Introduction
Comprehensive exercise-based cardiac rehabilitation (CR) is a secondary prevention tool used worldwide to improve prognosis in patients with various forms of cardiovascular disease (CVD). A key component of a comprehensive CR program is exercise training which has been shown to reduce the incidence of falls1 and mortality as well as improve quality of life, frailty, and cardiovascular fitness (defined as peak oxygen uptake [VO2]), which is an independent predictor of hospitalizations and mortality in patients with CVD.2 Moderate-intensity continuous training (MICT) has traditionally been a foundation of aerobic based exercise prescription resulting in short- and long-term clinical benefits for CVD patients.3
High-intensity interval training (HIIT) has recently emerged as an alternative or adjunct strategy to MICT and has been shown to result in similar or greater improvements in peak VO2 compared to MICT.4 Specifically, HIIT has been found to be as effective, if not superior, to MICT with respect to improving clinical outcomes for older patients with CVD, including quality of life (QoL),5 heart rate (HR) response to exercise,6 and myocardial function7. Importantly, HIIT also appears to be as safe as MICT for older CR patients.8,9 HIIT involves repeated bouts of relatively higher-intensity exercise interspersed with periods of lower-intensity recovery.10 Unfortunately, to date, there is no clear consensus on the optimal HIIT prescriptive variables that elicit the greatest benefits for patients at high risk of or with overt CVD.
The most common uncertainties surrounding the prescription and implementation of HIIT for older CVD patients include the specific exercise intensity for the high and low intervals, durations and ratio of high and low intervals, the method to prescribe exercise intensity (e.g. % peak HR, rating of perceived exertion, etc.), and patient safety. This review will discuss the principles of HIIT prescription and provide suggestions for the prescribing of HIIT for CVD patients in the CR setting. Further, we will discuss specific HIIT considerations in relation to frailty, falls, and other risks associated with older age in CVD patients. We will discuss the physiological mechanisms by which HIIT contributes to improvements in peak VO2. Finally, we discuss the impact and safety of HIIT in older patients with coronary artery disease and heart failure in the CR setting.
General Principles and Specific Considerations for Prescribing HIIT for Older Adults with CVD
i. Common Methods for Prescribing the Intensity of HIIT
The American College of Sports Medicine provides guidance on objective and subjective methods for prescribing exercise intensity, which result in improvements in peak VO2,11 some of which have been used to prescribe HIIT for older CR patients with CVD6,12–14. The most common objective metrics include the heart rate measured at peak exercise (peak HR), peak VO2, and metabolic equivalents (METs). Subjective measures that are commonly used include the Borg rating of perceived exertion (RPE, Borg: 6–20) and the perceived dyspnea on exertion (DoE: 0–10) scales. For patients with CVD, the methodology used to measure these objective and subjective measurements have been discussed previously.11 In this section, we discuss the advantages and disadvantages of these objective and subjective methods for prescribing exercise intensity during HIIT in older CR patients. In addition, we propose a guide for prescribing intensity for HIIT in older CR patients.
Peak VO2 is the gold standard measure of exercise capacity and/or physical fitness. Additionally, peak HR is a widely used metric to prescribe exercise intensity due to its relative ease of acquisition. The % peak VO2 and % peak HR methods for determining optimal exercise intensity during HIIT are the most widely researched and have the most robust evidence supporting their efficacy12,15,16; however, known limitations exist for using % peak VO2 and % peak HR. First, patients entering into CR who undergo baseline exercise stress testing may not reach a true maximum HR or VO2 due to early termination of the exercise stress test for a variety of reasons including heightened symptomology, early onset of peripheral fatigue, and/or anxiety.17,18 Additionally, some CR patients may have conditions for which maximal exercise stress testing may be contraindicated and thus only perform submaximal exercise testing because of specific clinical conditions such as advanced heart failure, known obstructive left main coronary artery stenosis, and moderate to severe aortic stenosis.17,18 Second, a large proportion of patients in CR are prescribed rate modulating pharmacotherapy (e.g. beta-blocker medication), which blunts the HR response at rest and during exercise and may lead to lower peak HR and VO2 values during the exercise stress test.19 Third, not all CR centers are equipped with cardiopulmonary exercise testing equipment, which would preclude the direct measurement of peak VO2. Finally, peak HR prediction equations (e.g. 220-age) can underestimate or overestimate measured peak HR20–22 leading to an inappropriate prescription of exercise intensity for exercise training purposes.
Although there are several considerations when using % peak VO2 and % peak HR for prescribing exercise intensity, they are the most widely used methods to prescribe exercise intensity for HIIT in older CR patients.12,15,16 Specifically, two recent multi-center randomized controlled trials, the Study of Aerobic Interval Exercise Training in CAD patients (SAINTEX-CAD, mean age: 58±9 years)5 and the Study of Myocardial Recovery after Exercise Training in Heart Failure (SMARTEX-HF, age range: 58–68 years),15 used % peak HR (i.e. 90–95% peak HR) to prescribe exercise intensity for HIIT. These studies found that although HIIT resulted in improved peak VO2 (~23%), not all patients were able to maintain the prescribed exercise intensity5,23 (i.e. 51% of the patients in the HIIT group exercised at a lower intensity than prescribed15). As a result, supplementary strategies may be advantageous to optimize exercise intensity prescription during HIIT in the CR setting particularly in older adults who may present with additional co-morbidities and/or musculoskeletal concerns.
The RPE (6–20) and DoE scales (0–10) are the two most common subjective methods used to prescribe exercise intensity24–26 and recent studies highlight the practical importance of incorporating subjective measures of intensity for older adults in the CR setting.5,23 Previous studies have shown that RPE is significantly related to HR, ventilation, and VO2 in patients with heart failure (HF),24,26 coronary artery disease (CAD), and atrial fibrillation,25 and is not influenced by beta-blocker mediation.26 The European Association for Cardiovascular Prevention and Rehabilitation, American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR), and Canadian Association of Cardiac Rehabilitation have published a joint position statement10 recommending the use of RPE and DoE scales as the primary prescription tool for exercise intensity or as an adjunct to objective measures in CR. Ferdinando et al. demonstrated that RPE is an easy-to-use and validated method for prescription of intensity in HIIT.24 In contrast, Aamot et al. recently demonstrated that when RPE was solely used in CR the exercise intensity was below target intensity during the HIIT bouts suggesting that the combination of both an objective and subjective metric may be needed to prescribe exercise intensity for HIIT in older adults with CVD.27 In attempt to provide clinicians and researchers with a framework for implementation of HIIT in CR, where the majority of participants are older patients with CVD, we recommend using a combination of objective and subjective measures to prescribe exercise intensity of HIIT. Specifically, the breadth of literature supports a protocol that prescribes high-intensity intervals at an exercise intensity between 85–95% peak HR and RPE between 15–17 (Borg) and low-intensity intervals at 50–75% peak HR and RPE between 12–14 (Borg) with differing high- and low-intensity interval durations dependent on the patients level of deconditioning, symptomology, disease severity, and comorbidity burden. While using objective and subjective measures to prescribe exercise intensity, there may be instances when discrepancies can occur between peak HR and RPE (e.g., peak HR is <85%, but RPE is >15). In this case, we recommend using RPE as the primary method because of limitations associated with peak HR and to optimize patient adherence to HIIT in the CR setting.
