Abstract
Polychlorinated biphenyls (PCBs) have been associated with neurodevelopmental disorders. Several neurotoxic congeners display axial chirality and atropselectively affect cellular targets implicated in PCB neurotoxicity. Only limited information is available regarding the atropselective metabolism of these congeners in humans and their atropselective effects on neurotoxic outcomes. Here we investigate the hypothesis that the oxidation of 2,2′,3,3′,4,6′-hexachlorobiphenyl (PCB 132) by human liver microsomes (HLMs) and their effects on dopaminergic cells in culture are atropselective. Racemic PCB 132 was incubated with pooled or single donor HLMs, and levels and enantiomeric fractions of PCB 132 and its metabolites were determined gas chromatographically. The major metabolite was either 2,2′,3,4,4′,6′-hexachlorobiphenyl-3′-ol (3′-140), a 1,2-shift product, or 2,2′,3,3′,4,6′-hexachlorobiphenyl-5′-ol (5′-132). The PCB 132 metabolite profiles displayed interindividual differences and depended on the PCB 132 atropisomer. Computational studies suggested that 3′-140 is formed via a 3,4-arene oxide intermediate. The second eluting atropisomer of PCB 132, first eluting atropisomer of 3′-140, and second eluting atropisomer of 5′-132 were enriched in all HLM incubations. Enantiomeric fractions of the PCB 132 metabolites differed only slightly between the single donor HLM preparations investigated. Reactive oxygen species and levels of dopamine and its metabolites were not significantly altered after a 24 h exposure of dopaminergic cells to pure PCB 132 atropisomers. These findings suggest that there are interindividual differences in the atropselective biotransformation of PCB 132 to its metabolites in humans; however, the resulting atropisomeric enrichment of PCB 132 is unlikely to affect neurotoxic outcomes associated with the endpoints investigated in the study.
Keywords: 1,2-shift; arene oxide; biotransformation; cytochrome P450 enzymes; dopamine metabolism; enantiomers; reactive oxygen species
Polychlorinated biphenyl (PCB) congeners with a 2,3,6-chlorine substitution pattern on 1 phenyl ring, such as 2,2′,3,3′,4,6′-hexachlorobiphenyl (PCB 132), are important components of commercial PCB mixtures (Kania-Korwel and Lehmler, 2016a). Food is the major source of exposure to these and other PCB congeners (Schecter et al., 2010; Su et al., 2012; Voorspoels et al., 2008). For example, PCB 132 has been detected in fish species caught for human consumption (Wong et al., 2001). PCB 132 is also present in the indoor air of U.S. schools (Thomas et al., 2012), raising concerns about inhalation exposure of school children, teachers, and staff to PCBs (Herrick et al., 2016). PCB 132 and structurally related PCB congeners are present in human blood (DeCaprio et al., 2005; Jursa et al., 2006; Whitcomb et al., 2005), breast milk (Bordajandi et al., 2008; Bucheli and Brandli, 2006; Glausch et al., 1995), and postmortem human tissue samples (Chu et al., 2003). Like several other PCB congeners with a 2,3,6-chlorine substitution pattern, PCB 132 is axially chiral because it exists as 2 stable rotational isomers, or atropisomers, which are nonsuperimposable mirror images of each other (Lehmler et al., 2010).
Exposure to PCBs has been implicated in the etiology of neurodevelopmental and neurodegenerative disorders (Hatcher-Martin et al., 2012; Jones and Miller, 2008; Pessah et al., 2010). In particular, PCB congeners with 2 or more ortho chlorine substituents are neurotoxic and, for example, have been associated with behavioral and cognitive deficits in animal models (Caudle et al., 2006; Schantz et al., 1995; Wayman et al., 2012). Similarly, animal studies with hydroxylated PCB metabolites reported impairments in behavioral and locomotor activity in rats and mice (Haijima et al., 2017; Lesmana et al., 2014). Mechanistic studies suggest that these PCBs affect the dopaminergic system. ortho-Chlorinated PCBs inhibited dopamine uptake in rat synaptosomes (Mariussen and Fonnum, 2001) and decreased the dopamine content in cells in culture, possibly due to inhibition of dopamine synthesis (Seegal, 1996). 2,2′,3,4′,6-Pentachlorobiphenyl (PCB 91) and 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) decreased dopamine content in rat synaptosomes by inhibiting vesicular monoamine transporter (VMAT) (Bemis and Seegal, 2004). In contrast, PCB 95 increased intracellular dopamine and decreased dopamine in the medium by down-regulating VMAT2 expression in PC12 cells (Enayah et al., 2018). Striatal dopamine levels in male mice exposed to PCB mixtures decreased due to a decrease in the expression of the dopamine transporter and VMAT2 (Richardson and Miller, 2004). Other studies suggest that, in addition to their effects on the dopaminergic system, PCB neurotoxicity can be mediated by altered intracellular Ca2+ signaling and/or disruption of thyroid and sex hormone homeostasis (reviewed by Kodavanti and Curras-Collazo, 2010; Mariussen and Fonnum, 2006; Pessah et al., 2010).
Neurotoxic PCBs are metabolized to potentially neurotoxic hydroxylated metabolites (OH-PCBs, hydroxylated polychlorinated biphenyls) in animal models and humans (Grimm et al., 2015; Kania-Korwel and Lehmler, 2016a). In general, PCB congeners with H-atoms in vicinal meta and para positions are readily metabolized by cytochrome P450 (P450) enzymes, whereas congeners without adjacent para and meta positions are metabolized more slowly (Grimm et al., 2015). The oxidation of PCBs by P450 enzymes occurs by direct insertion of an oxygen atom into an aromatic C–H bond or via an arene oxide intermediate (Forgue and Allen, 1982; Forgue et al., 1979; Preston et al., 1983). Several P450 isoforms, including CYP2A and CYP2B enzymes, are involved in the metabolism of ortho-chlorinated PCB congeners, such as PCB 132, in different species (Lu et al., 2013; McGraw and Waller, 2006; Nagayoshi et al., 2018; Ohta et al., 2012; Uwimana et al., 2019; Waller et al., 1999; Warner et al., 2009). Hydroxylated and methyl sulfone metabolites of PCB 132 have been detected in human blood, breast milk, and feces (Haraguchi et al., 2004, 2005). Similar to animal studies (Norstroem and Bergman, 2006), PCB 132 undergoes atropisomeric enrichment in humans (Bordajandi et al., 2008; Bucheli and Brandli, 2006; Chu et al., 2003; Glausch et al., 1995; Zheng et al., 2016) due to atropselective metabolism of PCB 132.
Biotransformation studies reveal species differences in the atropselective metabolism of PCBs to chiral hydroxylated metabolites (Kania-Korwel and Lehmler, 2016a); however, only a few PCB metabolism studies in humans have been reported (Schnellmann et al., 1983; Uwimana et al., 2016; 2018; Wu et al., 2014). Because PCB 132 is present in the environment, displays atropisomeric enrichment in human samples, and represents a largely unexplored environmental and human health concern, the objective of this study was to investigate the atropselective metabolism of PCB 132 to OH-PCB metabolites by human liver microsomes (HLMs) and assess if PCB 132 atropselectively affects neurotoxic outcomes in dopaminergic cells in culture.
MATERIALS AND METHODS
Chemicals and materials
Sources and purities of racemic PCB 132 (Supplementary Table 1), PCB metabolite standards, chemicals, and other reagents; information regarding the HLM preparations; and a description of the separation and characterization of PCB 132 atropisomers are presented in the Supplementary Material. Gas chromatograms and the corresponding mass spectrum of PCB 132 are shown in Supplementary Figures 1 and 2. The chemical structures and abbreviations of OH-PCB 132 metabolites are shown in Figure 1. Rat-tail collagen was obtained from Sigma Aldrich (St Louis, Missouri). All other cell culture components such as fetal bovine serum (FBS), horse serum (HS), RPMI 1640 medium, and penicillin-streptomycin were obtained from Life Technologies (Carlsbad, California). 3,4-Dihydroxyphenylacetic acid (DOPAC) was obtained from Sigma Aldrich. Dihydroxyphenyl ethanol (DOPET) was obtained by reducing 3,4-dihydroxyphenylaldehyde (DOPAL) in a 10-fold excess of sodium borohydride (Jinsmaa et al., 2009). DOPAL was obtained synthetically from epinephrine by the method of Fellman (Anderson et al., 2011; Fellman, 1958).
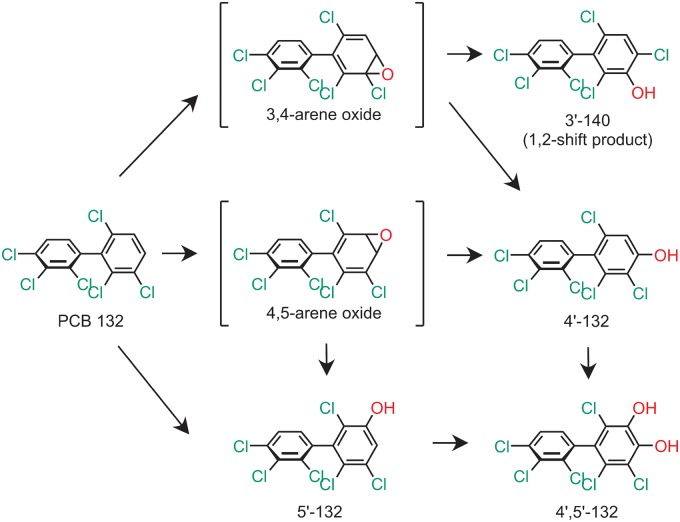
Figure 1.
Proposed metabolism scheme showing the chemical structures of metabolites of PCB 132 identified in incubations with human liver microsomes (HLMs). Only 1 atropisomer of each metabolite is shown for clarity reasons. Abbreviations: PCB 132—2,2′,3,3′,4,6′-hexachlorobiphenyl; 3′-140—2,2′,3,4,4′,6′-hexachlorobiphenyl-3-ol; 4′-132—2,2′,3,3′,4,6′-hexachlorobiphenyl-4′-ol; 5′-132—2,2′,3,3′,4,6′-hexachlorobiphenyl-5′-ol; P450—cytochrome P450 enzymes.
Microsomal incubations
The metabolism of PCB 132 was investigated in an incubation system containing sodium phosphate buffer (0.1 M, pH 7.4), magnesium chloride (3 mM), pooled human liver microsomes (pHLMs), or single donor HLMs (0.1 mg/ml), and NADPH (1 mM) (Kania-Korwel et al., 2011; Uwimana et al., 2017; Wu et al., 2011). The incubation mixtures were preincubated for 5 min, and PCB 132 (50 µM in DMSO; ≤ 0.5% vol/vol) was added to give a final volume of 2 ml. The mixtures were maintained for 10, 30, or 120 min at 37°C. Incubations with (−)-PCB 132, (+)-PCB132, or racemic PCB 132 (50 µM in DMSO; ≤ 0.5% vol/vol) were carried out analogously for 30 min at 37°C. The formation of PCB 132 metabolites was linear with time for up to 30 min. To terminate the enzymatic reaction, ice-cold sodium hydroxide (2 ml, 0.5 M) was added to each sample. Subsequently, the incubation mixtures were heated at 110°C for 10 min. Phosphate buffer blanks and control incubations without PCB accompanied each microsomal preparation. The following incubations were performed in parallel with each experiment to control for the abiotic transformation of PCB 132: Incubations without NADPH, without microsomes, and with heat-inactivated microsomes. No metabolites were detected in the control samples. All incubations were performed in triplicate, if not stated otherwise.
