Abstract
Cardiac rehabilitation (CR) is a structured exercise and lifestyle program that improves mortality and quality of life in patients with heart failure (HF) with reduced ejection fraction. However, significant gaps remain in optimizing CR for older adults with HF. This review summarizes the state of the science, and dives deeper into specific knowledge gaps regarding older adults with HF. The authors also discuss the importance of geriatric complexities (i.e. frailty, multimorbidity, cognitive impairment, depression and social support) in the design and implementation of CR. Finally, the authors summarize promising future research in this area, and provide a clinical framework for current CR clinicians to follow when considering the specific needs of older adults with HF.
Keywords: cardiac rehabilitation, older adults, heart failure, frailty, sarcopenia, cognitive impairment, multimorbidity
Introduction:
Cardiac rehabilitation (CR) is a structured exercise and lifestyle intervention program initially designed for patients with ischemic heart disease. CR program structure typically requires patients to attend group exercise and education courses at a medical center 3 times per week for 12 weeks. CR is successful in reducing cardiovascular mortality and rehospitalizations among patients with ischemic heart disease.1 The last decade has seen significant progress in the study of CR for patients with heart failure (HF), spearheaded by the NIH-sponsored HF-ACTION trial.2 HF-ACTION is the largest randomized clinical trial of CR to date, randomizing 2331 patients with symptomatic HF with reduced ejection fraction (≤ 35%; HFrEF) to CR or attention control. Notably, these patients were clinically stable outpatients who had not been hospitalized for at least 6 weeks and were on stable guideline-directed medical therapy. CR sessions occurred 3 times per week at the study center and consisted of traditional moderate-intensity aerobic exercise. Patients were also encouraged to exercise at home and were provided with home exercise equipment and frequent reminder phone calls to reinforce adherence.2
To some, the results of HF-ACTION were underwhelming, as the reduction in all-cause mortality and hospitalizations was only seen after pre-specified adjustment for highly prognostic baseline characteristics.2 However, HF-ACTION also showed clinically significant improvement in quality of life measures in the exercise intervention arm,3,4 a finding supported in smaller studies.5 Furthermore, HF-ACTION proved unequivocally that moderate intensity aerobic exercise is safe in patients with medically treated and stable HFrEF, an issue that was unresolved prior to the study. Ultimately, the results of HF-ACTION were instrumental to the decision by Centers for Medicare and Medicaid Services (CMS) to approve payment for CR for beneficiaries matching the HF-ACTION inclusion criteria. Specifically, this includes those with symptomatic HF with ejection fraction ≤ 35% who have not been hospitalized for at least 6 weeks and who did not have a planned major cardiovascular hospitalization or procedure in the past 6 months (https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=270). Most clinical CR programs now use these inclusion criteria when identifying which patients with HF should be enrolled.
Although HF-ACTION advanced the scientific understanding of aerobic exercise in patients with HFrEF in a significant way and led to important policy changes that made CR accessible to a large segment of the HF population, many clinically important questions remain unanswered. First, HF-ACTION did not include patients with heart failure with preserved ejection fraction (EF □45%; HFpEF). HFpEF is now recognized to account for about 50% of the total HF population in the United States, and is the most common type of HF among rapidly growing population of older adults. 6–8 While smaller studies have demonstrated improvement in exercise capacity, quality of life and possibly hospital admissions in patients with HFpEF who participate in structured endurance exercise training programs,9–11 such patients are typically excluded from CR by CMS and most other third party payers due to lack of evidence.
Second, HF-ACTION enrolled chronic stable HFrEF patients from the outpatient setting and excluded patients who had been hospitalized in the last 6 weeks. Consequently, recently hospitalized HF patients are explicitly excluded from CR participation under CMS policy. However, following hospitalization, older adults with HF are particularly susceptible to the “post-hospitalization syndrome.”12 Post-hospital syndrome is characterized by significant physical functional impairments beyond that expected in chronic stable HFrEF, leading to a period of high vulnerability for adverse clinical events such as rehospitalization.12 Rapid muscle loss and debility related to hospitalization, immobility and acute illness likely contribute this syndrome,13–16 and suggest opportunities for appropriately structured CR programs beginning early post-discharge to improve physical function and potentially other important outcomes, including mortality, hospitalization and quality of life.
