Abstract
Extensive efforts have been made to explore how the activities of multiple brain cells combine to alter physiology through imaging and cell-specific manipulation in different animal models. However, the temporal regulation of peripheral organs by the neuroendocrine factors released by the brain is poorly understood. We have established a suite of adaptable methodologies to interrogate in vivo the relationship of hypothalamic regulation with the secretory output of the pituitary gland, which has complex functional networks of multiple cell types intermingled with the vasculature. These allow imaging and optogenetic manipulation of cell activities in the pituitary gland in awake mouse models, in which both neuronal regulatory activity and hormonal output are preserved. These methodologies are now readily applicable for longitudinal studies of short-lived events (e.g., calcium signals controlling hormone exocytosis) and slowly evolving processes such as tissue remodeling in health and disease over a period of days to weeks.
In the past decade, there has been an exponential increase in the technical development of novel tools allowing interrogation of the functional interactions of the complex architecture of the mammalian brain in health and disease. These have principally been developed in mouse models, where both organization and function of the brain largely recapitulate that of higher mammals, including humans (1). The availability of a wide range of genetically modified mice, combined with novel virus-based approaches to infect specific mouse brain regions, has allowed identification of specific cell types, manipulation of neuronal circuits with optogenetic techniques, and in vivo monitoring of cell activity. Combining these with recently developed optical techniques, such as the use of a gradient-index (GRIN) lens for imaging deep brain regions (2), has resulted in rapid mapping of the activity and connectivity of neuronal networks (3). Although the mammalian brain is exceptionally complex, the increasing prevalence of neurologic and neuropsychiatric defects has recently inspired large-scale research programs, such as the National Institutes of Health Brain Research through Advancing Innovative Neurotechnologies Initiative (4, 5), to meet this challenge.
The brain does not simply work as an isolated unit but forms a functional continuum with other physiological processes (6), especially with the endocrine systems that control basic body functions (7, 8). These endocrine systems share complex functional features with the brain, such as hierarchal multicellular organization [e.g., presence of “hub” cells, which control neighbors (9, 10)], adaptive plasticity (11), and long-term memory (9), suggesting that studies of their function would benefit from application of the novel tools and techniques developed for neuroscience.
This is exemplified by the pituitary gland, which acts as an intermediate between the brain and the periphery, with endocrine and neural lobes (nerve terminals emanating from hypothalamic vasopressin and oxytocin neurons) connected to the brain by the pituitary stalk and surrounded by brain meninges (see Fig. 1). Interest in monitoring the in vivo function of this gland has recently been increased by large-scale ex vivo imaging, which has revealed three-dimensional cell networks that are structurally and functionally organized within the endocrine anterior pituitary (also called the pars distalis); this cell network connectivity is essential for normal gland development (14), coordination of gene expression (15), and pulsatile release of hormones to the periphery (8). To date, in vivo studies have been limited by the location of the pituitary on the ventral side of the brain, with extensive microsurgery required to expose the gland through the palate bone in terminally anesthetized mice to record and manipulate cell function (12). These surgical procedures preclude both longitudinal studies and functional investigation in awake mice.
Figure 1.