Extraction of PCB 132 and its hydroxylated metabolites
PCB 132 and its hydroxylated metabolites were extracted from the incubation mixtures as reported previously (Kania-Korwel et al., 2011; Uwimana et al., 2016; Wu et al., 2011). Briefly, PCB 117 (200 ng) and 4′-159 (68.5 ng) were added to each sample as surrogate recovery standards, followed by hydrochloric acid (6 M, 1 ml) and 2-propanol (5 ml). The samples were extracted with a hexane-MTBE mixture (1:1 vol/vol, 5 ml) and re-extracted once with hexane (3 ml). The organic layers were washed with aqueous potassium chloride (1%, 4 ml), the organic phase was transferred to a new vial, and the KCl mixture was re-extracted with hexane (3 ml). The organic layers were evaporated to dryness under a gentle stream of nitrogen and reconstituted with hexane (1 ml). After derivatization with diazomethane (0.5 ml in diethyl ether) for approximately 16 h at 4°C (Kania-Korwel et al., 2008), all extracts were subjected to sulfur and sulfuric acid clean-up steps before gas chromatographic analysis (Kania-Korwel et al., 2005, 2007).
Identification of PCB 132 metabolites
The identity of the hydroxylated PCB 132 metabolites was confirmed by high-resolution gas chromatography with time-of-flight mass spectrometry (GC-TOF/MS) (Uwimana et al., 2016; 2018). To obtain metabolite levels sufficient for GC-TOF/MS analysis, incubations were performed using the following experimental conditions: 50 µM racemic PCB 132, 0.3 mg/ml microsomal protein, and 1 mM NADPH for 90 min at 37°C. Metabolites were extracted and derivatized as described above before analysis on a Waters GCT Premier gas chromatograph (Waters Corporation, Milford, Massachusetts) combined with a time-of-flight mass spectrometer in the High-Resolution Mass Spectrometry Facility of the University of Iowa (Iowa City, Iowa) (Uwimana et al., 2016; 2018). Measurements were performed with and without heptacosafluorotributylamine as lock mass to determine the accurate mass of [M]+ and obtain mass spectra of the metabolites. PCB 132 metabolites were identified using the following criteria: The average relative retention times (RRT) of the OH-PCB 132 metabolites, calculated relative to PCB 51 as internal standard, were within 0.5% of the RRT of the authentic standard (European Commission, 2002); experimental accurate mass determinations were within 0.003 Da of the theoretical mass of [M]+; and the deviation between experimental and theoretical isotope pattern of [M]+ was < 20%.
Quantification of PCB 132 metabolite levels
Levels of OH-PCB 132 metabolites in extracts were quantified as methylated derivatives on an Agilent 7890A gas chromatograph with a 63Ni-micro electron capture detector (µECD) and a SPB-1 capillary column (60 m length, 250 µm inner diameter, 0.25 µm film thickness; Supelco, St Louis, Missouri) as reported earlier (Uwimana et al., 2016, 2017; Wu et al., 2013b). PCB 204 was added as internal standard (volume corrector) prior to analysis, and concentrations of OH-PCB 132 metabolites (as methylated derivatives) were determined using the internal standard method (Kania-Korwel et al., 2011; Wu and Lehmler, 2016; Wu et al., 2011). Levels of PCB and its metabolites were not adjusted for recovery to facilitate a comparison with earlier studies (Uwimana et al., 2016, 2018, 2019; Wu and Lehmler, 2016). The average RRTs of the metabolites, calculated relative to PCB 204, were within 0.5% of the RRT for the respective standard (European Commission, 2002). Metabolite levels and formation rates for all experiments described in the manuscript are summarized in the Supplementary Tables 2–4.
Ring opening calculations
The ring opening reactions of selected PCB arene oxide intermediates were modeled using density functional theory at the M11/def2-SVP level of theory (Peverati and Truhlar, 2011; Weigend and Ahlrichs, 2005), coupled with the SMD aqueous continuum solvation model (Marenich et al., 2009), to assess if the formation of specific OH-PCB metabolites is energetically favored. The reactions were examined under neutral conditions, with 2 explicit water molecules complexed to the system to serve as a proton shuttle. To ascertain steric and electronic effects, calculations were carried out on arene oxides formed from 1,2,4-trichlorobenzene (TCB), 2,3,6-trichlorobiphenyl, PCB 91, PCB 95, PCB 132, and PCB 136. Transition states were characterized by normal mode analysis and confirmed via intrinsic reaction coordinate (IRC) calculations. All calculations were performed using Gaussian 16, Rev. A.01 (Frisch et al., 2016). Additional details including computational data are available in the Supplementary Material.
Atropselective PCB analyses
Atropselective analyses were carried out with extracts from long-term incubations (ie, 5 or 50 µM PCB 132, 120 min at 37°C, 0.5 mg/ml protein, and 0.5 mM NADPH) and extracts from the 30 min incubations described above. Hydroxylated metabolites were analyzed as methylated derivatives after derivatization with diazomethane. Analyses were performed on an Agilent 6890 gas chromatograph equipped with a µECD detector and a CP-Chirasil-Dex CB (CD) (25 m length, 250 µm inner diameter, 0.12 µm film thickness; Agilent, Santa Clara, California) or a ChiralDex G-TA (GTA) capillary column (30 m length, 250 µm inner diameter, 0.12 µm film thickness; Supelco) (Kania-Korwel et al., 2011; Uwimana et al., 2017). The following temperature program was used for the atropselective analysis of PCB 132 and 5′-132 on a CD column: initial temperature was 50°C, hold for 1 min, ramped at 10°C/min to 160°C, hold for 220 min, ramped at 20°C/min to 200°C, and hold for 10 min. To analyze 3′-140 on a GTA column, the temperature program was as follows: initial temperature was 50°C, hold for 1 min, ramped at 10°C/min to 150°C, hold for 400 min, ramped at 10°C/min to 180°C, and hold for 40 min. The helium flow was 3 ml/min for all atropselective analyses. To facilitate a comparison with earlier studies (Kania-Korwel and Lehmler, 2016b; Uwimana et al., 2016, 2017; Wu et al., 2011), enantiomeric fractions (EFs) were calculated by the drop valley method (Asher et al., 2009) as EF = Area E1/(Area E1 + Area E2), with Area E1 and Area E2 denoting the peak area of the first (E1)and second (E2) eluting atropisomer on the respective column. All EF values are summarized in the Supplementary Table 6.
Quality assurance and quality control
The response of the µECDs used in this study was linear for all analytes up to concentrations of 1000 ng/ml (R2 ≥ 0.999). The recoveries of PCB 117 were 93 ± 13% (n = 120). Recoveries of 4′-159 could not be determined due to coelution with 4′,5′-132. The limits of detection (LOD) of the PCB 132 metabolites were calculated from blank buffer samples as LOD = mean of blank samples + k × standard deviation of blank samples, where k is the student’s t test value for a degree of freedom n − 1 = 5 at 99% confidence level (Kania-Korwel et al., 2011; Uwimana et al., 2016; Wu and Lehmler, 2016; Wu et al., 2011). The LODs were 0.03, 0.1, and 0.21 ng for 3′-140, 5′-132, and 4′-132, respectively. The background levels for 3′-140, 5′-132, and 4′-132 in control (DMSO) incubations with HLMs (n = 6) were 0.17, 0.13, and 0.19 ng, respectively. The resolution of the atropisomers of PCB 132 and 5′-132 on the CD column was 1.05 and 2.02, respectively. The resolution of the atropisomers of 3′-140 on the GTA column was 1.18. The EF values of the racemic standards of PCB 132, 3′-140, and 5′-132 were 0.51 ± 0.01 (n = 2), 0.50 ± 0.01 (n = 3), and 0.49 ± 0.01 (n = 3), respectively.
Cell culture
PC12 cells were purchased from American Tissue Culture Collection (Manassas, Virginia) and maintained in RPMI 1640 medium with 10% HS, 5% FBS, and 1% penicillin-streptomycin. Cells were kept in a humidified incubator at 37°C with 5% CO2 in a 100 mm2 tissue culture petri dish at a density of 5 × 106 cells. For experiments, cells were plated in 6-well plates in 2 ml medium at a density of 1.5 × 106 cells per well, or in a Collagen I coated 96-well plate at 50.5 × 103 cells per well. N27 cells, a generous gift from Dr Jau-Shyong at the National Institute of Environmental Health Sciences (Research Triangle Park, NC, USA), were maintained in RPMI 1640 with 10% HS, and 1% penicillin-streptomycin. Cells were maintained in a humidified environment at 37°C with 5% CO2 in a 100 mm2 collagen-coated tissue culture petri dish at a density of 1 × 106 cells. For experiments, cells were plated in collagen-coated 96-well plates at 5 × 103 cells per well.
Cell viability and production of reactive oxygen species
Cell viability was determined in N27 and PC12 cells using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (van Meerloo et al., 2011). The formation of reactive oxygen species (ROS) was measured in N27 cells using the DCFDA and dihydroethidium (DHE) fluorescent probes (Chen et al., 2010; Speisky et al., 2009; Zhao et al., 2003). Details regarding these assays are provided in the Supplementary Material.
Dopamine metabolite analysis using HPLC
Intracellular and extracellular dopamine metabolites were measured in PC12 lysate samples via an Agilent 1100 Series capillary HPLC system with an ESA Coulochem III coulometric electrochemical detector (Enayah et al., 2018). After seeding the cells in 6-well plates, they were kept under the same growth conditions for 48 h. Media was then removed and replaced with HEPES buffer (115 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5.5 mM glucose, 1 mM NaH2PO4, and 15 mM HEPES, pH = 7.4). The cells were then exposed to the PCB 132 atropisomers at 10 and 25 μM and incubated for 24 h at which point cell lysate was collected. After removing the extracellular buffer, 400 μl lysis buffer (10 mM potassium phosphate, 0.1% triton-X, pH = 7.4) was added to each well. Then a cell scraper was used to collect the cell lysate which was stored at −20°C until analysis. On the day of HPLC analysis, the samples were centrifuged at 10 000 g for 10 min, and 3 μl of supernatant injected into the HPLC to measure intracellular dopamine and its metabolites. Separation was achieved with a Phenomenex Synergi C18 column (2 × 150 mm, 80 Å) using an isocratic mobile phase (50 mM citric acid, 1.8 mM sodium heptane sulfonate, 0.2% trifluoroacetic acid, 2% acetonitrile, pH = 3.0) at 200 μl/min. For electrochemical detection of catechol-containing compounds, the following settings were used: guard = + 350, E1 = −150, and E2 = + 200 mV, respectively. The amount of dopamine, DOPAC, and DOPET was determined by comparison to a standard curve and presented as picomole of analyte per milligram of protein. Details regarding the measurement of dopamine and its metabolites in extracellular media are provided in the Supplementary Material. A bicinchoninic acid assay was used on the PC12 lysate samples as previously described to attain the protein concentration (Walker, 1994).
