exceed the total number of patients.
The majority of patients underwent genetic testing to classify lipodystrophy subtype. The proportion of patients known to have received genetic testing were similar across cohorts, at 55.6% in the GL subgroup (n = 45 out of 81), 57.7% in the PL subgroup (n = 86 out of 149), and 57% overall (n = 131 out of 230). Genetic testing and phenotype/subtype characteristics are presented in Table 5.
Table 5.
Genetic Testing and Phenotype/Subtype Characteristics
| Overall (N = 230) | GL (n = 81) | PL (n = 149) | |
|---|---|---|---|
| Genetic testing to identify lipodystrophy subtypea | |||
| Yes | 131 (57.0) | 45 (55.6) | 86 (57.7) |
| Not done | 37 (16.1) | 15 (18.5) | 22 (14.8) |
| Unknown or not documented | 62 (27.0) | 21 (25.9) | 41 (27.5) |
| Phenotype/subtypeb | |||
| APL/AGL | n = 35 | n = 7 | n = 28 |
| Lawrence syndrome (AGL) | 1 (2.9) | 1 (14.3) | — |
| Barraquer–Simons syndrome (APL) | 19 (54.3) | — | 19 (67.9) |
| Unknown or not documented | 10 (28.6) | 4 (57.1) | 6 (21.4) |
| Other | 5 (14.3) | 2 (28.6) | 3 (10.7) |
| CGLc | n = 72 | n = 72 | |
| CGL1, AGPAT2 mutations | 31 (43.1) | 31 (43.1) | — |
| CGL2, BSCL2 mutations | 18 (25.0) | 18 (25.0) | — |
| CGL4, PTRF mutations | 2 (2.8) | 2 (2.8) | — |
| Unknown or not documented | 19 (26.4) | 19 (26.4) | — |
| Other | 2 (2.8) | 2 (2.8) | — |
| Generalized progeroid lipodystrophy | 2 (0.9) | 2 (2.5) | — |
| FPLDc | n = 121 | n = 121 | |
| FPLD1, Köbberling variety | 10 (8.3) | — | 10 (8.3) |
| FPLD2, Dunnigan variety, LMNA mutations | 68 (56.2) | — | 68 (56.2) |
| FPLD3, PPARG mutations | 11 (9.1) | — | 11 (9.1) |
| Unknown or not documented | 18 (14.9) | — | 18 (14.9) |
| Other | 14 (11.6) | — | 14 (11.6) |
Data are n (%) unless otherwise noted.
Data abstractors were asked whether genetic testing was conducted to formally identify the subtype of lipodystrophy.
Includes diagnoses made without genetic testing.
No patients with mutations in CAV1 (CGL3), PLIN1 (FPLD4), or AKT2 (FPLD5) were identified. No patients with mutations in CIDEC or PCYT1a were identified.
Physical characteristics associated with lipodystrophy
Data on lipodystrophy-associated physical characteristics were available for a subset of patients. Muscular appearance (n = 156 out of 205; 76.1%), prominent veins (n = 110 out of 149; 73.8%), and lack of fat in face (n = 105 out of 190; 55.3%) were the three most frequently reported physical characteristics in the overall sample, and all three were more common among patients with GL than among patients with PL. Muscular appearance was the most commonly reported physical characteristic among patients with GL (n = 71 out of 77; 92.2%), whereas prominent veins was the most commonly reported physical characteristic among patients with PL (n = 63 out of 90; 70.0%). Notably, lack of fat in face was predominantly observed in patients with APL (n = 23 out of 26; 88.5%), CGL (n = 56 out of 64; 87.5%), and AGL (n = 3 out of 5; 60.0%), but was relatively uncommon among patients with FPLD (n = 22 out of 94; 23.4%). Lack of fat in face was also present in the one patient with generalized progeroid lipodystrophy evaluated for this physical characteristic.
Metabolic status and organ abnormalities in overall cohort
During the observation period, diabetes and/or insulin resistance was reported in more than half of patients (n = 134; 58.3%). Nearly all patients (n = 209; 90.9%) had records documenting at least one elevated laboratory value. Over half of patients had triglyceride levels >150 mg/dL (n = 190; 82.6%), HbA1c levels >5.7% (n = 143; 62.2%), and ALT levels >35 U/L (n = 140; 60.9%).
In the overall cohort, 182 (79.1%) patients had at least one organ abnormality. Prevalence was higher in patients with GL (n = 74; 91.4%; age-adjusted: 93.8%) than in patients with PL (n = 108; 72.5%; age-adjusted: 66.1%). Patients in the overall sample had, on average (SD), 1.6 organs (1.1) with abnormalities. Patients in the GL subgroup had, on average, 1.9 organs (1.0) with abnormalities, whereas those in the PL subgroup had, on average, 1.4 organs (1.1) with abnormalities. Liver abnormalities were the most frequently reported class of organ abnormalities (n = 165; 71.7%), followed by kidney abnormalities (n = 93; 40.4%), heart abnormalities (n = 70; 30.4%), and episodes of acute pancreatitis (n = 30; 13.0%).
Lifetime prevalence of metabolic involvement and organ abnormalities is presented by type of lipodystrophy in Table 6 and by country in Table 7(a).
Table 6.