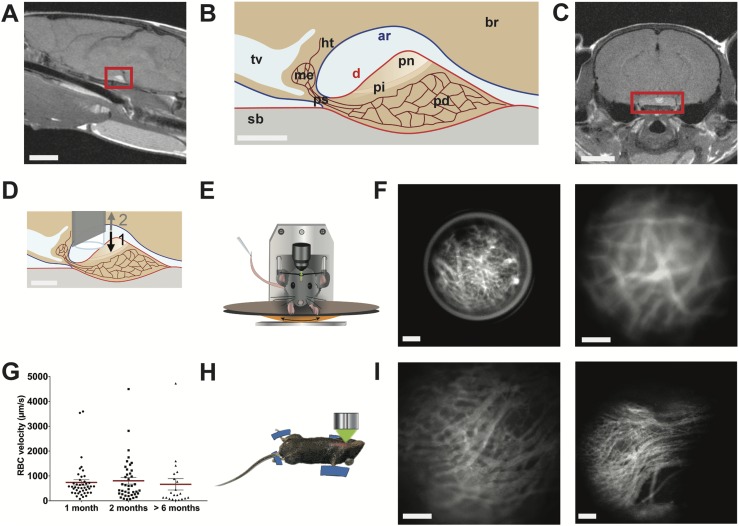
In vivo imaging of pituitary blood flow in the awake mouse. (A) Sagittal MRI view of the midbrain from a female mouse. Red rectangle indicates pituitary location below the ventral side of the brain. Scale bar, 3 mm. (B) Drawing of a sagittal view of the hypothalamic-pituitary system. Scale bar, 300 µm. (C) Coronal MRI view of the brain of a female mouse. Red rectangle indicates pituitary location. Scale bar, 3 mm. (D) Schema showing the GRIN lens implantation in the arachnoid matter region above the dorsal side of the pituitary. (1) Downward and (2) upward arrows indicate the sequential needle movements when the GRIN lens is positioned above the pituitary. Scale bar, 300 µm. (E) Head-fixed in vivo imaging of an awake mouse implanted with a GRIN lens, which provides an optical relay between the microscope and the pituitary gland. (F) Head-fixed in vivo imaging of the pituitary capillaries at low (left panel) and high (right panel) magnifications; representative image of n = 5 female mice. Scale bar, 100 µm. (G) Example of longitudinal monitoring of red blood cell (RBC) velocities in the same pituitary field viewed from 1 to 6 mo after GRIN lens implantation; n = 21 to 43 vessels analyzed per animal; n = 4 female mice. Also represented are means ± SEM (red lines). (H) Schematic arrangement of the ventral in vivo imaging approach in terminally anesthetized mice (12). (I) Ventral in vivo imaging of pituitary capillaries at the level of the pituitary parenchyma (left panel) and entrance (right panel) of different male mice. Scale bar, 100 µm. Data can be viewed in materials saved to an online repository (13). ar, arachnoid mater (in blue); br, brain; d, dura mater (in red); Ht, hypothalamus; me, median eminence; pd, pars distalis; pi, pars intermedia; pn, pars nervosa; ps, pituitary stalk; sb, sphenoidal bone; tv, third ventricle.
Here, we describe a tool kit for imaging and manipulating pituitary cells in vivo over periods of days to weeks in awake mouse models. We have used these tools to image the dynamics of pituitary microvascular function and cell signaling (calcium events), locally express exogenous proteins through injection of viral constructs within the parenchyma, and optogenetically manipulate specific cell networks while monitoring their secretory outputs into the bloodstream. This range of techniques allows analysis of the pituitary gland in awake mammalian models in unparalleled detail, complementing large-scale studies of the brain to further understand neural control of complex physiological systems via endocrine signals.
Materials and Methods
Animals
Tg(Gh1-cre)bKnmn (called GH-Cre; R. Kineman, Jesse Brown Veterans Administration Medical Center, Chicago, IL) (16), ROSA26-fl/fl-ChR2-dtTomato, and wild-type C57BL/6 mice (6 to 12 weeks old), as indicated in figure legends, were housed in a 12-hour light/12-hour dark cycle (lights on at 0800 hours and off at 2000 hours) with food and water available ad libitum. All animal procedures were approved by the local ethical committee under agreement CEEA-LR-12185 according to European Union Directive 2010/63/EU. Because this study included only one experimental group of animals, no randomization or blinding was required.
Stereotaxic injections of adeno-associated virus
Adult GH-Cre and wild-type C57BL/6 mice were anesthetized with ketamine/xylazine (0.1/0.02 mg/g), placed in a stereotaxic apparatus, and given bilateral 1-μL injections of adeno-associated virus (AAV) 5–cytomegalovirus β-actin β-globin (CAG)–dflox-GCaMP6s-WPRE-SV40 (2.52 × 1013 GC/mL; Penn Vector Core, University of Pennsylvania, Philadelphia, PA), AAV5-CAG-GCaMP6s-WPRE-SV40 (2.23 × 1013 GC/mL; Penn Vector Core), AAV2-CAG-GFP (gift from Margarita Arango, IGF, Montpellier, France), rAAV5/sspEMBOL-CBA-GFP (8 × 1012 GC/mL; UNC Vector Core, University of North Carolina, Chapel Hill, NC), rAAV8/sspEMBOL-CAG-GFP (8 × 1012 GC/mL; UNC Vector Core), or rAAV9/sspEMBOL-CAG-GFP (9.2 × 1012 GC/mL; UNC Vector Core) into the pituitary gland at a rate of 100 nL/min. Coordinates were −2.5 mm anteroposterior, ±0.4 mm lateral to midline, pointed as zero at the superior sagittal sinus. Two dorsoventral positions were used for injection, 50 µm and 400 µm over the sella turca, −6.15/5.75 mm for ventral injection and −5.6/5.3 mm for dorsal injection. Experiments were conducted from 4 weeks on after injection.