RESULTS
Identification and Quantification of PCB 132 Metabolites in Incubations With pHLMs
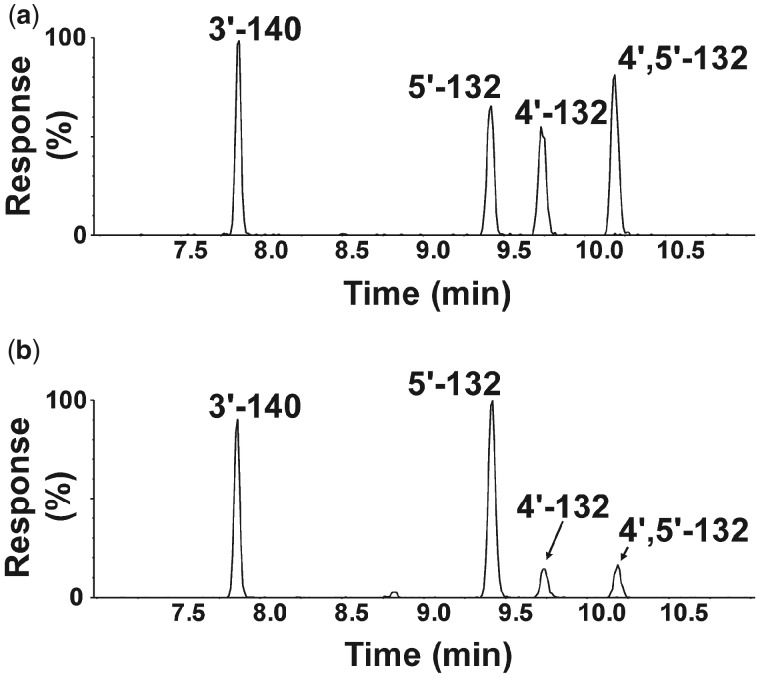
Several human biomonitoring studies have reported an atropisomeric enrichment of PCB 132 in human samples (Supplementary Table 8) (Lehmler et al., 2010); however, the atropselective oxidation of this PCB congener to hydroxylated metabolites by HLMs has not been studied to date (Grimm et al., 2015; Kania-Korwel and Lehmler, 2016a). Our GC-TOF/MS analyses revealed the formation of 3 monohydroxylated and 1 dihydroxylated PCB metabolite in incubations of racemic PCB 132 with pHLMs (Figure 2; Supplementary Figs. 4–11). The structure of these metabolites is shown in the simplified metabolism scheme in Figure 1. Their identification was based on accurate mass determinations, the chlorine isotope patterns of their molecular ion (analyzed as methylated derivatives) and their fragmentation patterns; for additional discussion, see the Supplementary Material. PCB 132 was oxidized by pooled and individual donor HLMs in the meta position, with 5′-132 and 3′-140 being major metabolites (Figs 3 and 4; Supplementary Table 2). 3′-140 is a 1,2-shift product (Guroff et al., 1967) that is formed via an arene oxide intermediate, followed by a 1,2-shift of the 3′-chlorine substituent to the para position. 4′-132 was only a minor metabolite. 4′,5′-132 could not be quantified in this study due to coelution with the recovery standard, 4′-159; however, this metabolite was only a minor metabolite. PCB 132 was not oxidized in the 2,3,4-chloro substituted ring.
Figure 2.
Three monohydroxylated (m/z 387.9) and 1 dihydroxylated (m/z 417.9) metabolite were identified in incubations of racemic 2,2′,3,3′,4,6′-hexachlorobiphenyl (PCB 132) with pooled human liver microsomes (pHLMs). Representative gas chromatograms showing (a) the reference standard containing 4 hydroxylated PCB 132 metabolites and (b) an extract from a representative incubation of racemic PCB 132 with pHLMs. All metabolites were analyzed as the corresponding methylated derivatives. Incubations were carried out with 50 µM racemic PCB 132, 0.3 mg/ml microsomal protein, and 1 mM NADPH for 90 min at 37°C (Uwimana et al., 2016, 2018). Analyses were performed by gas chromatography with time-of-flight mass spectrometry (GC-TOF/MS) as described under Materials and Methods. The metabolites were identified based on their retention times relative to the authentic standard and their m/z. For the corresponding mass spectra, see Supplementary Figures 4–11.
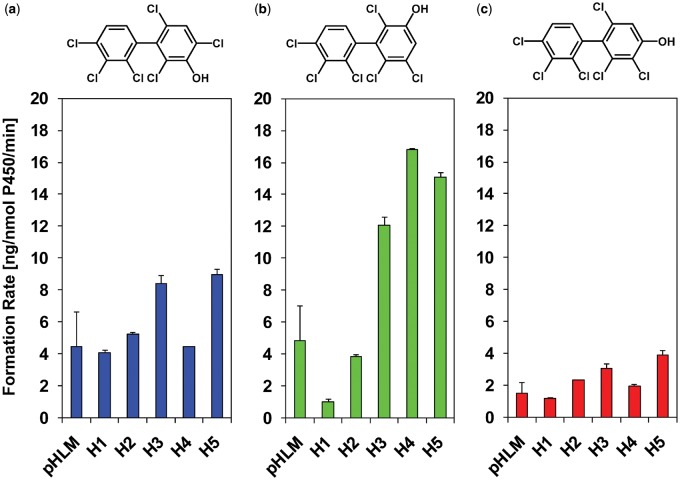
Figure 3.
Formation rates of 2,2′,3,3′,4,6′-hexachlorobiphenyl (PCB 132) metabolites by different human liver microsome (HLM) preparations display interindividual differences, with (a) the meta hydroxylated metabolites, 2,2′,3,4,4′,6′-hexachlorobiphenyl-3-ol (3′-140; 1,2-shift product), and (b) 2,2′,3,3′,4,6′-hexachlorobiphenyl-5′-ol (5′-132), being major metabolites, and (c) the para hydroxylated metabolite, 4′-132, being a minor metabolite. Incubations were carried out with 50 µM racemic PCB 132, 0.1 mg/ml microsomal protein, and 1 mM NADPH for 10 min at 37°C (Supplementary Tables 3 and 4) as reported earlier (Uwimana et al., 2016, 2018). Extracts from the microsomal incubations were derivatized with diazomethane and analyzed by GC-µECD; see Materials and Methods section for additional details. Data are presented as mean ± standard deviation, n = 3.
Metabolism of PCB 132 in Comparison to Structurally Related PCBs
As shown in Figure 4a, the PCB 132 metabolite profiles formed by HLMs differs considerably from the profiles of structurally related PCB congeners with a 2,3,6-trichlorophenyl group (ie, PCB 91, PCB 95, and 2,2′,3,3′,6,6′-hexachlorobiphenyl [PCB 136]). HLMs oxidized PCB 132 in the meta position to yield the 1,2-shift product, 3′-140, and 5′-132. PCB 91, which is structurally similar to PCB 132, was also primarily metabolized to a 1,2-shift product by HLMs (Uwimana et al., 2018). PCB 95 was preferentially oxidized by HLMs in the para position with the lower chlorinated, 2,5-dichloro substituted phenyl ring, and only traces of a 1,2-shift product were formed by different HLM preparations (Uwimana et al., 2016). PCB 136 was metabolized in both the meta and para position to yield comparable levels of 5′-136 and 4′-136 (Schnellmann et al., 1983; Wu et al., 2014).
Figure 4.
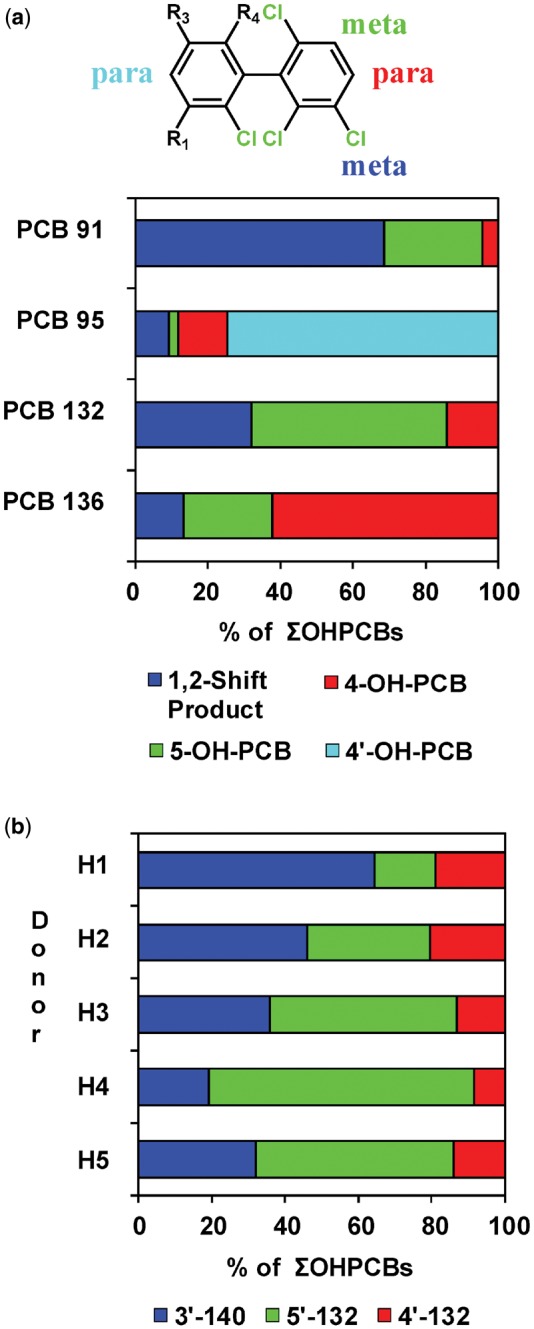
The OH-PCB metabolite profile of 2,2′,3,3′,4,6′-hexachlorobiphenyl (PCB 132) formed by pooled human liver microsomes (pHLMs) is (a) distinctively different from the published metabolite profiles of structurally related PCB congeners (ie, PCB 91, PCB 95, and PCB 136) and (b) shows considerable interindividual variability. a, Bar diagram comparing the profiles of hydroxylated metabolites of PCB 91, PCB 95, PCB 132, and PCB 136 formed in incubations with pHLMs. PCB 91 (Uwimana et al., 2018) and PCB 132 (this study) are preferentially oxidized in meta position, including the formation of 1,2-shift products, whereas PCB 95 (Uwimana et al., 2016) and PCB 136 (Wu et al., 2014) are hydroxylated in para position. In the case of PCB 95, the oxidation occurs preferentially in the para position of the lower chlorinated 2,5-dichlorophenyl ring (Uwimana et al., 2016). Incubations were carried out with 50 µM PCB, 0.1 mg/ml microsomal protein, and 1 mM NADPH for 5 min (PCB 91 and PCB 95) or 10 min (PCB 132 and PCB 136) at 37°C using the same pHLM preparation. b, Bar diagram showing interindividual differences in profiles of hydroxylated metabolites of PCB 132 formed in incubations with different single donor human liver microsome (HLM) preparations. Incubations were carried out with 50 µM PCB 132, 0.1 mg/ml microsomal protein and 1 mM NADPH for 10 min at 37°C (Uwimana et al., 2016, 2018). Extracts from the microsomal incubations were derivatized with diazomethane and analyzed by GC-µECD; see Materials and Methods section for additional details.
To assess if the congener-specific differences in OH-PCB metabolite profiles are due to different arene oxide intermediates or different substitution patterns in the second phenyl ring of chiral PCB congeners, the energetics of the 3,4-arene oxide ring opening reactions were determined for 1,2,4-trichlorobenzene, 2,3,6-trichlorobiphenyl, PCB 91, PCB 95, PCB 132, and PCB 136 (Figure 5; Supplementary Figure 12 and Table 5). Also, complete ring opening pathways were computed for the 3,4-, 4,5-, and 5,6-arene oxides of a single atropisomer of PCB 132 and PCB 136, another environmentally relevant PCB congener (Supplementary Table 5). Because the 3,4-, 4,5-, and 5,6-arene oxides may open in 2 directions, 6 pathways were computed for both PCB 132 and PCB 136.