Older patients with CVD present with predictable complexity that make specific considerations necessary when HIIT is prescribed in the CR setting. Specifically, older patients may exhibit frailty, multimorbidity, impaired balance and cognition as well as other liabilities with age.1 As a result, the approach to HIIT may need to be modified to best accommodate these factors and to facilitate HIIT adherence in this population. The primary strategies to alter the HIIT protocol are by modifying the exercise modality (especially for patients with musculoskeletal conditions and higher risk of falling) and utilizing subjective measures to prescribe exercise intensity (i.e. RPE) to a greater extent than objective measures.
Short-, Medium-, and Long-interval HIITs for Older CVD Patients
The duration and ratio of high-intensity and low-intensity intervals are key parameters that differentiate HIIT from MICT and contribute to the HIIT-enhanced physiological response and health benefits. 28,29 There are three classical categories for HIIT used for competitive sports that differ in both duration and exercise intensity: Long, Medium, and Short. Each category of HIIT protocols elicit specific physiological responses and can be sports-specific30,31 (see Table 1). Interval training for CVD patients in CR settings is often termed HIIT or aerobic interval training and defined as ‘near maximal’ efforts gene rally performed at an intensity below peak VO2 or peak power output that elicits ≥80% peak HR (often in the range of 85%−95%). It is important to note that for many deconditioned individuals, this may be similar to that encountered during activities of daily living.32 There are also three types of HIIT protocols widely used in CVD patients with varying durations of the high and low-intensity intervals, while the exercise intensity is typically constant at 85–95% peak VO2 or peak HR (see Table 1).
Table 1.
Short-, Medium- and Long-interval HIITs for Athletes and Older Patients with CVD
| Interval Duration Category | Duration (high-/low-intervals) | Intensity | Ratio of Interval Duration | Key Goals or Benefits | |
|---|---|---|---|---|---|
| Athletes30,31 | Long | 3–15/3–15 min | 85–90% peak HR or peak VO2 | 1:1 | Improving function of aerobic metabolism system |
| Medium | 1–3/1–3 min | 95–100% peak HR or peak VO2 | 1:1 | Improving functions of anaerobic and aerobic metabolism systems | |
| Short | 10–60/10–60 s | 100–120% peak HR or peak VO2 | 1:1 | Improving function of ATP-CP system | |
| Older Patients with CVD | Long | 3–4/3–4 mins | 85–95% peak HR or peak VO2 | 1:1 | VO2peak,VE/VCO2,VAT, QoL4–6,12,13,33–35 |
| Medium | 1–2/1–4 mins | 85–95% peak HR or peak VO2 | 1:1–4 | VO2peak, VO2/Pulse QoL15,36,67 | |
| Short | 15–60/15–120 s | 85–95% peak HR or peak VO2 | 1:1–8 | VO2peak,VO2/Pulse14,28,29,70 |
ATP-CP, adenosine triphosphate-creatine phosphate; CVD, cardiovascular disease; HR, heart rate; QoL, quality of life; VAT, ventilatory anaerobic threshold; VE, ventilation; VCO2 volume of carbon dioxide produced; VO2, volume of oxygen consumed.
Long-interval HIIT is the most widely used protocol for older patients with CVD. This may include 4 sets of high-intensity intervals, each lasting 4 minutes interspersed with 3 sets of low-intensity intervals, each lasting 3 minutes. 4–6,12,13,33–35 Medium-interval HIIT, such as 8×2 min high-intensity intervals interspersed with 7×2 min low-intensity intervals have also been used, albeit to a lesser extent, in older patients with CVD.36 For older patients with HF with reduced ejection fraction (HFrEF, NYHA II-III), medium- and short-interval protocols have been used14,23,37 such as 10×1 min high-intensity intervals interspe rsed with 9×2 min low-intensity intervals. All three protocols are safe and contribute to significant improvements in peak VO2 and QoL.5,6,8,9,23 At this time, there are no studies that have compared long, medium, and short-interval HIIT in CVD patients to determine the most appropriate duration. However, a recent meta-analysis found that long-interval HIIT may elicit greater improvements in peak VO2 compared to short-interval HIIT.38 However, as previous studies have found, some older CVD patients newly enrolled in CR may not be able to maintain the long-intervals at high-intensity. Thus, over the course of CR, health care providers or clinical exercise physiologists may recommend CR patients begin with short-, then progress to medium-, and finally progress to long-intervals as they accumulate the benefits of exercise training and increase their exercise tolerance. As detailed in Figure 1, based on the evidence described, we propose the use of short-interval protocols for CVD patients with low exercise capacity (<5 METs) or in the beginning stage of CR (0–4 weeks), and the use of medium- or long-interval protocols for CVD patients with intermediate or high exercise capacity (≥5 METs) as well as for those in the improvement (4–12 weeks) and maintenance stages (>12 weeks) of CR (see Figure 1).
Figure 1: Principles of HIIT Prescription and Progression.