Optical imaging through a GRIN lens in awake head-fixed mice
Adult mice were anesthetized with ketamine/xylazine (0.1/0.02 mg/g) and placed in a stereotaxic apparatus to implant a GRIN lens (0.6-mm diameter, 1.5 pitch, 7.5-mm length, and 150-µm working distance; GRINTECH, Jena, Germany) immediately above the pituitary gland. After a large part of the skull was exposed, the GRIN lens was placed in a 20G1/2 gauge needle (ultra-thin wall; Terumo, Somerset, NJ), with movement restricted by placing a metal rod above it. The needle was inserted at the coordinates −2.5 mm anteroposterior, ±0.4 mm lateral to midline pointed as zero at the superior sagittal sinus, and −5.5/5.1 mm dorsoventral. Then, the needle was removed with the metal rod kept in place so the GRIN lens stayed in place at the dorsal side of the pituitary. Finally, the metal rod was removed. The GRIN lens and a headplate were fixed with UV-retractable cement. Prior to and starting from 2 weeks after surgery, mice were habituated to the wheel and the headplate fixation system under the microscope every 2 to 3 days. Four weeks after surgery, the mice were placed on the wheel, the headplate was fixed, and fluorescence imaging was performed using a stereomicroscope (Zeiss Discovery V.12; Jena, Germany) that was fitted with a fluorescence lamp (Lambda LS; Sutter Instrument Company, Novato, CA), a shutter (Lambda 10-B Smart Shutter; Sutter Instrument Company), and a CMOS ORCA Flash 4.0 camera (C11440; Hamamatsu, Massy, France), all controlled with MetaMorph 7.8.9 software (Molecular Devices, Berkshire, UK).
In vivo imaging in terminally anesthetized mice
Details of the methods can be found in Lafont et al. (12). In brief, male 2- to 4-month-old transgenic GH-Cre mice and GH-ROSA26-fl/fl-ChR2-dtTomato mice on a C57Bl6 background were anesthetized by inhalation of isoflurane (1.5% in O2). After the mandibular symphysis was divided, the mucosa overlying the hard palate was parted by blunt dissection under a stereomicroscope to expose an area of palatal periosteal bone. This was thinned with a felt polisher (drill; World Precision Instruments, Sarasota, FL) and then removed with a hook and forceps. The exposed surface of the pituitary gland, visible through the hole in the bone, was continuously superfused with a physiological solution.
In vivo monitoring of blood flow and calcium signals
Mice underwent surgery (see previous section) to visualize either the ventral side (terminally anesthetized animals) or the dorsal side (awake animals) of the pituitary gland. With use of the ventral approach, 100 µL of tetramethylrhodamine isocyanate 150 kDa dextran (Sigma-Aldrich, St. Louis, MO) was injected into the jugular vein or in the retro-orbital sinus for GRIN lens approach. Imaging of blood flow was performed at 150 to 200 frames per second using 545-nm excitation and 570-nm emission filters. When calcium signals were recorded in vivo, experiments were performed as described previously 4 weeks after stereotaxic injection of GCAMP6s-expressing AAV5. Multicellular calcium imaging was typically performed at two to four frames per second, using 480-nm excitation and 520-nm emission filters.
Optogenetic photostimulation in awake mice
GH Cre × ROSA26-fl/fl-ChR2-dtTomato mice were anesthetized with ketamine/xylazine (0.1/0.02 mg/g) and placed in a stereotaxic apparatus to implant an optical fiber (diameter: 200 μm; Doric Lenses, Quebec, Canada) immediately above the pituitary gland (stereotaxic coordinates described previously). The optical fiber was fixed using UV-retractable cement. Two weeks later, an optical fiber was connected to the one previously implanted, and laser stimulation (488 nm) was delivered at 10 mW using various patterns (frequency: 1Hz; exposure time: 300 ms) while blood samples were collected as described in the following section.
