Abstract
Decreases in photosynthetic rate, stomatal conductance (gs), and mesophyll conductance (gm) are often observed under elevated CO2 conditions. However, which anatomical and/or physiological factors contribute to the decrease in gm is not fully understood. Arabidopsis thaliana wild-type and carbon-metabolism mutants (gwd1, pgm1, and cfbp1) with different accumulation patterns of non-structural carbohydrates were grown at ambient (400 ppm) and elevated (800 ppm) CO2. Anatomical and physiological traits of leaves were measured to investigate factors causing the changes in gm and in the mesophyll resistance (expressed as the reciprocal of mesophyll conductance per unit chloroplast surface area facing to intercellular space, Sc/gm). When grown at elevated CO2, all the lines showed increases in cell wall mass, cell wall thickness, and starch content, but not in leaf thickness. gm measured at 800 ppm CO2 was significantly lower than at 400 ppm CO2 in all the lines. Changes in Sc/gm were associated with thicker cell walls rather than with excess starch content. The results indicate that the changes in gm and Sc/gm that occur in response to elevated CO2 are independent of non-structural carbohydrates, and the cell wall represents a greater limitation factor for gm than starch.
Keywords: Arabidopsis thaliana, cell wall thickness, elevated CO2, mesophyll conductance, mesophyll resistance, non-structural carbohydrates, Rubisco
Changes in mesophyll conductance of Arabidopsis wild-type and carbohydrate-metabolism mutants grown at elevated CO2 are explained by changes in cell wall mass and thickness rather than by non-structural carbohydrates.
Introduction
Mesophyll conductance (gm) describes the diffusivity of CO2 from the intercellular space to the site of CO2 fixation in the chloroplast stroma as it passes through the cell wall, cell membrane, cytosol, and the chloroplast envelope. Three major methods have been used to estimate gm; A–Ci curve-fitting (Ethier and Livingston, 2004), chlorophyll fluorescence (Harley et al., 1992), and carbon isotope discrimination (Evans et al., 1986). The magnitude of the resulting value of gm expressed on a leaf-area basis is comparable to that of the stomatal conductance (gs), the measure of CO2 diffusion from the ambient air to the intercellular space (e.g. Tazoe et al., 2011; von Caemmerer and Evans 2015). Thus, gm represents a significant limitation to photosynthesis. Both gs and gm are known to change considerably depending on environmental conditions and plant growth status (Farquhar and Sharkey, 1982; von Caemmerer and Evans, 2015). Determining how the factors underlying gm respond to environmental conditions and their differences between species will increase our understanding of plant biomass production.
Among the components that influence gm, the cumulative surface area of the chloroplasts positioned along the cell membrane facing the intercellular space (Sc) and the cell wall thickness are the most crucial anatomical factors. Tholen et al. (2008) demonstrated that gm increases with an increase in Sc in photoreceptor mutants of Arabidopsis that showed marked differences in chloroplast positioning. Adachi et al. (2013) also found close relationships between Sc, gm, and maximum photosynthetic rate in inbred lines of Oryza sativa. In C3 plants, it is estimated that cell wall resistance accounts for 50% of the mesophyll resistance, the inverse of gm (Evans et al., 1994, 2009; Terashima et al., 2011). Scafaro et al. (2011) found that two wild Oryza relatives that had thicker cell walls than O. sativa showed greater draw-down of CO2 from the intercellular space to the chloroplast stroma. It has also been argued that extreme enlargement of starch grains would distort the chloroplasts (Cave et al., 1981; Delucia et al., 1985; Pritchard et al., 1997), causing suppression of photosynthesis by the hindrance of CO2 diffusion in the liquid phase (Nafziger and Koller, 1976; Nakano et al., 2000; Sawada et al., 2001).
Some species show decreases in gm in response to instantaneous increases in ambient CO2 concentration in the short-term (Flexas et al., 2007a; Tazoe et al., 2011); however, in other species including soybean and wheat gm remains virtually unchanged (Bernacchi et al., 2005; Tazoe et al., 2009). We have previously examined responses to elevated CO2 in Arabidopsis wild-type and stomatal-function mutants, and found that gs and gm respond independently to changes in CO2 concentration(Mizokami et al., 2019). The mechanisms associated with the decrease in gm in response to CO2 differ from those in response to water deficit, in which ABA plays a key role (Mizokami et al., 2015).
There are some reports that growth at elevated CO2 results in a decrease in gm in some species. It has been debated whether such interspecific differences in gm responses to the growth CO2 concentration can be explained by changes in cell wall thickness and/or starch content. For example, Zhu et al. (2012) reported that under free-air CO2 enrichment conditions, cell wall thickness significantly increased in rice but not in wheat, and a decrease in gm was found only in rice. Kitao et al. (2015) found a negative correlation between gm and starch content in the leaves of Betula platyphylla (Japanese white birch) grown under ambient or elevated CO2. Thus, an increase in cell wall thickness and/or starch accumulation may cause a decrease in gm at elevated CO2. However, the contribution of each of these factors to the increase in resistance to CO2 diffusion has not yet been fully elucidated since they usually occur simultaneously.
Teng et al. (2006) found that growth of Arabidopsis at elevated CO2 resulted in cell wall thickening, although the effects on Sc, gm, and photosynthesis were not reported. Given this fact, it should be possible to separately evaluate the effects of wall thickness and non-structural carbohydrates on gm by using Arabidopsis carbohydrate-metabolism mutants grown at ambient or elevated CO2. In this study, selected the Col-0 wild-type and three mutants of that are deficient in α-glucan/water dikinase (GWD1), plastidic phosphoglucomutase (PGM1), and cytosolic fructose 1, 6-bisphosphatase (CFBP1), which are key enzymes in starch breakdown, starch synthesis, and sucrose synthesis, respectively. Leaves of gwd1 show excess starch accumulation (Zeeman et al., 2004), leaves of pgm1 are starchless but show relatively high sugar concentrations (Periappuram et al., 2000), and leaves of cfbp1 are expected to show reduced sucrose content. The photosynthetic capacity of gwd1 is comparable to that of Col-0, whereas that of pgm1 and cfbp1 are significantly lower, especially when plants are grown at elevated CO2 (C.K.A. Watanabe et al., unpublished results). In addition to these mutants, we grew wild-type plants for an extended period with either low or high nitrogen availability at either ambient or elevated CO2 in order to obtain a wide range of control data. We also considered the reciprocal of mesophyll conductance per unit chloroplast surface area, i.e. mesophyll resistance per unit chloroplast surface area (Sc/gm), which might be directly related to the resistance associated with cell wall thickness (Evans et al., 2009). Sugars and starch tend to accumulate at elevated CO2, which may cause the down-regulation of photosynthesis due to a decrease in Rubisco content and activity (Sheen, 1994; Krapp and Stitt, 1995). We paid particular attention to the down-regulation of photosynthesis due to accumulation of non-structural carbohydrates.
Materials and methods
Plant material and growth conditions
We used Arabidopsis thaliana (L.) Heynh. accessions Col-0 (wild-type, WT), and the mutants CS3093 (At1g10760; gwd1/sex1), CS210 (At5g51820; pgm1), and SALK_064456C (At1g43670; cfbp1/fins1). cfbp1 corresponds to fins1 (Cho and Yoo, 2011). These mutants are each deficient in one of the enzymes related to carbohydrate metabolism, as follows: chloroplastic glucan water dikinase 1 (gwd1), chloroplastic phosphoglucomutase 1 (pgm1), and cytosolic fructose-1, 6-bisphosphatase 1 (cfbp1).
Seeds of all the accessions were sown in a mixture of autoclaved Metro Mix 350 (Sun Gro Horticulture, Bellevue, WA, USA) and vermiculite (1:1, v/v) in 200-ml plastic pots. The pots were placed in a cold room at 4 °C for two nights and transferred to two CO2-controlled growth chambers (LPH-0.5P-SH, Nippon Medical & Chemical Instruments).
Plants were grown at a photosynthetically active photon flux density (PFD) of 200 μmol m−2 s−1 provided by fluorescent lamps during an 8-h light period, with day/night temperatures of 23/21 °C, and a relative humidity of 60%. The CO2 concentrations in the growth chambers were controlled at either 400 ppm (ambient, aCO2) or 800 ppm (elevated, eCO2).
For the first week, plants were irrigated with deionized water. From the second week, they were irrigated with modified Hoagland solution 2–3 times a week. The solution contained 1.5 mM MgSO4, 1.35 mM NaH2PO4, 0.1 mM NaCl, 0.05 mM Fe-EDTA, 0.05 mM H3BO3, 0.01 mM MnSO4, 0.001 mM ZnSO4, 1 μM CuSO4, 0.5 μM Na2MoO4, 0. 2 μM CoSO4. High- and low-nitrogen solutions of 8 mM N and 1 mM N, respectively, with 5 mM K+ and 5 mM Ca2+ were obtained by adding KNO3, Ca(NO3)2, KCl, and CaCl2.