Lifetime Prevalence of Metabolic Involvement and Organ Abnormalities
| Overall (N = 230) | GL (n = 81) | PL (n = 149) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Percent | n | Percent | Adjusted Percentagea | n | Percent | Adjusted Percentagea | |
| Diabetes and/or IR | 134 | 58.3 | 47 | 58.0 | 75.7 | 87 | 58.4 | 51.8 |
| One or more elevated laboratory values | 209 | 90.9 | 75 | 92.6 | 96.5 | 134 | 89.9 | 85.5 |
| Tg >150 mg/dL | 190 | 82.6 | 66 | 81.5 | 86.5 | 124 | 83.2 | 77.2 |
| Tg >500 mg/dL | 93 | 40.4 | 41 | 50.6 | 63.4 | 52 | 34.9 | 32.1 |
| HbA1c >5.7% | 143 | 62.2 | 43 | 53.1 | 60.6 | 100 | 67.1 | 59.6 |
| HbA1c >6.5% | 114 | 49.6 | 35 | 43.2 | 54.1 | 79 | 53.0 | 45.6 |
| HbA1c >8.5% | 78 | 33.9 | 28 | 34.6 | 41.6 | 50 | 33.6 | 28.2 |
| ALT >35 U/L | 140 | 60.9 | 60 | 74.1 | 74.3 | 80 | 53.7 | 52.5 |
| ALT >55 U/L | 88 | 38.3 | 43 | 53.1 | 47.1 | 45 | 30.2 | 30.0 |
| AST >35 U/L | 112 | 48.7 | 50 | 61.7 | 58.5 | 62 | 41.6 | 42.3 |
| AST >48 U/L | 73 | 31.7 | 33 | 40.7 | 35.5 | 40 | 26.8 | 28.5 |
| One or more organ abnormalities | 182 | 79.1 | 74 | 91.4 | 93.8 | 108 | 72.5 | 66.1 |
| Liver abnormalities | 165 | 71.7 | 71 | 87.7 | 88.6 | 94 | 63.1 | 59.1 |
| Hepatic steatosisb | 142 | 61.7 | 55 | 67.9 | 74.9 | 87 | 58.4 | 54.6 |
| Hepatomegalyc | 99 | 43.0 | 57 | 70.4 | 70.6 | 42 | 28.2 | 28.0 |
| Cirrhosis | 8 | 3.5 | 5 | 6.2 | 5.9 | 3 | 2.0 | 1.5 |
| Kidney abnormalities | 93 | 40.4 | 44 | 54.3 | 67.5 | 49 | 32.9 | 28.3 |
| Nephropathyd | 74 | 32.2 | 36 | 44.4 | 59.7 | 38 | 25.5 | 22.0 |
| Chronic renal failuree | 10 | 4.3 | 5 | 6.2 | 11.4 | 5 | 3.4 | 3.4 |
| ESRD | 8 | 3.5 | 5 | 6.2 | 11.4 | 3 | 2.0 | 2.5 |
| Transplant | 1 | 0.4 | 1 | 1.2 | 2.4 | 0 | 0 | 0 |
| Otherf | 28 | 12.2 | 17 | 21.0 | 13.9 | 11 | 7.4 | 5.8 |
| Heart abnormalities | 70 | 30.4 | 28 | 34.6 | 51.3 | 42 | 28.2 | 21.8 |
| Coronary artery diseaseg | 22 | 9.6 | 6 | 7.4 | 20.9 | 16 | 10.7 | 8.1 |
| Cardiac arrhythmiah | 17 | 7.4 | 4 | 4.9 | 15.6 | 13 | 8.7 | 6.7 |
| Cardiomyopathyi | 15 | 6.5 | 13 | 16.0 | 15.9 | 2 | 1.3 | 1.0 |
| Heart failure | 9 | 3.9 | 0 | 0 | 0 | 9 | 6.0 | 4.6 |
| Transplant | 1 | 0.4 | 0 | 0 | 0 | 1 | 0.7 | 0.5 |
| Otherj | 27 | 11.7 | 10 | 12.3 | 12.4 | 17 | 11.4 | 9.4 |
| Pancreatitis | 30 | 13.0 | 8 | 9.9 | 11.3 | 22 | 14.8 | 11.9 |
Abbreviations: ESRD, end-stage renal disease; IR, insulin resistance; Tg, triglycerides.
Age-adjusted lifetime prevalence accounting for variations in the distribution of age groups between patients with GL and PL.
Includes nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and steatohepatitis.
Hepatic steatosis is the principle driver of hepatomegaly in lipodystrophy. However, a low percentage of patients may have hepatomegaly driven by other conditions.
Includes albuminuria, microalbuminuria, and proteinuria.
Includes mentions of chronic renal failure (recorded in open-text fields), kidney dysfunction (recorded in open-text fields), decreased kidney function (recorded in open-text fields), and ESRD (including kidney transplant).
Includes hematuria, kidney stones, nephromegaly, and renal hypoplasia.
Includes atherosclerosis, bypass surgery, ischemia, myocardial infarction, and probable anteroseptal infarct.
Includes atrial fibrillation, atrial flutter, bradycardia, and tachycardia.
Includes ventricular hypertrophy.
Includes aortic insufficiency, aortic outflow murmur, aortic regurgitation, aortic stenosis, ascending aorta dilated, asymmetric septal hypertrophy with a sigmoid septum, atrial-level shunt and ventricular dilation, AV malformation, AV shunt, cardiomegaly, dilated left atrium, effusion pericardial, grade II/VI midsystolic murmur at the base of the left sternal border, heart murmurs, left ventricular relaxation deficit, mild mitral insufficiency, mild mitral valve regurgitation, mild tricuspid insufficiency, mild tricuspid valve regurgitation, mitral insufficiency, mitral valve insufficiency, mitral valve prolapse, mitral valve regurgitation, moderate mitral insufficiency, pulmonary AV malformation, pulmonic valve regurgitation, subaortic stenosis, subaortic ventricular septal defect, tricuspid insufficiency, tricuspid valve regurgitation, valvular heart disease, and ventricle diastolic-systolic dysfunction.
Table 7.
Lifetime Prevalence of Metabolic Involvement and Organ Abnormalities by Country
| United States | Turkey | Brazil | Overall | |
|---|---|---|---|---|
| (a) GL and PL | n = 98 | n = 80 | n = 52 | N = 230 |
| Diabetes and/or IR | 71 (72.4) | 48 (60.0) | 15 (28.8) | 134 (58.3) |
| One or more elevated laboratory values | 89 (90.8) | 69 (86.3) | 51 (98.1) | 209 (90.9) |
| Tg >150 mg/dL | 79 (80.6) | 63 (78.8) | 48 (92.3) | 190 (82.6) |
| Tg >500 mg/dL | 30 (30.6) | 37 (46.3) | 26 (50.0) | 93 (40.4) |
| HbA1c >5.7% | 55 (56.1) | 51 (63.8) | 37 (71.2) | 143 (62.2) |
| HbA1c >6.5% | 42 (42.9) | 42 (52.5) | 30 (57.7) | 114 (49.6) |
| ALT >35 U/L | 62 (63.3) | 40 (50.0) | 38 (73.1) | 140 (60.9) |
| ALT >55 U/L | 34 (34.7) | 27 (33.8) | 27 (51.9) | 88 (38.3) |
| AST >35 U/L | 47 (48.0) | 31 (38.8) | 34 (65.4) | 112 (48.7) |
| AST >48 U/L | 29 (29.6) | 20 (25.0) | 24 (46.2) | 73 (31.7) |
| One or more organ abnormalities | 76 (77.6) | 69 (86.3) | 37 (71.2) | 182 (79.1) |