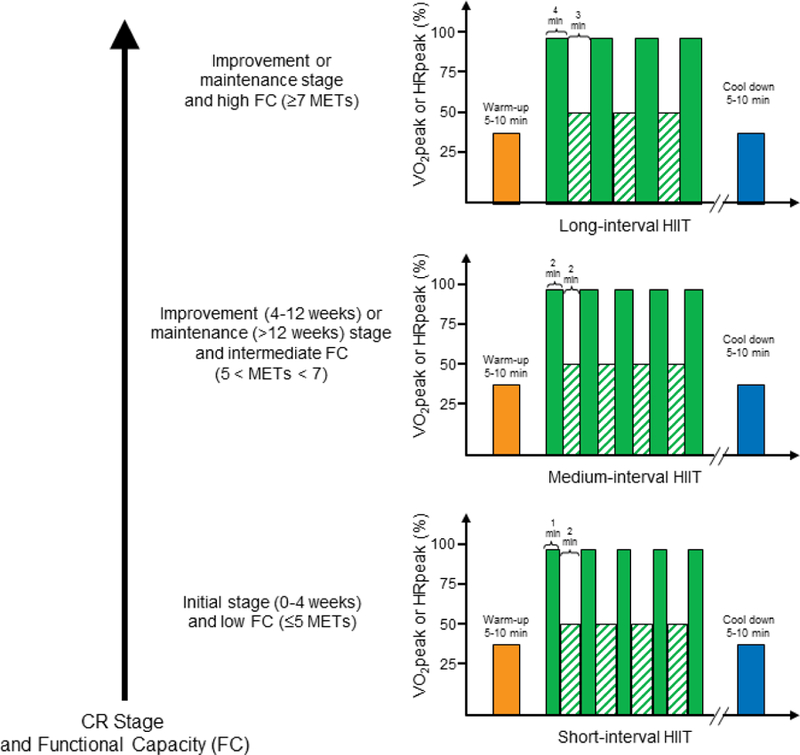
Representative examples of long-, medium- and short-interval HIITs. The short-interval HIIT may be appropriate for CVD patients with low functional capacity (<5 METs) or in the initiation stage of CR (0–4 weeks), and the medium- or long-interval HIIT protocols may be recommended for CVD patients with intermediate or high functional capacity (≥5 METs) and in the improvement (4–12 weeks) and/or maintenance stages (>12 week) of CR. The exercise intensity is constant for each of these HIIT protocols with the high and low-intensity intervals eliciting 85–95%HRpeak at RPE of 15–17 and 50–75% peak HR at RPE of 12–14, respectively. Abbreviation: CR, cardiac rehabilitation; FC, functional capacity; high-intensity interval training, HIIT.
Physiological Mechanisms by which HIIT contributes to Improved peak VO2
Despite compelling evidence that HIIT is a useful strategy to improve peak VO2 in individuals with and without CVD,12,38 the specific mechanisms underpinning the increased peak VO2 in these patients have not been well documented. Peak VO2 is primarily determined by the systems that transport and utilize oxygen including the respiratory (oxygen uptake from the atmosphere), heart (oxygen transport), peripheral vasculature (oxygen transport, tissue perfusion, tissue diffusion), and skeletal muscle (oxygen extraction and utilization) as highlighted in Figure 2.18 In this section, we review the physiological adaptations in response to HIIT in terms of these systems.
Figure 2: Key Physiologic Mechanisms of HIIT for Improvement of peak VO2.
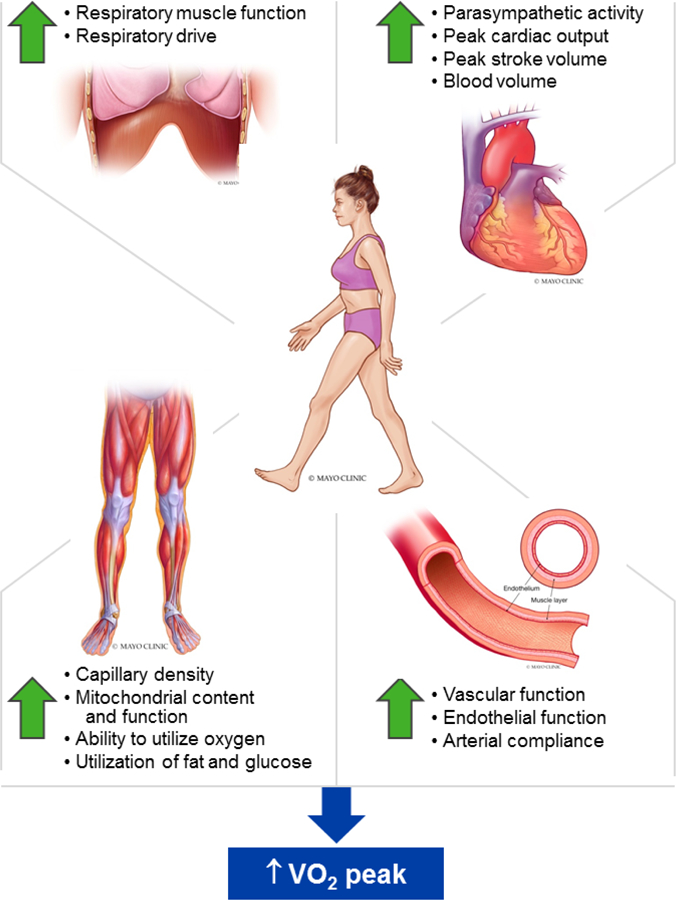
This summary figure illustrates the key physiologic systems that contribute to the increased VO2peak with HIIT. As discussed in the text, HIIT enhances the functions of the respiratory, cardiovascular and skeletal muscle systems contributing to the improvement in VO2peak. © Mayo Foundation for Medical Education and Researc h. All rights reserved.
i. The impact of HIIT on the respiratory system:
Respiratory muscle dysfunction is a common manifestation in patients with CVD, especially older patients with HF, and contributes to exercise intolerance.39–41 Tasoulis et al.42 demonstrated that 12 weeks of HIIT significantly improved respiratory muscle function in older patients with HF. Moreover, Dunham et al.43 demonstrated that 4 weeks of both HIIT and MICT elicited significant increases in respiratory muscle function (HIIT ~43%, MICT ~25%), with a greater increase with HIIT. Furthermore, Tasoulis42 and Christensen44 have shown that HIIT improves pulmonary VO2 kinetics, ventilatory drive (P0.1/ PImax), and ventilatory patterns (resting inspiratory flow (VT/TI) and VT/TI at identical exercise testing workloads) in patients with HF42 and healthy adults.44 Thus, HIIT has the potential to improve the pulmonary systems ability to take in oxygen for distribution to working skeletal muscle during exercise. This has important implications for overall exercise capacity/tolerance in older patients undergoing CR.
ii. The impact of HIIT on the cardiovascular system:
Peak stroke volume (SV), HR, and cardiac output (CO), as well as blood volume are cardinal parameters that influence peak VO2 according to the Fick equation.45 Astorino et al.46 recently showed that 10 sessions of short-interval HIIT increased peak CO. This finding is supported by previous studies demonstrating that 6 weeks of long-interval HIIT increased resting SV and CO, peak exercise CO, plasma volume and hemoglobin mass with greater improvement than47,48 or similar to49,50 MICT. Additionally, resting heart rate variability (HRV) is a predictor of peak VO2 and an independent predictor of all-cause mortality.51,52 An increase (improvement) in HRV has been identified as one of the early cardiac adaptations in response to exercise training likely due to improvement of intrinsic heart rate (SA node) and vagal activity (parasympathetic activity)53,54. Alansare et al. 55 demonstrated that 8 sessions of short-interval HIIT is superior to MICT at improving HRV in sedentary adults. These studies suggest that HIIT may have a greater effect on improving cardiovascular and autonomic nervous system function than MICT in sedentary adults; however, additional research is warranted to extend these findings to older patients with CVD in the CR setting. Collectively, HIIT appears to be more effective or at least equivalent to MICT with respect to increasing peak SV, HR, and CO, and improving cardiovascular and autonomic nervous system function, which together contributes to improved peak VO2.