WT plants irrigated with the high-nitrogen solution and grown for a total of 39–42 d are hereafter referred to as ColHN, whilst WT plants irrigated with the low-nitrogen solution and grown for 38–42 d are referred to as ColLN. To obtain leaves with different photosynthetic traits, some ColHN plants were grown for a further 2 weeks (a total of 50–52 d) and these are referred to as Col50. The gwd1, pgm1, and cfbp1 plants were irrigated with the high-nitrogen solution. ColHN, ColLN, and cfbp1 were grown for 38–42 d after sowing, whilst gwd1 and pgm1 were grown for 50–52 d after sowing due to their slower growth rates. Measurements of photosynthesis and the various traits were conducted on young mature leaves, which were the 11th–14th leaves to emerge.
Gas exchange and isotope measurements
Photosynthesis measurements were conducted during the second half of the light period. Gas exchange measurements were performed using a laboratory-made leaf cuvette (50×55×20 mm) designed for a single leaf of Arabidopsis (Tholen et al., 2008; Mizokami et al., 2015, 2019). All gas exchange measurements to evaluate photosynthetic traits were conducted at 1% O2 to minimize the effect of photorespiration on gm, using cylinders containing 100% N2, 100% O2, and 1% CO2, which were mixed using mass-flow controllers (MM-3102L-NN, LINTEC, Tokyo, Japan). The O2 concentration of the gas was checked using an oxygen sensor (3080-O2, Walz, Effeltrich, Germany).
Light was provided by a metal halide lamp (PCS-UMX250, NPI, Tokyo, Japan). The PFD at the leaf level was monitored using a GaAs photodiode (G1738, Hamamatsu Photonics, Hamamatsu, Japan) placed in the chamber during the measurements. The GaAs sensor was calibrated against a quantum sensor (LI-190SA, LI-COR, Lincoln, NE, USA). The concentrations of CO2 and H2O in the air entering and leaving the cuvette were monitored using an infrared gas analyser (LI-7000, Li-Cor). The O2 effect on the sensitivity of the infrared CO2/H2O analyser was corrected following Mizokami et al. (2015).
To measure photosynthesis at different levels of CO2, we placed two leaves from two different plants together in the leaf cuvette. The leaf temperature and vapor pressure deficit (VPD) were kept at 22.5 °C and 0.85 kPa, respectively, and PFD was set to 1000 µmol photons m–2 s–1. For the plants grown at aCO2, the first measurement was taken at an ambient CO2 concentration (Ca) of 400 ppm, and then Ca was switched to 800 ppm. For the plants grown at eCO2, the first measurement was taken at 800 ppm, and then Ca was switched to 400 ppm. Gas exchange parameters were recorded when the photosynthesis attained a steady-state rate, and the photosynthetic rates and stomatal conductance measured at Ca of 400 ppm (A400 and gs400) and 800 ppm (A800 and gs800) were obtained. The air entering and leaving the cuvette was collected in 30-ml Pyrex bottles with two stopcocks, and the 13C/12C ratios were measured with a mass spectrometer (IsoPrime 100, IsoPrime Ltd, Manchester, UK) to obtain mesophyll conductance at the two Ca levels (gm400 and gm800).
Calculation of mesophyll conductance
Mesophyll conductance was calculated as described by Tazoe et al. (2011):
where Ca is the ambient CO2 concentration, Cs is the CO2 at the leaf surface, Ci is the intercellular CO2, Cc is the CO2 in the chloroplast stroma, ab and as are the carbon isotope discriminations caused by diffusion through the boundary layer (2.9‰) and stomata (4.4‰), respectively, ai is the carbon isotope discrimination during CO2 diffusion/dissolution through water (1.8‰), b is the carbon isotope discrimination caused by the carboxylation reaction by Rubisco and phosphoenolpyruvate carboxylase (30‰), e is the discrimination in respiration (calculated as described by Tazoe et al., 2009, 2011), and Δ is the measured carbon isotope discrimination (Evans et al., 1986). The factor e required a correction because δ13C during plant growth was different to that during measurement (Wingate et al., 2007), its values were set to –24.4‰ and –17.5‰ for plants grown at aCO2 and eCO2, respectively, as described by Mizokami et al. (2019). f is the carbon isotope discrimination during photorespiration (11.6‰) and Γ* is the CO2 compensation point without day respiration (Lanigan et al., 2008). Rd is the day respiration rate. For Γ* and Rd at 1% O2, we assumed values for Arabidopsis Col-0 grown at aCO2 and eCO2 previously estimated by (Mizokami et al., 2019) using the Laisk method (Laisk, 1977). For the plants grown at aCO2, Γ* and Rd were 11.3 µmol mol−1 and 0.53 µmol m−2 s−1, respectively, whilst for the plants grown at eCO2, Γ* and Rd were 5.4 µmol mol−1 and 0.45 µmol m−2 s−1, respectively. Although the values of Γ* at 1% O2 were higher than those estimated by Walker and Cousins (2013) in a previous study, a sensitivity analysis has shown that variations in Γ* have a minor impact on gm (Mizokami et al., 2019). We did not apply the ternary correction (Farquhar and Cernusak, 2012) since the VPD (0.872±0.083 kPa) and transpiration rate (0.0018±0.0005 mol m−2 s−1) were sufficiently low in our present study: for Col-0 grown at aCO2 we determined that gm400 calculated with or without the ternary correction differed by only 0.005 mol m−2 s−1, and gm800 differed by only 0.0031 mol m−2 s−1.
Sampling
The leaves from the two plants that had been used for the photosynthesis measurements were subsequently sampled for microscopic and biochemical analyses. Two or three similarly aged leaves including leaves used for the photosynthesis measurements were sampled to determine leaf morphological traits, leaf nitrogen content, δ13C, and the content of non-structural carbohydrates (starch and soluble sugars). Discs were taken from the leaves and measurements were taken after drying. The other two or three similarly aged leaves were stored at –80 °C for later determination of Rubisco content and cell wall content. In total, 4–6 leaves were sampled from the two plants and the set of data obtained from them was dealt with as one biological replicate.
Light and transmission electron microscope analyses
Small lamina segments were cut with a razor blade, immediately immersed in 0.2 M sodium cacodylate buffer (pH 7.0) containing 2.5% paraformaldehyde and 2% glutaraldehyde, and vacuum-infiltrated until most of the segments sank. They were then stored at 4 °C overnight. The segments were post-fixed in 1% OsO4 for 1 h and dehydrated in an ethanol series. Some segments were embedded in Technovit 7100 (Heraus Holding, Hanau, Germany) and used for light-microscope analysis. The other segments were further dehydrated in a series of propylene oxide and embedded in Spurr’s resin for TEM analysis.
For light microscopy, leaf transverse sections of 1 µm thick were cut on an ultramicrotome (Reichert Ultracut S, Leica, Vienna, Austria) with a glass knife and stained with a 0.1% (w/v) Toluidine Blue solution in 1% (w/v) sodium borate. Leaf thickness, the surface area of mesophyll cell walls exposed to the intercellular space (Smes, m2 m−2), and the surface area of chloroplasts facing the intercellular space (Sc, m2 m−2) were determined for each replicate. Smes and Sc were calculated using the curvature correction factor following Thain (1983) and Evans et al. (1994). The mean value of the correction factor was 1.24 in the present study. For TEM, ultrathin sections of 70 nm thick were cut on the ultramicrotome with a diamond knife (Ultra 45°, Diatome AG, Switzerland) and placed on a 150-mesh copper grid. The grids were stained with a 2% uranyl acetate solution followed by a lead citrate solution. The sections were examined on a JEM-1010 TEM (JEOL, Japan). The wall thickness of mesophyll cells was measured on the ultrathin sections using the ImageJ software (Schneider et al., 2012). The thickness was calculated for randomly selected cells by dividing the cross-sectional area of the cell wall by its length. On average, 58 μm of cell wall length was analysed for each plant.
Leaf mass per unit area and non-structural carbohydrates
The 2–3 dried leaf discs were weighed to determine leaf mass per area (LMA, g m−2) and then ground with a Multi-beads Shocker (Yasui Kikai, Osaka, Japan). The ground samples (3–5 mg each) were then used to determine the contents of glucose, sucrose, and starch according to Araya et al. (2006). Soluble sugars were extracted with 80% ethanol, and sucrose was hydrolysed to glucose and fructose with an invertase solution (Wako Chemical, Osaka, Japan). The precipitate was treated with amyloglucosidase (A-9228, Sigma-Aldrich, St. Louis, MO) to break down starch into glucose. Finally, glucose, and glucose equivalents of sucrose and starch, were quantified using a Glucose CII test kit (Wako Chemicals). The content of sugars and starch were expressed on a leaf-area basis. Structural LMA (sLMA, g m−2) consisting of proteins, minerals, lipids, and soluble and insoluble phenolics (Poorter et al., 2006) was calculated by subtracting the content of non-structural carbohydrates from LMA (Bertin et al., 1999).