GH pulse profiling in mice and GH ELISA
A tail-tip blood collection procedure was used to sample blood from C57BL/6 adult mice or transgenic GH-Cre mice; 3-μL blood samples were analyzed for GH content by ELISA (17).
iDISCO+
Pituitary glands were removed and fixed by overnight immersion in 4% paraformaldehyde. For immunofluorescence labeling and clearing, an iDISCO+ clearing protocol was used as described in detail elsewhere (18). Primary antibodies were rat anti-Meca32 (catalog no. 550563; 1:100; BD Biosciences, San Jose, CA; RRID: AB_393754) (19); guinea pig anti-GH (catalog no. AFP12121390; 1:2500, National Institute of Diabetes and Digestive and Kidney Diseases–National Hormone and Pituitary Program (NIDDK-NHPP), Torrance, CA; RRID: AB_2756840) (20), and rabbit anti-GFP (catalog no. A-6455; 1:250; Molecular Probes–Thermo Fisher, Waltham, MA; RRID: AB_221570) (21). Secondary antibodies were anti-rat Alexa 647 (catalog no. 712-606-150; Jackson ImmunoResearch Laboratories, Ely, UK; RRID: AB_2340695) (22), anti‒guinea pig Alexa 510 (catalog no. 706-166-148; Jackson ImmunoResearch Laboratories; RRID: AB_2340461) (23), and anti-rabbit Alexa 488 (catalog no. A-21206; Molecular Probes–Thermo Fisher; RRID: AB_141708) (24) (1:2000). After clearing, transparent pituitary glands were mounted in well glass slides (065230; Dominique Dutscher, Brumath, France) in diBenzyl ether (Sigma-Aldrich). Coverslips were sealed with nail varnish.
Immunofluorescence staining in fixed pituitary slices
Pituitary glands were collected from terminally anesthetized mice and fixed by overnight immersion in 4% paraformaldehyde at 4°C; serial cuts were done at 40 µm‒thick tissue sections using a vibratome (Leica, Wetzlar, Germany). Combinations of the following antibodies were used: guinea pig anti-GH (catalog no. AFP12121390; NIDDK-NHPP; RRID: AB_2756840) (20), LH (catalog no. rLHb, also AFP571292393; NIDDK-NHPP; RRID: AB_2665511) (25), prolactin (catalog no. AFP65191; NIDDK-NHPP; RRID: AB_2756841) (26), TSH (catalog no. AFP9370793; NIDDK-NHPP; RRID: AB_2756856) (27), or ACTH (catalog no. AFP71111591; NIDDK-NHPP; RRID: AB_2756855) (28) (dilution: 1:2500), rabbit anti-GFP (catalog no. A-6455; 1:250; Molecular Probes; RRID: AB_221570) (21), and rabbit anti-RFP (catalog no. 600-401-379; 1:500; Rockland, Limerick, PA; RRID: AB_2209751) (29). Primary antibody incubation was performed in PBS, 0.1% Triton X-100 (Sigma-Aldrich), 2% BSA (Sigma-Aldrich) at 4°C for 48 hours. Sections were then incubated with secondary antibodies for 2 hours at room temperature.
Secondary antibodies were anti-rabbit Alexa 488 (catalog no. A-21206; Molecular Probes; RRID: AB_141708) (24), anti-guinea pig Alexa 510 (catalog no. 706-166-148; Jackson ImmunoResearch Laboratories; RRID: AB_2340461) (23), anti-rat Alexa 647 (catalog no. 712-606-150; Jackson ImmunoResearch Laboratories; RRID: AB_2340695) (22), anti‒guinea pig Alexa 488 (catalog no. 706-545-148; Jackson ImmunoResearch Laboratories; RRID: AB_2340472) (30), and anti-rabbit 510 (catalog no. 711-166-152; Jackson ImmunoResearch Laboratories; RRID: AB_2313568) (31) (1:2000 in PBS, 0.1% Triton X-100, 2% BSA).