| Liver abnormalities | 61 (62.2) | 67 (83.8) | 37 (71.2) | 165 (71.7) |
| Kidney abnormalities | 26 (26.5) | 42 (52.5) | 25 (48.1) | 93 (40.4) |
| Heart abnormalities | 37 (37.8) | 21 (26.3) | 12 (23.1) | 70 (30.4) |
| Pancreatitis | 12 (12.2) | 15 (18.8) | 3 (5.8) | 30 (13.0) |
| (b) GL subgroup | n = 24 | n = 32 | n = 25 | N = 81 |
| Diabetes and/or IR | 18 (75.0) | 18 (56.3) | 11 (44.0) | 47 (58.0) |
| One or more elevated laboratory values | 21 (87.5) | 30 (93.8) | 24 (96.0) | 75 (92.6) |
| Tg >150 mg/dL | 18 (75.0) | 26 (81.3) | 22 (88.0) | 66 (81.5) |
| Tg >500 mg/dL | 10 (41.7) | 20 (62.5) | 11 (44.0) | 41 (50.6) |
| HbA1c >5.7% | 8 (33.3) | 18 (56.3) | 17 (68.0) | 43 (53.1) |
| HbA1c >6.5% | 5 (20.8) | 16 (50.0) | 14 (56.0) | 35 (43.2) |
| ALT >35 U/L | 16 (66.7) | 22 (68.8) | 22 (88.0) | 60 (74.1) |
| ALT >55 U/L | 9 (37.5) | 18 (56.3) | 16 (64.0) | 43 (53.1) |
| AST >35 U/L | 14 (58.3) | 18 (56.3) | 18 (72.0) | 50 (61.7) |
| AST >48 U/L | 7 (29.2) | 13 (40.6) | 13 (52.0) | 33 (40.7) |
| One or more organ abnormalities | 22 (91.7) | 31 (96.9) | 21 (84.0) | 74 (91.4) |
| Liver abnormalities | 19 (79.2) | 31 (96.9) | 21 (84.0) | 71(87.7) |
| Kidney abnormalities | 9 (37.5) | 18 (56.3) | 17 (68.0) | 44 (54.3) |
| Heart abnormalities | 12 (50.0) | 10 (31.3) | 6 (24.0) | 28 (34.6) |
| Pancreatitis | 2 (8.3) | 5 (15.6) | 1 (4.0) | 8 (9.9) |
| (c) PL subgroup | n = 74 | n = 48 | n = 27 | N = 149 |
| Diabetes and/or IR | 53 (71.6) | 30 (62.5) | 4 (14.8) | 87 (58.4) |
| One or more elevated laboratory values | 68 (91.9) | 39 (81.3) | 27 (100.0) | 134 (89.9) |
| Tg >150 mg/dL | 61 (82.4) | 37 (77.1) | 26 (96.3) | 124 (83.2) |
| Tg >500 mg/dL | 20 (27.0) | 17 (35.4) | 15 (55.6) | 52 (34.9) |
| HbA1c >5.7% | 47 (63.5) | 33 (68.8) | 20 (74.1) | 100 (67.1) |
| HbA1c >6.5% | 37 (50.0) | 26 (54.2) | 16 (59.3) | 79 (53.0) |
| ALT >35 U/L | 46 (62.2) | 18 (37.5) | 16 (59.3) | 80 (53.7) |
| ALT >55 U/L | 25 (33.8) | 9 (18.8) | 11 (40.7) | 45 (30.2) |
| AST >35 U/L | 33 (44.6) | 13 (27.1) | 16 (59.3) | 62 (41.6) |
| AST >48 U/L | 22 (29.7) | 7 (14.6) | 11 (40.7) | 40 (26.8) |
| One or more organ abnormalities | 54 (73.0) | 38 (79.2) | 16 (59.3) | 108 (72.5) |
| Liver abnormalities | 42 (56.8) | 36 (75.0) | 16 (59.3) | 94 (63.1) |
| Kidney abnormalities | 17 (23.0) | 24 (50.0) | 8 (29.6) | 49 (32.9) |
| Heart abnormalities | 25 (33.8) | 11 (22.9) | 6 (22.2) | 42 (28.2) |
| Pancreatitis | 10 (13.5) | 10 (20.8) | 2 (7.4) | 22 (14.8) |
Data are n (%) unless otherwise noted.
Abbreviations: IR, insulin resistance; Tg, triglycerides.
Patients with GL
In the GL subgroup (n = 81), diabetes and/or insulin resistance was identified in 47 patients (58.0%; age-adjusted: 75.7%). The mean (SD) age at first diagnosis of diabetes and/or insulin resistance was 19.3 years (12.6). At least one elevated laboratory value was identified in 75 patients (92.6%; age-adjusted: 96.5%), with triglyceride levels >150 mg/dL being most common and occurring in 66 patients (81.5%; age-adjusted: 86.5%). One or more organ abnormalities was identified in 74 patients (91.4%; age-adjusted: 93.8%), and the mean age at diagnosis of the first organ abnormality was 15.2 years (13.7). Liver abnormalities were identified in 71 patients (87.7%; age-adjusted: 88.6%). Hepatomegaly and hepatic steatosis were the most common liver abnormalities identified, affecting 57 patients (70.4%; age-adjusted: 70.6%) and 55 patients (67.9%; age-adjusted: 74.9%), respectively. Kidney abnormalities were identified in 44 patients (54.3%; age-adjusted: 67.5%), with nephropathy being the most common and affecting 36 patients (44.4%; age-adjusted: 59.7%). Heart abnormalities were identified in 28 patients (34.6%; age-adjusted: 51.3%), and episodes of acute pancreatitis were identified in 8 patients (9.9%; age-adjusted: 11.3%). Lifetime prevalence of metabolic involvement and organ abnormalities in the GL subgroup are presented by country in Table 7(b).
Patients with PL
In the PL subgroup (n = 149), diabetes and/or insulin resistance was identified in 87 patients (58.4%; age-adjusted: 51.8%). The mean (SD) age at first diagnosis of diabetes and/or insulin resistance was 35.7 years (14.9). At least one elevated laboratory value was identified in 134 patients (89.9%; age-adjusted: 85.5%), with triglyceride levels >150 mg/dL being most common and occurring in 124 patients (83.2%; age-adjusted: 77.2%). One or more organ abnormalities was identified in 108 patients (72.5%; age-adjusted: 66.1%), and the mean age at diagnosis of the first organ abnormality was 36.5 years (15.8). Liver abnormalities were identified in 94 patients (63.1%; age-adjusted: 59.1%), with hepatic steatosis being the most common and affecting 87 patients (58.4%; age-adjusted: 54.6%). Kidney abnormalities were identified in 49 patients (32.9%; age-adjusted: 28.3%), with nephropathy being the most common and affecting 38 patients (25.5%; age-adjusted: 22.0%). Heart abnormalities were identified in 42 patients (28.2%; age-adjusted: 21.8%), and episodes of acute pancreatitis were identified in 22 patients (14.8%; age-adjusted: 11.9%). Lifetime prevalence of metabolic involvement and organ abnormalities in the PL subgroup are presented by country in Table 7(c).