Flow-mediated dilation (FMD), an indicator of endothelial function, is closely associated with peak VO2 where individuals with lower FMD exhibit lower peak VO2.56 Van et al. reported that both MICT and HIIT improved peak VO2 and FMD in CVD patients undergoing CR, with a close relationship between the improvement in peak VO2 and FMD. A meta-analysis by Ramos57 reported 12 weeks of MICT and long-interval HIIT increased brachial artery FMD by 2.15 and 4.31%, respectively, with a greater improvement demonstrated in the HIIT group. Moreover, Mora et al.58 recently demonstrated 6 months of long-interval HIIT reduced arterial stiffness in patients with metabolic syndrome. Thus, while the available research is suggestive that HIIT has the capacity to improve vascular function, more studies are necessary to fully elucidate the impact of HIIT on vascular function in older patients undergoing CR.
iii. The impact of HIIT on the skeletal muscle system:
Skeletal muscle total fiber amount and type proportions, capillary density, mitochondrial content, and function all play a role in regulating the efficiencies of oxygen extraction and utilization of energy substrates, such as fat and glucose, and as a result significantly contribute to exercise tolerance.59 Early studies investigating the effect of HIIT on skeletal muscle fiber type changes date back 30 years to a landmark study by Simoneau60 who showed that HIIT significantly increased total muscle fiber quantity and the proportion of type I fibers, and decreased the proportion of type IIb fibers, while the proportion of type IIa remained unchanged in the vastus lateralis muscle of healthy adults. A recent study by Tan61 further showed that 18 sessions of short-interval HIIT over 6 weeks increased the total amount of type I and II muscle fibers, capillary density and the protein expression of cytochrome oxidase IV (a marker of skeletal muscle oxidative capacity), in overweight women. Besides skeletal muscle structure alterations induced by HIIT, several studies also62–64 demonstrate that HIIT improves skeletal muscle deoxygenation, indicative of oxygen extraction, as well as the content and activity of glucose and fat oxidative metabolism markers in patients with obesity62,63 and HF.64 In summary, HIIT is a powerful strategy to improve skeletal muscle total fiber amount and type proportions, capillary density, as well as mitochondrial content and function. However, very few studies have been conducted in this area specific to older patients with CVD and thus additional research is critical to extend these findings to the older patients in the CR setting.
Application of HIIT for Older Patients with Cardiovascular Disease
i. HIIT for Older Patients with CAD
Guiraud et al.65 and Ribeiro et al.66 reviewed the application of HIIT in CR globally and patients with CAD, respectively in 2012 and 2017. To further evaluate the effects of HIIT exclusively in older patients with CAD using recent data, we reviewed randomized controlled trials from the last 5 years that compared the effects of HIIT and MICT in older patients with CAD (Table 2). All reviewed studies demonstrated that both MICT and HIIT led to improvements in peak VO2,4–6,12,13,33,36 oxygen pulse,36 ventilatory efficiency (i.e. VE/VCO2 slope),36 oxygen uptake efficiency slope (OUES),13,36, QoL,5 HR recovery,6 and submaximal HR during cardiopulmonary exercise stress testing.4
Table 2.
Study Characteristics of Randomized Control Trials Comparing HIIT and MICT for Older Patients with Coronary Artery Disease and Heart Failure
| Author(year) | No. of randomized patients (HIIT/MICT) | Age (years) and sex (male %) (HIIT/MICT) | Average exercise capacity (HIIT/MICT) | Intervention (frequency/duration) | HIIT (intensity/duration/mode) | MICT (intensity/duration/mode) | Cardiovascular AEs (HIIT/MICT) | Delta of main effects (HIIT vs. MICT) |
|---|---|---|---|---|---|---|---|---|
| Coronary Artery Disease | ||||||||
| Van De Heyning et al. (2018)12 | 100/100 | 60/57 91/89% |
NS | F: 3 × week D: 12 weeks |
I: 4 × 4 min 85–95% peak HR Rec: 3 × 3 min 50–70% HR peak D: 25 min Mode: bicycle |
I: 70–75% peak HR D: 32 min M: bicycle |
NS | peak VO2: 23% vs. 21%. |
| Prado et al. (2016)13 | 17/18 | 57/61 82/77% |
medium/medium | F: 3 × week D: 12 weeks |
I: 7 × 3 min VAT Rec: 7 × 3 min RCP D: 42 min Mode: treadmill |
I: VAT D: 50 min M: treadmill |
NS | peak VO2: 25% vs. 22%. AT: 14% vs. 20% |
| Conraads et al. (2015) 5 | 100/100 | 57/59 91/89 % |
medium/medium | F: 3 × week D: 12 weeks |
I: 4 × 4 min 90–95% peak HR Rec: 3 × 3 min 50–70% HR peak D: 38 min Mode: bicycle |
I: 70–75% peak HR D: 30 min M: bicycle |
No AEs during training sessions MICT: 1 AMI, after the last session (PCI was performed; 2 significant ST- depression during the exercise test at 6 weeks (2 PCI performed) | VO2 peak: 23% vs. 20%. No effect on BP |
| Cardozo et al. (2015) 36 | 23/24 | 56/62 63/66% |
medium/medium | F: 3 × week D: 16 weeks |
I: 2 min × 90% peak HR Rec: 2 min at 60% peak HR D: 30 min Mode: treadmill |
I: 70–75% HR peak D: 30 min M: treadmill |
0/0 | peak VO2: 18% vs. 0.5%. No effect on BP |
| Kim et al. (2015)6 | 14/14 | 57/60 86/71% |
high/high | F: 3 × week D: 6 weeks |
I: 4 × 4 min 85–95% HRR Rec: 3 × 3 min 50–70% HRR D: 25 min Mode: treadmill |
I: 70–85% HRR D: 25 min M: treadmill |
0/0 | peak VO2: 22% vs 9% |
| Eadssen et al. (2014)33 | 15/21 | 56/60 93/71% |
high/high | F: 3 × week D: 12 weeks |
I: 4 × 4 min 85–95% peak HR Rec: 3 × 3 min 70% peak HR D: 28 min Mode: treadmill |
I: 60% peak HR D: 46 min M: treadmill |
HIIT: cerebral hemorrhage | peak VO2: 11% vs 7% |
| Keteyian et al. (2014)4 | 21/18 | 60/58 73/92% |
low/low | F: 3 × week D: 10 weeks |