Nitrogen and δ13C
The nitrogen content (Nmass, g N g–1) and δ13C of the ground leaf samples were determined with a CN analyser (Vario Micro, Elementar Analyzensysteme GmbH, Hanau, Germany) connected to an isotopic ratio mass spectrometer (IsoPrime100, IsoPrime, Manchester, UK). N content per area (Narea, g N m–2) was calculated as a product of Nmass and LMA. δ13C (‰) as follows:
where Rsample and Rstandard are the 13C/12C ratios of the sample and the standard (PDB, 0.011180).
Rubisco and cell wall contents
The two or three frozen leaf discs were used to determine the contents of Rubisco and cell wall materials as described previously (Mizokami et al., 2015; Sugiura et al., 2017). The frozen discs were homogenized in Tris–HCl buffer (62.5 mM, pH 6.8) using a Multi-beads Shocker (Yasui Kikai) to extract Rubisco. After extraction, SDS–PAGE, Coomassie Brilliant Blue staining, and formamide (NACALAI TESQUE, Kyoto, Japan) extraction were conducted and the Rubisco content was determined by measuring absorbance of the extract at 595 nm. From the residual pellet after the Rubisco extraction, starch was removed using amyloglucosidase and the cytoplasmic protein was removed using 1 M NaCl, and the resultant pellet was assumed to contain the cell wall materials. The pellet was weighed after drying, and cell wall mass per area (CMA, g m−2) was calculated.
Δ(Sc/gm400), Δcell wall thickness, and Δstarch
Because gm is proportional to the surface area available for diffusion, it is sometimes expressed as per unit chloroplast surface area adjacent to the intercellular airspaces (gm/Sc; Terashima et al., 2011). Because it is the resistance that is expected to be linearly related to cell wall thickness, we used the reciprocal of the conductance per Sc in our analyses, i.e. Sc/gm400. Sc/gm400 and the draw-down of CO2 from the intercellular space to the site of carboxylation in the chloroplast (Ci–Cc) were then related to cell wall thickness and starch content. To quantify the effect of growth CO2 on Sc/gm400, we used ΔX (%), the percent change in the trait X between the plants grown at eCO2 and those grown at aCO2 normalized to those grown at aCO2, as follows:
ΔX was calculated for Sc/gm400, cell wall thickness, and starch content.
Statistical analysis
Statistical tests were performed using Systat13 (Systat Software, Richmond, CA, USA). The effects of growth CO2 on anatomical, morphological, physiological, and photosynthetic traits were evaluated by ANOVA followed by Tukey’s multiple comparison test. δ13C of the leaves was compared only by Tukey’s test at each growth CO2 level since δ13C in the air differed depending on the growth chambers. Photosynthetic traits were also compared between the CO2 concentrations during the measurements by Student’s t-test.
Results
Leaf anatomical traits
Leaf anatomical traits of the different Arabidopsis lines were analysed using light and electron micrographs of leaf transverse sections (Supplementary Figs S1, S2 at JXB online) and were found to differ markedly (Table 1). Only the cell wall thickness was increased significantly by growth at eCO2. The thickness was greatest in Col50 and lowest in cfbp1 at both aCO2 and eCO2 (Table 1, Supplementary Fig. S2). Thickness was greater in eCO2 by ~20–35% in the WT and pgm1, whereas it did not differ in gwd1 or cfbp1. Leaf thickness, Sc, Sm, and Sc/Sm were greatest in pgm1, but the differences between the mutants and the WT were small.
Table 1.
Anatomical and physiological traits of Arabidopsis Col-0 and carbohydrate-mutants grown under ambient CO2 (aCO2, 400 ppm) or elevated CO2 (eCO2, 800 ppm)
| Growth CO2 | ColHN | Col50 | ColLN | gwd1 | pgm1 | cfbp1 | |
|---|---|---|---|---|---|---|---|
| Leaf thickness (μm) | aCO2 (ns) | 201.8±28.3b | 216.6±32.7b | 221.6±13.8b | 223.6±20.7b | 306.0±39.6a | 184.4±6.0b |
| eCO2 | 202.4±24.0B | 191.9±19.5B | 215.5±23.0B | 233.1±17.4B | 355.2±69.4A | 193.2±25.0B | |
| Cell wall thickness (μm) | aCO2** | 0.233±0.023ab | 0.286±0.032a | 0.221±0.032ab | 0.247±0.012ab | 0.246±0.012ab | 0.210±0.059b |
| eCO2 | 0.317±0.033A | 0.336±0.063A | 0.257±0.024AB | 0.246±0.052AB | 0.295±0.059AB | 0.195±0.015B | |
| S c (m2 m−2) | aCO2 (ns) | 6.52b±0.49b | 8.29±1.26b | 7.59±0.52b | 7.76±1.52b | 12.82±1.22b | 6.54±1.12b |
| eCO2 | 7.15±1.21B | 8.19±0.80B | 8.22±0.85B | 7.88±2.09B | 14.63±3.21A | 6.78±0.34B | |
| S m (m2 m−2) | aCO2 (ns) | 8.41±0.58b | 10.13±1.72b | 9.53±0.91b | 10.19±1.47b | 14.15±1.63a | 8.37±1.54b |
| eCO2 | 9.19±1.43B | 10.29±1.00B | 9.98±1.34B | 9.90±2.24B | 16.17±3.57A | 8.31±0.30B | |
| S c/Sm (m2 m−2) | aCO2 (ns) | 0.776±0.054b | 0.820±0.038ab | 0.798±0.022b | 0.757±0.057b | 0.908±0.032a | 0.784±0.056b |
| eCO2 | 0.777±0.017B | 0.797±0.044B | 0.828±0.065AB | 0.790±0.050B | 0.904±0.031A | 0.816±0.033AB | |
| Narea (g N m−2) | aCO2** | 1.167±0.078c | 1.391±0.060b | 0.881±0.139d | 1.194±0.039c | 1.731±0.085a | 1.071±0.070c |
| eCO2 | 1.227±0.066BC | 1.427±0.133B | 0.813±0.123D | 1.342±0.049B | 1.979±0.110A | 1.120±0.033C | |
| Rubisco (g m−2) | aCO2** | 1.050±0.278a | 1.061±0.111a | 0.852±0.112a | 1.015±0.045a | 1.183±0.076a | 1.175±0.148a |
| eCO2 | 1.271±0.078A | 1.091±0.148A | 0.695±0.110B | 1.128±0.044A | 1.151±0.093A | 1.158±0.149A | |
| δ13C (‰) | aCO2 | –32.5±0.34b | –33.0±0.19ab | –32.5±0.29bc | –32.0±0.19c | –33.5±0.14a | –32.6±0.23b |
| eCO2 | –40.4±0.48AB | –39.3±0.24C | –39.6±0.10BC | –38.9±0.28C | –41.2±0.56A | –40.9±0.65A |
S c is the chloroplast surface area exposed to the intercellular space; Sm is the mesophyll surface area exposed to the intercellular space; and Narea is leaf nitrogen content per area. Values are means (±SD), n=4. The overall effect of growth CO2 was evaluated by ANOVA (**P<0.01; ns, P>0.05), except for δ13C (see Methods). Significant differences within each CO2 treatment were determined using ANOVA followed by Tukey’s test (P<0.05), and are indicated by different letters (lower-case for aCO2, upper-case for eCO2).
Leaf morphological and physiological traits
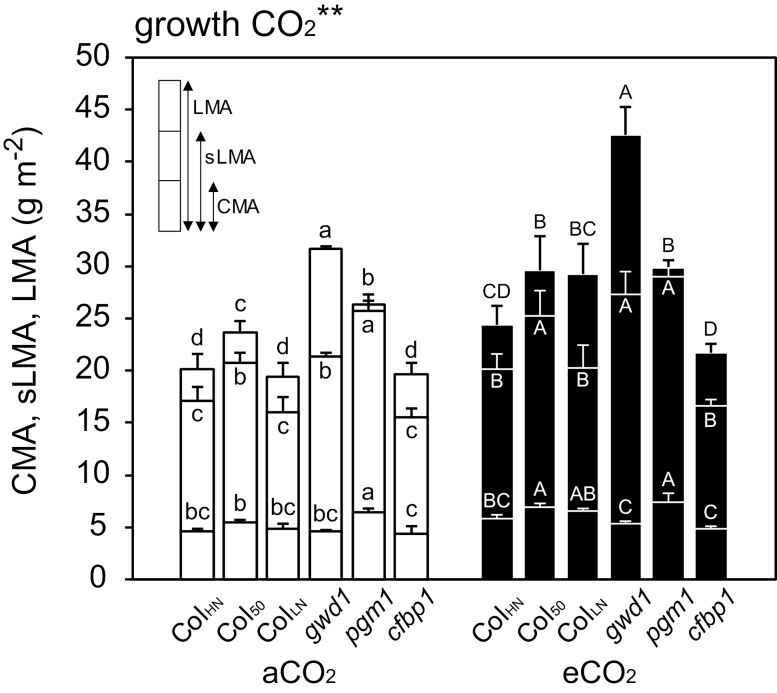
Leaf morphological and physiological traits were determined for the leaves that had been used for the photosynthesis measurements. LMA, sLMA, and CMA were greater in the eCO2 samples, and they differed significantly among the lines (Fig. 1). In both aCO2 and eCO2 plants LMA was highest in gwd1 whilst sLMA and CMA were highest in pgm1. These traits tended to be greater in Col50 than ColHN.