Third, HF-ACTION, as well as most other CR studies to date, did not take into account the complexities of aging such as frailty, multimorbidity and polypharmacy, which are increasingly prevalent among older patients with HF. For example, the average age of patients enrolled in HF-ACTION was 59 years, while the majority of patients hospitalized for heart failure are in their 60s, 70s and 80s.6 Although HF-ACTION enrolled 477 patients 70 years and older, the study may have selected for a relatively robust group able to perform maximal exercise testing to establish peak oxygen consumption and a 6 minute walk test as part of enrollment and with fewer comorbidities due to exclusion criteria. HF is a disease of aging, with incidence of HF increasing dramatically in the 8th and 9th decades of life.17 Comorbiditities frequently contribute to functional impairments and ability to participate in traditional endurance-based training modalities (e.g. treadmill walking). Aging-related considerations may warrant a different approach than that of traditional CR, as discussed further below.
Finally, HF-ACTION achieved only weak adherence to the exercise intervention despite tremendous efforts by the study investigators to promote regular participation.18 Less than 50% of patients in the intervention arm of HF-ACTION reached their target number of exercise hours in a given week.2 Non-adherence is common among patients with HF,18 and issues of old age, multimorbidity and debilitating symptoms are all relevant.18 Such limitations in the current CR landscape will guide our discussion of the geriatric-specific complexities in CR for older adults with HF, and the clinical and research implications of those complexities.
Geriatric complexities and CR for older adults with HF (Figure 1)
Figure 1.
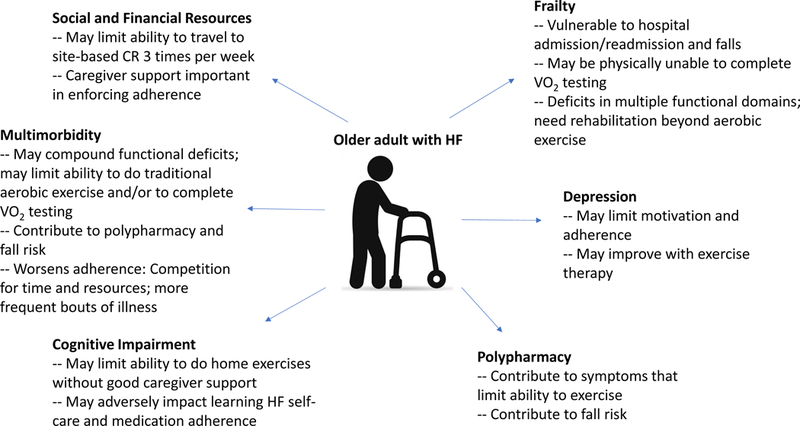
Summary of the geriatric complexities often present among older adults with HF. CR, cardiac rehabilitation;
Frailty and its Implications for CR
Frailty is the accumulation of deficits across multiple organ systems, leading to vulnerability in the face of stress. Frailty is highly prevalent among patients with HF, and even more prevalent among older adults hospitalized for HF.19–21 Physical frailty is characterized by a phenotype of activity intolerance beyond what would be expected for aging or HF alone. Among older frail patients with HF, this manifests as severe physical function impairments across multiple domains (e.g. balance, mobility) adversely impacting functional mobility.20
Frailty is an independent risk factor for adverse outcomes among older patients with HF and exercise training has potential to mitigate the risk of all-cause and cardiovascular death associated with frailty.22 Furthermore, CR is associated with better outcomes among frail older adults who suffered an acute myocardial infarction.23 Therefore, frailty should not preclude structured exercise therapy; rather it may help to identify a higher-risk population with greater potential to benefit from this intervention, especially if it can be structured to meet the specific needs of this patient population.
There is no clear “gold standard” for frailty measurement in HF. However, it would seem reasonable to choose frailty measure(s) based on which outcomes are most actionable or important to their patient population. Therefore, careful assessments of physical frailty such as the Fried criteria,24 Short Physical Performance Battery (a well-validated measure of strength, mobility and balance that predicts morbidity and mortality in older adults)25 or individual measures such as gait speed or handgrip strength would seem appropriate options. The Clinical Frailty Scale26 was proposed as a sensitive and specific screening tool for frailty in HF,27 and may help identify patients who warrant closer evaluation for significant barriers to conventional CR participation. Frail patients are also at risk for loss of functional independence. Maintaining or regaining functional independence is often an important goal for most older adults that may be addressed through rehabilitation interventions or other components of a comprehensive CR program. Assessment of basic and instrumental activities of daily living in conjunction with frailty assessments may be helpful in structuring a rehabilation intervention that may be particularly meaningful to the participant, potentially improving adherence and having a greater impact on QOL.