I: 4 × 4 min 80–90% HRR Rec: 3 × 3 min 60–70% HRR D: 25 min Mode: treadmill |
I: 60–80% HRR D: 30 min M: treadmill |
1 keen pain(HIIT) 1 leg pain (MICT) No events that required hospitalization during or within 3 h after exercise | peak VO2: 16% vs. 8%. AT: 21% vs. 5% No effect on BP |
| Heart failure | ||||||||
| Ellingsen et al. (2017)23 | 77/65 | 65/65 82/81% EF:29/29 NYHA:II-III |
low/low | F: 3 × week D: 12 weeks |
I: 4 × 4 min 90–95% peak HR Rec: 3 × 3 min 60–70% peak HR D: 25 min Mode: bicycle |
I: 60–70% peak HR D: 32 min M: bicycle |
HIIT: 2 ventricular arrhythmia, 4 worsening HF, 3 other cardiovascular Events MICT: 1 fatal cardiovascular event, 1 ventricular arrhythmia, 3 worsening HF, 1 other cardiovascular events |
peak VO2: 8% vs. 5%. LVEF 2% vs. −2% |
| Ulbrich et al. (2016)67 | 12/10 | 53/54 100%/100% NYHA:II-III |
medium/medium | F: 3 × week D: 12 weeks |
I: 6 × 3min ~ 90% peak HR Rec: 5 × 3 min 50% peak HR D: 33 min Mode: treadmill |
I: 70–75% peak HR D: 40 min M: treadmill |
NS | peak VO2: 11% vs. 8%. |
| Benda et al. (2015) 14 | 10/10 | 63/64 90%/100% EF:37/38% NYHA:II-III |
medium/medium | F: 3 × week D: 12 weeks |
I: 10 × 1 min 90% PPO peak (RPE 15–17) Rec: 9 × 2.5 min 30% PPO D: 35 min Mode: bicycle |
I: 60–70% HR PPO (RPE 12–14) D: 30 min M: bicycle |
NS | peak VO2: 7% vs. 1%. |
| Angadi et al. (2015)34 | 9/6 | 69/71 89/50% HFpEF |
medium/medium | F: 3 × week D: 4 weeks |
I: 4 × 4min 85–90% peak HR Rec: 3 × 3 min 30–50% peak HR D: 25 min Mode: treadmill |
I: 70% peak HR D: 40 min M: treadmill |
No AEs during exercise | peak VO2: 9% vs. 0%. |
| Dall et al. (2015)37 | 16/16 | 51/51 75/75% Heart transplant |
medium/medium | F: 3 × week D: 12 weeks |
I: 4, 2, 4,2 plus 4 × 1 min > 80% VO2 peak Rec: 7 × 2 min 70% peak VO 2 D: 30 min Mode: bicycle |
I: 60% peak HR D: 45 min M: bicycle |
NS | peak VO2: 21% vs 11% |
| Koufaki et al. (2014)35 | 16/17 | 60/60 88/76% EF :42/35 NYHA:II-III |
low/low | F: 3 × week D: 12 weeks |
I: 4 × 4 min 90–95% peak HR Rec: 3 × 3 min 50–70% peak HR D: 38 min Mode: bicycle |
I: 60–70% peak HR D: 47 min M: bicycle |
NS | peak VO2: 13% vs. 13%. |
AE, adverse event; BP, blood pressure; HIIT, high-intensity interval training; HR, heart rate; HRpEF, heart failure with preserved ejection fraction; EF, ejection fraction; MICT, moderate-intensity continuous training; NS: data not shown; NYHA, New York Heart Association classification; PPO: peak power output; RPE, rating of perceived exertion (Borg 6–20 scale); RCP: respiratory compensation point; VAT, ventilatory anaerobic threshold; VO2, volume of oxygen consumed.
Exercise capacities were classified as low-, medium- and high-levels according to the stratification for cardiac events during exercise participation exercise risk classification guidelines of ACCVPR, Low level, Mets ≤ 5; medium level, 5 < Mets < 7; high level, Mets ≥ 7; 1 Met = 3.5 ml/kg/min oxygen uptake.
Of these seven studies, four studies reported a superior effect of HIIT over MICT in improving peak VO24,6,33 and oxygen pulse.36 For example, Keteyian et al.4 found that long-interval HIIT and MICT resulted in significant decreases in resting HR, systolic blood pressure, and increases in peak VO2 in older patients with CAD, with greater improvements observed in the HIIT group. These results are consistent with the result of Kim et al.6 who demonstrated that the 6 weeks of HIIT resulted in greater increases in peak VO2 and HR recovery after exercise in CR patients with CVD compared to MICT.
Three studies5,13,33 demonstrated that HIIT and MICT elicited numerous physiologic benefits for patients with CAD to a similar degree. For example, a multicenter randomized controlled trial, SAINTEX-CAD,5 included 200 older patients with CAD and examined the impact of 12 weeks of either HIIT (4×4 min at 90–95 % peak HR, with 3 min active recovery) or MICT (32 min at 70%−75% peak HR) on peak VO2, peripheral endothelial function, cardiovascular risk factors, and QoL. The improvements in peak VO2, endothelial function, QoL, and resting diastolic blood pressure were similar for HIIT and MICT groups.
Collectively, HIIT appears to be more effective or at least equally beneficial compared to MICT in terms of improving peak VO2 for older patients with CAD. However, future studies are needed to determine the long-term effect of HIIT on mortality, morbidity, re-hospitalization and recurrent MI in older patients with CAD.
ii. HIIT for Older Patients with HF
We reviewed randomized controlled trials for the last five years that compared the effects of HIIT and MICT in older patients with HF (Table 2). Among all reviewed studies, four studies recruited HF patients with reduced ejection fraction (HFrEF),14,23,35,67 one study recruited HF patients with preserved ejection fraction (HFpEF),34 and one study recruited heart transplant patients.37
With respect to HFrEF patients, a multi-center randomized controlled trial, SMARTEX-HF15, included 210 older patients with HFrEF and examined the impact of 12 weeks of either HIIT (4×4 min at 90–95% peak HR, with 3 min active recovery) or MICT (32 min at 60%−70% peak HR) on left ventricular end-diastolic diameter (LVEDD) and peak VO2. The authors report a significant decrease in LVEDD and increase in peak VO2 in both HIIT and MICT groups compared to the control group from pre to post-training, with no differences between the HIIT and MICT groups.
In HFpEF patients, Andadi et al.34 compared the effects of 4 weeks of HIIT (4×4 min a t 85–90% peak HR, with 3 min active recovery) versus MICT (30 min at 70% peak HR) on peak VO2, left ventricular diastolic dysfunction, and endothelial function in older patients with HFpEF. HIIT improved peak VO2 and left ventricular diastolic dysfunction, while no changes were observed following MICT.