Confocal imaging
Fluorescence images of both sliced pituitaries and whole clarified pituitaries were acquired on a Zeiss LSM 780 confocal microscope with 20×, 40×, and 63× objectives. Images were analyzed using Imaris (Bitplane, Zurich, Switzerland).
MRI image acquisition from the mouse brain
Animals were scanned on a 9.4T Agilent Varian MRI scanner (Agilent, Santa Clara, CA). A volumic RF43 antenna (Rapid Biomedical, Rimpar, Germany) was used. For image acquisition, mice were anesthetized with isoflurane, and their heads were secured with bite and ear bars. Respiration rate and heart rate were monitored. Animals were scanned using a spin-echo sequence with the following parameters: repetition time, 500 ms; echo time, 10 ms; 1 echo, averaging 16 times, matrix of 256 × 256 pixels in a field of view of 30 × 30 mm, slice thickness 0.5 mm. Total imaging time was 34 minutes.
Analysis
Blood flow changes were estimated from red blood cell velocities as previously described (12) and analyzed using a two-tailed variance ratio test followed by a Mann-Whitney U test for any differences directly attributable to treatment application. Estimation of decay time (τ = 5 seconds) from calcium signals (27 single calcium transients) recorded in vivo was used to generate simulated calcium rises due to trains of calcium spikes firing at frequencies of either 0.4 or 1 Hz. Spike frequencies high enough (1 Hz) to generate robust plateau rises in cytosolic calcium (13) then guided selection of appropriate frequencies of laser light pulses during optogenetic experiments.
Results
Longitudinal optical monitoring of pituitary blood flow in awake mice
Unraveling the intricacies of pituitary function with cellular in vivo imaging studies lasting days to weeks requires optical access to the gland while maintaining both its integrity and that of surrounding tissue. The location of the pituitary (Fig. 1A‒1C; sagittal and coronal MRI sections of mouse heads and relative schemas, respectively) suggested that the least invasive strategy would be insertion of a GRIN lens through the cortex toward the dorsal side of the pituitary using a stereotaxic frame in anesthetized animals. To overcome the major challenge of crossing the meninges covering the ventral brain without damaging the nearby pituitary tissue (Fig. 1B), the GRIN lens was inserted into the lumen of a needle, which was then retracted once the GRIN lens was located correctly (Fig. 1D). The GRIN lens was then fixed to the cranium with UV-retractable cement, and a titanium bar with a central opening for the lens was attached to the skull.
After at least 3 to 4 weeks of mouse habituation to being head-fixed under a stereomicroscope fitted with a 20× objective, with the body and limbs able to move on a treadmill (Fig. 1E), pituitary blood flow was imaged for 0.5 to 2 hours in animals preinjected in the retro-orbital sinus with fluorescent 150-kDa dextran (Fig. 1F) (13). These in vivo imaging sessions were repeatable every 3 to 4 days and up to several months after GRIN lens implantation, with no alteration in blood flow as assessed by measurements of red blood cell velocities (Fig. 1G). Imaging pituitary blood flow in awake mice using a GRIN lens with a numerical aperture of 0.5 provided image resolution similar to that obtained in terminally anesthetized animals with ventral surgery and imaged with a long-range (2 cm working distance, numerical aperture 0.5) objective (12) (Fig. 1H and 1I). All imaging sessions were performed between 1 and 6 months after GRIN lens implantation without noticeable changes in pituitary function, according to preservation of endogenous hormone rhythms (13). Thus implantation of thin GRIN lenses through two layers of meninges, one at the level of the cortex and the other covering the ventral side of the brain, allowed long-lasting in vivo imaging of the dorsal side of the pituitary while preserving characteristic features of pituitary function.
Selective viral delivery and fluorescent protein expression in the pituitary parenchyma
Local stereotaxic delivery for expression of specific genes [e.g., by viral transduction (2)], has been an important tool for monitoring the activities of cells in selective brain regions. Although this approach has been applied to very large pituitary tumors by trans-auricular injection (32, 33), it has not been described in the pituitary of healthy mice. We developed stereotaxic delivery of viral particles that could easily be combined with in vivo imaging using GRIN lenses with minimal pituitary damage. We first inserted vertically the AAV-containing needle via the cortex and then positioned the needle tip to touch the palate bone. After a wait of 5 minutes, the needle was retracted by 50 µm and 400 µm to target the ventral and dorsal regions of the pituitary, respectively (Fig. 2A). AAV particles were then injected using a controlled pneumatic pump to transduce cells with an expression cassette encoding the calcium sensor GCAMP6s (34) or GFP under the control of the strong ubiquitous CAG promoter.