CR clinicians must be innovative in designing an exercise program for patients with deficits across multiple physical domains and associated loss of functional independence as these patients may not tolerate conventional CR strategies. In fact, many frail patients will have rehabilitation needs that exceed resources typically available (or reimbursed) in contemporary CR. Further study of alternative CR delivery models is needed to establish an evidence base for such comprehensive rehabilitation care.
Multimorbidity and its Implications for CR
Multimorbidity is pervasive among older patients with HF, many of whom have at least 5 or more comorbid illnesses, a large portion of which are non-cardiovascular conditions.20,28 This geriatric ailment is associated with to reduce adherence to CR among patients with HF,18 complicating CR participation in a number of ways. Even when chronic and stable, comorbid conditions can “compete” for patients’ attention and resources.29 Multimorbidity may also increase patients’ risk for polypharmacy, which has its own attendant adverse outcomes. Furthermore, multimorbidity increases risk of acute illness and hospitalization, both non-HF and HF-related,30 which may prevent participation. Comorbid illnesses also contribute to more severe functional impairments which may further limit participation. For example, chronic conditions such as CKD and COPD contribute to muscle wasting, weakness and exertional intolerance.31,32 Orthopedic and neurologic conditions in particular may impact patients’ ability to participate in traditional CR modalities, such as treadmill walking. CR clinicians must be alert to these conditions and prepared to alter exercise prescriptions accordingly.
Polypharmacy and its Implications for CR
Polypharmacy may contribute to symptoms of fatigue, cognitive impairment and depression. Alleviating these medication side effects may improve patients’ adherence to CR. Therefore, a thoughtful protocol for reviewing patients’ medications as part of CR may be beneficial. For example, stopping beta blockers in patients with HFpEF and no coronary artery disease may improve fatigue or orthostatic hypotension. Medications on the BEERS list should receive close scrutiny as many of these may cause symptoms that interfere with meaningful CR participation.33 Multidisciplinary review including a clinical pharmacist can also be helpful in identifying drug-drug interactions and dosing adjustments related to declining renal or liver function. Any de-prescribing should be done a) under the guidance of a qualified clinician who is trained in geriatrics, cardiology and the medication management of HF in older adults, and b) either by or in consultation with the prescribing provider. This team-based approach is necessary to avoid miscommunications and ensure referring providers maintain control over their patients’ medication regimen.
Cognitive Impairment and its Implications for CR
Cognitive impairment is common in HF, with an estimated prevalence of 43%−80% depending on HF population (e.g., acute vs chronic) and sensitivity of the instrument used.20,34 Cognitive impairment is often mild and commonly urecognized in clinical practice. However, even when recognized, cognitive impairment alone should not preclude CR participation; cognitive impairment did not diminish adherence to a supervised exercise program designed for community-dwelling older adults with impaired mobility.35 Caregiver support can be especially important for older adults with HF and cognitive impairment. For example, patients who are unable to remember instructions for safe exercise participation or who have processing impairments will likely not be appropriate for a home-based program without sufficient caregiver support. All patients with cognitive impairment will be at risk for medication errors and poorer HF self-care; therefore caregiver support can be helpful in these areas as well. In addition, direct communication of any newly identified cognitive impairment to the patient’s cardiovascular and/or primary care provider is important for long term management.
Depression and its Implications for CR
Depression is present in approximately 22% of patients with HF,36 and prevalence of depressive symptoms are nearly twice that among older adults hospitalized for HF.20 Depression may complicate CR by reducing patients’ motivation and adherence,18 and can masquerade as cognitive impairment. Fortunately, exercise improves depressive symptoms in HF;37 therefore depression alone should not preclude CR participation. In fact, the social aspect of site-based CR may improve patients’ mood. As with cognitive impairment, patients’ cardiovascular and/or primary care clinicians should always be notified of a positive depression screen in patients where depression has previously not been identified.
Social and Financial Resources and their Implications for CR
Involvement, or bolstering, of the patient’s social support network and use of community-based resources is integral to encouraging CR participation and long-term exercise adherence. The support of caregivers, formal and/or informal, may be required for transportation and supporting continued exercise adherence at home, both during and after completion of the CR program. Patients with cognitive impairment and/or depression will require significant support from a caregiver(s) when it comes to attending sessions regularly and retaining information taught by the CR staff. Patients who do not have a mode of transportation to the CR center are particularly challenged, and connecting patients with community-based resources may be necessary. There is a growing interest in home-based or hybrid CR programs in part because they reduce or obviate the need for transportation. However, patients should be carefully assessed for fall risk, poor physical function and potential need for physical or occupational therapy (i.e., home health or outpatient therapy) as a bridge to commencing either a home-based or outpatient CR program.