In heart transplant patients, Dall et al.37 used a randomized, controlled crossover trial to study the impact of 12 weeks of HIIT and MICT on peak VO2, endothelial function, arterial stiffness, QoL, anxiety, depression, and biomarkers, such as glucose, insulin, IL-6, adiponectin. There were significant improvements in peak VO2, SF-36 physical function score, and depression score in HIIT compared to MICT. In contrast, arterial stiffness, biomarkers, and endothelial function did not change following HIIT or MICT.
In summary, HIIT can elicit numerous physiologic benefits in older patients with HF, such as peak VO2, QoL, left ventricular diastolic function, and endothelial function. However, future large prospective studies are needed to determine if HIIT is superior to MICT with respect to different types of HF patients, (i.e. HFrEF, HFpEF and patients following heart transplant).
Safety Considerations of Using HIIT for Older Patients with CVD in CR Settings
The safety of HIIT for clinical populations is an important topic, especially for older patients with CVD where the potential for adverse events is heighted.68 It must be noted that HIIT protocols have been modified for clinical populations to include less strenuous exercise intensities (i.e. usually 85–95% of HRpeak) compared to those used for athletes. Rognmo Ø et al.9 examined the risk of cardiovascular events during HIIT and MICT among 4,846 CR patients with CVD (mean age of 58 years). These authors report only 1 fatal cardiac arrest during MICT and 2 nonfatal cardiac arrests during HIIT. Further, the rate of complications to number of patient-exercise hours was 1 per 129,456 hours of MICT and 1 per 23,182 hours of HIIT. The SMARTEX-HF Study23 demonstrated no differences between the HIIT and MICT groups in terms of total number of serious adverse events during the 12-week intervention and follow-up period (i.e. from weeks 13 to 52) in older HFrEF patients. Thus, current studies suggest the risk of a cardiovascular event is low for both HIIT and MICT in older patients with CVD in the CR setting.
As always, it is important to recognize standard CR procedures whenever developing an exercise prescription using either HIIT or MICT. These procedures, as described in the joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, AACVPR and Canadian Association of Cardiac Rehabilitation,69 include performing a pre-exercise evaluation, recognizing the relative and absolute indications for avoiding and terminating exercise, as well as taking into account special considerations for older CVD patients who may present with various co-morbidities attributable to ageing (frailty, sarcopenia, balance disorders, cognitive decline, etc.).
Conclusions and Perspectives
As part of a comprehensive CR program, HIIT results in similar or even superior physiologic exercise training adaptations compared to MICT. These physiological adaptations contribute to greater improvements in risk factors and exercise capacity/tolerance for these patients. It should be recognized that numerous studies have demonstrated that HIIT is a safe exercise training strategy. Because solely using an objective or subjective method for determining the appropriate intensity of exercise is prone to misrepresent actual exercise intensity, it may be more appropriate to use a combination of objective and subjective methods when prescribing HIIT in clinical populations. Exercise training using high-intensity (85–95% peak HR or peak VO2 and RPE 15–17) with low-intensity intervals (50–75% peak HR or peak VO2 and RPE 12–14) is proposed for older patients undergoing CR. With respect to the duration of HIIT, we propose short-interval HIIT for patients with low exercise capacity or in the initial stage of CR (0–4 week), and medium- or long-interval HIIT for patients with intermediate or high exercise capacity (≥ 5 METs) and in the improvement (4–12 week) and maintenance stages (> 12 week) of CR.
SYNOPSIS.
Recently, high-intensity interval training (HIIT) has been recognized as a safe and effective alternative to moderate-intensity continuous training (MICT) for older patients with CVD in cardiac rehabilitation (CR) settings in an effort to improve health outcomes. The effect of HIIT and specific HIIT protocols for older patients with CVD, and the contributing mechanisms underlying the improvements in peak oxygen uptake (VO2), an independent predictor of all-cause and cardiovascular mortality, have not been adequately reviewed. This brief review firstly considers general principles and suggestions for prescription of HIIT for older patients with CVD. Further, specific challenges pertaining to older adults will be discussed, including complexities related to frailty and other physiological limitations that are common in older patients with CVD. Second, we discuss the physiological mechanisms by which HIIT contributes to improvements in peak VO2. Third, we report the effects of HIIT on cardiovascular health in older patients with coronary artery disease and heart failure.
KEY POINTS.
High-intensity interval training has been shown to result in greater improvements in peak oxygen uptake (VO2) when compared to moderate-intensity continuous training for patients at high risk of developing and those with overt cardiovascular disease (CVD).
The presence of CVD and frailty increase with advanced age. High-intensity interval training has shown positive effects in improving cardiovascular outcomes and frailty in older adults.
High-intensity interval training can be prescribed using a combination of objective and subjective measures of exercise intensity with similar results for older CVD patients in cardiac rehabilitation settings.
Multisystem integrative physiologic adaptations in respiratory, cardiovascular, and skeletal muscle systems induced by high-intensity interval training contribute to improvements in peak VO2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The Authors have nothing to disclose.