Figure 2.
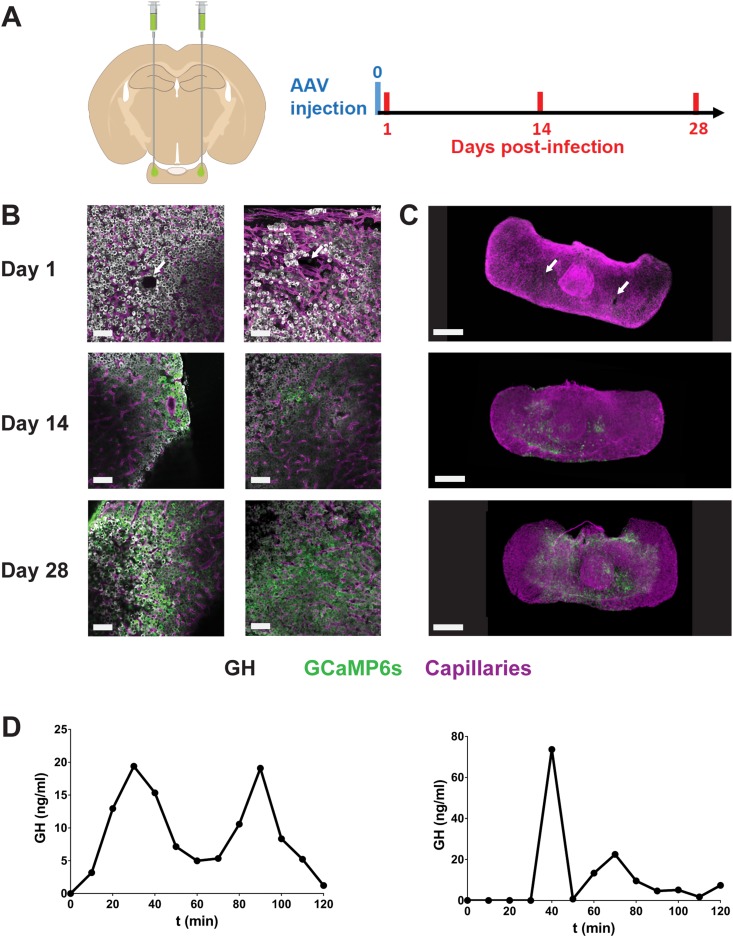
AAV injection into the pituitary. (A) Following bilateral AAV injection in anesthetized mice, pituitaries were dissected from terminally anesthetized animals from 1 to 28 d after GCAMP6s-expressing AAV5 injection, fixed, and subjected to immunostaining and imaging. (B) Pituitary sections and (C) whole gland (iDISCO+ protocol) are shown. Immunostaining for GH (cells pseudocolored in white), GCAMP6s (green), and MECA32 (a marker of fenestrated capillaries; magenta) was performed; representative images of n = 2 to 4 male mice per condition. White arrows indicate presumed needle tissue damage. Scale bars, 50 µm (left panels) and 300 µm (right panels). (D) Endogenous GH pulses before and 1 mo after AAV5 injection in the same animal. Blood samples (3 µL) were collected every 5 min at the tail-tip, and GH content was then measured using a high-sensitivity ELISA assay. Data can be viewed in materials saved to an online repository (13).