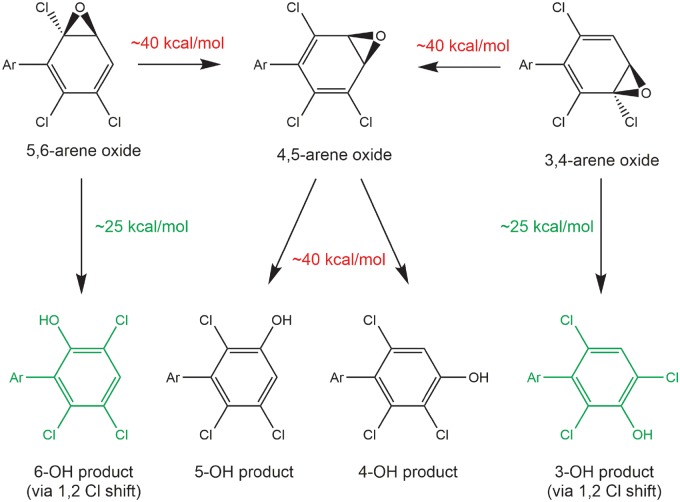
Figure 5.
The formation of 1,2-chlorine shift metabolites via arene oxide intermediates of chiral polychlorinated biphenyls (PCBs) is energetically favored. Energies shown are free energies of activation at the M11/def2-SVP level of theory with the SMD aqueous continuum solvation model. Abbreviations: Ar—2′,4′-dichlorophenyl for PCB 91; PCB 95—2′,5′-dichlorophenyl; PCB 132—2,3,4-trichlorophenyl; PCB 136—2′,3′,6′-dichlorophenyl.
In the case of the 3,4- and 5,6-arene oxides, the arene oxide opening toward the C–Cl group and the 1,2-shift of the chlorine were concerted, with barriers to the opening of approximately 25 kcal/mol (Supplementary Figure 12). The proton transfer step to form the phenol and restore aromaticity proceeded with a negligible barrier and was essentially spontaneous. Partial pathways opening toward C–Cl were further explored for the 3,4-arene oxides of 1,2,4-trichlorobenzene, 2,3,6-trichlorobiphenyl, PCB 91 and PCB 95. The presence or absence of the substituent aryl ring had no effect on the computed mechanism or energetics for an opening toward C–Cl, nor did the substitution pattern on the aryl substituent.
The energy barrier to arene oxide opening was approximately 40 kcal/mol for both arene oxides when the 3,4- and 5,6-arene oxides open toward the aromatic C–H bond (Figure 5). For both the 3,4- and 5,6-arene oxides, this mode of opening leads to formation of the 4,5-arene oxide as confirmed by IRC calculations, and not the formation of OH-PCB. Because both possible openings of the 4,5-arene oxide also proceed toward C–H, it is not surprising that the barriers for 4,5-arene oxide opening were also approximately 40 kcal/mol. Here, IRC calculations revealed concerted 1,2-hydride shifts analogous to the 1,2-chloride shift observed for 3,4-arene oxide opening toward C–Cl. Overall, the computational results suggest that the formation of 1,2-shift products, such as 3′-140, is energetically favored for all PCB arene oxides investigated.
Interindividual Differences in PCB 132 Metabolism by HLMs
In experiments with single donor HLMs, the formation rates of the monohydroxylated PCB 132 metabolites displayed interindividual variation (Figs 3 and 4b; Supplementary Tables 3 and 4). The sum of OH-PCBs (ΣOH-PCBs) formed by single donor HLM preparations followed the following rank order: donor H5 > H4 ∼ H3 > H2 ∼ pHLM > H1. Notably, levels of ΣOH-PCB were 4.5-fold higher in incubations with HLMs from donors H5 versus H1. The rate of 5′-132 formation differed 16-fold for incubations with HLM preparations from donors H1 versus H4. The rate of 3′-140 and 4′-132 formation differed 2.2- and 3.3-fold, respectively, for incubations with HLM preparations from donors H1 versus H5. As a consequence, the OH-PCB metabolite profiles differed across the HLM preparations investigated (Figure 4b). Briefly, 3′-140 and 5′-132 were formed in a 1:1 ratio by pHLMs. In incubations with HLMs from donors H1 and H2, the 3′-140 to 5′-132 ratios were 3.9:1 and 1.4:1 for donors H1 and H2, respectively. In contrast, 5′-132, and not 3′-140, were major in incubations with HLMs from donors H3, H4, and H5 (3′-140 to 5′-132 ratios were 0.7:1, 0.3:1 and 0.6:1 for donors H3, H4, and H5, respectively). Although only a small number of single donor HLM preparations were investigated in this and other studies, the profiles of OH-PCB metabolite formed in the liver likely display considerable variability in humans.
Atropisomeric Enrichment of PCB 132
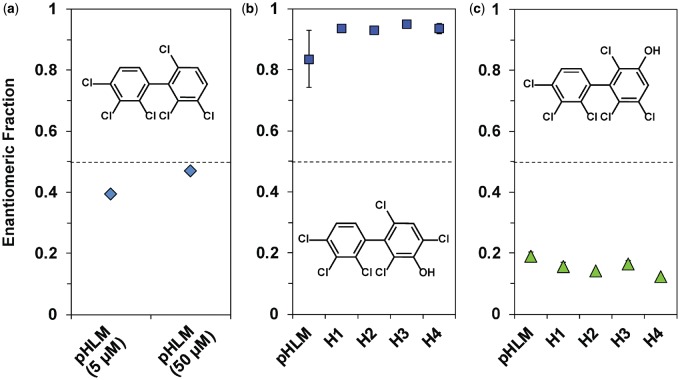
Because chiral PCBs undergo atropisomeric enrichment in vivo and affect toxic endpoints in an atropselective manner (Kania-Korwel and Lehmler, 2016a; Lehmler et al., 2010), the enrichment of PCB 132 atropisomers in incubations of racemic PCB 132 with HLMs was investigated with atropselective gas chromatography. E2-PCB 132 (EF = 0.39), which corresponds to (+)-PCB 132 (Haglund and Wiberg, 1996), was enriched in experiments with low PCB 132 concentration (5 µM) and long incubation time (120 min) (Figure 6, Supplementary Table 6). EF values were near racemic in incubations with higher PCB 132 concentrations (50 µM) because the large amount of racemic PCB 132 masked the atropselective depletion of one PCB 132 atropisomer over the other (Uwimana et al., 2016, 2018).
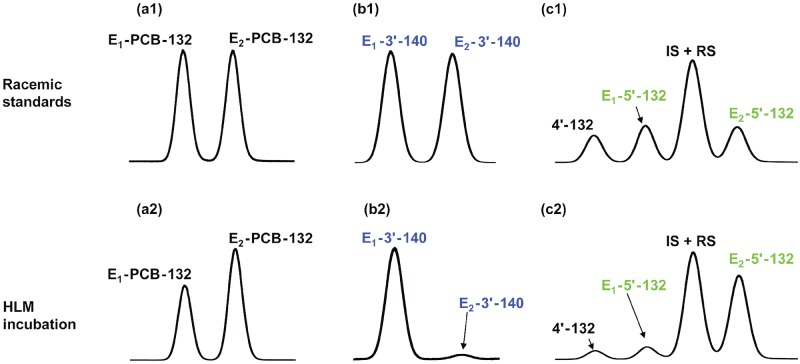
Figure 6.
Atropselective gas chromatographic analysis revealed the atropselective metabolism of racemic 2,2′,3,3′,4,6′-hexachlorobiphenyl (PCB 132) to 2,2′,3,3′,4,6′-hexachlorobiphenyl-5′-ol (5′-132) and 2,2′,3,4,4′,6′-hexachlorobiphenyl-3-ol (3′-140) in incubations with pooled human liver microsomes (pHLMs). Representative gas chromatograms of racemic standards (top panels) versus PCB 132, 5′-132, and 3′-140 in incubations of racemic PCB 132 with human liver microsomes (HLMs) (bottom panels) show a depletion of E1-PCB 132 (panels a1 vs a2) and atropselective formation of E1-3′-140 (panels b1 vs b2) and E2-5′-132 (panels c1 vs c2). To assess the atropselective depletion of PCB 132, incubations were carried out with 5 µM PCB 132, 0.5 mg/ml microsomal protein (pHLMs only), and 0.5 mM NADPH for 120 min at 37°C. To study the atropselective formation of the PCB 132 metabolites, incubations were carried out with 50 µM racemic PCB 132, 0.1 mg/ml microsomal protein, and 1 mM NADPH for 30 min at 37°C (donor H3 shown here; see Figure 7 for results from incubations using other HLM preparations) (Uwimana et al., 2016, 2018). Metabolites were analyzed as the corresponding methylated derivatives after derivatization with diazomethane. Atropselective analyses of 3′-140 were performed with a ChiralDex G-TA (GTA) column at 150°C, and atropselective analyses of PCB 132 and 5′-132 were carried out with a CP-Chirasil-Dex CB (CD) column at 160°C (Kania-Korwel et al., 2011).
Atropselective Formation of OH-PCB 132 Metabolites
Several studies have reported the atropselective formation of chiral OH-PCB metabolites, both from chiral and prochiral PCBs, in in vitro and in vivo studies (Kania-Korwel and Lehmler, 2016a; Lehmler et al., 2010; Uwimana et al., 2017). Consistent with these earlier studies, we observed the atropselective formation of different OH-PCB 132 metabolites in incubations with HLMs. Specifically, the atropselective analysis on the GTA column revealed the atropselective formation of E1-3′-140, with EF values > 0.8 (range: 0.84–0.95) (Figs 6 and 7). E2-5′-132 was significantly enriched with EF values < 0.2 (range: 0.12–0.18) in incubations with HLMs. As reported previously, the atropisomers of 4′-132 could not be resolved on any of the chiral columns used (Kania-Korwel et al., 2011).
Figure 7.
Enantiomeric fractions (EFs) of (a) parent 2,2′,3,3′,4,6′-hexachlorobiphenyl (PCB 132), (b) 2,2′,3,4,4′,6′-hexachlorobiphenyl-3-ol (3′-140), and (c) 2,2′,3,3′,4,6′-hexachlorobiphenyl-5′-ol (5′-132) reveal only small interindividual differences in the atropselective formation of both OH-PCB metabolites. a, To assess the atropselective depletion of PCB 132, incubations were carried out with 5 µM or 50 µM PCB 132, 0.5 mg/ml microsomal protein (using pooled human liver microsomes [pHLMs] only), 0.5 mM NADPH for 120 min at 37°C (see Figure 6 for a representative chromatogram). To study the atropselective formation of (b) 3′-140 and (c) 5′-132, microsomal incubations were carried out with 50 µM racemic PCB 132, 0.1 mg/ml microsomal protein, 1 mM NADPH for 30 min at 37°C (Uwimana et al., 2016, 2018). Metabolites were analyzed as the corresponding methylated derivatives. Atropselective analyses of 3′-140 were performed with a ChiralDex G-TA (GTA) column at 150°C, and atropselective analyses of PCB 132 and 5′-132 were carried out with a CP-Chirasil-Dex CB (CD) column at 160°C (Kania-Korwel et al., 2011). EF values could not be determined in incubations with human liver microsomes (HLMs) from donor H5 due to the low metabolite levels. Data are presented as mean ± standard deviation, n = 3. The dotted line indicates the EF values of the racemic standards.
