Abstract
The tachykinin neurokinin B (NKB, Tac2) is critical for proper GnRH release in mammals, however, the role of the other tachykinins, such as substance P (SP) and neurokinin A (NKA) in reproduction, is still not well understood. In this study, we demonstrate that NKA controls the timing of puberty onset (similar to NKB and SP) and stimulates LH release in adulthood through NKB-independent (but kisspeptin-dependent) mechanisms in the presence of sex steroids. Furthermore, this is achieved, at least in part, through the autosynaptic activation of Tac1 neurons, which express NK2R (Tacr2), the receptor for NKA. Conversely, in the absence of sex steroids, as observed in ovariectomy, NKA inhibits LH through a mechanism that requires the presence of functional receptors for NKB and dynorphin (NK3R and KOR, respectively). Moreover, the ability of NKA to modulate LH secretion is absent in Kiss1KO mice, suggesting that its action occurs upstream of Kiss1 neurons. Overall, we demonstrate that NKA signaling is a critical component in the central control of reproduction, by contributing to the indirect regulation of kisspeptin release.
Tachykinins (TACs) are a large family of peptides that include neurokinin (NK) A, substance P (SP; encoded by TAC1), and NKB (encoded by TAC3 or Tac2 in rodents) (1). TACs act preferentially on different G protein–coupled receptors: NK1R (encoded by Tacr1, the receptor for SP), NK2R (encoded by Tacr2, the receptor for NKA), and NK3R (encoded by Tacr3, the receptor for NKB). These TAC systems are expressed throughout the central nervous system, where they participate in a variety of physiological functions, e.g., nociception and fear conditioning (1, 2).
The NKB/NK3R signaling system has emerged as a critical neuroendocrine regulator of reproductive function. A growing body of evidence from our laboratory and others has documented the stimulatory role of NKB on GnRH release, in an estradiol- and kisspeptin-dependent manner, in all studied species including humans (3). In addition to NKB, the SP/NK1R signaling system also participates in the central regulation of the gonadotropic axis, as supported by the following studies: (i) the central administration of SP induces LH release in rabbits and rats (4–6), (ii) electrophysiology studies showed activation of Kiss1 neurons by SP (7), (iii) SP mRNA and protein have been found in the arcuate nucleus (ARC) of rodents (8, 9), and (iv) SP immunoreactivity has been detected within Kiss1 and NKB neurons in the human infundibular nucleus (10). Interestingly, we have recently shown that long-term SP administration advances puberty onset in rodents (11) and that Tac1KO mice with congenital absence of SP display delayed puberty onset and reproductive impairments (11, 12). However, Tac1 encodes both SP and NKA and thus, the reproductive defects observed in Tac1KO mice may be, at least in part, because of the absence of NKA signaling. Importantly, we and others have documented that NKA induces LH release in mice and rats (9, 13, 14) in a kisspeptin-dependent manner (9). Furthermore, the stimulatory action of NKA on LH release is dependent on the presence of physiological levels of circulating sex steroids (9) and in their absence, such as after ovariectomy (OVX), NKA inhibits LH release, similar to NKB (9). However, unlike NKB, the receptor for NKA (NK2R) is not present on Kiss1 or GnRH neurons (9). Therefore, we hypothesize that NKA must act upstream of Kiss1 neurons upon an unknown population of neurons that in turn control NKB release. Alternatively, all TAC ligand-receptor systems have been reported to display cross-reactivity (15), suggesting that cross-activation of NK3R could account for the NKB-like action of NKA.
Overall, there is compelling evidence that all TACs (not only NKB) participate in the control of GnRH release. Thus, deciphering individual and/or potential synergistic mechanisms of action of TAC could provide important insight into the neuroendocrine control of reproduction.
Interestingly, a number of human patients with TAC3/TACR3 mutations have been reported to overcome initial pubertal failure and central hypogonadotropic hypogonadism (HH) (16). These patients present an awakening of GnRH secretion and hypogonadism reversal (16), a phenotype that resembles that of Tacr3KO mice, which are subfertile (17). This further suggests that in the absence of NKB signaling, compensation by NKA (and/or SP) may restore GnRH/LH secretion.
Thus, in this study, we aim to characterize the role of NKA in the control of GnRH release during puberty onset and adulthood in a series of pharmacological and genetic experiments in wild-type (WT), Tac2KO, and Kiss1KO female mice, with special interest in the interactions between the NKA/NK2R and NKB/NK3R systems.
Materials and Methods
Mice
WT female C57Bl/6 mice were purchased from Charles River Laboratories International, Inc. Tac2 knockout (KO) mice were obtained from Dr. Seminara (Massachusetts General Hospital, Boston, MA) (18). Kiss1KO were obtained from Dr. Richard Palmiter (University of Washington, Seattle, WA) (19). Tac2KO and Kiss1KO mice were compared with their respective WT littermates. All animal studies were approved by the Harvard Medical Area Standing Committee on the Use of Animals in Research and Teaching in the Harvard Medical School Center for Animal Resources and Comparative Medicine. Mice were maintained in a 12-hour light/12-hour dark cycle and were fed a standard rodent chow diet and water ad libitum. Genotyping was conducted by PCR analyses on isolated genomic DNA from tail biopsies.
Reagents
The agonists for NK1R (GR73632), NK2R (GR64349), and NK3R (senktide) and the antagonists for NK3R (SB 222200) and NK1R (RP67580) were purchased from Tocris. Naloxone hydrochloride (opioid receptor antagonist) and GnRH were purchased from Sigma-Aldrich. Mouse kisspeptin-10 was purchased from Phoenix Pharmaceutical. All drugs were dissolved in saline [0.9% sodium chloride (NaCl)], except for SB 222200 and RP67580, which were dissolved in 12% dimethyl sulfoxide. Doses and timing for hormonal analyses were selected based on previous studies (9, 20, 21).