Virus was routinely injected in both pituitary “wings” (lateral regions are 500 to 700 µm thick). Pituitaries were then dissected and fixed 1, 14, and 28 days after viral injection (Fig. 2B and 2C). Although a small region of tissue damage was apparent 1 day after AAV injection using a needle with an outer diameter of 210 µm, this was markedly reduced or absent 2 weeks postinjection and apparently fully repaired after 4 weeks. Pituitary tissues were immunostained for fenestrated vessel markers (MECA32), pituitary hormones (e.g., GH), and GCAMP6s in thick pituitary sections (Fig. 2B; top left panels), and tissue was clarified with the iDISCO+ protocol (Fig. 2C; top right panel) (18). This showed that expression of AAV-CAG‒expressed GCAMP6s could be detected 2 weeks postinjection (Fig. 2B and 2C; middle panels) but was increased and more extensive after 4 weeks (Fig. 2B and 2C; bottom panels). Consistent with the apparently complete tissue recovery 1 month after AAV injection (Fig. 2B and 2C), endogenous (Fig. 2D) and hormonal responses to hypothalamic agonists (13) were unaltered after stereotaxic injection of AAV.
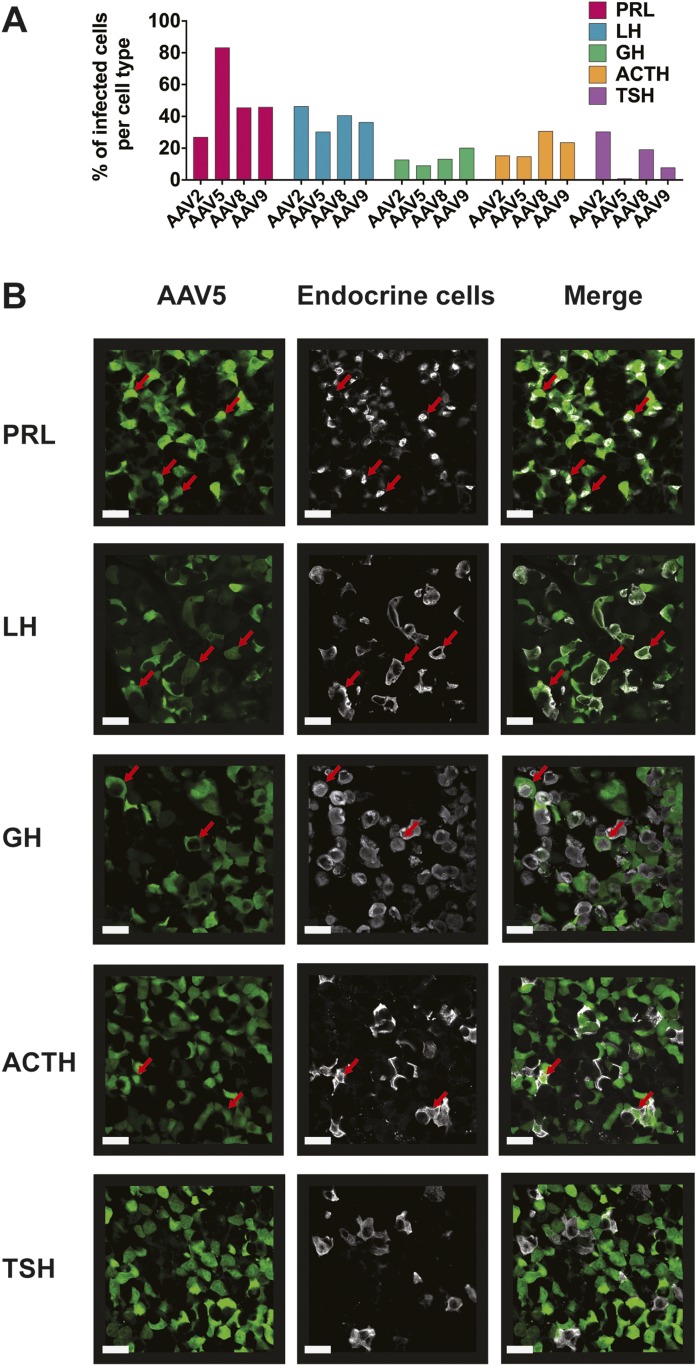
Because the pituitary gland contains five endocrine cell types that secret specific hormones (prolactin, LH/FSH, GH, ACTH, and TSH), we tested the efficiency of viral transduction in each of these by a range of AAV serotypes expressing CAG promoter‒driven GFP. All pituitary hormonal cell types were transduced with variable efficiency depending on AAV serotype (Fig. 3) (13). For all AAV serotypes, expression of GFP could readily be detected by immunostaining from constructs utilizing a CAG promoter but not those with a cytomegalovirus promoter (data not shown).
