While female sex is a risk factor for torsades de pointes,1 29-44% of drug-induced cases occur in men.2,3 Older age is also a risk factor.1 Preclinical studies have shown that testosterone and progesterone protect against drug-induced prolongation of ventricular repolarization, early afterdepolarizations and arrhythmias.4 Oral progesterone shortens QT intervals and attenuates drug-induced QT lengthening in young women.5 We hypothesized that transdermal testosterone and oral progesterone attenuate drug-induced QT interval lengthening in older men.
This prospective, randomized, double-blind, placebo-controlled three-way crossover-design study was approved by the Indiana University IRB and conducted from July 2015 – October 2017 (ClinicalTrial.gov Identifier: ). Exclusion criteria: history of prostate or breast cancer; benign prostatic hyperplasia; weight <60 or >135 kg; potassium <3.6 mEq/L; magnesium <1.8 mg/dL; hematocrit <26%; hepatic transaminases >3x ULN; baseline Bazett’s-corrected QTc >450 ms; heart failure (LVEF <40%); family/personal history of long QT syndrome, arrhythmias or sudden cardiac death; permanently paced ventricular rhythm; taking QT-prolonging drugs or strong CYP3A inhibitors. Subjects provided written informed consent.
Men ≥ 65 years of age received, for 7 days, in randomized order: a) Transdermal testosterone 100mg (1% Androgel®) every morning and 2 placebo capsules every evening, b) Oral progesterone 400mg (2×200mg capsules, Teva Pharmaceuticals) every evening and placebo transdermal gel every morning, or c) Placebo transdermal gel every morning and 2 placebo capsules every evening.
On the morning after the 7th day, subjects received a 10-minute intravenous infusion of the QT-lengthening drug ibutilide (0.003 mg/kg). Three 12-lead ECGs were obtained ~ 1 minute apart at baseline (pre-ibutilide), end-of-infusion, and at 5, 10, 15, 20, 30, and 45 minutes and 1, 2, 4, 6, and 8 hours post-infusion. Lead II QT intervals were measured by one investigator (blinded to treatment phases) using computerized high-resolution electronic calipers and the tangent method. QT and RR intervals were averaged over 3 consecutive complexes. QT intervals were Fridericia (QTF)- and Framingham (QTFram)-corrected. Linear mixed effects modeling was performed, where treatment arms and time periods were fixed effects and the subjects were random effects. When overall p values were significant, pairwise comparisons were performed. Serum concentration data were log-transformed.
Seventy-seven subjects were screened; 16 declined and 39 met ≥1 exclusion criterion. Twenty-two subjects consented; 8 subsequently met ≥1 exclusion criterion. Fourteen subjects were enrolled and completed all study phases. Mean age: 73±6 years (65-86); 13 white, 1 black. Mean weight: 90±16 kg; mean ibutilide dose: 0.27±0.05 mg. There was no significant difference in median (IQR) maximum serum ibutilide concentration between the testosterone, progesterone and placebo phases [955 (679, 1921) vs 1135 (632, 1526) vs 885 (646, 1430) pg/mL, p=0.82]. Median serum testosterone concentration was higher during the testosterone phase [678 (430,1017) vs 258 (227, 276) vs 272 (219,335) ng/dL, p<0.001]. Median serum progesterone concentration was higher during the progesterone phase [18.9 (15.3,23.7) vs 0.45 (0.30,0.60) vs 0.40 (0.30,0.60) ng/mL, p<0.001].
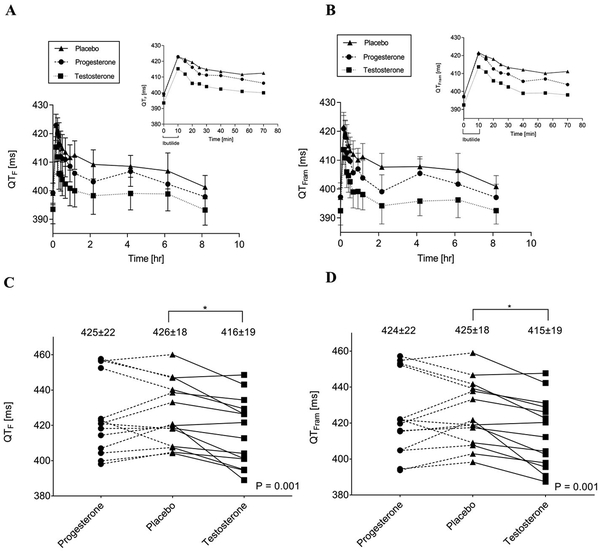
Median pre-ibutilide heart rates (HRs) during testosterone, progesterone and placebo phases were 57 (54, 67) vs 60 (53, 65) vs 58 (55, 66) bpm (p=0.99). HRs were similar, and not significantly different across treatment phases, at the end-of-infusion and one-hour post-infusion. Post-ibutilide QTF and QTFram during testosterone, progesterone and placebo phases are presented in Figure 1, panels A&B. Pre-ibutilide QTF and QTFram were not significantly different between the testosterone, progesterone or placebo phases (QTF: 393±19 vs 399±16 vs 399±13 ms, p=0.09; QTFram: 392±19 vs 397±12 vs 397±18 ms, p=0.22). Maximum post-ibutilide QTF and QTFram intervals were lowest during the testosterone phase (Figure 1, panels C&D). There was no significant difference in maximum QTF or QTFram between progesterone and placebo.