The Future is Bright: Ongoing or Future Studies of CR in Older Adults with Heart Failure
Older adults hospitalized for HF have severe deficits in strength, balance, mobility and endurance, likely as a result of geriatric complexities such as frailty, multimorbidity and polypharmacy.20 However, as discussed previously, such patients are currently not immediately eligible for CR based on CMS criteria and lack of evidence. Even if these patients were eligible for CR, their rehabilitation needs immediately following hospitalization would frequently exceed the resources typically available at conventional CR. The REHAB-HF study is currently testing a rehabilitation intervention in this patient population. The intervention arm receives an individualized, progressive, multi-domain exercise intervention that addresses strength, balance, mobility and endurance among patients ≤ 60 years old who are hospitalized for HF.38,39 Akin to traditional CR, patients attend exercise sessions at the study site 3 times per week for 12 weeks.38 The REHAB-HF intervention in the pilot study was well tolerated and feasible when enrolling patients who were living independently prior to hospitalization and were discharged home without home health rehabilitation.40
The REHAB-HF protocol differs significantly from traditional CR in that investigators are not relying on maximal or sub-maximal exercise testing to guide exercise prescription; rather investigators are relying on a functional assessment25 to guide exercise prescription. This is because in patients with profound impairments in strength, mobility or balance, exercise testing aimed at assessing aerobic capacity/anaerobic threshold may not be possible or may not yield valuable information. Targeting endurance (walking) training without also addressing deficits in balance, mobility and strength may increase risk of falls. Furthermore, patients can track their progress through attainment of independence in activities of daily living which may improve adherence, and clinicians can observe progress through serial objective measurements, such as the Short Physical Performance Battery and the 6-Minute Walk Test.
A novel CR program tailored to the older adult with cardiovascular disease (including, but not limited to HF) is being tested in the NIH-sponsored Modified Application of Cardiac Rehabilitaion for Older Adults (MACRO) study (ClinicalTrials.gov: ) This trial will test the a comprehensive intervention that will specifically 1) broadens risk assessment of older cardiovascular disease patients with functional, psychosocial as well as cardiac domains; 2) enhances cardiac rehabilitation care transitions (e.g. inpatient cardiac rehabilitation to home-based cardiac rehabilitation, with outpatient options then including site-based, home-based, and hybrid models of care); 3) assesses the home environment for home exercise safety; 4) augments motivation with a novel behavioral strategy; 5) integrates de-prescribing of medications that impede functional recovery. The MACRO study results may be anticipated in the next 3–5 years.
The COMPASS trial is testing a multi-dimensional intervention designed to identify and meet the post-acute care needs of patients hospitalized for stroke.41 The COMPASS intervention is centered around the patient and caregiver, and attempts to organize the various components of the patient’s care (i.e., stroke specialist, primary care, home health care) around their needs. COMPASS investigators are now working to develop a similar intervention for patients hospitalized for HF. Incorporating the physical rehabilitation needs into a comprehensive, patient- and caregiver-centered intervention for patients hospitalized for HF is another promising approach for meeting the unique needs of older adults hospitalized for HF.
Guidance for the CR clinician
While CR clinicians await the results of the above studies, they may still apply these geriatric principles to current practice based on existing data (Figure 2). Home-based cardiac rehabilitation for patients meeting traditional CR criteria are considered safe and effective, 42 therefore CR programs could consider incorporate a hybrid or home-based CR program for patients with barriers to transportation. Routine screening for all the geriatric complexities discussed above should be considered in CR programs currently. However, programs must carefully plan for the care coordination required for handling new diagnoses of depression and cognitive impairment and for addressing polypharmacy.
Figure 2.