Experimental Design
General procedures
For intracerebroventricular (icv) injections, the mice were briefly anesthetized with isoflurance two to three days before the experiment and a small hole was bored in the skull (1 mm lateral and 0.5 mm posterior to bregma) using a Hamilton syringe (27-gauge needle fitted with polyethylene tubing, leaving 3.5 mm of the needle tip exposed). All subsequent injections were made through this site. For icv injections, mice were anesthetized with isoflurane for a total of 2 to 3 minutes, during which time 5 µL of solution was slowly and continuously injected into the lateral ventricle. The needle remained inserted for approximately 60 seconds after the injection to minimize backflow up the needle track. Mice typically recovered from the anesthesia within 3 minutes after the injection. For hormonal analyses, blood samples (4 µL) were obtained from the tail at 0, 25, and 60 minutes after an icv injection and stored at −80°C until further processing. The dose and time of blood sampling were selected based on our previous studies (9).
Experiment 1
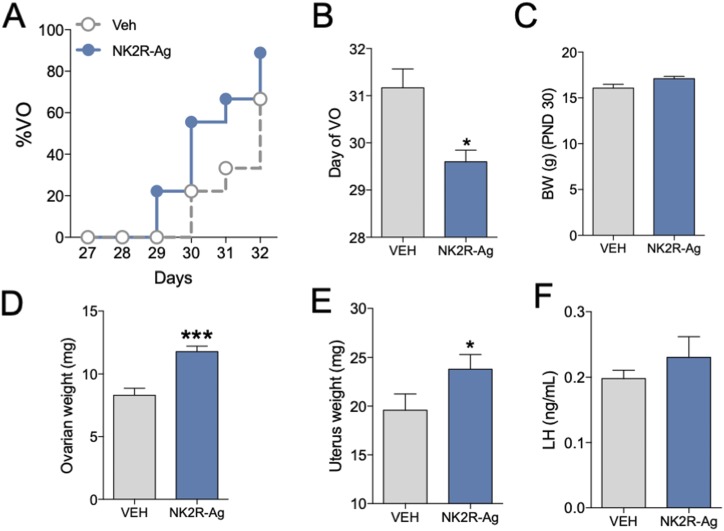
To investigate whether NKA/NK2R signaling plays a role in puberty onset, we performed a systemic long-term (from 23 days to 32 days) administration of NK2R-Ag (3 nmol/100 μL IP every 12 hours) or vehicle (0.9% NaCl) to WT female mice (n ≥ 6 per group). Reproductive maturation [i.e., progression of vaginal opening (VO)] was monitored daily. Body weight (BW) was recorded at day 30 when 50% of control females showed VO. Last, uterine and ovarian weights, as well as LH concentrations, were determined at day 32, the final day of NK2R-Ag administration.
Experiment 2
Interaction between NKA, NKB, and SP for the stimulation of LH release in the presence of estradiol
In this experiment, adult WT female mice were subjected to bilateral OVX under light isofluorane anesthesia, one week before pharmacological tests. Immediately after OVX, capsules filled with diluted crystalline of 17β-estradiol (E2) or vehicle (sesame seed oil) were implanted subcutaneously (SC) via a small midscapular incision at the base of the neck (OVX+E2). Silastic tubing (15 mm long, 0.078 inner diameter, 0.125 outer diameter; Dow Corning) was used for capsule preparation. A low dose of crystalline E2 (50 μg/mL in sesame oil) was used to fill the capsules that were sealed with silicone cement and allowed to cure overnight. The day before surgery, implants were washed twice for 10 minutes in changes of 100% ethanol and then placed in sterile physiological saline overnight.
First, we aimed to investigate the potential additive effect of the NK2R-Ag and senktide on LH secretion in the presence of sex steroids. To this end, LH levels were measured in WT OVX+E2 females (n ≥ 5 per group), 25 and 60 minutes after an icv injection of NK2R-Ag (600 pmol), senktide (600 pmol), or the coadministration of both drugs. Next, WT OVX+E2 females (n ≥ 5 per group) were pretreated with the NK3R antagonist SB222200 (7 nmol), 60 minutes prior to the icv injection of NK2R-Ag (600 pmol, senktinde, or vehicle 0.9% NaCl). Blood samples were collected before SB222200 injection (basal) and at 25 and 60 minutes after injection of the agonists. Additionally, we further investigated the action of NK2R-Ag in the absence of NKB signaling using Tac2KO OVX+E2 females and their corresponding WT littermate controls (WT OVX+E2; n ≥ 5 per group). Both groups of females were injected with NK2R-Ag and blood samples were collected before and 25 and 60 minutes after injection. Finally, to evaluate whether the action of NKA requires kisspeptin to stimulate LH release, we used Kiss1KO OVX+E2 females and their corresponding WT littermate controls (WT OVX+E2; n ≥ 5 per group) and LH levels were measured 25 minutes after icv injection of NK2R-Ag.
Experiment 3
Interaction between NKA, NKB, and SP for the inhibition of LH release in ovariectomized female mice
Adult WT females were subjected to bilateral OVX under light isoflurane anesthesia one week before pharmacological tests. First, we aimed to investigate the potential additive effect of NK2R-Ag and senktide in the inhibition of LH secretion in the absence of sex steroids. Thus, LH levels were measured in WT OVX females (n ≥ 5 per group), 25 and 60 minutes after an icv injection of NK2R-Ag (600 pmol), senktide (600 pmol), or the coadministration of both drugs. In the next experiment, we intended to assess the role of NKB in the inhibition of LH secretion achieved by NKA, in WT OVX females. To this end, LH responses to the NK2R-Ag were evaluated after blockade of the effects of NKB using SB222200 (7 nmol) as a selective antagonist for NK3R. For this purpose, adult WT OVX female mice (n ≥ 5 per group) were pretreated with SB222200 60 minutes prior to the icv injection of NK2R-Ag (600 pmol). Blood samples were collected before SB222200 injection (basal) and at 25 and 60 minutes after vehicle or NK2R-Ag injection. In addition, we investigated the role of endogenous opioids in the control of LH secretion and in the modulation of LH responses to NK2R, using adult WT OVX females. To this end, LH responses to NK2R-Ag were measured after the blockade of the κ and μ opioid receptors (KOR and MOR) using naloxone (5 mg/kg per 100 μL IP). All animals (WT OVX females; n ≥ 5 per group) were injected with naloxone, 12 hours and then 60 minutes prior to the icv injection of NK2R-Ag (600 pmol). Blood samples were collected before naloxone injection and at 25 and 60 minutes after NK2R-Ag injection. We further investigated the action of NK2R-Ag in the absence of NKB signaling and sex steroids using Tac2KO OVX and WT OVX females (n ≥ 5 per group). Both groups of animals were injected with NK2R-Ag (600 pmol) and blood samples were collected before and then 25 and 60 minutes after injection. In addition, we evaluated the role of SP in the NK2R-Ag induced inhibition of LH in WT OVX and Tac2KO OVX mice (n ≥ 5 per group). Both groups of females were injected with NK1R-Antg (2 nmol), 30 minutes before the administration of NK2R-Ag (600 pmol), NK1R-Ag (600 pmol), or vehicle. Blood samples were collected before and then 25 and 60 minutes after injection. Finally, we assessed the ability of NK2R signaling to modulate LH release in the absence of kisspeptin and sex steroids using Kiss1KO OVX and WT OVX females (n ≥ 5 per group), which were injected with NK2R-Ag (600 pmol) and LH levels were measured at 25 and 60 minutes after icv injection.