Figure 1.
Effect of transdermal testosterone and oral progesterone on drug-induced QT interval lengthening.
Panel A. Mean QTF intervals during and for 8 hours (± SEM) and 1 hour (inset) after a 10-minute infusion of ibutilide 0.003 mg/kg in testosterone, progesterone and placebo phases, Panel B. Mean QTFram intervals during and for 8 hours (± SEM) and 1 hour (inset) after a 10-minute infusion of ibutilide 0.003 mg/kg in testosterone, progesterone and placebo phases. Panel C. Lead II maximum QTF interval after ibutilide 0.003 mg/kg during progesterone, placebo and testosterone phases (mean ± SD), and Panel D. Lead II maximum QTFram interval after ibutilide 0.003 mg/kg during progesterone, placebo and testosterone phases (mean ± SD).
QTF = Fridericia-corrected QT interval; QTFram = Framingham-corrected QT interval; SD = Standard deviation; SEM = Standard error of the mean
* p<0.002, testosterone vs placebo and testosterone vs progesterone
 Testosterone
Testosterone
 Progesterone
Progesterone
 Placebo
Placebo
Transdermal testosterone decreased the area under the QT interval effect curves (AUEC) one-hour post-ibutilide (AUEC0-1.17, accounting for the 10-minute infusion) compared to progesterone and placebo (QTF: 471±24 vs 480±24 vs 483±18 ms•hr; overall p=0.0003, p≤0.002, testosterone vs placebo and testosterone vs progesterone; QTFram: 469±23 vs 477±25 vs 482±17 ms•hr, overall p=0.0005; p≤0.007 testosterone vs placebo and testosterone vs progesterone). Progesterone did not significantly affect QTF or QTFram AUEC0-1.17 vs placebo.
Transdermal testosterone also attenuated the AUEC0-8.17, indicating a more prolonged effect (QTF: 3255±173 vs 3304±145 vs 3335±142 ms•hr; overall p=0.001, p≤0.02, testosterone vs placebo and testosterone vs progesterone; QTFram: 3234±160 vs 3289±146 vs 3328±130 ms•hr, overall p<0.0001; p≤0.003 testosterone vs placebo and testosterone vs progesterone). Progesterone did not significantly influence QTF AUEC0-8.17 versus placebo (p=0.10). However, the difference between progesterone and placebo on QTFram AUEC0-8.17 was significant (p=0.03). Adverse effects included fatigue (progesterone, n=1) and mild rash (transdermal placebo, n=1).
In conclusion, despite a small sample, our results suggest that transdermal testosterone attenuates drug-induced QT lengthening in older men. We cannot rule out an effect of oral progesterone on attenuation of drug-induced QT lengthening. These findings support larger studies investigating the efficacy, safety and feasibility of transdermal testosterone and oral progesterone for attenuating drug-induced QT interval lengthening in older men with risk factors who require therapy with QT-prolonging drugs.
Acknowledgments
SOURCES OF FUNDING
This study was supported by a Grant-in-Aid from the American Heart Association Midwest Affiliate (15GRNT24470168). This investigation was also made possible in part with support from the Indiana Clinical and Translational Sciences Institute (CTSI) funded in part by Award Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Biospecimens were stored in the CTSI Specimen Storage Facility which is supported, in part, by grant NIH/NCRR RR020128. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Analytical work was performed by the Clinical Pharmacology Analytical Laboratory, a core laboratory of the Indiana University Melvin and Bren Simon Cancer Center, supported by National Cancer Institute Grant P30 CA082709.
Footnotes
Data sharing: Study materials and summary data are available at ClinicalTrials.gov and can be accessed at https://clinicaltrials.gov/ct2/show/NCT02513940?term=Tisdale&rank=2
DISCLOSURES
The authors report no relevant financial, personal or professional relationships with other people or organizations.
REFERENCES
- 1.Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, Kovacs RJ. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/circoutcomes.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore). 2003;82:282–290. [DOI] [PubMed] [Google Scholar]
- 3.Pratt CM, Al-Khalidi HR, Brum JM, Holroyde MJ, Schwartz PJ, Marcello SR, Borggrefe M, Dorian P, Camm AJ. Cumulative experience of azimilide-associated torsades de pointes ventricular tachycardia in the 19 clinical studies comprising the azimilide database. J Am Coll Cardiol. 2006;48:471–477. [DOI] [PubMed] [Google Scholar]
- 4.Kurokawa J, Kodama M, Clancy CE, Furukawa T. Sex hormonal regulation of cardiac ion channels in drug-induced QT syndromes. Pharmacol Ther. 2016;168:23–28. doi: 10.1016/j.pharmther.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisdale JE, Jaynes HA, Overholser BR, Sowinski KM, Flockhart DA, Kovacs RJ. Influence of oral progesterone administration on drug-induced QT interval lengthening. A randomized, double-blind, placebo-controlled crossover study. JACC Clin Electrophysiol. 2016;2:765–774. doi: 10.1016/j.jacep.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]