Fig. 1.
Leaf mass per area (LMA), structural leaf mass per area (sLMA), and cell wall mass per area (CMA) in leaves of Arabidopsis Col-0 grown with high nitrogen for 39–42 d (ColHN), with low nitrogen for 38–42 d (ColLN), or with high nitrogen for 50–52 d (Col50), and the carbohydrate-metabolism mutants gwd1, pgm1, and cfbp1 grown under either ambient CO2 (aCO2, 400 ppm) or elevated CO2 (eCO2, 800 ppm). Data are means (±SD), n=4. The overall effect of growth CO2 was evaluated by ANOVA and is indicated above the graph (**P<0.01). Significant differences among the lines within each CO2 treatment were determined using ANOVA followed by Tukey’s test (P<0.05), and are indicated by different letters (lower-case for aCO2, upper-case for eCO2).
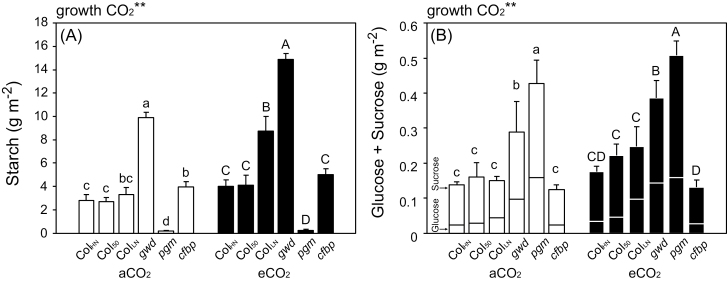
The level of non-structural carbohydrates was also increased by elevated growth CO2. The starch content was highest in gwd1 (the starch-excess mutant) and lowest in pgm1 (Fig. 2A). Within the WT, the starch content was highest in ColLN in both aCO2 and eCO2. Soluble sugars were highest in pgm1, followed by gwd1 (Fig. 2B). Chloroplasts with excess starch were clearly visible in light and electron micrographs of gwd1, whilst starchless chloroplasts could be seen in pgm1 (Supplementary Figs S1, S2).
Fig. 2.
(A) Starch and (B) soluble sugars in the leaves of Arabidopsis Col-0 grown with high nitrogen for 39–42 d (ColHN), with low nitrogen for 38–42 d (ColLN), or with high nitrogen for 50–52 d (Col50), and the carbohydrate-metabolism mutants gwd1, pgm1, and cfbp1 grown under ambient CO2 (aCO2, 400 ppm) or elevated CO2 (eCO2, 800 ppm). Data are means (±SD), n=4. The overall effect of growth CO2 was evaluated by ANOVA and is indicated above the graph (**P<0.01). Significant differences among the lines within each CO2 treatment were determined using ANOVA followed by Tukey’s test (P<0.05), and are indicated by different letters (lower-case for aCO2, upper-case for eCO2).
Narea was slightly increased by elevated growth CO2, whereas Rubisco content was not (Table 1). Narea was highest in pgm1 and lowest in ColLN in both aCO2 and eCO2. The Rubisco content in ColLN grown at eCO2 was significantly lower than in the other lines, but otherwise no other differences were observed. There was a positive correlation between the Rubisco content and Narea among the WT and mutants gwd1 and cfbp1 grown at aCO2 and eCO2 (Supplementary Fig. S3). However, pgm1 did not fit this pattern, having relatively low Rubisco content despite having the highest Narea.
δ13C differed significantly among the lines (Table 1), with the highest value in gwd1 and lowest in pgm1 in both aCO2 and eCO2. The WT showed intermediate values for both aCO2 and eCO2. There were negative relationships between δ13C and Cc (Supplementary Fig. S4A, B) and positive correlations between δ13C value and starch content for all the lines (Supplementary Fig. S4C, D).
Leaf photosynthetic characteristics
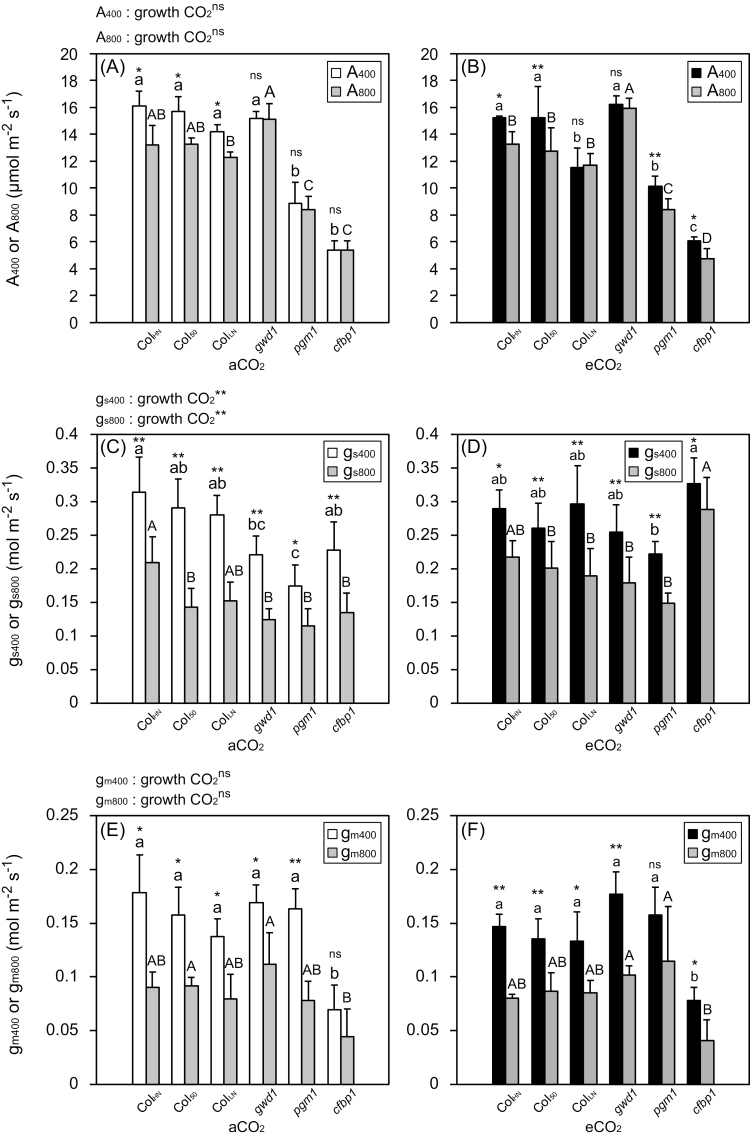
Photosynthesis measurements were conducted at 400 ppm CO2 (A400, gs400, and gm400) and 800 ppm (A800, gs800, and gm800) for plants grown at aCO2 and eCO2.
A 400 and A800 were higher in the WT and gwd1 than in pgm1 and cfbp1 at both aCO2 and eCO2 (Fig. 3A, B). A800 was significantly lower than A400 in ColHN, Col50, and ColLN at aCO2 (Fig. 3A), and also significantly lower in ColHN, Col50, pgm1, and cfbp1 at eCO2 (Fig. 3B). gs400 and gs800 were lowest in pgm1 at both aCO2 and eCO2 (Fig. 3C, D). gs800 was significantly lower than gs400 in all the lines at both aCO2 and eCO2. gm400 and gm800 were lowest in cfbp1 at both aCO2 and eCO2 (Fig. 3E, F). gm800 was significantly lower than gm400 in all the lines at both aCO2 and eCO2 (Fig. 3C, D) except for cfbp1 at aCO2 and pgm1 at eCO2. Although the photosynthetic rates (A400 and A800) and mesophyll conductances (gm400 and gm800) were not significantly affected by growth CO2 conditions, gm400 was slightly lower in the WT and pgm1 grown at eCO2. Stomatal conductance (gs400 and gs800) was significantly increased by elevated growth CO2, especially in gwd1, pgm1, and cfbp1.
Fig. 3.