Experiment 4
Expression of Tacr1, Tacr2, Tacr3, Kiss1, and Pdyn in the mediobasal hypothalamus of female mice
We aimed to determine if there are changes in the expression of Tacr1, Tacr2, Tacr3, Kiss1, and Pdyn in the mediobasal hypothalamus (MBH), the site that includes the ARC between WT (n = 7) and Tac2 KO (n = 9) ovary-intact females.
Total RNA from the MBH was isolated using TRIzol reagent (Invitrogen) followed by chloroform/isopropanol extraction. RNA was quantified using NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific), and 1 μm of RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR assays were performed on an ABI Prism 7000 sequence detection system and analyzed using ABI Prism 7000 SDS software (Applied Biosystems). The cycling conditions were the following: 2 minutes incubation at 95°C (hot start), 45 amplification cycles (95°C for 30 seconds, 60°C for 30 seconds, and 45 seconds at 75°C, with fluorescence detection at the end of each cycle), followed by melting curve of the amplified products obtained by ramped increase of the temperature from 55°C to 95°C to confirm the presence of single amplification product per reaction. For data analysis, relative standard curves were constructed from serial dilutions of one reference sample cDNA and the input value of the target gene was standardized to Hprt levels in each sample. The primers used are listed in Table 1.
Table 1.
List of Primers Used for PCR Determination
| Gene Name | Primer Sequence | Gene Accession Number |
|---|---|---|
| Hprt | F: CCTGCTGGATTACATTAAAGCGCTG | NM_013556.2 |
| R: GTCAAGGGCATATCCAACAACAAAC | ||
| Tacr1 | F: GTCTGCCAAGAGCCAAGAAC | NM_009313 |
| R: CCAGCCACATCTGAGAGACA | ||
| Tacr2 | F: TCAACTTCATCTATGCCAGTCAC | NM_009314 |
| R: ATGACAGCAATAACCGCCTTG | ||
| Tacr3 | F: GCCATTGCAGTGGACAGGTAT | NM_021382.6 |
| R: ACGGCCTGGCATGACTTTTA | ||
| Kiss1 | F: CTCTGTGTCGCCACCTATGC | AF472576.1 |
| R: TTCCCAGGCATTAACGAGTTC | ||
| Pdyn | F: ACAGGGGGAGACTCTCATCT | NM_018863.4 |
| R: GGGGATGAATGACCTGCTTACT |
Abbreviations: F, forward; R, reverse.
In situ hybridization
To determine the presence of coexpression between Tac2r and Tac1 mRNA in key areas [ventromedial nucleus (VMN) and ARC], dual fluorescence in situ hybridization (ISH) was performed in tissue samples from OVX+sham and OVX+E2 mice. We used probes for Tac2r-C1 and Tac1-C2 obtained from ACDBio and used the RNAscope method per their protocol (ACDBio). The brains were removed for ISH, fresh-frozen on dry ice, and then stored at −80°C until sectioning. Five sets of 20-μm sections in the coronal plane were cut on a cryostat, from the diagonal band of Broca to the mammillary bodies, thaw-mounted onto SuperFrost Plus slides (VWR Scientific), and stored at −80°C. A single set was used for the ISH experiment (adjacent sections 100 μm apart).
Hormone measurements
LH was measured by a sensitive sandwich ELISA for the assessment of whole-blood LH concentrations (22). A 96-well high-affinity binding microplate (9018; Corning) was coated with 50 μL of capture antibody [monoclonal antibody, antibovine LH β subunit, 518B7 (23); University of California] at a final dilution of 1:1000 [in 1× PBS, 1.09 g of disodium phosphate (Na2HPO4; anhydrous), 0.32 g of NaH2PO4 (anhydrous), and 9 g of NaCl in 1000 mL of distilled water] and incubated overnight at 4°C. To minimize unspecific binding of the capture antibody, wells were incubated with 200 μL of blocking buffer [5% (w/v) skim milk powder in 1× PBS-T (1× PBS with 0.05% Tween 20) for 2 hours at room temperature (RT). A standard curve was generated using a twofold serial dilution of LH [reference preparation, AFP-5306A; National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Pituitary Program (NIDDK-NHPP)] in 0.2% (w/v) BSA-1× PBS-T. The LH standards and blood samples were incubated with 50 μL of detection antibody [polyclonal antibody, rabbit LH antiserum, AFP240580Rb (24); NIDDK-NHPP] at a final dilution of 1:10,000 for 1.5 hours (at RT). Each well containing bound substrate was incubated with 50 μL of horseradish peroxidase conjugated antibody [polyclonal goat anti-rabbit, D048701-2 (25); DakoCytomation] at a final dilution of 1:2000. After a 1.5-hour incubation, 100 mL of o-phenylenediamine (002003; Invitrogen), substrate containing 0.1% hydrogen peroxide was added to each well and left at RT for 30 minutes. The reaction was stopped by addition of 50 μL of 3 M hydrochloric acid to each well, and absorbance of each well was read at a wavelength of 490 nm (Sunrise; Tecan Group). The concentration of LH in whole blood samples was determined by interpolating the OD values of unknowns against a nonlinear regression of the LH standard curve (22).
Statistical analysis
All data are expressed as the mean ± SEM for each group. A two-tailed unpaired Student t test or a one- or two-way ANOVA test followed by a Tukey or Newman Kleus post hoc test was used to assess variation among experimental groups. Significance level was set at P < 0.05. All analyses were performed with GraphPad Prism Software, Inc.