Patients by length of observation
The prevalence of organ abnormalities by median length of patient follow-up periods (i.e., time between diagnosis and end of follow-up) was assessed. In the overall cohort, median duration of the follow-up period was 4.5 years, with 115 patients having follow-up periods ≤4.5 years and 115 patients having follow-up periods >4.5 years. In the overall cohort, 74.8% of patients with follow-up periods ≤4.5 years had at least one organ abnormality compared with 83.5% of patients with follow-up periods >4.5 years (P = 0.143). Patients with follow-up periods ≤4.5 years had an average (SD) of 1.4 organs (1.0) with abnormalities, whereas those with follow-up periods >4.5 years had an average of 1.8 organs (1.1) with abnormalities (P = 0.006). In the GL subgroup, median duration of the follow-up period was 7.7 years, with 41 patients having follow-up periods ≤7.7 years and 40 patients having follow-up periods >7.7 years. In the GL subgroup, 90.2% of patients with follow-up periods ≤7.7 years had at least one organ abnormality compared with 92.5% of patients with follow-up periods >7.7 years (P = 1.000). Patients with GL and follow-up periods ≤7.7 years had an average of 1.7 organs (1.0) with abnormalities, whereas those with follow-up periods >7.7 years had an average of 2.0 organs (1.0) with abnormalities (P = 0.175). In the PL subgroup, median duration of the follow-up period was 3.4 years, with 74 patients having follow-up periods ≤3.4 years and 75 patients having follow-up periods >3.4 years. In the PL subgroup, 68.9% of patients with follow-up periods ≤3.4 years had at least one organ abnormality compared with 76.0% of patients with follow-up periods >3.4 years (P = 0.363). Patients with PL and follow-up periods ≤3.4 years had an average of 1.2 organs (1.0) with abnormalities, whereas those with follow-up periods >3.4 years had an average of 1.6 organs (1.2) with abnormalities (P = 0.075).
The prevalence of organ abnormalities in the time period up to and including the date of diagnosis was also assessed. The proportion of patients with at least one organ abnormality over this time period was 32.2% in the overall cohort (n = 74 out of 230), 35.8% in the GL subgroup (n = 29 out of 81), and 30.2% in the PL subgroup (n = 45 out of 149). The average number of organs with abnormalities over this time period was 0.4 (0.7) in the overall cohort, 0.5 (0.7) in the GL subgroup, and 0.4 (0.6) in the PL subgroup.
Patients by genetic subtype
The prevalence of organ abnormalities among patients with CGL and FPLD with a known genetic diagnosis was compared with respective patients with CGL and FPLD in whom the genetic basis was unknown. No considerable differences in the prevalence of patients with at least one organ abnormality and the average number of organ abnormalities between the respective CGL and FPLD cohorts were observed (P > 0.05 for both). Organ abnormality rates across lipodystrophy subtypes are summarized in Table 8.
Table 8.
Organ Abnormalities by Phenotype/Subtype
| Phenotype/Subtype | Total Number of Patients | Patients With More Than One Organ Abnormality, n (%) | Organs With Abnormalities, Mean (SD) |
|---|---|---|---|
| AGL | |||
| Lawrence syndrome | 1 | 1 (100.0) | 1.0 (—) |
| Unknown or not documented | 4 | 2 (50.0) | 1.0 (1.4) |
| Other | 2 | 2 (100.0) | 3.5 (0.7) |
| APL | |||
| Barraquer-Simons syndrome | 19 | 10 (52.6) | 1.0 (1.1) |
| Unknown or not documented | 6 | 4 (66.7) | 1.2 (1.5) |
| Other | 3 | 2 (66.7) | 1.0 (1.0) |
| CGLa | |||
| CGL1, AGPAT2 mutations | 31 | 29 (93.5) | 1.9 (1.0) |
| CGL2, BSCL2 mutations | 18 | 18 (100.0) | 2.1 (0.8) |
| CGL4, PTRF mutations | 2 | 2 (100.0) | 2.0 (0.0) |
| Unknown or not documented | 19 | 16 (84.2) | 1.5 (0.9) |
| Other | 2 | 2 (100.0) | 2.5 (0.7) |
| Generalized progeroid lipodystrophy | 2 | 2 (100.0) | 2.0 (0.0) |
| FPLDa | |||
| FPLD1, Köbberling variety | 10 | 8 (80.0) | 1.7 (1.2) |
| FPLD2, Dunnigan variety, LMNA mutations | 68 | 49 (72.1) | 1.5 (1.2) |
| FPLD3, PPARG mutations | 11 | 11 (100.0) | 1.6 (0.7) |
| Unknown or not documented | 18 | 12 (66.7) | 1.2 (1.0) |
| Other | 14 | 12 (85.7) | 1.6 (1.0) |
No patients with mutations in CAV1 (CGL3), PLIN1 (FPLD4), or AKT2 (FPLD5) were identified. No patients with mutations in CIDEC or PCYT1a were identified.
Time to diabetes, organ abnormality, and disease progression
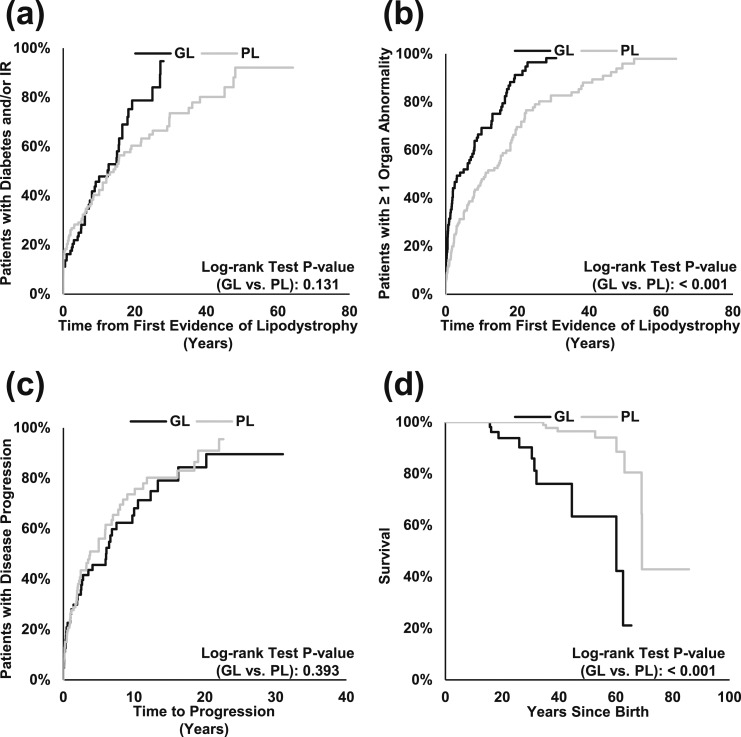
Kaplan-Meier estimates of mean (SE) time to diagnosis of diabetes and/or insulin resistance was 17.3 years (1.3) in the overall cohort, 12.7 years (1.2) in patients with GL, and 19.1 years (1.7) in patients with PL [P = 0.131; Fig. 2(a)]. Kaplan-Meier estimates of mean time to first organ abnormality in the overall sample was 12.9 years (1.0), and patients with GL developed their initial organ abnormality sooner than patients with PL. The mean time to first organ abnormality was 7.7 years (0.9) among patients with GL compared with 16.1 years (1.5) among patients with PL [P < 0.001; Fig. 2(b)]. However, mean time to disease progression was 7.6 years (0.8) in the overall sample, and there was no statistically significant difference between the GL and PL subgroups [P = 0.393; Fig. 2(c)].