Photosynthetic characteristics of Arabidopsis Col-0 grown with high nitrogen for 39–42 d (ColHN), with low nitrogen for 38–42 d (ColLN), or with high nitrogen for 50–52 d (Col50), and the carbohydrate-metabolism mutants gwd1, pgm1, and cfbp1 grown under ambient CO2 (aCO2, 400 ppm) or elevated CO2 (eCO2, 800 ppm). (A, B) Photosynthetic rates measured at 400 ppm CO2 (A400) and 800 ppm CO2 (A800) for plants grown in (A) aCO2 and (B) eCO2. (C, D) Stomatal conductance measured at 400 ppm CO2 (gs400) and 800 ppm CO2 (gs800) for plants grown in (A) aCO2 and (B) eCO2. (E, F) Mesophyll conductance measured at 400 ppm CO2 (gm400) and 800 ppm CO2 (gm800) for plants grown in (A) aCO2 and (B) eCO2. Data are means (±SD), n=4. The overall effect of growth CO2 was evaluated by ANOVA (**P<0.01; ns, P>0.05). Significant differences among the lines within each growth CO2 treatment were determined using ANOVA followed by Tukey’s test (P<0.05), and are indicated by different letters (lower-case for aCO2, upper-case for eCO2). Significant differences between measurements at 400 ppm CO2 and 800 ppm CO2 within each line were determined using Student’s t-test (**P<0.01; *P<0.05; ns, P>0.05).
Factors that determine photosynthetic characteristics
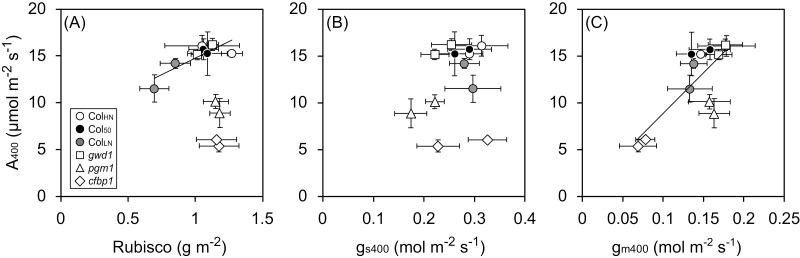
Relationships among photosynthetic characteristics and various morphological and physiological traits were analysed to determine which traits were involved in the responses to growth CO2 conditions. Here, we focus on the photosynthetic characteristics measured at 400 ppm CO2.
There was a positive correlation between A400 and Rubisco content for the WT and gwd1, whereas pgm1 and cfbp1 showed lower photosynthetic rates despite having high Rubisco contents (Fig. 4A). The relationship between A400 and gs400 was not significant (Fig. 4B); however, A400 was positively correlated with gm400 for all the lines except for pgm1 (Fig. 4C).
Fig. 4.
Relationships between photosynthetic rate, Rubisco content per area, stomatal conductance, and mesophyll conductance measured at 400 ppm CO2 for Arabidopsis lines grown under ambient CO2 (aCO2, 400 ppm) or elevated CO2 (eCO2, 800 ppm). The lines, as indicated in the key in (A), were Col-0 grown with high nitrogen for 39–42 d (ColHN), with low nitrogen for 38–42 d (ColLN), or with high nitrogen for 50–52 d (Col50), and the carbohydrate-metabolism mutants gwd1, pgm1, and cfbp1. (A) Photosynthetic rate (A400) and Rubisco content per area, (B) A400 and stomatal conductance (gs400), and (C) A400 and mesophyll conductance (gm400). Data are means (±SD), n=4. Regression lines are shown in (A) for ColHN, Col50, ColLN, and gwd1 (R2=0.68), and in (C) for ColHN, Col50, ColLN, gwd1, and cfbp1 (R2=0.60).
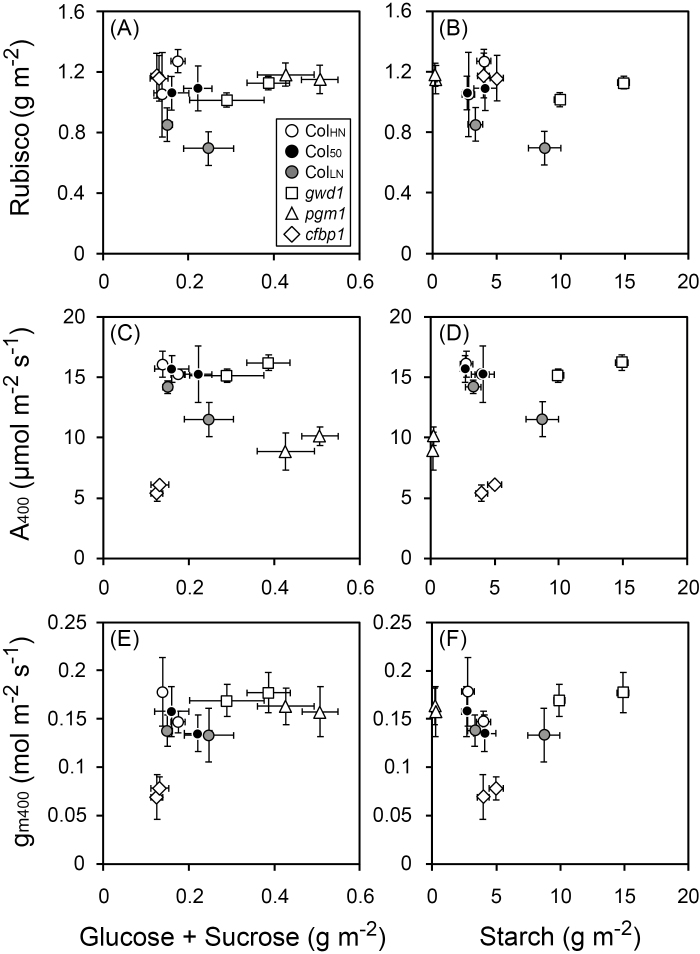
Relationships among photosynthetic characteristics and accumulation of sugars and starch were investigated to determine whether feedback regulation was occurring (Fig. 5). No negative correlations with the soluble sugar or starch contents were found for either Rubisco content (Fig. 5A, B), A400 (Fig. 5C, D), or gm400 (Fig. 5E, F).
Fig. 5.
Relationships between photosynthetic characteristics measured at 400 ppm CO2 and content of soluble sugars (glucose and sucrose) and starch for Arabidopsis lines grown under ambient CO2 (400 ppm) or elevated CO2 (800 ppm). The lines, as indicated in the key in (A), were Col-0 grown with high nitrogen for 39–42 d (ColHN), with low nitrogen for 38–42 d (ColLN), or with high nitrogen for 50–52 d (Col50), and the carbohydrate-metabolism mutants gwd1, pgm1, and cfbp1. Rubisco versus contents of glucose and sucrose (A) and versus starch (B), photosynthetic rate (A400) versus contents of glucose and sucrose (C) and versus starch (D), and mesophyll conductance (gm400) versus contents of glucose and sucrose (E) and versus starch (F). Data are means (±SD), n=4.
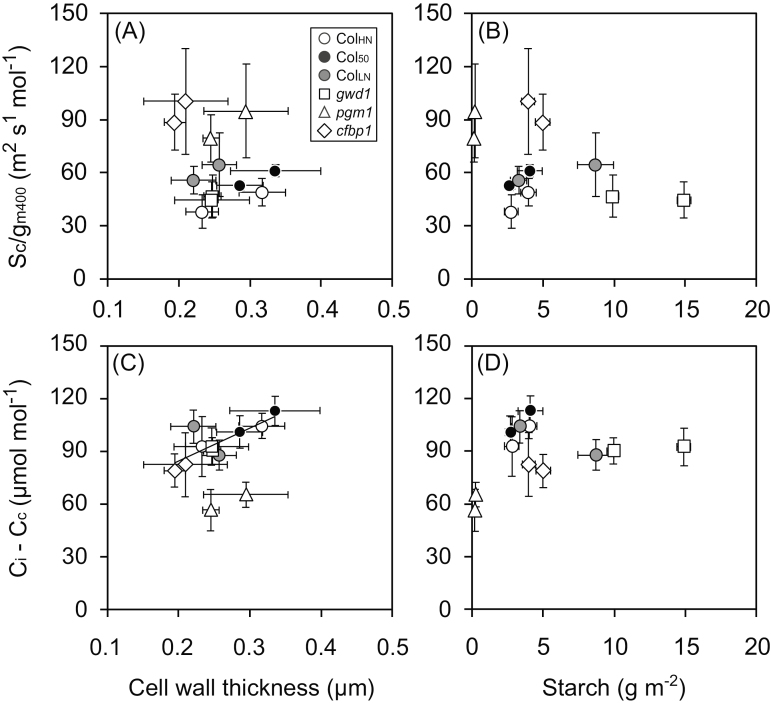
Relationships between the mesophyll conductance and morphological and anatomical features were investigated. There was no overall relationship between Sc/gm400 and cell wall thickness among the lines (Fig. 6A); however, within each line Sc/gm400 increased with the increase in cell wall thickness. No relationship was observed between Sc/gm400 and starch content among the lines (Fig. 6B). The draw-down of CO2 from the intercellular space to the site of carboxylation in the chloroplast (Ci–Cc) was positively correlated with cell wall thickness for all the lines except for pgm1 (Fig. 6C). There was no clear relationship between Ci–Cc and starch content among the lines (Fig. 6D). Cell wall thickness was positively correlated with cell wall mass per area (CMA) for all the lines and CMA was also positively correlated with sLMA (Supplementary Fig. S5B, C). We also expected a positive correlation between mesophyll conductance and Sc, but no clear relationship was found (Supplementary Fig. S6).