Results
Advancement of puberty onset after long-term activation of NK2R in female mice
Our previous studies have demonstrated that long-term administration of specific agonists for the NK1R and NK3R receptor are able to advance puberty onset in mice and rats (11, 20), indicating that these systems are in place before puberty and likely participate in the proper timing of puberty onset. However, whether NKA/NK2R signaling is also involved in the awakening of the gonadotropic axis at the time of puberty is unknown. To address this question, we treated WT females long-term (every 12 hours) with a specific agonist of NK2R from weaning age (22 days) to 32 days. We observed that this treatment was able to advance puberty onset as evidenced by the advanced timing of VO and increased uterine and ovarian weight compared with controls [uterine weight: 19.55 ± 1.89 mg vs 23.79 ± 1.61 mg in control and NK2R-Ag treated females, respectively (*P < 0.05) and ovarian weight: 8.3 ± 0.56 mg vs 11.7 ± 0.44 mg in control and NK2R-Ag treated females, respectively (***P < 0.001); Fig. 1A–1F]. BW at 30 days was not different between groups.
Figure 1.
Advancement of puberty onset after long-term activation of NK2R in female mice. Repeated stimulation (every 12 h) of WT female mice with GR64349 (NK2R-A, 3 nmol/100 μL IP) or vehicle (0.9% NaCl/100 μL IP) from p23 to p32 (n ≥ 6 per group). (A) Progression of VO, (B) mean postnatal day of VO, and (C) BW the day that 50% of control animals displayed VO. (D) Uterine weight, (E) ovarian weight, and (F) serum LH levels at p32. Statistical analysis was performed using a two-tailed t test: *P < 0.05; ***P < 0.001.
The receptor for NKA (NK2R) is expressed in ventromedial hypothalamus Tac1 neurons
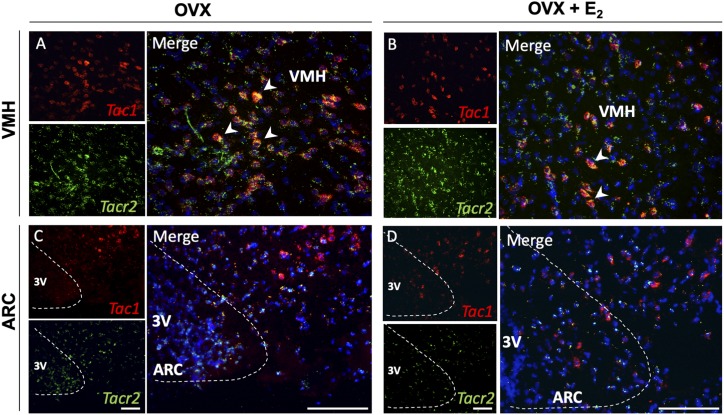
We have documented previously the existence of mRNA for the NKB receptor (NK3R) and SP receptor (NK1R) in Kiss1 neurons in the ARC, whereas the receptor for NKA (NK2R) was undetectable in Kiss1 or GnRH neurons (9). We therefore aimed to assess if NK2R (encoded by Tacr2) is expressed in other neurons located in the ARC or VMH and whether it colocalizes with Tac1 neurons in these areas as the source of SP and NKA. Our ISH (RNAscope) results showed that Tacr2 is expressed in both ARC and VMH nuclei. Virtually all Tac1 neurons in the VMH of adult WT mice coexpressed Tacr2 regardless of the E2 milieu, however, Tacr2 was also present in non-Tac1 neurons of both nuclei (Fig. 2).
Figure 2.
Tacr2 (NK2R) is expressed in VMH Tac1 neurons. Representative double-label ISH depicting colocalization of Tac1 (red) and Tacr2 (green) mRNA. (A) VMH and (C) ARC of female WT C57Bl/6 mice after 1 wk of OVX. (B) VMH and (D) ARC of female WT C57Bl/6 OVX mice after 1 wk of E2 replacement. Scale bars: left (small) panels, 100 μm; right panels, 20 μm.
The stimulatory action of NKA is independent of NKB but dependent on kisspeptin
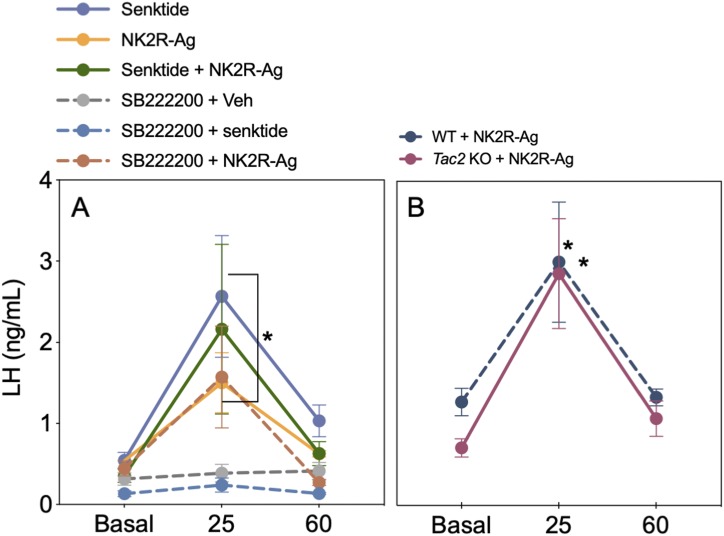
We reported previously that the action of NKA and NKB on LH release is largely equivalent—that is, both increase LH release in the presence of physiological circulating levels of E2 but inhibit LH in the absence of sex steroids (9). It was, therefore, tentative to speculate that NKA could induce LH release through the stimulation of NKB given the absence of NK2R in Kiss1 and GnRH neurons. To test this hypothesis, first we coadministered NK2R and NK3R agonists in OVX+E2 WT mice and observed that the increase in LH was similar in groups injected with an individual dose of each agonist or the combination of both (Fig. 3A), eliminating the possibility of an additive effect of NKA and NKB on LH release and suggesting a possible common pathway. Next, to evaluate if NKA requires NKB signaling to induce LH release, the LH response to NK2R-Ag was tested in the presence of an NK3R antagonist (Fig. 3A) or in NKB-deficient (Tac2KO) mice (Fig. 3B). In both cases, NK2R-Ag was able to stimulate LH release indicating that NK2R activation induces LH release independently of the presence of NKB or its receptor NK3R. However, NK2R signaling requires the presence of kisspeptin because Kiss1KO mice replaced with E2 did not have any effect on LH release (WT basal: 0.27 ± 0.06 ng/mL; WT NK2R-Ag: 0.57 ± 0.09 ng/mL; *P < 0.05; Kiss1KO basal: 0.21 ± 0.02 ng/mL; Kiss1KO NK2R-Ag: 0.29 ± 0.04 ng/mL; not significant).