Figure 2.
Time to diabetes and/or insulin resistance, organ abnormalities, and overall survival, stratified by GL and PL. Time to diagnosis of (a) diabetes and/or insulin resistance (IR), (b) first organ abnormality, and (c) abnormality in subsequent organ (proxy for disease progression), stratified by GL and PL are shown. (d) Overall survival stratified by GL and PL is also shown.
Overall survival
Kaplan-Meier estimates of mean (SE) time to death was 63.9 years (1.2) in the overall sample [Fig. 2(d)]. Mean time to death was shorter among patients with GL compared with patients with PL (51.2 [3.5] vs 66.6 [1.0] years; P < 0.001).
A total of 18 patients (7.8%) died during the observation period, including 10 patients (4.3%) with GL and 8 (3.5%) patients with PL. Cardiovascular events were reported as potential contributing factors to death in six patients (33.3%). Mortality events by contributing factors and by lipodystrophy subtype are presented in Table 9. Details of individual mortality events are presented in Table 10. Mortality status at the last observation date was unknown for 17 patients with GL and for 15 patients with PL.
Table 9.
Mortality Events by Contributing Factors and Patient Genetics
| Category | GL (n = 10) | PL (n = 8) |
|---|---|---|
| Age at death in y, mean (SD) | 33.8 (17.0) | 52.9 (14.7) |
| Contributing factora | ||
| Bone marrow/hematologic abnormalities | 1 | 0 |
| Cancer | 0 | 0 |
| Cardiovascular event | 4 | 2 |
| Cerebrovascular disease | 1 | 2 |
| Immunosuppression | 1 | 0 |
| Infection (viral) | 0 | 0 |
| Infection (bacterial) | 3 | 0 |
| Liver disease | 3 | 1 |
| Pancreatitis | 2 | 0 |
| Pneumonia | 2 | 0 |
| Renal failure | 1 | 1 |
| Sepsis | 1 | 0 |
| Unknown | 1 | 4 |
| Otherb | 1 | 0 |
| Subtype | ||
| CGL1, AGPAT2 mutations | 4 | 0 |
| CGL2, BSCL2 mutations | 4 | 0 |
| FPLD2, Dunnigan variety, LMNA mutations | 0 | 4 |
| FPLD3, PPARG mutations | 0 | 1 |
| Unknown or not documented | 1 | 3 |
| Otherc | 1 | 0 |
More than one contributing factor may be selected for each mortality event and may be different from the reported cause(s) of death noted in patient records.
Other potential contributing factors of death included mentions of pancytopenia, steatohepatitis, and chronic renal insufficiency.
Patient had symptoms consistent with CGL1 and CGL2 but lacked definitive testing or documentation.
Table 10.
Causes of Mortality by Patient
| Patient Number | Type of Lipodystrophy | Sex | Country | Age at Death, y | Cause(s) of Deatha |
|---|---|---|---|---|---|
| 1 | CGL | Male | United States | 31 | Probable end-stage liver disease |
| 2 | CGL | Male | United States | 32 | Atypical interstitial pneumonitis; respiratory failure |
| 3 | CGL | Male | Turkey | 44 | Died after coronary artery bypass grafting operation |
| 4 | CGL | Female | Turkey | 62 | Myocardial infarction |
| 5 | CGL | Female | Turkey | 26 | Diabetic foot infection |
| 6 | CGL | Female | United States | 30 | Cardiac arrest due to underlying nonischemic cardiomyopathy |
| 7 | CGL | Female | Brazil | 16 | Sepsis |
| 8 | CGL | Female | United States | 18 | Heart failure related to valvular stenosis |
| 9 | CGL | Female | Brazil | 15 | Septic shock |
| 10 | CGL | Female | Turkey | 60 | Stroke |
| 11 | FPLD | Male | Turkey | 35 | Not documented |
| 12 | FPLD | Male | United States | 69 | Not documented |
| 13 | FPLD | Male | United States | 63 | Not documented |
| 14 | FPLD | Female | United States | 39 | Not documented |
| 15 | FPLD | Female | United States | 69 | Probable kidney failure |
| 16 | FPLD | Female | Brazil | 60 | Hepatic cirrhosis |
| 17 | FPLD | Female | Brazil | 34 | Hypovolemic shock |
| 18 | FPLD | Female | United States | 52 | Possible cardiac episode |
As reported in patient medical records and by study investigators.
Discussion
The current real-world study describes the natural history of patients with non-HIV–related lipodystrophy syndromes in a large multinational sample. Our analysis of data from the medical charts of 230 patients who have never initiated LT or other lipodystrophy-specific therapies explores the disease natural history over the life of patients with lipodystrophy and highlights differences between patients with GL and PL, including time to initial organ abnormality and survival. The findings from our retrospective analysis provide an initial point of comparison for upcoming results from ongoing prospective natural history studies of patients with lipodystrophy syndromes, such as the LD Lync study (NCT03087253) in the United States and the ECLip Registry study (NCT03553420) involving sites in Europe and Turkey (17, 18). The current study also provides a comparator data set for an ongoing analysis to estimate the treatment effect of metreleptin therapy on long-term clinical outcomes, including mortality.
The current analysis included 81 patients with GL and 149 patients with PL from five study treatment centers in the United States, Turkey, and Brazil. In an effort to describe the impact of the physical changes seen in the lipodystrophy syndromes, we reported the prevalence of physical characteristics. Lipodystrophy-associated changes in physical appearance can cause substantial psychological distress and general physical discomfort and have a negative impact on quality of life (19, 20). We found that the majority of patients in our study had at least one hallmark physical characteristic of lipodystrophy, which confirms that physical changes in lipodystrophy syndromes are an important component contributing to the burden of illness. Notably, the lifetime prevalence of pancreatitis was 13.0% in the overall cohort (9.9% in GL; 14.8% in PL) and considerably higher than in the general population (21, 22).