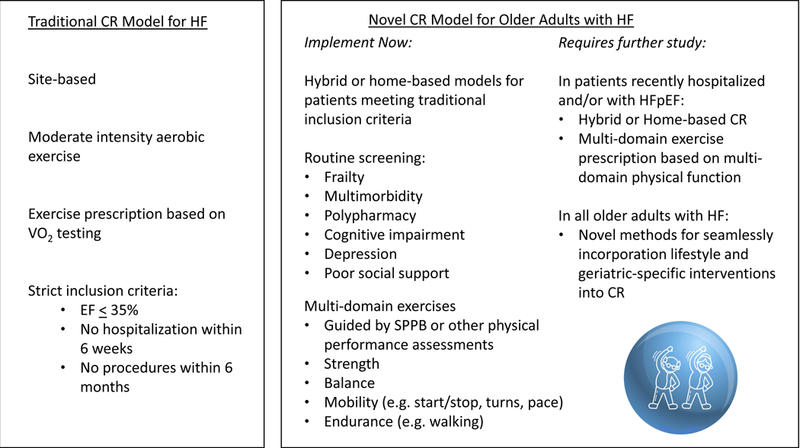
Comparison of current, traditional cardiac rehabilitation (CR) programs and the potential direction CR programs can take currently, and changes that may be soon be supported by evidence. EF, ejection fraction; SPPB, short physical performance battery; HFpEF, heart failure with preserved ejection fraction; HF, heart failure
CR programs could also consider routine testing of multiple domains of physical function (e.g., SPPB) rather than simply assessing aerobic capacity. In addition to prognosis, such assessments can help inform prescription of multi-domain exercise interventions, which have shown benefit in frail older patients in general. For example, the LIFE study tested a supervised, multi-domain exercise intervention for sedentary adults aged 70–89 years at risk for mobility-disability (defined as SPPB score </= 10; scores range from 0–12 with lower scores indicating worse function). The LIFE study physical activity domains included walking as the primary intervention (target 30 minutes/day), supplemented with strength training targeting most major muscle groups and balance training, each done 3x/week for 10 minutes. The intervention was successful in delaying major mobility disability (defined as inability to complete a 400 meter walk test without stopping or assistance) after one year (HR 0.72, 95% CI 0.57 – 0.91) and was safe (RR of adverse events in intervention vs. control arms 1.08 95% CI 0.98–1.20).43 A systematic review of frail older adults also supported the choice of a multidomain exercise intervention44 like the one prescribed in the LIFE study and the ongoing REHAB-HF study. Therefore, CR clinicians may consider designing exercise prescriptions for older adults with HF with multiple physical domains in mind. Initial physical assessment tools, such as the SPPB, can help inform whether patients have greater deficit in strength, mobility or balance, therefore guiding which domain(s) should receive the most emphasis, primarily early on in the CR program.
Conclusion
Exercise has tremendous potential to improve mortality, hospitalization and quality of life among older adults with HF. Traditional CR may be appropriate for robust older adults with HF, however important modifications are needed to accommodate the age-related intricacies that typically occur. Although CR clinicians can make important modifications for this patient population now, additional research, including results of ongoing trials, are needed to implement evidenced-based novel CR models designed to meet the specific needs of this population.
Key points:
The last decade has seen significant progress in the study of cardiac rehabilitation (CR) for patients with heart failure (HF), spearheaded by the NIH-sponsored HF-ACTION trial.
Although HF-ACTION advanced the scientific understanding of aerobic exercise in patients with HFrEF in a significant way and led to important policy changes that made CR accessible to a large segment of the HF population, many clinically important questions remain unanswered.
Such limitations in the current CR landscape will guide our discussion of the geriatric-specific complexities in CR for older adults with HF, and the clinical and research implications of those complexities.
Acknowledgments
FUNDING:
Dr. Reeves receives funding from NIH grant R01AG045551. Dr. Pastva receives funding from NIH R01 AG045551, PCORI PCS-1403-14532 and NIH P30 AG028716. Dr. Flint has no funding sources to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The Authors have nothing to disclose.