References
- 1.Schopfer DW, Forman DE. Cardiac Rehabilitation in Older Adults. Can J Cardiol 2016;32(9):1088–1096. [DOI] [PubMed] [Google Scholar]
- 2.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346(11):793–801. [DOI] [PubMed] [Google Scholar]
- 3.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37(29):2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keteyian SJ, Hibner BA, Bronsteen K, et al. Greater improvement in cardiorespiratory fitness using higher-intensity interval training in the standard cardiac rehabilitation setting. J Cardiopulm Rehabil Prev 2014;34(2):98–105. [DOI] [PubMed] [Google Scholar]
- 5.Conraads VM, Pattyn N, De Maeyer C, et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol 2015;179(1):203–210. [DOI] [PubMed] [Google Scholar]
- 6.Kim C, Choi HE, Lim MH. Effect of High Interval Training in Acute Myocardial Infarction Patients with Drug-Eluting Stent. Am J Phys Med Rehabil 2015;94(10 Suppl 1):879–886. [DOI] [PubMed] [Google Scholar]
- 7.Molmen-Hansen HE, Stolen T, Tjonna AE, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol 2012;19(2):151–160. [DOI] [PubMed] [Google Scholar]
- 8.Hannan AL, Hing W, Simas V, et al. High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: a systematic review and meta-analysis. Open Access J Sports Med 2018;9(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rognmo O, Moholdt T, Bakken H, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012;126(12):1436–1440. [DOI] [PubMed] [Google Scholar]
- 10.Mezzani A, Hamm LF, Jones AM, et al. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol 2013;20(3):442–467. [DOI] [PubMed] [Google Scholar]
- 11.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43(7):1334–1359. [DOI] [PubMed] [Google Scholar]
- 12.Van De Heyning CM, De Maeyer C, Pattyn N, et al. Impact of aerobic interval training and continuous training on left ventricular geometry and function: a SAINTEX-CAD substudy. Int J Cardiol 2018;257:193–198. [DOI] [PubMed] [Google Scholar]
- 13.Prado DM, Rocco EA, Silva AG, et al. Effects of continuous vs interval exercise training on oxygen uptake efficiency slope in patients with coronary artery disease. Braz J Med Biol Res 2016;49(2):e4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benda NM, Seeger JP, Stevens GG, et al. Effects of High-Intensity Interval Training versus Continuous Training on Physical Fitness, Cardiovascular Function and Quality of Life in Heart Failure Patients. PLoS One 2015;10(10):e0141256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellingsen O, Halle M, Conraads V, et al. High-Intensity Interval Training in Patients With Heart Failure With Reduced Ejection Fraction. Circulation 2017;135(9):839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor JL, Holland DJ, Spathis JG, et al. Guidelines for the Delivery and Monitoring of High Intensity Interval Training in Clinicsal Populations. Prog Cardiovasc Dis 2019;9(1):1–17. [DOI] [PubMed] [Google Scholar]
- 17.Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012;126(18):2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013;128(8):873–934. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Buschmann I, Jaureguizar KV, Calero MJ, Aquino RS. Programming exercise intensity in patients on beta-blocker treatment: the importance of choosing an appropriate method. Eur J Prev Cardiol 2014;21(12):1474–1480. [DOI] [PubMed] [Google Scholar]
- 20.Zhu N, Suarez-Lopez JR, Sidney S, et al. Longitudinal examination of age-predicted symptom-limited exercise maximum HR. Med Sci Sports Exerc 2010;42(8):1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulati M, Shaw LJ, Thisted RA, Black HR, Merz CN Bairey, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: the st. James women take heart project. Circulation 2010;122(2):130–137. [DOI] [PubMed] [Google Scholar]
- 22.Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK. Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc 2007;39(5):822–829. [DOI] [PubMed] [Google Scholar]
- 23.Ellingsen O, Halle M, Conraads V, et al. High-Intensity Interval Training in Patients With Heart Failure With Reduced Ejection Fraction. Circulation 2017;135(9):839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iellamo F, Manzi V, Caminiti G, et al. Validation of rate of perceived exertion-based exercise training in patients with heart failure: insights from autonomic nervous system adaptations. Int J Cardiol 2014;176(2):394–398. [DOI] [PubMed] [Google Scholar]
- 25.Tang LH, Zwisler AD, Taylor RS, et al. Self-rating level of perceived exertion for guiding exercise intensity during a 12-week cardiac rehabilitation programme and the influence of heart rate reducing medication. J Sci Med Sport 2016;19(8):611–615. [DOI] [PubMed] [Google Scholar]
- 26.Levinger I, Bronks R, Cody DV, Linton I, Davie A. Perceived exertion as an exercise intensity indicator in chronic heart failure patients on Beta-blockers. J Sports Sci Med 2004;3(YISI 1):23–27. [PMC free article] [PubMed] [Google Scholar]
- 27.Aamot IL, Forbord SH, Karlsen T, Stoylen A. Does rating of perceived exertion result in target exercise intensity during interval training in cardiac rehabilitation? A study of the Borg scale versus a heart rate monitor. J Sci Med Sport 2014;17(5):541–545. [DOI] [PubMed] [Google Scholar]
- 28.Townsend LK, Islam H, Dunn E, Eys M, Robertson-Wilson J, Hazell TJ. Modified sprint interval training protocols. Part II. Psychological responses. Applied Physiology Nutrition and Metabolism 2017;42(4):347–353. [DOI] [PubMed] [Google Scholar]
- 29.Islam H, Townsend LK, Hazell TJ. Modified sprint interval training protocols. Part I. Physiological responses. Applied Physiology Nutrition and Metabolism 2017;42(4):339–346. [DOI] [PubMed] [Google Scholar]
- 30.Billat VL, Flechet B, Petit B, Muriaux G, Koralsztein JP. Interval training at VO2max: effects on aerobic performance and overtraining markers. Med Sci Sports Exerc 1999;31(1):156–163. [DOI] [PubMed] [Google Scholar]
- 31.MacDougall D, Sale D. Continuous vs. interval training: a review for the athlete and the coach. Can J Appl Sport Sci 1981;6(2):93–97. [PubMed] [Google Scholar]
- 32.Haykowsky MJ, Daniel KM, Bhella PS, Sarma S, Kitzman DW. Heart Failure: Exercise-Based Cardiac Rehabilitation: Who, When, and How Intense? Can J Cardiol 2016;32(10 Suppl 2):S382–S387. [DOI] [PubMed] [Google Scholar]
- 33.Madssen E, Moholdt T, Videm V, Wisloff U, Hegbom K, Wiseth R. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am J Cardiol 2014;114(10):1504–1511. [DOI] [PubMed] [Google Scholar]
- 34.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol (1985) 2015;119(6):753–758. [DOI] [PubMed] [Google Scholar]
- 35.Koufaki P, Mercer TH, George KP, Nolan J. Low-volume high-intensity interval training vs continuous aerobic cycling in patients with chronic heart failure: a pragmatic randomised clinical trial of feasibility and effectiveness. J Rehabil Med 2014;46(4):348–356. [DOI] [PubMed] [Google Scholar]
- 36.Cardozo GG, Oliveira RB, Farinatti PT. Effects of high intensity interval versus moderate continuous training on markers of ventilatory and cardiac efficiency in coronary heart disease patients. ScientificWorldJournal 2015;2015(2):e192479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dall CH, Gustafsson F, Christensen SB, Dela F, Langberg H, Prescott E. Effect of moderate- versus high-intensity exercise on vascular function, biomarkers and quality of life in heart transplant recipients: A randomized, crossover trial. J Heart Lung Transplant 2015;34(8):1033–1041. [DOI] [PubMed] [Google Scholar]