First symptoms were typically identified during childhood for patients with GL and during adulthood for patients with PL. Although we reported the time to development of a single-organ abnormality as well as progression of the number of organ abnormalities, we recognize that these data may underestimate the presence of the organ abnormalities. Because these patients were not followed using a specific screening protocol and care evolved in the context of clinical standards before a consensus statement was created for diagnosis and treatment of patients with lipodystrophy, this underestimation is justified from the nature of this study. Clinical practice guidelines specific to lipodystrophy syndromes first became available in 2016 and recommend annual screening for diabetes, dyslipidemia, and liver, kidney, and heart disease for patients with most lipodystrophy syndromes (1). Despite these limitations, we still found that patients with GL developed their first lipodystrophy-related organ abnormality sooner than patients with PL. Although there were a limited number of deaths over the observation period, the available data suggest that patients with GL experience shorter lifespans compared with patients with PL. These results are supportive of the view that, on average, patients with generalized forms of lipodystrophy have more severe complications of lipodystrophy than patients with partial forms (1, 6, 9, 20). However, the manifestation of lipodystrophy syndromes is heterogeneous, and cases of PL with comparable or more severe symptomology than cases of GL have been documented (23–25).
Many patients in the current study were undiagnosed or lacked a definitive diagnosis of lipodystrophy until they were referred to a specialist treatment center, which is reflected in the high reported ages at first symptoms and at diagnosis. In Brazil and Turkey, there remains a general lack of recognition and understanding of lipodystrophy by physicians outside of the specialist treatment center setting. Furthermore, more patients from the US cohort had a diagnosis of PL, which is typically diagnosed at a later age, and a greater proportion of US patients were born and diagnosed in a time period when lipodystrophy syndromes were less well-recognized by the medical community. A recent systematic review has reported lower mean ages of onset for lipodystrophy syndromes (specifically fat loss and diabetes) but the results may be not directly comparable because the study population was limited to patients ≤18 years of age (26).
Analysis of organ abnormality rates stratified by length of observation and by genetic diagnosis were also conducted to determine how such parameters could affect reported prevalence rates. In the overall cohort, as expected, longer follow-up period was associated with significantly higher number of organs with abnormalities. We also find that the median length of the follow-up period in the GL subgroup (7.7 years) is more than double that of the PL subgroup (3.4 years). These differences suggest reported lifetime prevalence rates of metabolic involvement and organ abnormalities may not be directly comparable across the GL and PL subgroups. Although the proportion of patients with one or more organ abnormalities and the mean number of organs with abnormalities were numerically higher among cases of CGL and FLPD with a known genetic basis when compared with cases in which the genetic basis was unknown, the differences were not statistically significant.
An analysis of the time to diagnosis of diabetes and/or insulin resistance was conducted as a proxy for visualizing the development of metabolic abnormalities over the study observation period in patients with GL and PL. We did not observe a noteworthy difference in the mean time to diagnosis of diabetes and/or insulin resistance among patients with GL when compared with patients with PL. However, we did observe that some patients in our sample had a diagnosis of diabetes prior to their diagnosis of GL or PL, suggesting a need to improve awareness of lipodystrophy syndromes among the physician community. Limitations in study design and available data precluded a more robust analysis, such as the time to development of any metabolic abnormality over the study observation period.
Organ abnormality rates from start of observation through the date of diagnosis were, as expected, lower than rates reported over the entire observation period, potentially highlighting the progressive nature of lipodystrophy syndromes. However, it is recognized that the diagnosis of lipodystrophy itself may trigger more rigorous disease monitoring and the discovery of previously undiagnosed abnormalities. In the time period up to and including date of diagnosis, 32.2% of patients had one or more organ abnormalities, and there was an average of 0.4 organs with abnormalities. When assessed over the entire observation period, these figures increased to 79.1% of patients and an average of 1.6 organs with abnormalities. A separate analysis of the length of time between diagnosis of the first organ abnormality and the diagnosis of an abnormality in another organ was also conducted as a proxy for visualizing disease progression. We observed this length of time to be comparable between the GL and PL subgroups. Though our findings can be confounded from our retrospective design and more rigorous disease monitoring, this observation is similar to the findings from a prospective clinical study from Diker-Cohen et al. (25) that reported patients with PL were older at study entry, but their disease activity at study entry was comparable to that of patients with GL. Although the authors acknowledged that their study patients with PL were likely more severe than typical due to the metabolic inclusion criteria, the findings demonstrated that patients with PL can experience severe metabolic consequences similar to patients with GL. A more robust analysis of disease progression could not be conducted due to inherent limitations in study design. Select clinical parameters such as the severity and clinical impact of abnormalities in individual organs are recognized as markers of disease progression but could not be reliably captured from the patient medical charts. Therefore, the current study can only provide a simplified view of disease progression in lipodystrophy syndromes. Anticipated data from the ongoing prospective LD Lync and EClip Registry natural history studies are expected enable more robust analyses of disease progression, including the severity of impairment in individual metabolic parameters and organs over time (17, 18).
The inclusion of patients from the United States at a time when metreleptin was available to select patients with GL and PL (as an approved therapy for GL and through clinical trials for both GL and PL) could have created selection bias in our sample population toward patients with less severe disease who have never received leptin or other lipodystrophy-specific therapies. However, metreleptin was generally unavailable in Turkey and Brazil when the study was conducted. Thus, the patients from Turkey and Brazil comprising over half our study population would not be subject to such selection bias. Furthermore, no obvious differences that could be attributed to treatment selection bias were identified in the subgroup of US patients with GL and PL. Thus, it is reasonable to conclude that treatment selection bias likely has only a small impact on the study findings.
Although data on medication use at baseline were collected, only 89 of the 230 patient medical charts contained such data, reflecting the inherent heterogeneity of real-world data sources. Data abstraction was conducted using patient medical charts at the specialist treatment centers, which often lacked information about patient care in the primary care setting. Although certain background medications can delay the development of select abnormalities, their impact on the study findings could not be assessed.