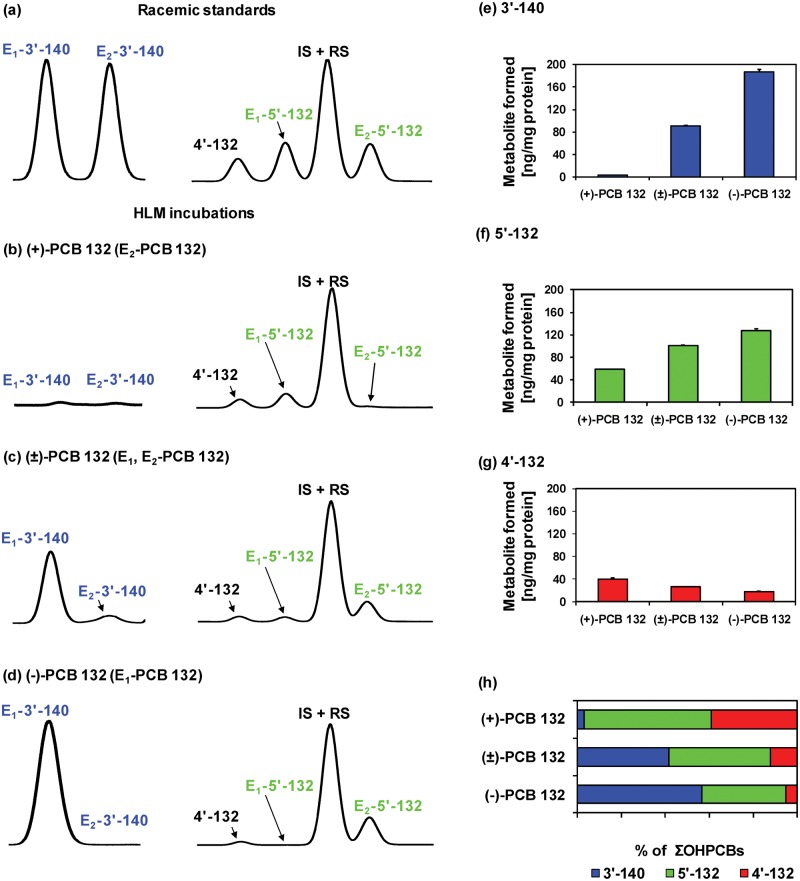
To determine which of E1-OH-PCB and E2-OH-PCB atropisomer is formed from (−)- or (+)-PCB 132, we investigated the metabolism of (−)-, (±)-, and (+)-PCB 132 by pHLMs (Figure 8, Supplementary Table 7). The atropselective analysis showed that E1-5′-132 is formed from (+)-PCB 132. Conversely, E1-3′-140 and E2-5′-132 are formed from (−)-PCB 132. The ΣOH-PCBs formed from (−)-PCB 132 was 3-times the ΣOH-PCBs formed from (+)-PCB 132 (Supplementary Table 7). A more complex picture emerges when individual OH-PCB 132 metabolites are analyzed (Figs. 8e–g). The levels of both major metabolites, 3′-140 and 5′-132, decreased in the order of (−)-PCB 132 > (±)-PCB 132 > (+)-PCB 132. In contrast, levels of the minor metabolite, 4′-132, decreased in the reverse order. As a consequence, the OH-PCB 132 metabolite profiles formed by HLMs differ considerably for incubations with (−)-, (±)-, and (+)-PCB 132 (Figure 8h).
Figure 8.
Comparison of representative gas chromatograms of (a) racemic OH-PCB metabolite standards with OH-PCB metabolites formed in incubations of (b) (+)-PCB 132 (2,2′,3,3′,4,6′-hexachlorobiphenyl), (c) (±)-PCB 132, or (d) (−)-PCB 132 with pooled human liver microsomes (pHLMs) reveals that E1-5′-132 (2,2′,3,3′,4,6′-hexachlorobiphenyl-5′-ol) is formed from (+)-PCB 132 and E1-3′-140 (2,2′,3,4,4′,6′-hexachlorobiphenyl-3-ol), and E2-5′-132 are formed from (−)-PCB. Moreover, (e) 3′-140 and (f) 5′-132, but not (g) 2,2′,3,3′,4,6′-hexachlorobiphenyl-4′-ol (4′-132) are formed more rapidly from (−)-PCB 132 than (+)-PCB 132, resulting (h) in distinct OH-PCB metabolite profiles formed from (+)-, (±)-, and (−)-PCB 132 in incubations with pHLMs. Incubations were carried out with 50 µM (+)-PCB 132, racemic PCB 132, or (−)-PCB 132, 0.1 mg/ml microsomal protein and 1 mM NADPH for 30 min at 37°C (Uwimana et al., 2016). Metabolites were analyzed as the corresponding methylated derivatives after derivatization with diazomethane. Atropselective analyses of 3′-140 were performed with a ChiralDex G-TA (GTA) column at 150°C, and atropselective analyses of PCB 132 and 5′-132 were carried out with a CP-Chirasil-Dex CB (CD) column at 160°C. 4′-132 was not resolved on any of the columns used in this study (Kania-Korwel et al., 2011). Data are presented as mean ± standard deviation, n = 3.
Cytotoxicity and Oxidative Stress of PCB 132 Atropisomers
It is currently unknown if pure PCB atropisomers differentially cause cytotoxicity or atropselectively increase the production of ROS in dopaminergic cells. In our study, PCB 132 atropisomers did not elicit overt cytotoxicity in immortalized dopaminergic cell lines as determined by MTT analysis (Supplementary Figure 13). Both (+)-PCB 132 and (−)-PCB 132 exerted measurable toxicity to N27 cells only at the highest dose (100 μM) with a 24 h exposure. Analogously, PC12 cells showed no significant cell loss following PCB 132 atropisomer exposure. We observed a slight increase in overall ROS and hydrogen peroxide, measured with DCFDA and DHE, respectively, after 24 h exposure of N27 cells to PCB 132 atropisomers; however, these changes did not reach statistical significance (Supplementary Figure 14). Similarly, no statistically significant, time-dependent changes in the production of ROS were observed at earlier time intervals (data not shown). There were also no significant differences in the effects of both PCB 132 atropisomers on dopaminergic cellular toxicity and levels of ROS.
Effect of PCB 132 on Levels of Dopamine and Its Metabolites
PCBs, including chiral PCB 95, have been shown to affect the dopaminergic system, including by altering the expression of dopamine receptors and by changing dopamine levels (Bavithra et al., 2012; Enayah et al., 2018; Seegal et al., 2002); however, it is unknown if PCBs atropselectively affect the dopaminergic system and dopamine metabolism. PC12 cells were exposed to PCB atropisomers for 24 h, and intracellular dopamine and its metabolites were measured via HPLC analysis. Dopamine is converted by monoamine oxidase to DOPAL which is further metabolized by aldehyde dehydrogenase to DOPAC or by aldehyde reductase to DOPET. Intracellular levels of dopamine, DOPAC, and DOPET showed no significant differences from control (Figure 9). Extracellular levels of dopamine and DOPAC was also measured in media with similar results (Supplementary Figure 15).
Figure 9.
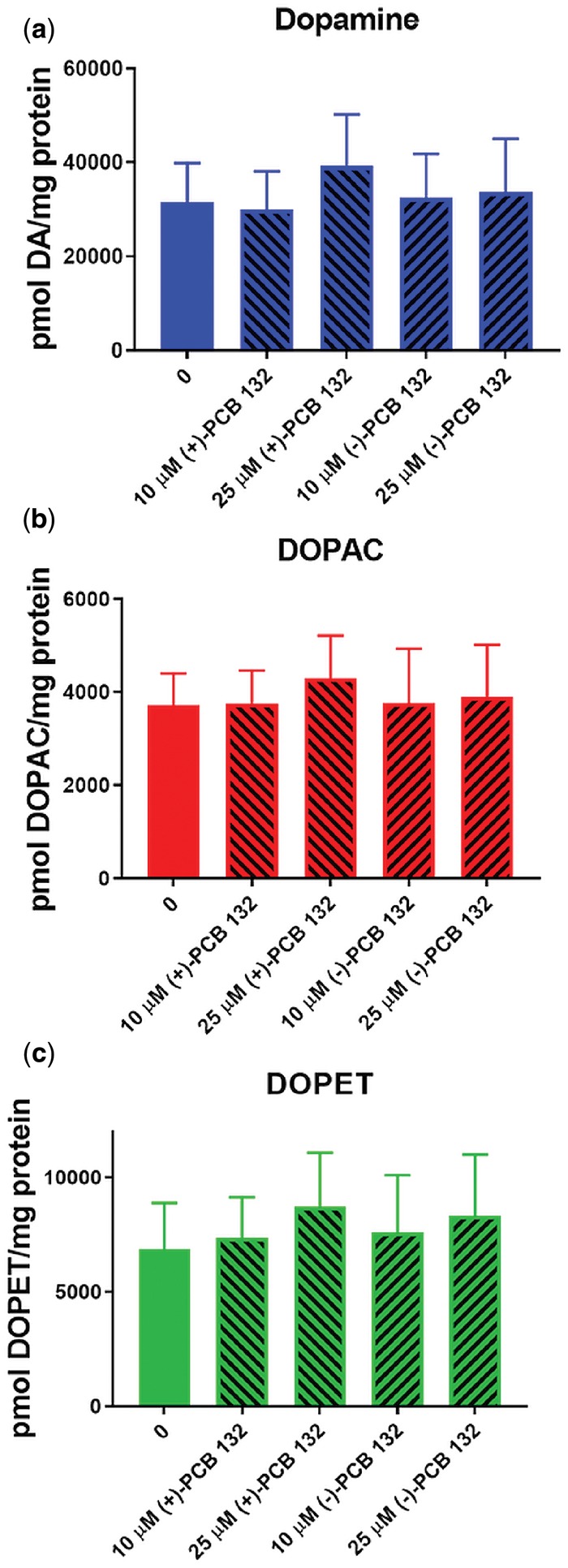
Exposure of PC12 cells for 24 h to (+)- or (−)-PCB 132 (2,2′,3,3′,4,6′-hexachlorobiphenyl) did not significantly alter intracellular levels of (a) dopamine, (b) 3,4-dihydroxyphenylacetic acid (DOPAC), or (c) 3,4-dihydroxyphenyl ethanol (DOPET). Intracellular measurements of dopamine and its metabolites were performed by HPLC analysis with electrochemical detection. Data are represented as mean ± standard error, n = 4.
DISCUSSION
PCB 132 is readily metabolized by HLMs to a 1,2-shift product, 3′-140, and a second meta hydroxylated metabolite, 5′-132. Several structurally related PCB congeners with multiple ortho chlorine substituents are also metabolized by HLMs to OH-PCBs (Nagayoshi et al., 2018; Schnellmann et al., 1983; Wu et al., 2014). For example, PCB 91 is primarily metabolized to a 1,2-shift product by HLMs (Uwimana et al., 2018), whereas PCB 95 and PCB 136 are oxidized by HLMs with different regioselectivity (Schnellmann et al., 1983; Uwimana et al., 2016; Wu et al., 2014). Based on published structure-metabolism relationships (Grimm et al., 2015), these congener-specific differences in their metabolism are likely due to the presence of a para chlorine substituent in PCB 91 and PCB 132, but not PCB 95 and PCB 136. The formation of a 1,2-shift product indicates that similar to PCB 91, PCB 132 is oxidized by human P450 enzymes to an arene oxide intermediate that subsequently undergoes a 1,2-chlorine shift to 3′-140. Importantly, different OH-PCB profiles have been reported for the metabolism of these PCB congeners in rodent models used to study the neurotoxicity of PCBs (Kania-Korwel et al., 2017; Schantz et al., 1997; Wayman et al., 2012). For example, 5′-132, and not 3′-140, was the major metabolite formed from PCB 132 in experiments with rat liver microsomes (Kania-Korwel et al., 2011), recombinant rat CYP2B1 (Lu et al., 2013), and precision-cut mouse liver tissue slices (Wu et al., 2013a). 1,2-Shift products were only minor metabolites of PCB 132 and other chiral PCBs in in vitro and in vivo studies in rodent models (Kania-Korwel et al., 2011; Lu et al., 2013; Wu et al., 2013a).