Figure 3.
The stimulatory action of NKA is independent of NKB in the presence of physiological circulating levels of E2. (A) LH release before (basal), 25 and 60 min after the icv injection of NK2R-Ag, senktide, or the coadministration of both (600 pmol/5 μL icv) in WT OVX+E2 females (n ≥ 5 per group). *P < 0.05 vs corresponding basal levels (two-way ANOVA followed by Tukey post hoc test). For SB222200-treated mice, LH levels before (basal) SB222200 (7 nmol/5 μL icv) administration and at 25 and 60 min after injection of NK2R-Ag (600 pmol/5 μL icv), senktide (600 pmol/5 μL icv), or vehicle (0.9% NaCl/5 μL icv) in WT OVX+E2 females (n ≥ 4 per group). *P < 0.05 vs corresponding basal levels. (B) LH before (basal) and at 25 and 60 min after injection of NK2R-Ag (600 pmol/5 μL icv) in OVX+E2 WT and Tac2KO females (n ≥ 5 per group). *P < 0.05 vs corresponding basal levels (two-way ANOVA followed by Tukey post hoc test).
The inhibitory action of NKA is NKB- and dynorphin-dependent
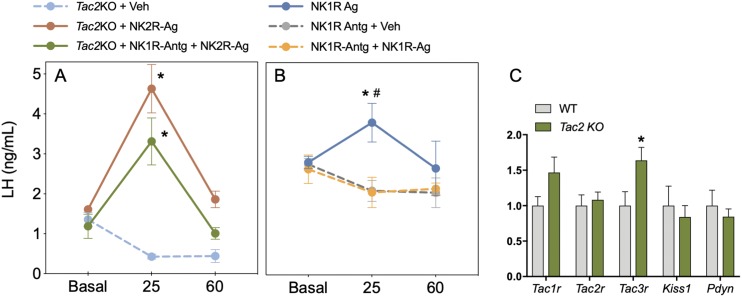
In the next set of experiments, we sought to determine whether the inhibitory action of NKA/NK2R on LH release in the absence of sex steroids (i.e., OVX) is mediated by NKB or dynorphin. First, we showed that the inhibitory action of NK2R-Ag plus senktide was similar to that of senktide alone, suggesting (as in the presence of E2) that there is no additive effect of both tachykinins in the inhibition of LH (Fig. 4A). The use of the specific NK3R antagonist alone decreased LH in OVX animals, in line with recent literature showing that blockade of NK3R decreases LH pulsatility (26–29). However, coadministration of the NK3R antagonist and NK2R-Ag failed to induce a further decrease in LH, suggesting that NK3R signaling is required for the inhibitory action of NKA in the absence of E2 (Fig. 4A). Moreover, as previously described in rats, NK2R signaling requires dynorphin to inhibit LH (30), which is prevented after blockade of the KOR and MOR using naloxone (Fig. 4B). Of note, naloxone alone also inhibited LH release in OVX mice, in line with our previous reports in OVX PdynKO and Oprk1KO mice (dynorphin KO and KOR KO mice, respectively) (31), suggesting that the absence of the inhibitory signal of dynorphin leads to a decrease in the ability of the mouse to secrete LH, probably resulting from disruption of the LH pulse generator mechanism (32). Next, we assessed the action of NK2R-Ag in the congenital absence of NKB (OVX Tac2KO mice) to further confirm the data obtained after NK3R blockade. Unexpectedly, we observed that the congenital absence of NKB in Tac2KO OVX mice leads to a robust induction of LH release (Fig. 5A), revealing an action that is not present in WT OVX regardless of whether a functional NK3R is present or antagonized.
Figure 4.
The inhibitory action of NKA is NK3R and KOR dependent. (A) LH release before (basal), 25 and 60 min after icv injection of NK2R-Ag, senktide, or the coadministration of both (600 pmol/5 μL icv) in WT OVX females (n ≥ 5 per group). *P < 0.05 vs corresponding basal levels; For SB222200-treated mice, LH levels before (Basal) SB222200 (7 nmol/5 μL icv) injection and at 25 and 60 min after injection of NK2R-Ag (600 pmol/5 μL icv), senktide (600 pmol/5 μL icv), or vehicle (0.9% NaCl/5 μL icv) in WT OVX females (n ≥ 5 per group). *P < 0.05 vs corresponding basal levels (two-way ANOVA followed by Tukey post hoc test). (B) LH levels before (basal), 25 and 60 min after injection of naloxone (5 mg/kg/100 μL IP) or vehicle (0.9% NaCl/100 μL IP) in WT OVX females (n ≥ 5 per group). *P < 0.05 vs corresponding basal levels (two-way ANOVA followed by Tukey post hoc test).
Figure 5.
The inhibitory action of NKA is NKB independent and partially SP dependent. (A) LH levels before (Basal) [vehicle or NK1R-Antg (2 nmol/5 μL icv)] and at 25 and 60 min after injection of NK2R-Ag (600 pmol/5 μL icv) or vehicle (0.9% NaCl/5 μL icv) in OVX Tac2KO females (n ≥ 5 per group). *P < 0.05 vs corresponding basal levels (two-way ANOVA followed by Tukey post hoc test). (B) LH levels before (Basal) [vehicle or NK1R-Antg (2 nmol/5 μL icv)] and at 25 and 60 min after injection of NK1R-Ag (600 pmol/5 μL icv) or vehicle (0.9% NaCl/5 μL icv) in WT OVX females (n ≥ 5 per group). *P < 0.05 vs corresponding basal levels (two-way ANOVA followed by Tukey post hoc test); #P < 0.05 vs NK1R-Antg + vehicle injected mice levels. (C) Expression of Tac1r, Tacr2, Tacr3, Kiss1, and Pdyn in the mediobasal hypothalamus of Tac2KO females (n = 9) and their WT controls (n = 7). *P < 0.05 Student t test.