Fig. 6.
Relationships between mesophyll resistance and draw-down of CO2 from intercellular space to the chloroplast (Ci–Cc) measured at 400 ppm CO2 and cell wall thickness and starch content for Arabidopsis lines grown under ambient CO2 (400 ppm) or elevated CO2 (800 ppm). The lines, as indicated in the key in (A), were Col-0 grown with high nitrogen for 39–42 d (ColHN), with low nitrogen for 38–42 d (ColLN), or with high nitrogen for 50–52 d (Col50), and the carbohydrate-metabolism mutants gwd1, pgm1, and cfbp1. Mesophyll resistance (Sc/gm400) versus cell wall thickness (A) and versus starch content (B), and draw-down of CO2 versus cell wall thickness (C) and versus starch content (D). Data are means (±SD), n=4. The regression line for ColHN, Col50, ColLN, gwd1, and cfbp1 is shown in (C) (R2=0.65).
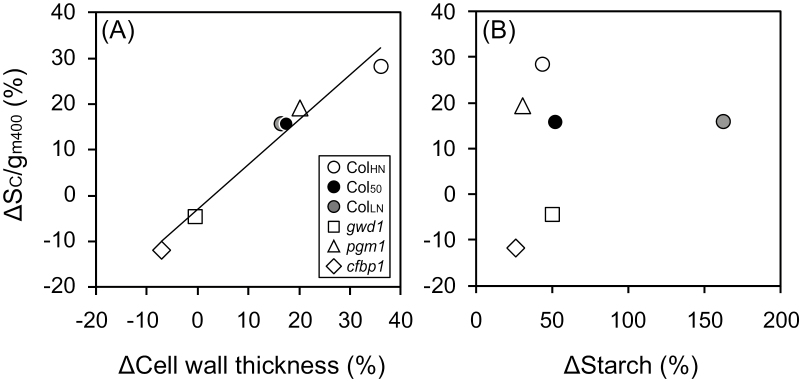
We examined how the changes in the cell wall thickness and starch content in response to growth CO2 conditions affected mesophyll resistance using ΔSc/gm400, the percentage change in a given line between the plants grown at eCO2 and those grown at aCO2 normalised to aCO2. ΔSc/gm400 showed a strong positive correlation with ΔCell wall thickness but there was no clear correlation between Δ Sc/gm400 and ΔStarch content (Fig. 7A, B).
Fig. 7.
Relationships between changes in mesophyll resistance and changes in cell wall thickness and starch content in response to CO2 growth conditions for Arabidopsis lines grown under ambient CO2 (400 ppm) or elevated CO2 (800 ppm). The lines, as indicated in the key in (A), were Col-0 grown with high nitrogen for 39–42 d (ColHN), with low nitrogen for 38–42 d (ColLN), or with high nitrogen for 50–52 d (Col50), and the carbohydrate-metabolism mutants gwd1, pgm1, and cfbp1. Percentage change in mesophyll resistance (ΔSc/gm400) measured at 400 ppm CO2 versus (A) percentage change in cell wall thickness (ΔCell wall thickness) and versus percentage change in starch content (ΔStarch). For calculation of the percentage changes see Methods. Data are means (±SD), n=4. The regression line for all the data is shown in (A) (R2=0.97).
Discussion
The cell wall is a greater limiting factor for gm than starch content
Our results indicated that among the changes in various morphological, anatomical, and physiological traits that we observed, the increases in cell wall mass and thickness in Arabidopsis had the major impact on mesophyll conductance. The excess accumulation of starch was not linked to a decrease in gm (Fig. 5F), whereas within each line the increase in cell wall thickness was associated with an increase in mesophyll resistance, Sc/gm (Fig. 6A), and a greater draw-down of CO2 from the intercellular space to the chloroplast, Ci−Cc (Fig. 6C). It was clear that the percentage change in mesophyll resistance between eCO2 and aCO2 plants (ΔSc/gm400) was associated with the percentage change in cell wall thickness (ΔCell wall thickness) across all lines (Fig. 7A). The fact that there was no clear correlation between ΔSc/gm400 and ΔStarch indicated the lack of effect of the latter (Fig. 7B). The measurement of cell wall thickness requires observations with an electron microscope and is therefore laborious, whereas measurement of cell wall mass is relatively easy. Since cell wall thickness was highly correlated with cell wall mass per area (CMA, Supplementary Fig. S5A), the latter could be an alternative indicator of cell wall thickness when investigating the environmental responses of gm in the same species. As also shown in a previous study (Sugiura et al., 2017), there was a strong positive correlation between CMA and sLMA (Supplementary Fig. S5B), indicating that sLMA could also be an efficient parameter within a plant species that reflects the thickness and mass of the cell wall.
When plants are grown under elevated CO2 conditions, increases are observed in the thickness and mass of cell walls (Teng et al., 2006) and in the accumulation of non-structural carbohydrates (Kitao et al., 2015; Sugiura et al., 2017), whilst decreases are observed in gm (Zhu et al., 2012). It has been proposed that excess accumulation of starch in mesophyll cells could hinder CO2 diffusion and result in decreased gm (Nafziger and Koller, 1976; Nakano et al., 2000; Sawada et al., 2001). Zhu et al. (2012) reported a decrease in gm and an increase in starch content in flag-leaves of rice grown with free-air CO2 enrichment); however, inter-relationships among the traits were not examined in detail. In our present study, although the starch-excess mutant gwd1 accumulated starch at both aCO2 and eCO2 (Fig. 2A), gm did not decrease (Fig. 3E, F). Our results suggested that direct effects of non-structural carbohydrates on CO2 diffusion and gm were minor, whereas the increased cell wall mass and thickness caused a decrease in gm and an increase in Sc/gm under elevated CO2 conditions (Table 1, Figs 1, 7A). Thus, we conclude that the increases in cell wall mass and thickness contributed more to the decrease in gm and the increase in Sc/gm than starch has been observed to do in previous studies.
Although we found a simultaneous increase in Sc/gm and cell wall thickness in each individual line, there was not a positive correlation between them overall across all lines (Fig. 6A). The fact that ColLN, pgm1, and cfbp1 showed different patterns suggests that variation in other physiological processes and anatomical features could also be involved. Mesophyll conductance can be separated into gaseous and liquid phases, and the liquid phase can be further separated into five phases, namely cell wall, plasma membrane, cytosol, chloroplast envelope, and stroma (Evans et al., 2009). It is considered that CO2 diffusion across the cell wall and across the plasma membrane are the major limiting steps (Terashima et al., 2011). In recent years, it has been suggested that aquaporins—proteins involved in water transport in the plasma membrane—may affect the permeability of CO2 through membranes (Terashima et al., 2006; Mori et al., 2014; Groszmann et al., 2017). Therefore, it is possible that ColLN grown under low N availability showed higher Sc/gm400 regardless of the thinner cell wall due to a lower expression of aquaporins in the plasma membranes. The expression levels of aquaporins are significantly decreased in roots of Oryza sativa grown under low N availability (Ishikawa-Sakurai et al., 2014), so it is possible that a similar effect may occur in the leaf mesophyll cells in Arabidopsis. In cfbp1, in which the sucrose synthesis pathway is impaired, the photosynthetic rate, A400, was limited by the low gm (Figs 3, 4A) even though this line showed a high value of Narea, high Rubisco content, and the thinnest cell walls (Table 1). Therefore, factors other than cell wall thickness, such as the plasma membrane, cytoplasm, chloroplast envelope, might be markedly changed in cfbp1. Furthermore, since the composition of the cell wall is markedly different among carbohydrate-metabolism mutants of Arabidopsis, including pgm1 and gwd1 (Engelsdorf et al., 2017), it is possible that differences in the porosity of the cell wall affected gm in these mutants, as discussed by Evans et al. (2009). Future metabolomic, transcriptomic, and micro-anatomical studies will be required to elucidate the mechanisms underlying the changes in gm in these mutants.
It is reported that gm in plants within the same functional group, such as annuals, broad-leaved deciduous trees, and broad-leaved evergreen trees, is roughly proportional to Sc (Terashima et al., 2006). Such a positive correlation between gm and Sc has been found for WT and chloroplast-positioning mutants of Arabidopsis (Tholen et al., 2008). However, there was no correlation between gm400 and Sc for the lines used in our present study (Supplementary Fig. S6), possibly due to the fact that the positioning of chloroplasts was normal in the lines that we used and that the range of variation in Sc was not large enough.