The preferential formation of different PCB arene oxide intermediates in rodents versus humans is one explanation for the species differences in the oxidation of PCB 132 and structurally related PCB congeners. We employed a computational approach to study the energetics of the ring opening of the 3 possible arene oxides on the 2,3,6-trichloro-substituted phenyl ring to explore this possibility. Independent of the position of the arene oxide, our calculations suggested that (1) the ring opening leading to a 1,2-shift of chlorine substituents is energetically favorable over a ring opening toward C–H and (2) the presence of the second aromatic ring does not perturb the energetics of the arene oxide ring opening. Based on our computational results, the 1,2-shift products of PCB 132 (this study) and PCB 91 (Uwimana et al., 2018) are formed via a 3,4-arene oxide intermediate which opens toward C–Cl to form the 1,2-shift metabolite. The presence or absence of the substituent aryl ring had no effect on the computed mechanism or energetics for an opening toward C–Cl, nor did the substitution pattern on the aryl substituent. This is expected given the lack of conjugation between the 2 aryl rings due to their (near) orthogonality. The computational results therefore suggest that the formation of para-substituted metabolites of PCB 95 and PCB 136 is not merely due to the energetically favored opening of the 3,4- or 4,5-arene oxide intermediates. Instead, congener-specific differences in the steric or electronic interaction of both PCB congeners with P450 isoforms involved in their metabolism (Uwimana et al., 2019) likely affect the ring opening of the putative arene oxide intermediates to OH-PCBs. In contrast, the formation of OH-PCB metabolites of PCB 132 and related PCB congeners in rodents may or may not involve an arene oxide intermediate. For example, the meta oxidation of PCB 52 by rat CYP2B1 does not involve an arene oxide but occurs via the insertion of an oxygen atom into the aromatic C–H bond (Preston et al., 1983). Other PCB congeners are readily metabolized via arene oxide intermediates by P450 enzymes (Kennedy et al., 1981; Schnellmann et al., 1983).
In addition to congener and species-dependent differences in the OH-PCB metabolite profiles, we observed considerable interindividual variability in the formation of PCB 132 metabolites by HLM preparations from different donors. For example, differences as high as 16-fold were found in the formation of 5′-132 by different HLM preparations. Similarly, we reported interindividual differences in the formation of OH-PCB metabolites of PCB 91 and PCB 95 with the same HLM preparations (Uwimana et al., 2016, 2018). This observation is not surprising considering the well-documented variability of P450 enzyme activities among humans (Guengerich, 2015). As we reported recently, both CYP2A6 and CYP2B6 are involved in the metabolism of PCB 132 (Uwimana et al., 2019). CYP2A6 primarily formed 3′-140, with 5′-132 and 4′-132 being minor metabolites. CYP2B6 primarily yielded 5′-132, with the 1,2-shift product being a minor metabolite. Differences in the levels of CYP2A6 and CYP2B6 in the HLM preparations investigated are one explanation for the interindividual differences in the OH-PCB formation rates in this and our earlier studies. Moreover, both CYP2A6 and CYP2B6 are highly polymorphic enzymes (PharmWar, 2012, 2013). As has been shown for active site mutants of CYP2B1 (Waller et al., 1999), the rat ortholog of CYP2B6, polymorphisms can alter the regioselectivity of the oxidation of PCBs.
The biotransformation of PCB 132 by P450 enzymes results in nonracemic chiral signatures of both the parent PCB and its metabolites in environmental samples and mammals, including humans (Kania-Korwel and Lehmler, 2016b; Lehmler et al., 2010). Importantly, the enrichment of PCB atropisomers observed in in vitro studies typically predicts the atropisomeric enrichment in animal studies. (+)-PCB 132 was enriched in in vitro metabolism studies with precision-cut mouse liver tissue slices (Wu et al., 2013a), rat liver microsomes (Kania-Korwel et al., 2011), and recombinant rat CYP2B1 and human CYP2B6 (Lu et al., 2013; Warner et al., 2009). Consistent with these in vitro metabolism studies, (+)-PCB 132 was enriched in female mice (Kania-Korwel et al., 2010; Milanowski et al., 2010) and male Wistar rats (Norstrom et al., 2006). (−)-PCB 132 had a shorter half-life in a disposition study in mice (Kania-Korwel et al., 2010). In rats, levels of methyl sulfone metabolites of PCB 132 were higher in (−)-PCB 132 compared (+)-PCB 132 exposed animals, suggesting that (−)-PCB 132 is more rapidly metabolized in rats (Norstrom et al., 2006). Analogous to the laboratory studies, the preferential oxidation of (−)-PCB 132 by HLMs observed in this study is consistent with the enrichment of (+)-PCB 132 found in most human samples (Supplementary Table 8). However, other factors may also contribute to the atropisomeric enrichment of PCB 132 in humans. For example, we cannot dismiss the possibility that exposure to atropisomerically enriched PCB 132 via the diet also contributes to the atropisomeric enrichment of PCB 132 in humans (Harrad et al., 2006; Vetter, 2016). More in-depth studies are therefore needed to characterize which P450 isoforms contribute to the atropselective metabolism of PCB 132, and to determine whether or not genetic polymorphisms affect the stereoselectivity of its oxidation.
In addition to the parent PCB, and consistent with earlier studies reporting the atropselective formation of chiral OH-PCB metabolites from PCBs (Kania-Korwel and Lehmler, 2016a; Lehmler et al., 2010; Uwimana et al., 2017), we observed the atropselective formation of OH-PCB 132 metabolites in incubations with HLMs. Specifically, we noted the atropselective formation of E1-3′-140. This 3′-140 atropisomer was also enriched in experiments with rat liver microsomes (Kania-Korwel et al., 2011). E2-5′-132 was significantly enriched in incubations with HLMs. Similarly, metabolism of racemic PCB 132 resulted in an enrichment of E2-5′-132 in incubations with rat liver microsomes (Kania-Korwel et al., 2011) and mouse liver tissue slices (Wu et al., 2013a). In contrast, rat CYP2B1 metabolized PCB 132 preferentially to E1-5′-132, which indicates the involvement of other P450 isoforms in the atropselective oxidation of PCB 132 in incubations with rat liver microsomes (Lu et al., 2013). The atropselective formation of OH-PCBs by HLMs is important in the context of several findings from laboratory studies: Chiral OH-PCBs present in the developing brain of mice exposed developmentally to racemic PCBs (Kania-Korwel et al., 2017). Moreover, the position of the OH-group has a profound effect on the interaction of OH-PCBs with cellular targets implicated in PCB neurotoxicity (Kodavanti et al., 2003; Niknam et al., 2013). It is therefore likely that interindividual differences in (1) PCB and OH-PCB levels and (2) chiral signatures influence toxic outcomes in humans.
Several studies have shown that PCB atropisomers atropselectively affect endpoints relevant to PCB neurotoxicity, including altered calcium homeostasis, estrogen receptor activation, and ROS, linked to PCB developmental neurotoxicity (Chai et al., 2016; Feng et al., 2017; Lehmler et al., 2005; Pěnčíková et al., 2018; Yang et al., 2014). In our preliminary assessment of the effects of PCB 132 atropisomers on endpoints implicated in PCB neurotoxicity, we saw increased general ROS levels, which are known to damage lipids and proteins, at subtoxic PCB 132 concentrations in dopaminergic cells in culture; however, this effect did not reach statistical significance at the PCB 132 concentrations investigated using cultured dopaminergic cell lines. Toxicity for 2 dopaminergic cell lines was only observed at the highest concentration used (ie, 100 μM). These findings suggest that the PCB 132 atropisomers are not toxic over the periods examined (ie, 4 and 24 h) to yield alterations in dopamine metabolism, cell loss or increased oxidative stress. Exposure of rats to a PCB mixture containing racemic PCB 132 for 4 weeks leads to changes in levels of dopamine and increased oxidative damage and loss of cells in dopaminergic regions (Lee et al., 2012); therefore, it is possible that more time or PCB 132 metabolism (eg, hydroxylated metabolites) are required before toxicity to dopaminergic systems is apparent (Kodavanti et al., 2003; Niknam et al., 2013). Future work should address these issues.
In addition, it is conceivable that PCB 132 lacks the structural requirements for neurotoxicity. A detailed structure-activity investigation in the same cell line (ie, PC12) showed that PCB congeners with ortho substituents result in a decrease in intracellular, total dopamine levels, but that chlorine substituents in meta position counteract this effect. The lack of an effect of PCB 132, which contains a meta chlorine substituent in both phenyl rings, on cellular dopamine metabolism is therefore not entirely surprising. It is noteworthy that exposure of PC12 cells to racemic PCB 95 decreases dopamine concentrations and increases levels of toxic dopamine metabolites (Enayah et al., 2018). A systematic study of other PCB atropisomers is therefore warranted to assess if PCBs atropselectively cause oxidative stress and alter dopamine homeostasis in the brain and, ultimately, if the atropselective metabolism of PCBs plays a currently unexplored role in PCB-mediated neurotoxicity.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr S. Joshi, Dr S. Vyas, and Dr Y. Song (University of Iowa) for synthesizing the PCB standards and Mr V. Parcel from the University of Iowa HRMS Facility for help with the GC-TOF/MS analyses.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences/National Institutes of Health (ES05605, ES013661, and ES027169 to H.L.J., ES029035 to J.A.D.); and the National Science Foundation (CHE-1609669, CHE-1229354 to the MERCURY consortium [http://mercuryconsortium.org/ Accessed July 7, 2019], CHE-1662030 to E.V.P.). The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health or the National Science Foundation.
REFERENCES
- Anderson D. G., Mariappan S. V., Buettner G. R., Doorn J. A. (2011). Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J. Biol. Chem. 286, 26978–26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher B. J., D’Agostino L. A., Way J. D., Wong C. S., Harynuk J. J. (2009). Comparison of peak integration methods for the determination of enantiomeric fraction in environmental samples. Chemosphere 75, 1042–1048. [DOI] [PubMed] [Google Scholar]
- Bavithra S., Selvakumar K., Pratheepa Kumari R., Krishnamoorthy G., Venkataraman P., Arunakaran J. (2012). Polychlorinated biphenyl (PCBs)-induced oxidative stress plays a critical role on cerebellar dopaminergic receptor expression: Ameliorative role of quercetin. Neurotox. Res. 21, 149–159. [DOI] [PubMed] [Google Scholar]
- Bemis J. C., Seegal R. F. (2004). PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol. Sci. 80, 288–295. [DOI] [PubMed] [Google Scholar]
- Bordajandi L. R., Abad E., Gonzalez M. J. (2008). Occurrence of PCBs, PCDD/Fs, PBDEs and DDTs in spanish breast milk: Enantiomeric fraction of chiral PCBs. Chemosphere 70, 567–575. [DOI] [PubMed] [Google Scholar]
- Bucheli T. D., Brandli R. C. (2006). Two-dimensional gas chromatography coupled to triple quadrupole mass spectrometry for the unambiguous determination of atropisomeric polychlorinated biphenyls in environmental samples. J. Chromatogr. A 1110, 156–164. [DOI] [PubMed] [Google Scholar]
- Caudle W. M., Richardson J. R., Delea K. C., Guillot T. S., Wang M., Pennell K. D., Miller G. W. (2006). Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson's disease-associated dopamine toxicity. Toxicol. Sci. 92, 490–499. [DOI] [PubMed] [Google Scholar]
- Chai T., Cui F., Mu X., Yang Y., Qi S., Zhu L., Wang C., Qiu J. (2016). Stereoselective induction by 2,2′,3,4′,6-pentachlorobiphenyl in adult zebrafish (Danio rerio): Implication of chirality in oxidative stress and bioaccumulation. Environ. Pollut. 215, 66–76. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhong Z., Xu Z., Chen L., Wang Y. (2010). 2′,7′-dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 44, 587–604. [DOI] [PubMed] [Google Scholar]
- Chu S., Covaci A., Schepens P. (2003). Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ. Res. 93, 167–176. [DOI] [PubMed] [Google Scholar]
- CYP2A6 pharmacogene variation consortium (pharmvar) at www.Pharmvar.Org. 2012. Available at: https://www.pharmvar.org/gene/CYP2A6. Accessed February 28, 2019.