The current retrospective study is subject to multiple limitations. First, the study population was limited to patients who have never received metreleptin or other lipodystrophy-specific therapies. Thus, the reported lifetime prevalence rates of metabolic abnormalities and organ system involvement are likely to be an underestimate for the broader patient population that would include patients who have received LT or enrolled in clinical trials evaluating other lipodystrophy-specific therapies. Furthermore, the current study design was conceptualized in 2015, prior to the publication of consensus screening protocols for patients with lipodystrophy syndromes, and data collection was not conducted in a manner that robustly captured the multisystem involvement of lipodystrophy syndromes extending beyond the key organ systems, such as thyroid dysfunction, hirsutism, psychological distress, fatigue, and pain. Although the current study focused on capturing abnormalities associated with insufficient storage capacity for nutrients and spillover to ectopic sites, it does not attempt to determine the underlying cause of the observed metabolic and organ abnormalities, which can also include nonmetabolic disease mechanisms. In addition, observed variables in the current study (organ abnormalities, diabetes and/or insulin resistance, elevated laboratory values, physical characteristics, and medication use) were identified as present or absent based on documentation within patient medical records. The availability of data on these variables can be affected by center-specific variations in data collection, clinical protocols, documentation styles, and measurement methodologies, which were not standardized nor harmonized prior to the study design. Such factors may have contributed to observed differences in the prevalence of abnormalities between patients in the current study and those enrolled in clinical trials (14, 25) and a previously published prospective observational cohort study (15). Moreover, only retrospectively available data were collected. Data that did not fall within the scope of interest at the specialty center may not have been available and accessible. In addition, data from primary care physicians (including data on comorbidities, laboratory results and organ abnormalities, and medication use prior to intake at the centers) were not captured. These limitations in data collection methodology might affect the accuracy of the reported clinical burden estimates for lipodystrophy. In addition, the current study relied on the accuracy of data recorded in patient medical charts and entered into an online form by abstractors. Therefore, errors or omissions in data entry that occurred during medical charting or online entry may affect the accuracy of the results. Lastly, an inherent limitation of observational studies is that the analyses can only account for observable factors a posteriori. The influence of unobserved factors is not accounted for in the study design.
Despite these limitations, results of the current study augment the understanding of the natural history of GL and PL and may address several perceived limitations in previously published studies, such as those related to sample size, study geography, patient population, and reported outcomes. The current study presents the natural history of non-HIV–related lipodystrophy syndromes based on an analysis of data from >200 patients managed at 5 treatment centers across 3 countries, encompassing both generalized and partial forms of the disorder, and includes longitudinal outcomes related to progression and survival. These results provide a preliminary resource for ongoing and future studies to understand the burden of lipodystrophy syndromes and could serve as a reference point in studies to estimate the treatment effect of approved and investigational therapies on long-term clinical outcomes.
Acknowledgments
We thank Melda Sonmez, Huseyin Onay, Samim Ozen, Tahir Atik, Tevfik Demir, Beyhan Tuysuz, Hulya Kayserili, Mehmet Nuri Ozbek, Gulcin Akinci, Ilgin Yildirim Simsir, Ulku Aybuke, Ela Temeloglu, Banu Sarer Yurekli, Nilufer Ozdemir Kutbay, Tugba Arkan, Ramazan Gen, Fatos Dilan Koseoglu, Guzin Fidan Yaylali, Mehmet Sercan Erturk, Habib Bilen, and all of the other collaborating physicians who referred patients to the treatment centers.
Financial Support: This work was supported by Aegerion Pharmaceuticals, the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases and the Lipodystrophy Fund at the University of Michigan (generously provided by the Sopha family and the White Point Foundation).
Glossary
Abbreviations:
- AGL
acquired generalized lipodystrophy
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AV
arteriovenous
- CGL
congenital generalized lipodystrophy
- FPLD
familiar partial lipodystrophy
- GL
generalized lipodystrophy
- LT
leptin therapy
- PL
partial lipodystrophy
Additional Information
Current Affiliation: P. Bradt’s current affiliation is the Institute for Clinical and Economic Review, Boston, Massachusetts 02109.
Disclosure Summary: B.A. is or has been on the advisory board of Aegerion Pharmaceuticals and a speaker for AstraZeneca, Eli Lilly and Company, Novartis, Novo Nordisk, Boehringer Ingelheim, Servier, and Sanofi Aventis. E.A.O. has received consulting fees indirectly through fees paid to the University of Michigan from Aegerion Pharmaceuticals, Akcea Therapeutics, and Regeneron Pharmaceuticals, has received grants from Akcea Therapeutics, Ionis Pharmaceuticals, Inc., Aegerion Pharmaceuticals, Gemphire Therapeutics, GI Dynamics, and Regeneron Pharmaceuticals, and had writing support relationships with Aegerion Pharmaceuticals. A.N., D.R., M.C.F.d.F., V.O.F., E.C., and R.J.B. have writing support relationships with Aegerion Pharmaceuticals. W.Y.C. and P.T.-L. are employees of Analysis Group, which has received consulting fees from Aegerion Pharmaceuticals to conduct this study. P.B. is a former employee of Aegerion Pharmaceuticals. R.M.M. serves as consultant and speaker for and received a research grant from Aegerion Pharmaceuticals.
Data Availability:
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References and Notes
- 1. Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiquette E, Oral EA, Garg A, Araújo-Vilar D, Dhankhar P. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obes. 2017;10:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garg A. Acquired lipodystrophy. Available at: https://rarediseases.org/rare-diseases/acquired-lipodystrophy/. Accessed 1 October 2018.
- 5. Lake JE, Currier JS. Metabolic disease in HIV infection. Lancet Infect Dis. 2013;13(11):964–975. [DOI] [PubMed] [Google Scholar]
- 6. Garg A. The physician’s guide to lipodystrophy disorders. Available at: https://rarediseases.org/physician-guide/lipodystrophy-disorders/. Accessed 20 August 2018.
- 7. Araujo-Vilar D, Santini F. Diagnosis and treatment of lipodystrophy: a step-by-step approach. J Endocrinol Invest. 2019;42(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US National Library of Medicine. Congenital generalized lipodystrophy. Available at: https://ghr.nlm.nih.gov/condition/congenital-generalized-lipodystrophy. Accessed 11 May 2018.