Because we have observed that VMH Tac1 neurons coexpress Tacr2 (NK2R) (Fig. 2), we hypothesized that NKA could be inducing LH release in Tac2KO OVX mice through the stimulation of SP from Tac1 neurons that, in turn, would activate Kiss1 neurons (9). To test this hypothesis, we administered the NK2R-Ag in the presence of a NK1R antagonist (Fig. 5A) [proven to efficiently block the action of a NK1R- agonist (Fig. 5B)] in Tac2KO OVX mice. NK2R-Ag was still able to induce LH release after NK1R blockade although the magnitude of this increase tended to be lower than in the absence of the NK1R antagonist (Fig. 5A). Last, we confirmed that this action is kisspeptin-dependent by showing complete absence of LH response after the administration of NK2R-Ag in Kiss1KO mice (WT: basal 2.90 ± 0.42 ng/mL, NK1R-Ag 2.08 ± 0.32 ng/mL, *P < 0.05; Kiss1KO: basal 0.32 ± 0.06 ng/mL, NK1R-Ag 0.30 ± 0.02 ng/mL, not significant). To assess whether this difference between the response to NK2R agonists after the pharmacological blockade of NK3R and the congenital absence of the NK3R ligand (NKB) is caused by compensation in the expression of any of the ligand-receptor components of the tachykinin systems or in dynorphin, we evaluated the expression of these genes in the MBH of WT vs Tac2KO female mice. We observed an increase in the expression of Tacr3 (NK3R) in Tac2KO mice compared with controls (Fig. 5C).
Discussion
The neuroendocrine mechanisms controlling the timing of puberty onset remain largely unknown. To date, a number of stimulatory signals responsible for the awakening of the reproductive axis during the late juvenile period have been identified (33). Among these, the increased synthesis and release of kisspeptin has been shown to play a pivotal role (34). Inactivating mutations in the KISS1/KISS1R genes lead to HH and absent puberty onset (35, 36), whereas gain-of-function mutations in KISS1R advance puberty onset in humans (37). Similarly, long-term administration of kisspeptin-10 advances puberty onset in rodents (38). However, the pattern of kisspeptin release is dependent on upstream regulators, such as the tachykinin peptides (3). For example, the tachykinin NKB acts autosynaptically on ARC Kiss1 neurons and its release precedes that of kisspeptin to allow for the proper timing of puberty onset (20, 31, 39–41). Moreover, we have recently documented that the tachykinin SP, originating from Tac1 neurons and located upstream of Kiss1, is also involved in puberty initiation (11, 12). In this study, we extend these findings to include NKA, and demonstrate that prepubertal female mice exhibit a premature activation of the reproductive axis in response to this tachykinin. Specifically, chronic activation of the NKA receptor (NK2R) during this developmental period led to the advancement of puberty onset; and this was evidenced by an earlier vaginal opening and an increase in ovarian and uterine weights. Further studies can help to pinpoint the precise role of NKA on the initiation of GnRH release and the awakening of the reproductive axis. Nonetheless, this study demonstrates that female mice are able to respond to NKA prepubertally, which further suggests that the delay in pubertal onset observed in Tac1KO mice (11) may be caused by the loss of the stimulatory action of both SP and NKA on kisspeptin release.
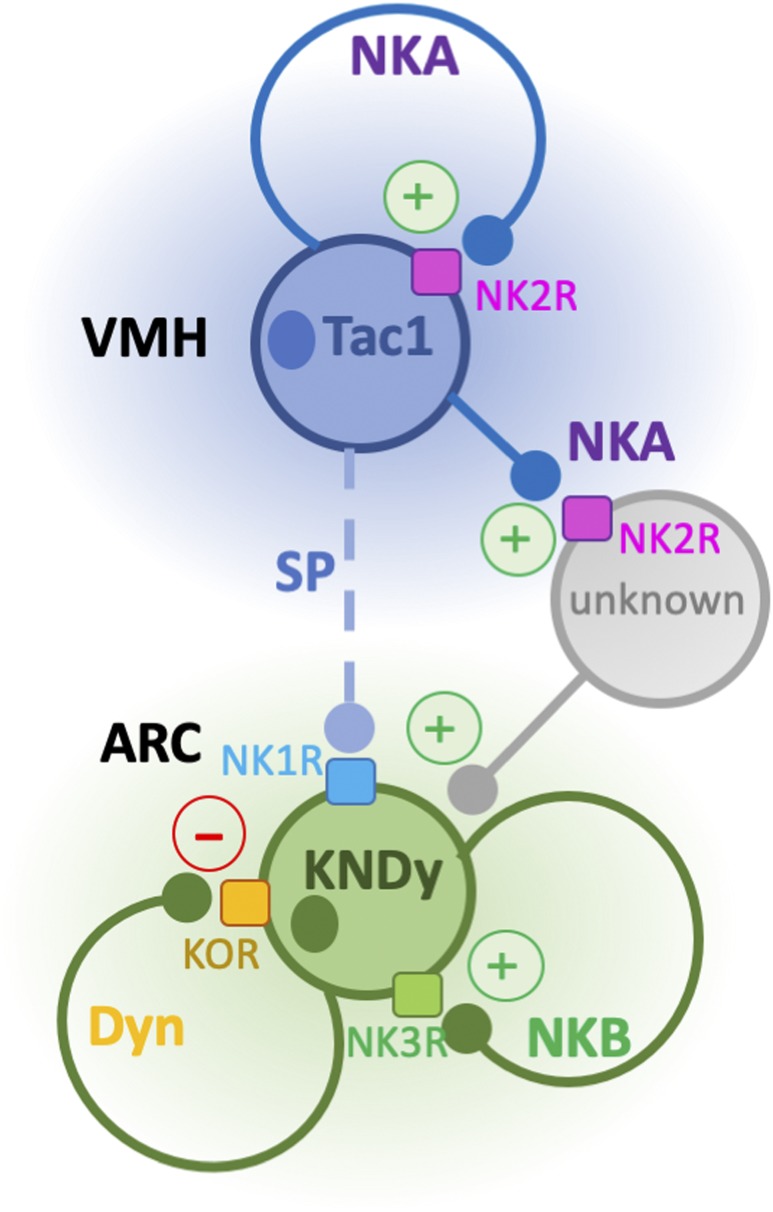
Interestingly, although the vast majority of ARC Kiss1 neurons express NK3R (Tacr3) and approximately half express NK1R (Tacr1), neither Kiss1 nor GnRH neurons have been shown to express NK2R (Tacr2) (9). This suggests that NKA stimulates kisspeptin release indirectly, via the initial activation of unknown intermediate neurons. In this study, we identified Tacr2 in Tac1 neurons located in the VMH. It is reasonable to speculate that this population of Tac1 neurons is the main source of NKA eliciting kisspeptin, and hence GnRH/LH release, however, this requires further investigation. Nonetheless, the high degree of colocalization between the ligand (NKA; encoded by Tac1) and its receptor (Tacr2), as observed in the VMH is reminiscent of the NKB/NK3R signaling mechanism observed in ARC Kiss1 neurons and suggests the existence of an autosynaptic loop that may modulate the release of SP onto Kiss1 neurons. Of note, a scarce population of Tac1 neurons is also present in the ARC and adjacent to Kiss1 neurons (9). However, these neurons do not express Tacr2 and their role, if any, in the control of Kiss1 neurons remains to be characterized. Importantly, the population of Tacr2-expressing neurons is larger than that of Tac1 neurons in the VMH and scattered non-Tac1 neurons expressing Tacr2 are also found in the ARC. Therefore, a Tac1 neuron-independent action from these unknown neurons in the control of kisspeptin release cannot be ruled out.