- CYP2B6 pharmacogene variation consortium (pharmvar) at www.Pharmvar.Org. 2013. Available at: https://www.pharmvar.org/gene/CYP2B6. Accessed February 28, 2019.
- DeCaprio A. P., Johnson G. W., Tarbell A. M., Carpenter D. O., Chiarenzelli J. R., Morse G. S., Santiago-Rivera A. L., Schymura M. J. Akwesasne Task Force on the Environment. (2005). Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult native american population. Environ. Res. 98, 284–302. [DOI] [PubMed] [Google Scholar]
- Enayah S. H., Vanle B. C., Fuortes L. J., Doorn J. A., Ludewig G. (2018). PCB95 and PCB153 change dopamine levels and turn-over in PC12 cells. Toxicology 394, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. (2002). Commission decision EC 2002/657 of 12 August 2002 implementing council directive 96/23/ec concerning the performance of analytical methods and the interpretation of results. Off. J. Eur Communities 221, 0008–0036. [Google Scholar]
- Fellman J. H. (1958). The rearrangement of epinephrine. Nature 182, 311–312. [DOI] [PubMed] [Google Scholar]
- Feng W., Zheng J., Robin G., Dong Y., Ichikawa M., Inoue Y., Mori T., Nakano T., Pessah I. N. (2017). Enantioselectivity of 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) atropisomers toward ryanodine receptors (RyRs) and their influences on hippocampal neuronal networks. Environ. Sci. Technol. 51, 14406–14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgue S. T., Allen J. R. (1982). Identification of an arene oxide metabolite of 2,2′,5,5′-tetrachlorobiphenyl by gas chromatography-mass spectroscopy. Chem. Biol. Interact. 40, 233–245. [DOI] [PubMed] [Google Scholar]
- Forgue S. T., Preston B. D., Hargraves W. A., Reich I. L., Allen J. R. (1979). Direct evidence that an arene oxide is a metabolic intermediate of 2,2′,5,5′-tetrachlorobiphenyl. Biochem. Biophys. Res. Commun. 91, 475–483. [DOI] [PubMed] [Google Scholar]
- Frisch M., Trucks G., Schlegel H., Scuseria G., Robb M., Cheeseman J., Scalmani G., Barone V., Petersson G., Nakatsuji H., et al. 2016. Gaussian 16, Revision a. 03. Gaussian Inc, Wallingford, CT. [Google Scholar]
- Glausch A., Hahn J., Schurig V. (1995). Enantioselective determination of chiral 2,2′,3,3′,4,6′-hexachlorobiphenyl (PCB 132) in human milk samples by multidimensional gas chromatography/electron capture detection and by mass spectrometry. Chemosphere 30, 2079–2085. [DOI] [PubMed] [Google Scholar]
- Grimm F. A., Hu D., Kania-Korwel I., Lehmler H. J., Ludewig G., Hornbuckle K. C., Duffel M. W., Bergman A., Robertson L. W. (2015). Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol. 45, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P. 2015. Human cytochrome P450 enzymes In Cytochrome P450 (Ortiz de Montellano P. R., Ed.), pp. 523–785. Springer US, Boston, MA. [Google Scholar]
- Guroff G., Daly J. W., Jerina D. M., Renson J., Witkop B., Udenfriend S. (1967). Hydroxylation-induced migration: The NIH shift. Recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds. Science 157, 1524–1530. [DOI] [PubMed] [Google Scholar]
- Haglund P., Wiberg K. (1996). Determination of the gas chromatographic elution sequences of the (+) and (−) enantiomers of stable enantiomeric PCBs on Chirasil-Dex. J. High Resolut. Chromatogr. 19, 373–376. [Google Scholar]
- Haijima A., Lesmana R., Shimokawa N., Amano I., Takatsuru Y., Koibuchi N. (2017). Differential neurotoxic effects of in utero and lactational exposure to hydroxylated polychlorinated biphenyl (OH-PCB 106) on spontaneous locomotor activity and motor coordination in young adult male mice. J. Toxicol. Sci. 42, 407–416. [DOI] [PubMed] [Google Scholar]
- Haraguchi K., Koga N., Kato Y. (2005). Comparative metabolism of polychlorinated biphenyls and tissue distribution of persistent metabolites in rats, hamsters, and guinea pigs. Drug Metab. Dispos. 33, 373–380. [DOI] [PubMed] [Google Scholar]
- Haraguchi K., Kato Y., Koga N., Degawa M. (2004). Metabolism of polychlorinated biphenyls by Gunn rats: Identification and serum retention of catechol metabolites. Chem. Res. Toxicol. 17, 1684–1691. [DOI] [PubMed] [Google Scholar]
- Harrad S., Ren J., Hazrati S., Robson M. (2006). Chiral signatures of PCB#s 95 and 149 in indoor air, grass, duplicate diets and human faeces. Chemosphere 63, 1368–1376. [DOI] [PubMed] [Google Scholar]
- Hatcher-Martin J. M., Gearing M., Steenland K., Levey A. I., Miller G. W., Pennell K. D. (2012). Association between polychlorinated biphenyls and Parkinson's disease neuropathology. Neurotoxicology 33, 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick R. F., Stewart J. H., Allen J. G. (2016). Review of PCBs in US schools: A brief history, an estimate of the number of impacted schools, and an approach for evaluating indoor air samples. Environ. Sci. Pollut. Res. Int. 23, 1975–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinsmaa Y., Florang V. R., Rees J. N., Anderson D. G., Strack S., Doorn J. A. (2009). Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem. Res. Toxicol. 22, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. C., Miller G. W. (2008). The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem. Pharmacol. 76, 569–581. [DOI] [PubMed] [Google Scholar]
- Jursa S., Chovancova J., Petrik J., Loksa J. (2006). Dioxin-like and non-dioxin-like PCBs in human serum of Slovak population. Chemosphere 64, 686–691. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I., Duffel M. W., Lehmler H. J. (2011). Gas chromatographic analysis with chiral cyclodextrin phases reveals the enantioselective formation of hydroxylated polychlorinated biphenyls by rat liver microsomes. Environ. Sci. Technol. 45, 9590–9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., El-Komy M. H., Veng-Pedersen P., Lehmler H. J. (2010). Clearance of polychlorinated biphenyl atropisomers is enantioselective in female C57BL/6 mice. Environ. Sci. Technol. 44, 2828–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., Hornbuckle K. C., Peck A., Ludewig G., Robertson L. W., Sulkowski W. W., Espandiari P., Gairola C. G., Lehmler H. J. (2005). Congener-specific tissue distribution of Aroclor 1254 and a highly chlorinated environmental PCB mixture in rats. Environ. Sci. Technol. 39, 3513–3520. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I., Lehmler H. J. (2016a). Chiral polychlorinated biphenyls: Absorption, metabolism and excretion—A review. Environ. Sci. Pollut. Res. Int. 23, 2042–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., Lehmler H. J. (2016b). Toxicokinetics of chiral polychlorinated biphenyls across different species—A review. Environ. Sci. Pollut. Res. Int. 23, 2058–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., Lukasiewicz T., Barnhart C. D., Stamou M., Chung H., Kelly K. M., Bandiera S., Lein P. J., Lehmler H. J. (2017). Editor's highlight: Congener-specific disposition of chiral polychlorinated biphenyls in lactating mice and their offspring: Implications for PCB developmental neurotoxicity. Toxicol. Sci. 158, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., Shaikh N. S., Hornbuckle K. C., Robertson L. W., Lehmler H. J. (2007). Enantioselective disposition of PCB 136 (2,2′,3,3′,6,6′-hexachlorobiphenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality 19, 56–66. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I., Zhao H., Norstrom K., Li X., Hornbuckle K. C., Lehmler H. J. (2008). Simultaneous extraction and clean-up of polychlorinated biphenyls and their metabolites from small tissue samples using pressurized liquid extraction. J. Chromatogr. A 1214, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. W., Carpentier N. K., Dymerski P. P., Kaminsky L. S. (1981). Metabolism of dichlorobiphenyls by hepatic microsomal cytochrome P-450. Biochem. Pharmacol. 30, 577–588. [DOI] [PubMed] [Google Scholar]
- Kodavanti P. R., Curras-Collazo M. C. (2010). Neuroendocrine actions of organohalogens: Thyroid hormones, arginine vasopressin, and neuroplasticity. Front. Neuroendocrinol. 31, 479–496. [DOI] [PubMed] [Google Scholar]
- Kodavanti P. R., Ward T. R., Derr-Yellin E. C., McKinney J. D., Tilson H. A. (2003). Increased [3H]phorbol ester binding in rat cerebellar granule cells and inhibition of 45Ca2+ buffering in rat cerebellum by hydroxylated polychlorinated biphenyls. Neurotoxicology 24, 187–198. [DOI] [PubMed] [Google Scholar]
- Lee D. W., Notter S. A., Thiruchelvam M., Dever D. P., Fitzpatrick R., Kostyniak P. J., Cory-Slechta D. A., Opanashuk L. A. (2012). Subchronic polychlorinated biphenyl (Aroclor 1254) exposure produces oxidative damage and neuronal death of ventral midbrain dopaminergic systems. Toxicol. Sci. 125, 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler H. J., Harrad S. J., Huhnerfuss H., Kania-Korwel I., Lee C. M., Lu Z., Wong C. S. (2010). Chiral polychlorinated biphenyl transport, metabolism, and distribution: A review. Environ. Sci. Technol. 44, 2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler H. J., Robertson L. W., Garrison A. W., Kodavanti P. R. (2005). Effects of PCB 84 enantiomers on [3H]-phorbol ester binding in rat cerebellar granule cells and 45Ca2+-uptake in rat cerebellum. Toxicol. Lett. 156, 391–400. [DOI] [PubMed] [Google Scholar]
- Lesmana R., Shimokawa N., Takatsuru Y., Iwasaki T., Koibuchi N. (2014). Lactational exposure to hydroxylated polychlorinated biphenyl (OH-PCB 106) causes hyperactivity in male rat pups by aberrant increase in dopamine and its receptor. Environ. Toxicol. 29, 876–883. [DOI] [PubMed] [Google Scholar]
- Lu Z., Kania-Korwel I., Lehmler H.-J., Wong C. S. (2013). Stereoselective formation of mono- and di-hydroxylated polychlorinated biphenyls by rat cytochrome P450 2B1. Environ. Sci. Technol. 47, 12184–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenich A. V., Cramer C. J., Truhlar D. G. (2009). Performance of SM6, SM8, and SMD on the SAMPL1 test set for the prediction of small-molecule solvation free energies. J. Phys. Chem. B 113, 4538–4543. [DOI] [PubMed] [Google Scholar]
- Mariussen E., Fonnum F. (2001). The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology 159, 11–21. [DOI] [PubMed] [Google Scholar]
- Mariussen E., Fonnum F. (2006). Neurochemical targets and behavioral effects of organohalogen compounds: An update. Crit. Rev. Toxicol. 36, 253–289. [DOI] [PubMed] [Google Scholar]
- McGraw J. E. Sr, Waller D. P. (2006). Specific human CYP 450 isoform metabolism of a pentachlorobiphenyl (PCB-IUPAC# 101). Biochem. Biophys. Res. Commun. 344, 129–133. [DOI] [PubMed] [Google Scholar]