- 38.Bacon AP, Carter RE, Ogle EA, Joyner MJ. VO2max trainability and high intensity interval training in humans: a meta-analysis. PLoS One 2013;8(9):e73182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer FJ, Borst MM, Zugck C, et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation 2001;103(17):2153–2158. [DOI] [PubMed] [Google Scholar]
- 40.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 2010;588(13):2487–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith JR, Hageman KS, Harms CA, Poole DC, Musch TI. Effect of chronic heart failure in older rats on respiratory muscle and hindlimb blood flow during submaximal exercise. Respir Physiol Neurobiol 2017;243(9):20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tasoulis A, Papazachou O, Dimopoulos S, et al. Effects of interval exercise training on respiratory drive in patients with chronic heart failure. Respiratory Medicine 2010;104(10):1557–1565. [DOI] [PubMed] [Google Scholar]
- 43.Dunham C, Harms CA. Effects of high-intensity interval training on pulmonary function. European Journal of Applied Physiology 2012;112(8):3061–3068. [DOI] [PubMed] [Google Scholar]
- 44.Christensen PM, Jacobs RA, Bonne T, Fluck D, Bangsbo J, Lundby C. A short period of high-intensity interval training improves skeletal muscle mitochondrial function and pulmonary oxygen uptake kinetics. Journal of Applied Physiology 2016;120(11):1319–1327. [DOI] [PubMed] [Google Scholar]
- 45.Boyle J 3rd. Graphic analysis of the Fick equation to evaluate oxygen transport. Respiration 1984;45(4):353–359. [DOI] [PubMed] [Google Scholar]
- 46.Astorino TA, Edmunds RM, Clark A, et al. Increased cardiac output and maximal oxygen uptake in response to ten sessions of high intensity interval training. The Journal of sports medicine and physical fitness 2018;58(1):164–171. [DOI] [PubMed] [Google Scholar]
- 47.Matsuo T, Saotome K, Seino S, et al. Effects of a Low-Volume Aerobic-Type Interval Exercise on VO2max and Cardiac Mass. Medicine and Science in Sports and Exercise 2014;46(1):42–50. [DOI] [PubMed] [Google Scholar]
- 48.Baekkerud FH, Solberg F, Leinan IM, Wisloff U, Karlsen T, Rognmo O. Comparison of Three Popular Exercise Modalities on V O2max in Overweight and Obese. Med Sci Sports Exerc 2016;48(3):491–498. [DOI] [PubMed] [Google Scholar]
- 49.Warburton DE, Haykowsky MJ, Quinney HA, et al. Blood volume expansion and cardiorespiratory function: effects of training modality. Med Sci Sports Exerc 2004;36(6):991–1000. [DOI] [PubMed] [Google Scholar]
- 50.Esfandiari S, Sasson Z, Goodman JM. Short-term high-intensity interval and continuous moderate-intensity training improve maximal aerobic power and diastolic filling during exercise. Eur J Appl Physiol 2014;114(2):331–343. [DOI] [PubMed] [Google Scholar]
- 51.Jensen MT, Suadicani P, Hein HO, Gyntelberg F. Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart 2013;99(12):882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nauman J, Aspenes ST, Nilsen TI, Vatten LJ, Wisloff U. A prospective population study of resting heart rate and peak oxygen uptake (the HUNT Study, Norway). PLoS One 2012;7(9):e45021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rave G, Fortrat JO. Heart rate variability in the standing position reflects training adaptation in professional soccer players. Eur J Appl Physiol 2016;116(8):1575–1582. [DOI] [PubMed] [Google Scholar]
- 54.da Silva VP, de Oliveira NA, Silveira H, Mello RG, Deslandes AC. Heart rate variability indexes as a marker of chronic adaptation in athletes: a systematic review. Ann Noninvasive Electrocardiol 2015;20(2):108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alansare A, Alford K, Lee S, Church T, Jung HC. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Heart Rate Variability in Physically Inactive Adults. Int J Environ Res Public Health 2018;15(7):e1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buscemi S, Canino B, Batsis JA, et al. Relationships between maximal oxygen uptake and endothelial function in healthy male adults: a preliminary study. Acta Diabetol 2013;50(2):135–141. [DOI] [PubMed] [Google Scholar]
- 57.Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The Impact of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Vascular Function: a Systematic Review and Meta-Analysis. Sports Medicine 2015;45(5):679–692. [DOI] [PubMed] [Google Scholar]
- 58.Mora-Rodriguez R, Ramirez-Jimenez M, Fernandez-Elias VE, et al. Effects of aerobic interval training on arterial stiffness and microvascular function in patients with metabolic syndrome. Journal of Clinical Hypertension 2018;20(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baum O, Torchetti E, Malik C, et al. Capillary ultrastructure and mitochondrial volume density in skeletal muscle in relation to reduced exercise capacity of patients with intermittent claudication. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 2016;310(10):R943–R951. [DOI] [PubMed] [Google Scholar]
- 60.Simoneau JA, Lortie G, Boulay MR, Marcotte M, Thibault MC, Bouchard C. Human skeletal muscle fiber type alteration with high-intensity intermittent training. Eur J Appl Physiol Occup Physiol 1985;54(3):250–253. [DOI] [PubMed] [Google Scholar]
- 61.Tan R, Nederveen JP, Gillen JB, et al. Skeletal muscle fiber-type-specific changes in markers of capillary and mitochondrial content after low-volume interval training in overweight women. Physiological Reports 2018;6(5):e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guadalupe-Grau A, Fernandez-Elias VE, Ortega JF, Dela F, Helge JW, Mora-Rodriguez R. Effects of 6-month aerobic interval training on skeletal muscle metabolism in middle-aged metabolic syndrome patients. Scandinavian Journal of Medicine & Science in Sports 2018;28(2):585–595. [DOI] [PubMed] [Google Scholar]
- 63.De Matos MA, Vieira DV, Pinhal KC, et al. High-Intensity Interval Training Improves Markers of Oxidative Metabolism in Skeletal Muscle of Individuals With Obesity and Insulin Resistance. Frontiers in Physiology 2018;9(10):e1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spee RF, Niemeijer VM, Wijn PF, Doevendans PA, Kemps HM. Effects of high-intensity interval training on central haemodynamics and skeletal muscle oxygenation during exercise in patients with chronic heart failure. Eur J Prev Cardiol 2016;23(18):1943–1952. [DOI] [PubMed] [Google Scholar]
- 65.Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports medicine (Auckland, NZ) 2012;42(7):587–605. [DOI] [PubMed] [Google Scholar]
- 66.Ribeiro PAB, Boidin M, Juneau M, Nigam A, Gayda M. High-intensity interval training in patients with coronary heart disease: Prescription models and perspectives. Annals of Physical and Rehabilitation Medicine 2017;60(1):50–57. [DOI] [PubMed] [Google Scholar]
- 67.Ulbrich AZ, Angarten VG, Netto A Schmitt, et al. Comparative effects of high intensity interval training versus moderate intensity continuous training on quality of life in patients with heart failure: Study protocol for a randomized controlled trial. Clinical Trials and Regulatory Science in Cardiology 2016;13:21–28. [Google Scholar]
- 68.Thompson PD, Franklin BA, Balady GJ, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 2007;115(17):2358–2368. [DOI] [PubMed] [Google Scholar]
- 69.Mezzani A, Hamm LF, Jones AM, et al. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol 2013;20(3):442–467. [DOI] [PubMed] [Google Scholar]
- 70.Astorino TA, Vella CA. Predictors of change in affect in response to high intensity interval exercise (HIIE) and sprint interval exercise (SIE). Physiol Behav 2018;196:211–217. [DOI] [PubMed] [Google Scholar]