In light of the analogous action of NKA and NKB in the regulation of LH release [i.e., stimulation in the presence of E2 and inhibition in its absence (9)], the existence of convergence of the NKA mechanism of action onto NKB signaling was assessed. Despite the aforementioned functional similarities, our data using an NK3R antagonist and Tac2KO mice after OVX or OVX and E2 replacement clearly demonstrate that the stimulatory action of NKA on LH is NKB independent but kisspeptin dependent. This suggests the existence of a yet unknown population of NKA-responsive neurons that in turn activate Kiss1 neurons to induce kisspeptin/GnRH release (Fig. 6). In contrast, NKA has been shown to inhibit LH via a mechanism that involves dynorphin in the rat (14, 30) similar to what has been described for NKB in the absence of E2 (42). Here, we show that the inhibitory action of NKA is weaker than the one exerted by NKB. This conclusion is derived from the fact that animals treated with an NK2R agonist showed a quicker recovery in LH levels (within 60 minutes after injection) compared with animals treated with senktide. Furthermore, we demonstrate that the inhibitory action of NKA is mediated by the activation of the NKB–dynorphin signaling pathway because blockade of both NK3R and KOR receptors prevented the inhibition of LH induced by the NK2R agonist. Importantly, the action of stimulating or inhibiting NK3R and KOR in both cases leads to the inhibition of LH release in the absence of sex steroids likely as a result of the disruption of the GnRH pulse generator at the level of the ARC Kiss1 neuron. However, we unexpectedly observed that in the congenital absence of NKB (Tac2KO mice), the NK2R agonist stimulates LH release in the absence of E2. These data suggest that the action of NKA is inherently stimulatory, NKB independent, and kisspeptin dependent, as NK2R agonists did not induce any LH release in Kiss1KO mice regardless of the sex steroid milieu, unlike our recent findings on the kisspeptin-independent action of NKB (19). Thus, in Tac2KO mice, in which NK3R and KOR are not blocked, the absence of NKB leads to a lower basal activation of Kiss1 neurons after OVX (see basal LH levels in OVX Tac2KO mice in Fig. 5A compared with basal OVX levels in WT mice, Fig. 5B). In this context, the administration of NKA to Tac2KO mice is able to further stimulate Kiss1 neurons beyond their basal OVX levels, leading to an increase in LH release. Interestingly, the congenital absence of Tac2 led to the compensatory increase of Tacr3 and a noticeable trend to increase Tacr1. This is reminiscent of the increase in Tacr2 observed in the absence of Tac1 [Tac1KO mice (11)] and could also account, at least partially, for the increase in LH by NK2R agonists in Tac2KO mice regardless of the sex steroid milieu. Altogether, these data suggest that NKA contributes to the activation of ARC Kiss1 neurons through a process that may involve auto-synaptic signaling on VMH Tac1 neurons to induce SP release, as well as the activation of yet unknown NKA-responsive neurons that eventually further regulate ARC Kiss1 neurons (Fig. 6).
Figure 6.
Schematic representation of the proposed mechanism of action of NKA. NKA, expressed by Tac1 neurons in the VMH, regulates the activity of ARC Kiss1 neurons through two potential mechanisms: through autosynaptic loops that regulate the release of SP onto Kiss1 neurons and through the action of NKA on nearby (unidentified) NKA-responsive neurons that eventually contact Kiss1 neurons.
Overall, in this study we have demonstrated that NKA is able to advance the timing of puberty onset in females, along with SP, NKB, and kisspeptin. Moreover, it offers insights into the interaction and mechanism of action of tachykinins in the control of LH release, especially related to NKA-NKB interaction, which remained largely unexplored. Importantly, this study suggests that in the absence of NKB, the derived hypogonadism could be compensated (and potentially reversed) by NKA, which may account for the reversal of the HH phenotype frequently observed in TAC3 deficient patients. Thus, the exogenous activation of the NKA signaling pathway may offer a novel approach for treating these patients in the clinic.
Acknowledgments
Financial Support: This work was supported by Grant R01 HD090151, funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Institute of Health (NIH), and the Women’s Brain Initiative of the Brigham and Women’s Hospital to V.M.N. and by the International Brain Research Organization (IBRO) Research Fellowship to R.T. The authors declare no competing financial interests.
Author Contributions: S.L. and V.M.N. conceived and designed the research. S.L., C.F., R.T., S.S., C.A.M., and A.G. conducted experiments. S.L. and V.M.N. contributed to data analysis. S.L. and V.M.N. wrote the manuscript, and all authors contributed to manuscript editing.