- Milanowski B., Lulek J., Lehmler H. J., Kania-Korwel I. (2010). Assessment of the disposition of chiral polychlorinated biphenyls in female mdr 1a/b knockout versus wild-type mice using multivariate analyses. Environ. Int. 36, 884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayoshi H., Kakimoto K., Konishi Y., Kajimura K., Nakano T. (2018). Determination of the human cytochrome P450 monooxygenase catalyzing the enantioselective oxidation of 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) and 2,2′,3,4,4′,5′,6-heptachlorobiphenyl (PCB 183). Environ. Sci. Pollut. Res. Int. 25, 16420–16426. [DOI] [PubMed] [Google Scholar]
- Niknam Y., Feng W., Cherednichenko G., Dong Y., Joshi S. N., Vyas S. M., Lehmler H. J., Pessah I. N. (2013). Structure-activity relationship of selected meta- and para-hydroxylated non-dioxin like polychlorinated biphenyls: From single RyR1 channels to muscle dysfunction. Toxicol. Sci. 136, 500–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norstroem K., Bergman A. (2006). Chiral PCB methyl sulfones and their metabolic formation. Organohalogen Compd. 68, 21–24. [Google Scholar]
- Norstrom K., Eriksson J., Haglund J., Silvari V., Bergman A. (2006). Enantioselective formation of methyl sulfone metabolites of 2,2′,3,3′,4,6′-hexachlorobiphenyl in rat. Environ. Sci. Technol. 40, 7649–7655. [DOI] [PubMed] [Google Scholar]
- Ohta C., Haraguchi K., Kato Y., Endo T., Koga N. (2012). Involvement of rat CYP3A enzymes in the metabolism of 2,2′,3,4′,5′,6-hexachlorobiphenyl (CB149). Organohalogen Compd. 74, 1475–1478. [Google Scholar]
- Pěnčíková K., Brenerová P., Svržková L., Hrubá E., Pálková L., Vondráček J., Lehmler H.-J., Machala M. (2018). Atropisomers of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) exhibit stereoselective effects on activation of nuclear receptors in vitro. Environ. Sci. Pollut. Res. Int. 25, 16411–16419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I. N., Cherednichenko G., Lein P. J. (2010). Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 125, 260–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverati R., Truhlar D. G. (2011). Improving the accuracy of hybrid meta-GGA density functionals by range separation. J. Phys. Chem. Lett. 2, 2810–2817. [Google Scholar]
- PharmVar [Pharmacogene Variation Consortium]. 2012. CYP2A6 allele nomenclature Available at: https://www.pharmvar.org/gene/CYP2A6. Accessed July 7, 2019. [Google Scholar]
- PharmVar [Pharmacogene Variation Consortium]. 2013. CYP2A6 allele nomenclature Available at: https://www.pharmvar.org/gene/CYP2A6. Accessed July 7, 2019. [Google Scholar]
- Preston B. D., Miller J. A., Miller E. C. (1983). Non-arene oxide aromatic ring hydroxylation of 2,2′,5,5′-tetrachlorobiphenyl as the major metabolic pathway catalyzed by phenobarbital-induced rat liver microsomes. J. Biol. Chem. 258, 8304–8311. [PubMed] [Google Scholar]
- Richardson J. R., Miller G. W. (2004). Acute exposure to Aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol. Lett. 148, 29–40. [DOI] [PubMed] [Google Scholar]
- Schantz S. L., Moshtaghian J., Ness D. K. (1995). Spatial learning deficits in adult rats exposed to ortho-substituted PCB congeners during gestation and lactation. Fundam. Appl. Toxicol. 26, 117–126. [DOI] [PubMed] [Google Scholar]
- Schantz S. L., Seo B. W., Wong P. W., Pessah I. N. (1997). Long-term effects of developmental exposure to 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) on locomotor activity, spatial learning and memory and brain ryanodine binding. Neurotoxicology 18, 457–467. [PubMed] [Google Scholar]
- Schecter A., Colacino J., Haffner D., Patel K., Opel M., Papke O., Birnbaum L. (2010). Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ. Health Perspect. 118, 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnellmann R. G., Putnam C. W., Sipes I. G. (1983). Metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl by human hepatic microsomes. Biochem. Pharmacol. 32, 3233–3239. [DOI] [PubMed] [Google Scholar]
- Seegal R. F. (1996). Epidemiological and laboratory evidence of PCB induced neurotoxicity. Crit. Rev. Toxicol. 26, 709–737. [DOI] [PubMed] [Google Scholar]
- Seegal R. F., Okoniewski R. J., Brosch K. O., Bemis J. C. (2002). Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: An in vivo microdialysis study. Environ. Health Perspect. 110, 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speisky H., Gomez M., Burgos-Bravo F., Lopez-Alarcon C., Jullian C., Olea-Azar C., Aliaga M. E. (2009). Generation of superoxide radicals by copper-glutathione complexes: Redox-consequences associated with their interaction with reduced glutathione. Bioorg. Med. Chem. 17, 1803–1810. [DOI] [PubMed] [Google Scholar]
- Su G., Liu X., Gao Z., Xian Q., Feng J., Zhang X., Giesy J. P., Wei S., Liu H., Yu H. (2012). Dietary intake of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) from fish and meat by residents of Nanjing, China. Environ. Int. 42, 138–143. [DOI] [PubMed] [Google Scholar]
- Thomas K., Xue J., Williams R., Jones P., Whitaker D.. 2012. Polychlorinated Biphenyls (PCBs) in School Buildings: Sources, Environmental Levels, and Exposures. United States Environmental Protection Agency, Office of Research and Development, National Exposure Research Laboratory. [Google Scholar]
- Uwimana E., Li X., Lehmler H. J. (2016). 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) is atropselectively metabolized to para hydroxylated metabolites by human liver microsomes. Chem. Res. Toxicol. 29, 2108–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwimana E., Li X., Lehmler H. J. (2018). Human liver microsomes atropselectively metabolize 2,2′,3,4′,6-pentachlorobiphenyl (PCB 91) to a 1,2-shift product as the major metabolite. Environ. Sci. Technol. 52, 6000–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwimana E., Maiers A., Li X., Lehmler H. J. (2017). Microsomal metabolism of prochiral polychlorinated biphenyls results in the enantioselective formation of chiral metabolites. Environ. Sci. Technol. 51, 1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwimana E., Ruiz P., Li X., Lehmler H. J. (2019). Human CYP2A6, CYP2B6 and CYP2E1 atropselectively metabolize polychlorinated biphenyls to hydroxylated metabolites. Environ. Sci. Technol. 53, 2114–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meerloo J., Kaspers G. J., Cloos J. (2011). Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 731, 237–245. [DOI] [PubMed] [Google Scholar]
- Vetter W. (2016). Gas chromatographic enantiomer separation of polychlorinated biphenyls (PCBs): Methods, metabolisms, enantiomeric composition in environmental samples and their interpretation. Isr. J. Chem. 56, 940–957. [Google Scholar]
- Voorspoels S., Covaci A., Neels H. (2008). Dietary PCB intake in Belgium. Environ. Toxicol. Pharmacol. 25, 179–182. [DOI] [PubMed] [Google Scholar]
- Walker J. M. (1994). The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 32, 5–8. [DOI] [PubMed] [Google Scholar]
- Waller S. C., He Y. A., Harlow G. R., He Y. Q., Mash E. A., Halpert J. R. (1999). 2,2′,3,3′,6,6′-Hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem. Res. Toxicol. 12, 690–699. [DOI] [PubMed] [Google Scholar]
- Warner N. A., Martin J. W., Wong C. S. (2009). Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ. Sci. Technol. 43, 114–121. [DOI] [PubMed] [Google Scholar]
- Wayman G. A., Yang D., Bose D. D., Lesiak A., Ledoux V., Bruun D., Pessah I. N., Lein P. J. (2012). PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ. Health Perspect. 120, 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigend F., Ahlrichs R. (2005). Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for h to rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305. [DOI] [PubMed] [Google Scholar]
- Whitcomb B. W., Schisterman E. F., Buck G. M., Weiner J. M., Greizerstein H., Kostyniak P. J. (2005). Relative concentrations of organochlorines in adipose tissue and serum among reproductive age women. Environ. Toxicol. Pharmacol. 19, 203–213. [DOI] [PubMed] [Google Scholar]
- Wong C. S., Garrison A. W., Smith P. D., Foreman W. T. (2001). Enantiomeric composition of chiral polychlorinated biphenyl atropisomers in aquatic and riparian biota. Environ. Sci. Technol. 35, 2448–2454. [DOI] [PubMed] [Google Scholar]
- Wu X., Duffel M., Lehmler H. J. (2013). Oxidation of polychlorinated biphenyls by liver tissue slices from phenobarbital-pretreated mice is congener-specific and atropselective. Chem. Res. Toxicol. 26, 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Kammerer A., Lehmler H. J. (2014). Microsomal oxidation of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) results in species-dependent chiral signatures of the hydroxylated metabolites. Environ. Sci. Technol. 48, 2436–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Kania-Korwel I., Chen H., Stamou M., Dammanahalli K. J., Duffel M., Lein P. J., Lehmler H. J. (2013). Metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) atropisomers in tissue slices from phenobarbital or dexamethasone-induced rats is sex-dependent. Xenobiotica 43, 933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Lehmler H. J. (2016). Effects of thiol antioxidants on the atropselective oxidation of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) by rat liver microsomes. Environ. Sci. Pollut. Res. Int. 23, 2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Pramanik A., Duffel M. W., Hrycay E. G., Bandiera S. M., Lehmler H. J., Kania-Korwel I. (2011). 2,2′,3,3′,6,6′-Hexachlorobiphenyl (PCB 136) is enantioselectively oxidized to hydroxylated metabolites by rat liver microsomes. Chem. Res. Toxicol. 24, 2249–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Kania-Korwel I., Ghogha A., Chen H., Stamou M., Bose D. D., Pessah I. N., Lehmler H. J., Lein P. J. (2014). PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol. Sci. 138, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Kalivendi S., Zhang H., Joseph J., Nithipatikom K., Vasquez-Vivar J., Kalyanaraman B. (2003). Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: Potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 34, 1359–1368. [DOI] [PubMed] [Google Scholar]
- Zheng J., Yu L.-H., Chen S.-J., Hu G.-C., Chen K.-H., Yan X., Luo X.-J., Zhang S., Yu Y.-J., Yang Z.-Y., et al. (2016). Polychlorinated biphenyls (PCBs) in human hair and serum from e-waste recycling workers in southern China: Concentrations, chiral signatures, correlations, and source identification. Environ. Sci. Technol. 50, 1579–1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.