REFERENCES
- 1.Anderson L, Oldridge N, Thompson DR, et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol 2016;67(1):1–12. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301(14):1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosy AP, Cerbin LP, DeVore AD, et al. Aerobic exercise training and general health status in ambulatory heart failure patients with a reduced ejection fraction-Findings from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION)trial. Am Heart J 2017;186:130–138. [DOI] [PubMed] [Google Scholar]
- 4.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301(14):1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;1:CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012;126(1):65–75. [DOI] [PubMed] [Google Scholar]
- 7.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014;168(5):721–730. [DOI] [PubMed] [Google Scholar]
- 8.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355(3):251–259. [DOI] [PubMed] [Google Scholar]
- 9.Lang CC, Smith K, Wingham J, et al. A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF Pilot Study. BMJ Open 2018;8(4):e019649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitzman DW, Brubaker P, Morgan T, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016;315(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey A, Kitzman DW, Brubaker P, et al. Response to Endurance Exercise Training in Older Adults with Heart Failure with Preserved or Reduced Ejection Fraction. J Am Geriatr Soc 2017;65(8):1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med 2013;368(2):100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welch C, KH-S Z, AG C, ML J, AJ T. Acute Sarcopenia Secondary to Hospitalisation - An Emerging Condition Affecting Older Adults. Aging Dis 2018;9(1):151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization: A Randomized Clinical Trial. JAMA Intern Med 2018. [DOI] [PMC free article] [PubMed]
- 15.Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of Structured Exercise Interventions for Older Adults Hospitalized With Acute Medical Illness: A Systematic Review. J Aging Phys Act 2018;26(2):284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood SS, Wang TJ. The epidemiology of congestive heart failure: the Framingham Heart Study perspective. Glob Heart 2013;8(1):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conraads VM, Deaton C, Piotrowicz E, et al. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2012;14(5):451–458. [DOI] [PubMed] [Google Scholar]
- 19.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail 2016;18(7):869–875. [DOI] [PubMed] [Google Scholar]
- 20.Warraich HK, DJ; Duncan PW; Mentz RJ; Pastva AM; Nelson MB; Upadhya B; Reeves GR. Physical Function, Frailty, Cognition, Depression and Quality-of-Life in Hospitalized Adults ≥60 Years with Acute Decompensated Heart Failure with Preserved versus Reduced Ejection Fraction: Insights from the REHAB-HF Trial. Circulation: Heart Failure 2018;11:e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol 2017;236:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higueras-Fresnillo S, Cabanas-Sanchez V, Lopez-Garcia E, et al. Physical Activity and Association Between Frailty and All-Cause and Cardiovascular Mortality in Older Adults: Population-Based Prospective Cohort Study. J Am Geriatr Soc 2018;66(11):2097–2103. [DOI] [PubMed] [Google Scholar]
- 23.Flint K, Kennedy K, Arnold SV, Dodson JA, Cresci S, Alexander KP. Slow Gait Speed and Cardiac Rehabilitation Participation in Older Adults After Acute Myocardial Infarction. J Am Heart Assoc 2018;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55(4):M221–231. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173(5):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of Frailty in Chronic Heart Failure. JACC Heart Fail 2019. [DOI] [PubMed]
- 28.Manemann SM, Chamberlain AM, Boyd CM, et al. Multimorbidity in Heart Failure: Effect on Outcomes. J Am Geriatr Soc 2016;64(7):1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint KM, Forman DE. Lessons From the First 202 REHAB-HF Participants. Circ Heart Fail 2018;11(11):e005611. [DOI] [PubMed] [Google Scholar]
- 30.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. Journal of the American College of Cardiology 2003;42(7):1226–1233. [DOI] [PubMed] [Google Scholar]
- 31.Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015;70(3):213–218. [DOI] [PubMed] [Google Scholar]
- 32.Moorthi RN, Avin KG. Clinical relevance of sarcopenia in chronic kidney disease. Current Opinion in Nephrology and Hypertension 2017;26(3):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2015;63(11):2227–2246. [DOI] [PubMed] [Google Scholar]
- 34.Cannon JA, Moffitt P, Perez-Moreno AC, et al. Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J Card Fail 2017;23(6):464–475. [DOI] [PubMed] [Google Scholar]
- 35.Reid KF, Walkup MP, Katula JA, et al. Cognitive Performance Does not Limit Physical Activity Participation in the Lifestyle Interventions and Independence for Elders Pilot Study (LIFE-P). J Prev Alzheimers Dis 2017;4(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006;48(8):1527–1537. [DOI] [PubMed] [Google Scholar]
- 37.Blumenthal JA, Babyak MA, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA 2012;308(5):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeves GR, Whellan DJ, Duncan P, et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. Am Heart J 2017;185:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastva AM, Duncan PW, Reeves GR, et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial. Contemp Clin Trials 2018;64:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeves GR, Whellan DJ, O’Connor CM, et al. A Novel Rehabilitation Intervention for Older Patients With Acute Decompensated Heart Failure: The REHAB-HF Pilot Study. JACC Heart Fail 2017;5(5):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bushnell CD, Duncan PW, Lycan SL, et al. A Person-Centered Approach to Poststroke Care: The COMprehensive Post-Acute Stroke Services Model. J Am Geriatr Soc 2018;66(5):1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwisler AD, Norton RJ, Dean SG, et al. Home-based cardiac rehabilitation for people with heart failure: A systematic review and meta-analysis. Int J Cardiol 2016;221:963–969. [DOI] [PubMed] [Google Scholar]
- 43.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014;311(23):2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millan-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr 2015;15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]