Glossary
Abbreviations:
- ARC
arcuate nucleus
- BW
body weight
- E2
17β-estradiol
- HH
hypogonadotropic hypogonadism
- icv
intracerebroventricular
- ISH
in situ hybridization
- KO
knockout
- KOR
κ opioid receptor
- MBH
mediobasal hypothalamus
- MOR
μ opioid receptor
- NaCl
sodium chloride
- Na2HPO4
disodium phosphate
- NK
neurokinin
- OVX
ovariectomy
- RT
room temperature
- SC
subcutaneously
- SP
substance P
- TAC
tachykinin
- VMH
ventromedial hypothalamus
- VO
vaginal opening
- WT
wild-type
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Lasaga M, Debeljuk L. Tachykinins and the hypothalamo-pituitary-gonadal axis: an update. Peptides. 2011;32(9):1972–1978. [DOI] [PubMed] [Google Scholar]
- 2. Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392(6674):390–394. [DOI] [PubMed] [Google Scholar]
- 3. Fergani C, Navarro VM. Expanding the role of tachykinins in the neuroendocrine control of reproduction. Reproduction. 2016;153(1):R1–R14. [DOI] [PubMed] [Google Scholar]
- 4. Arisawa M, De Palatis L, Ho R, Snyder GD, Yu WH, Pan G, McCann SM. Stimulatory role of substance P on gonadotropin release in ovariectomized rats. Neuroendocrinology. 1990;51(5):523–529. [DOI] [PubMed] [Google Scholar]
- 5. Coiro V, Volpi R, Capretti L, Caiazza A, Marcato A, Bocchi R, Colla R, Rossi G, Chiodera P. Luteinizing hormone response to an intravenous infusion of substance P in normal men. Metabolism. 1992;41(7):689–691. [DOI] [PubMed] [Google Scholar]
- 6. Traczyk WZ, Pau KY, Kaynard AH, Spies HG. Modulatory role of substance P on gonadotropin and prolactin secretion in the rabbit. J Physiol Pharmacol. 1992;43(3):279–297. [PubMed] [Google Scholar]
- 7. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 8. Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60(4):337–345. [DOI] [PubMed] [Google Scholar]
- 9. Navarro VM, Bosch MA, León S, Simavli S, True C, Pinilla L, Carroll RS, Seminara SB, Tena-Sempere M, Rønnekleiv OK, Kaiser UB. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hrabovszky E, Borsay BA, Rácz K, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Substance P immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PLoS One. 2013;8(8):e72369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simavli S, Thompson IR, Maguire CA, Gill JC, Carroll RS, Wolfe A, Kaiser UB, Navarro VM. Substance p regulates puberty onset and fertility in the female mouse. Endocrinology. 2015;156(6):2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maguire CA, Song YB, Wu M, León S, Carroll RS, Alreja M, Kaiser UB, Navarro VM. Tac1 signaling is required for sexual maturation and responsiveness of GnRH neurons to kisspeptin in the male mouse. Endocrinology. 2017;158(7):2319–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruiz-Pino F, Garcia-Galiano D, Manfredi-Lozano M, Leon S, Sánchez-Garrido MA, Roa J, Pinilla L, Navarro VM, Tena-Sempere M. Effects and interactions of tachykinins and dynorphin on FSH and LH secretion in developing and adult rats. Endocrinology. 2015;156(2):576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sahu A, Kalra SP. Effects of tachykinins on luteinizing hormone release in female rats: potent inhibitory action of neuropeptide K. Endocrinology. 1992;130(3):1571–1577. [DOI] [PubMed] [Google Scholar]
- 15. Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94(1):265–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015;156(4):1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fergani C, Leon S, Padilla SL, Verstegen AM, Palmiter RD, Navarro VM. NKB signaling in the posterodorsal medial amygdala stimulates gonadotropin release in a kisspeptin-independent manner in female mice. eLife. 2018;7:e40476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, García-Galiano D, Hobbs SJ, Manfredi-Lozano M, León S, Sangiao-Alvarellos S, Castellano JM, Clifton DK, Pinilla L, Steiner RA, Tena-Sempere M. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32(7):2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. León S, Barroso A, Vázquez MJ, García-Galiano D, Manfredi-Lozano M, Ruiz-Pino F, Heras V, Romero-Ruiz A, Roa J, Schutz G, Kirilov M, Gaytan F, Pinilla L, Tena-Sempere M. Direct actions of kisspeptins on GnRH neurons permit attainment of fertility but are insufficient to fully preserve gonadotropic axis activity. Sci Rep. 2016;6(1):19206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RRID:AB_2756886, https://scicrunch.org/resolver/AB_2756886.
- 24.RRID:AB_2665533, https://scicrunch.org/resolver/AB_2665533.
- 25.RRID:AB_2617144, https://scicrunch.org/resolver/AB_2617144.
- 26. George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S, Webber L.. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4313–4321. [DOI] [PubMed] [Google Scholar]
- 27. Li SY, Li XF, Hu MH, Shao B, Poston L, Lightman SL, O’Byrne KT. Neurokinin B receptor antagonism decreases luteinising hormone pulse frequency and amplitude and delays puberty onset in the female rat. J Neuroendocrinol. 2014;26(8):521–527. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura S, Wakabayashi Y, Yamamura T, Ohkura S, Matsuyama S. A neurokinin 3 receptor-selective agonist accelerates pulsatile luteinizing hormone secretion in lactating cattle. Biol Reprod. 2017;97(1):81–90. [DOI] [PubMed] [Google Scholar]
- 29. Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K, Tsukamura H. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev. 2011;57(3):409–415. [DOI] [PubMed] [Google Scholar]
- 30. Kalra PS, Sahu A, Bonavera JJ, Kalra SP. Diverse effects of tachykinins on luteinizing hormone release in male rats: mechanism of action. Endocrinology. 1992;131(3):1195–1201. [DOI] [PubMed] [Google Scholar]
- 31. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ojeda SR, Lomniczi A. Puberty in 2013: unravelling the mystery of puberty. Nat Rev Endocrinol. 2014;10(2):67–69. [DOI] [PubMed] [Google Scholar]
- 34. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–1316. [DOI] [PubMed] [Google Scholar]
- 35. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 37. Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico ACA. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Navarro VM, Fernández-Fernández R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561(Pt 2):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300(1):E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95(5):2287–2295. [DOI] [PubMed] [Google Scholar]
- 42. Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O’Byrne KT. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.