Short-term changes in gm in response to elevated CO2 are independent of non-structural carbohydrates
Short-term changes in gm in response to the increase in the CO2 concentration from 400 ppm to 800 ppm during our photosynthesis measurements were observed in all the carbohydrate-metabolism mutants as well as in the WT (Fig. 3E, F). This suggests that the amount of non-structural carbohydrates was not involved in the short-term changes in gm. A previous study had shown that gs and gm were independently regulated in response to CO2 concentration during gas-exchange measurements in Arabidopsis WT plants and two mutants of stomatal function, open stomata 1 (ost1) and slow-type anion channel 1–2 (slac1-2) (Mizokami et al., 2019). Since these plants showed stable gs and gm over an hour, the observed changes when the ambient CO2 concentration was changed from either 400 ppm to 800 ppm or vice versa were not caused by the stress of long-term measurements. In the present study, pgm1 showed the lowest gs whereas cfbp1 showed the lowest gm. This also suggests that gs and gm are not necessarily coordinated well in Arabidopsis. In addition to the short-term changes in gm, the positive correlations between A400 and Rubisco and gm400 that we observed were consistent with previous reports (Fig. 4; Flexas et al., 2007b; Tholen et al., 2008; José Javier et al., 2017).
Ecophysiological significance of the down-regulation of photosynthesis
The photosynthetic rate measured at 800 ppm (A800) was significantly lower than that measured at 400 ppm (A400) (Fig. 3A, B), which can be explained by a limitation in triose phosphate use (Sharkey, 1985; Harley and Sharkey, 1991). According to these studies, CO2 assimilation rate measured at low oxygen concentration reaches a maximum at lower Ci than that measured at normal concentration. In our present study, all the gas-exchange measurements were performed at 1% O2, which led to a decrease in photosynthetic rate with the increase in Ca and Ci (Woo and Wong, 1983).
The decrease in Rubisco was not observed in gwd1, which showed the highest starch content, and in pgm1, which showed the highest soluble sugars (Table 1, Fig. 5A, B). This strongly supports the idea that accumulation of soluble sugars and starch does not cause the down-regulation of photosynthesis through the decrease in Rubisco content in Arabidopsis. Although an increase in the content of starch and decreases in Rubisco and A400 were found simultaneously only in ColLN (Fig. 5A–D), this would be related to the promotion of leaf senescence and nitrogen remobilization to other organs. Ludewig and Sonnewald (2000) reported that the down-regulation of photosynthesis in tobacco was found in senescing leaves only, and there was no correlation between the transcript levels of photosynthesis-related genes and soluble sugar contents.
It has been argued that the sensitivity of maximum photosynthetic capacity to carbohydrate varies greatly among plant species (Sugiura et al., 2018). Sugiura et al. (2015, 2017) showed that Raphanus sativus, which in common with Arabidopsis belongs to Brassicaceae, can be classified as a carbohydrate insensitive species. They reciprocally grafted plants with different sink activities and found that neither the maximum photosynthesis nor the Rubisco content was down-regulated even though excessive non-structural carbohydrates were accumulated in the source leaves in shoots grafted to the stock of a variety with a low sink activity. Therefore, it is possible that Brassicaceae can be classified as having carbohydrate-insensitive species. Since carbohydrate-insensitive species such as R. sativus and Glycine max show increases in CMA in response to accumulation of non-structural carbohydrates, it is possible that the cell wall is also an important carbohydrate sink in the response to changes in the sink–source balance (Sugiura et al., 2017, 2018). Thus, these plants might down-regulated photosynthetic capacity through the suppression of CO2 conductance by increasing cell wall thickness in order to avoid excess accumulation of non-structural carbohydrates.
Since Rubisco discriminates less against 13C at low concentrations of CO2 in the chloroplast stroma (Cc), we expected that the δ13C values of the leaves would reflect Cc, and indeed we found negative correlations between them (Supplementary Fig. S4A, B). Meanwhile, positive correlations were found between δ13C and starch content for all the lines at both aCO2 and eCO2 (Supplementary Fig. S4C, D). Brugnoli et al. (1988) found higher δ13C values in starch than in soluble sugars. They considered that the slower turnover rate of starch is a possible reason, since it would not be available for isotopic exchange for several days, thus resulting in a higher concentration of 13C. Hence, attention should be paid when treating the δ13C value as an indicator of Cc when non-structural carbohydrates are accumulated excessively.
The Arabidopsis lines that we grew under aCO2 or eCO2 showed cell wall thicknesses ranging from 0.195–0.336 μm (Table 1). To confirm our findings, it would be necessary to investigate the relationship between gm and cell wall thickness in plants over a wider range of wall thickness. It would be possible to obtain plants with thinner cell walls by growing them under low-light conditions (Conn et al., 2011; Lehmeier et al., 2017) and with thicker cell walls by growing them under high-light or more elevated CO2 conditions (Teng et al., 2006). It would also be interesting to determine the extent to which cell walls become thickened depending on the CO2 growth conditions. Another issue is to determine interspecific differences in how plastically cell wall thickness and gm are regulated in the field in response to micro-environmental changes such as temperature (von Caemmerer and Evans, 2015), light intensity, and CO2 concentration (Tazoe et al., 2009). This would reveal the ecological significance of the down-regulation of photosynthesis.
Conclusions
Our results suggest that elevated CO2 conditions could decrease mesophyll conductance, gm, and increase mesophyll resistance, Sc/gm, through increases in cell wall mass and thickness in Arabidopsis in the long-term. On the other hand, excess starch accumulation had minor effects on gm and Sc/gm. We also demonstrated that short-term changes in gm in response to elevated CO2 are independent of non-structural carbohydrates in Arabidopsis. Our study provides clues to the ecophysiological significance of the down-regulation of photosynthesis that are observed under elevated CO2 conditions.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Cross-sections of leaves of Arabidopsis grown under 400 ppm or 800 ppm CO2.
Fig. S2. Electron microscopy of leaves of Arabidopsis grown under 400 ppm or 800 ppm CO2.
Fig. S3. Relationship between Rubisco content per area and leaf nitrogen content per area in leaves of Arabidopsis grown under 400 ppm or 800 ppm CO2.
Fig. S4. Relationships between δ13C and CO2 concentration in the chloroplast stroma and starch content in leaves of Arabidopsis grown under 400 ppm or 800 ppm CO2.
Fig. S5. Relationships between cell wall thickness and cell wall mass per area (CMA) and that between CMA and structural leaf mass per area in leaves of Arabidopsis grown under 400 ppm or 800 ppm CO2.
Fig. S6. Relationship between mesophyll conductance measured at 400 ppm CO2 and the chloroplast surface area exposed to intercellular space in leaves of Arabidopsis grown under 400 ppm or 800 ppm CO2.
Acknowledgements
We greatly thank the members of the Laboratory of Plant Ecology in the University of Tokyo and the Laboratory of Crop Science in Nagoya University for their valuable comments and encouragement. This study was supported by Grant-in-Aid for JSPS Research Fellows (nos 25·10531 and 14J07443), and CREST (Creation of essential technologies to utilize carbon dioxide as a resource through the enhancement of plant productivity and the exploitation of plant products).