- 9. Akinci B, Oral E, Neidert A, Rus D, Cheng W, Thompson-Leduc P, Salinard T, Cochran E, Brown R. Burden of illness associated with generalized lipodystrophy in leptin replacement therapy-naïve patients: A longitudinal medical chart review study. Poster presented at ENDO 2018; 17–20 March 2018; Chicago, IL. [Google Scholar]
- 10. Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7(3):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polyzos SA, Mantzoros CS. Lipodystrophy: Time for a global registry and randomized clinical trials to assess efficacy, safety and cost-effectiveness of established and novel medications. Metabolism. 2017;72:A4–A10. [DOI] [PubMed] [Google Scholar]
- 12. Akinci B, Onay H, Demir T, Ozen S, Kayserili H, Akinci G, Nur B, Tuysuz B, Nuri Ozbek M, Gungor A, Yildirim Simsir I, Altay C, Demir L, Simsek E, Atmaca M, Topaloglu H, Bilen H, Atmaca H, Atik T, Cavdar U, Altunoglu U, Aslanger A, Mihci E, Secil M, Saygili F, Comlekci A, Garg A. Natural history of congenital generalized lipodystrophy: a nationwide study from Turkey. J Clin Endocrinol Metab. 2016;101(7):2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore). 2004;83(1):18–34. [DOI] [PubMed] [Google Scholar]
- 14. Ajluni N, Meral R, Neidert AH, Brady GF, Buras E, McKenna B, DiPaola F, Chenevert TL, Horowitz JF, Buggs-Saxton C, Rupani AR, Thomas PE, Tayeh MK, Innis JW, Omary MB, Conjeevaram H, Oral EA. Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort. Clin Endocrinol (Oxf). 2017;86(5):698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akinci B, Onay H, Demir T, Savas-Erdeve Ş, Gen R, Simsir IY, Keskin FE, Erturk MS, Uzum AK, Yaylali GF, Ozdemir NK, Atik T, Ozen S, Yurekli BS, Apaydin T, Altay C, Akinci G, Demir L, Comlekci A, Secil M, Oral EA. Clinical presentations, metabolic abnormalities and end-organ complications in patients with familial partial lipodystrophy. Metabolism. 2017;72:109–119. [DOI] [PubMed] [Google Scholar]
- 16. Lima JG, Nobrega LHC, Lima NN, Dos Santos MCF, Silva PHD, Baracho MFP, Lima DN, de Melo Campos JTA, Ferreira LC, Freire Neto FP, Mendes-Aguiar CO, Jeronimo SMB. Causes of death in patients with Berardinelli-Seip congenital generalized lipodystrophy. PLoS One. 2018;13(6):e0199052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. University of Michigan. The LD Lync Study–Natural History Study of Genetic Lipodystrophy Syndromes. Available at: https://clinicaltrials.gov/ct2/show/NCT03087253. Accessed 15 November 2018.
- 18. University of Ulm. Registry for Patients With Lipodystrophy (ECLip Registry). Available at: https://clinicaltrials.gov/ct2/show/NCT03553420. Accessed 15 November 2018.
- 19. Leclercq P, Goujard C, Duracinsky M, Allaert F, L’henaff M, Hellet M, Meunier JP, Carret S, Thevenon J, Ngo Van P, Pialoux G. High prevalence and impact on the quality of life of facial lipoatrophy and other abnormalities in fat tissue distribution in HIV-infected patients treated with antiretroviral therapy. AIDS Res Hum Retroviruses. 2013;29(5):761–768. [DOI] [PubMed] [Google Scholar]
- 20. Hussain I, Garg A. Lipodystrophy syndromes. Endocrinol Metab Clin North Am. 2016;45(4):783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lévy P, Domínguez-Muñoz E, Imrie C, Löhr M, Maisonneuve P. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterol J. 2014;2(5):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown RJ, Oral EA, Cochran E, Araújo-Vilar D, Savage DB, Long A, Fine G, Salinardi T, Gorden P. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine. 2018;60(3):479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oral EA, Gorden P, Cochran E, Araújo-Vilar D, Savage DB, Long A, Fine G, Salinardi T, Brown RJ. Long-term effectiveness and safety of metreleptin in the treatment of patients with partial lipodystrophy. Endocrine. 2019;64(3):500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta N, Asi N, Farah W, Almasri J, Barrionuevo P, Alsawas M, Wang Z, Haymond MW, Brown RJ, Murad MH. Clinical features and management of non-HIV-related lipodystrophy in children: a systematic review. J Clin Endocrinol Metab. 2017;102(2):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Medical charts of 230 patients with a diagnosis of GL or PL meeting eligibility criteria were included, and available data over the observation period (i.e., baseline plus follow up) for each chart were extracted. The mean (SD) duration of the baseline period was 26.2 years (18.4) in the overall study cohort, 12.3 years (13.7) in the GL subgroup, and 33.7 years (16.1) in the PL subgroup. The mean duration of the follow up period was 7.6 years (7.9) in the overall cohort, 9.5 years (8.1) in the GL subgroup, and 6.5 years (7.6) in the PL subgroup. By country, the mean duration of the baseline period was 32.8 years (18.5) for patients in the US, 24.5 years (16.4) for patients in Turkey, and 16.2 years (16.1) for patients in Brazil, whereas the mean duration of the follow-up period was 8.0 years (9.9) for patients in the United States, 5.0 years (4.9) for patients in Turkey, and 10.5 years (6.0) for patients in Brazil. The start of the observation period ranged from 1977 to 2017 for patients in the United States, from 1992 to 2016 for patients in Turkey, and from 1979 to 2014 for patients in Brazil.
A tabular summary of the data availability metrics, including the number of medical evaluations across patient charts and the proportion with data on mortality status, physical characteristics, laboratory values, and medication use, is presented in Table 2. The proportion of patients with fewer than three medical evaluations over the observation period was 11.1% in the GL subgroup (n = 9 out of 81), 15.4% in the PL subgroup (n = 23 out of 149), and 13.9% overall (n = 32 out of 230). Data on medication use at baseline were only available from 38.7% (n = 89 out of 230) of patient records. The extent of missing data on the prevalence of diabetes and/or insulin resistance and organ abnormalities could not be assessed.
Table 2.
Data Availability Metrics
| Parameter | Overall (N = 230) | GL (n = 81) | PL (n = 149) |
|---|---|---|---|
| Number of medical evaluations, n (%) | |||
| ≥3 | 198 (86.1) | 72 (88.9) | 126 (84.6) |
| <3 | 32 (13.9) | 9 (11.1) | 23 (15.4) |
| Survival status available at last observation date, n (%) | 198 (86.1) | 64 (79.0) | 134 (89.9) |
| Physical characteristics data available at last observation date, n (%) | |||
| Acanthosis nigricans | 186 (80.9) | 72 (88.9) | 114 (76.5) |
| Acromegaloid features | 149 (64.8) | 64 (79.0) | 85 (57.0) |
| Lack of fat in face | 190 (82.6) | 70 (86.4) | 120 (80.5) |
| Muscular appearance | 205 (89.1) | 77 (95.1) | 128 (85.9) |
| Prominent veins | 149 (64.8) | 59 (72.8) | 90 (60.4) |
| Splenomegaly | 168 (73.0) | 62 (76.5) | 106 (71.1) |
| One or more laboratory value measurements available over entire observation period, n (%) | |||
| HbA1c | 200 (87.0) | 67 (82.7) | 133 (89.3) |
| Tg | 225 (97.8) | 79 (97.5) | 146 (98.0) |
| ALT | 221 (96.1) | 77 (95.1) | 144 (96.6) |
| AST | 211 (91.7) | 76 (93.8) | 135 (90.6) |
| Medication use data at baseline available, n (%) | 89 (38.7) | 30 (37.0) | 59 (39.6) |
Abbreviation: Tg, triglycerides.
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.