References
- Adachi S, Nakae T, Uchida M, et al.. 2013. The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. Journal of Experimental Botany 64, 1061–1072. [DOI] [PubMed] [Google Scholar]
- Araya T, Noguchi K, Terashima I. 2006. Effects of carbohydrate accumulation on photosynthesis differ between sink and source leaves of Phaseolus vulgaris L. Plant & Cell Physiology 47, 644–652. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Morgan PB, Ort DR, Long SP. 2005. The growth of soybean under free air [CO2] enrichment (FACE) stimulates photosynthesis while decreasing in vivo Rubisco capacity. Planta 220, 434–446. [DOI] [PubMed] [Google Scholar]
- Bertin N, Tchamitchian M, Baldet P, Devaux C, Brunel B, Gary C. 1999. Contribution of carbohydrate pools to the variations in leaf mass per area within a tomato plant. New Phytologist 143, 53– 61. [Google Scholar]
- Brugnoli E, Hubick KT, von Caemmerer S, Wong SC, Farquhar GD. 1988. Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ratio of intercellular and atmospheric partial pressures of carbon dioxide. Plant Physiology 88, 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave G, Tolley LC, Strain BR. 1981. Effect of carbon dioxide enrichment on chlorophyll content, starch content and starch grain structure in Trifolium subterraneum leaves. Physiologia Plantarum 51, 171–174. [Google Scholar]
- Cho YH, Yoo SD. 2011. Signaling role of fructose mediated by FINS1/FBP in Arabidopsis thaliana. PLoS Genetics 7, e1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, et al.. 2011. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. The Plant Cell 23, 240–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delucia EH, Sasek TW, Strain BR. 1985. Photosynthetic inhibition after long-term exposure to elevated levels of atmospheric carbon dioxide. Photosynthesis Research 7, 175–184. [DOI] [PubMed] [Google Scholar]
- Engelsdorf T, Will C, Hofmann J, et al.. 2017. Cell wall composition and penetration resistance against the fungal pathogen Colletotrichum higginsianum are affected by impaired starch turnover in Arabidopsis mutants. Journal of Experimental Botany 68, 701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier GJ, Livingston NJ. 2004. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant, Cell & Environment 27, 137–153. [Google Scholar]
- Evans JR, Caemmerer SV, Setchell BA, Hudson GS. 1994. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Functional Plant Biology 21, 475–495. [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. 2009. Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60, 2235–2248. [DOI] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. 1986. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Functional Plant Biology 13, 281–292. [Google Scholar]
- Farquhar GD, Cernusak LA. 2012. Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant, Cell & Environment 35, 1221–1231. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD. 1982. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33, 317–345. [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M. 2007a. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell & Environment 30, 1284–1298. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ortuño MF, Ribas-Carbo M, Diaz-Espejo A, Flórez-Sarasa ID, Medrano H. 2007b. Mesophyll conductance to CO2 in Arabidopsis thaliana. New Phytologist 175, 501–511. [DOI] [PubMed] [Google Scholar]
- Groszmann M, Osborn HL, Evans JR. 2017. Carbon dioxide and water transport through plant aquaporins. Plant, Cell & Environment 40, 938–961. [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology 98, 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley PC, Sharkey TD. 1991. An improved model of C3 photosynthesis at high CO2: reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynthesis Research 27, 169–178. [DOI] [PubMed] [Google Scholar]
- Ishikawa-Sakurai J, Hayashi H, Murai-Hatano M. 2014. Nitrogen availability affects hydraulic conductivity of rice roots, possibly through changes in aquaporin gene expression. Plant and Soil 379, 289–300. [Google Scholar]
- José Javier P-P, Sergio S, Jaume F, Jeroni G. 2017. Cell-level anatomical characteristics explain high mesophyll conductance and photosynthetic capacity in sclerophyllous Mediterranean oaks. New Phytologist 214, 585–596. [DOI] [PubMed] [Google Scholar]
- Kitao M, Yazaki K, Kitaoka S, Fukatsu E, Tobita H, Komatsu M, Maruyama Y, Koike T. 2015. Mesophyll conductance in leaves of Japanese white birch (Betula platyphylla var. japonica) seedlings grown under elevated CO2 concentration and low N availability. Physiologia Plantarum 155, 435–445. [DOI] [PubMed] [Google Scholar]
- Krapp A, Stitt M. 1995. An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195, 313–323. [Google Scholar]
- Laisk AK. 1977. Kinetics of photosynthesis and photorespiration of C3 in plants. Moscow: Nauka. [Google Scholar]
- Lanigan GJ, Betson N, Griffiths H, Seibt U. 2008. Carbon isotope fractionation during photorespiration and carboxylation in Senecio. Plant Physiology 148, 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmeier C, Pajor R, Lungren MR, et al.. 2017. Cell density and airspace patterning in the leaf can be manipulated to increase leaf photosynthetic capacity. The Plant Journal 92, 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig F, Sonnewald U. 2000. High CO2-mediated down-regulation of photosynthetic gene transcripts is caused by accelerated leaf senescence rather than sugar accumulation. FEBS Letters 479, 19–24. [DOI] [PubMed] [Google Scholar]
- Mizokami Y, Noguchi K, Kojima M, Sakakibara H, Terashima I. 2015. Mesophyll conductance decreases in the wild type but not in an ABA-deficient mutant (aba1) of Nicotiana plumbaginifolia under drought conditions. Plant, Cell & Environment 38, 388–398. [DOI] [PubMed] [Google Scholar]
- Mizokami Y, Noguchi K, Kojima M, Sakakibara H, Terashima I. 2019. Effects of instantaneous and growth CO2 levels, and ABA on stomatal and mesophyll conductances. Plant, Cell &, Environment 42, 1257–1269. [DOI] [PubMed] [Google Scholar]
- Mori IC, Rhee J, Shibasaka M, Sasano S, Kaneko T, Horie T, Katsuhara M. 2014. CO2 transport by PIP2 aquaporins of barley. Plant & Cell Physiology 55, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafziger ED, Koller HR. 1976. Influence of leaf starch concentration on CO2 assimilation in soybean. Plant Physiology 57, 560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Muramatsu S, Makino A, Mae T. 2000. Relationship between the suppression of photosynthesis and starch accumulation in the pod-removed bean. Functional Plant Biology 27, 167–173. [Google Scholar]
- Periappuram C, Steinhauer L, Barton DL, Taylor DC, Chatson B, Zou J. 2000. The plastidic phosphoglucomutase from Arabidopsis. A reversible enzyme reaction with an important role in metabolic control. Plant Physiology 122, 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Pepin S, Rijkers T, de Jong Y, Evans JR, Körner C. 2006. Construction costs, chemical composition and payback time of high- and low-irradiance leaves. Journal of Experimental Botany 57, 355–371. [DOI] [PubMed] [Google Scholar]
- Pritchard SG, Peterson CM, Prior SA, Rogers HH. 1997. Elevated atmospheric CO2 differentially affects needle chloroplast ultrastructure and phloem anatomy in Pinus palustris: interactions with soil resource availability. Plant, Cell & Environment 20, 461–471. [Google Scholar]
- Sawada S, Kuninaka M, Watanabe K, Sato A, Kawamura H, Komine K, Sakamoto T, Kasai M. 2001. The mechanism to suppress photosynthesis through end-product inhibition in single-rooted soybean leaves during acclimation to CO2 enrichment. Plant & Cell Physiology 42, 1093–1102. [DOI] [PubMed] [Google Scholar]
- Scafaro AP, Von Caemmerer S, Evans JR, Atwell BJ. 2011. Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant, Cell & Environment 34, 1999–2008. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. 1994. Feedback control of gene expression. Photosynthesis Research 39, 427–438. [DOI] [PubMed] [Google Scholar]
- Sharkey TD. 1985. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. The Botanical Review 51, 53–105. [Google Scholar]
- Sugiura D, Betsuyaku E, Terashima I. 2015. Manipulation of the hypocotyl sink activity by reciprocal grafting of two Raphanus sativus varieties: its effects on morphological and physiological traits of source leaves and whole-plant growth. Plant Cell & Environment 38, 2629–2640. [DOI] [PubMed] [Google Scholar]
- Sugiura D, Betsuyaku E, Terashima I. 2018. Interspecific differences in how sink–source imbalance causes photosynthetic downregulation among three legume species. Annals of Botany 123, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura D, Watanabe CKA, Betsuyaku E, Terashima I. 2017. Sink–source balance and down-regulation of photosynthesis in Raphanus sativus: effects of grafting, N and CO2. Plant & Cell Physiology 58, 2043–2056. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Badger MR, Evans JR. 2009. Light and CO2 do not affect the mesophyll conductance to CO2 diffusion in wheat leaves. Journal of Experimental Botany 60, 2291–2301. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Estavillo GM, Evans JR. 2011. Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant, Cell & Environment 34, 580–591. [DOI] [PubMed] [Google Scholar]
- Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J. 2006. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytologist 172, 92–103. [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S. 2006. Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. Journal of Experimental Botany 57, 343–354. [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets Ü. 2011. Leaf functional anatomy in relation to photosynthesis. Plant Physiology 155, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thain JF. 1983. Curvature correction factors in the measurement of cell surface areas in plant tissues. Journal of Experimental Botany 34, 87–94. [Google Scholar]
- Tholen D, Boom C, Noguchi K, Ueda S, Katase T, Terashima I. 2008. The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant, Cell & Environment 31, 1688–1700. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR. 2015. Temperature responses of mesophyll conductance differ greatly between species. Plant, Cell & Environment 38, 629–637. [DOI] [PubMed] [Google Scholar]
- Walker BJ, Cousins AB. 2013. Influence of temperature on measurements of the CO2 compensation point: differences between the Laisk and O2-exchange methods. Journal of Experimental Botany 64, 1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate L, Seibt U, Moncrieff JB, Jarvis PG, Lloyd J. 2007. Variations in 13C discrimination during CO2 exchange by Picea sitchensis branches in the field. Plant, Cell & Environment 30, 600–616. [DOI] [PubMed] [Google Scholar]
- Woo KC, Wong SC. 1983. Inhibition of CO2 assimilation by supraoptimal CO2: effect of light and temperature. Functional Plant Biology 10, 75–85. [Google Scholar]
- Zeeman SC, Smith SM, Smith AM. 2004. The breakdown of starch in leaves. New Phytologist 163, 247–261. [DOI] [PubMed] [Google Scholar]
- Zhu C, Ziska L, Zhu J, Zeng Q, Xie Z, Tang H, Jia X, Hasegawa T. 2012. The temporal and species dynamics of photosynthetic acclimation in flag leaves of rice (Oryza sativa) and wheat (Triticum aestivum) under elevated carbon dioxide. Physiologia Plantarum 145, 395–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.