Figure 3.
Percentage of infected cells per pituitary cell type. (A) Infection efficiency by different AAV serotypes (2, 5, 8, 9) of endocrine cell types (six tissue sections per pituitary; n = 3 female mice). The percentage of infected cells was counted under microscopic observation within each field of infected cells. (B) Examples of colabeling of endocrine pituitary cells infected by AAV5-CAG-GFP particles (fixed pituitary sections followed by dual immunostaining against hormones and GFP). Scale bars, 20 µm. Data can be viewed in materials saved to an online repository (13). Red arrows indicate infected cells that were immunopositive for a hormone.
Pituitary calcium signals in awake mice
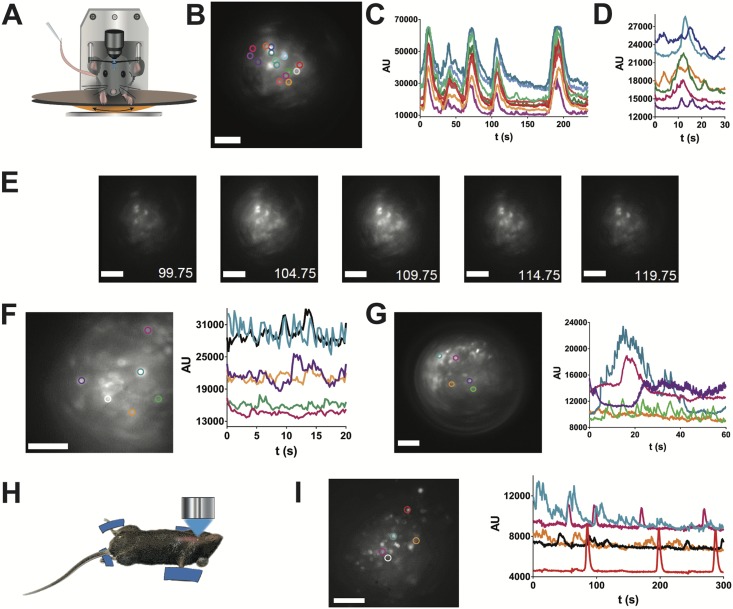
Having successfully transduced pituitary cells with constructs expressing GCAMP6s by stereotaxic injection of AAV5-CAG-GCAMP6s, we then explored whether this could be used to monitor multicellular calcium signals in awake mice after AAV injection. GRIN lenses were implanted above the dorsal pituitary at the site where AAVs had previously been injected stereotaxically. One month after GRIN lens implantation, it was possible to monitor a wide range of profiles of pituitary calcium transients in awake mice (Fig. 4A‒4G)(13), with evidence of cell-cell coordination (Fig. 4B‒4E) (13), similar to that previously reported in ex vivo studies on pituitary slice preparations (9, 11, 35). Of note, similar calcium activity was detected in vivo using a ventral imaging approach in anesthetized mice (12) that had been injected with AAV5-CAG-GCAMP6s (Fig. 4H and 4I), suggesting that GRIN lens implantation does not affect calcium signaling.
Figure 4.
In vivo calcium imaging in pituitary cells in the awake mouse. (A) Schematic shows the arrangement of calcium imaging in head-fixed animals injected with AAV5-CAG-GCAMP6s particles into the pituitary. (B) Field of GCAMP6s cells viewed from the dorsal pituitary side with the selection of cells as regions of interest (ROIs) shown in colored circles. Scale bar, 40 µm; representative image of n = 3 female mice. (C) Coordinated calcium spikes recorded in the cells shown in (B). (D) Calcium spikes recorded at 10 frames per second in the cells shown in (B). (E) Mosaic of GCAMP6s images (bottom right, recording time in seconds) shows a coordinated increase in calcium spike firing. Scale bar, 50 µm. (F and G) Two examples of calcium recordings in other female animals injected with AAV5-CAG-GCAMP6s particles. ROIs are shown in colored circles. Scale bar, 100 µm. (I) Calcium signals in (H) pituitary cells imaged from the ventral side in a terminally anesthetized animal; representative image and traces of n = 2 male mice. ROIs are shown in colored circles in (I). Scale bar, 100 μm.
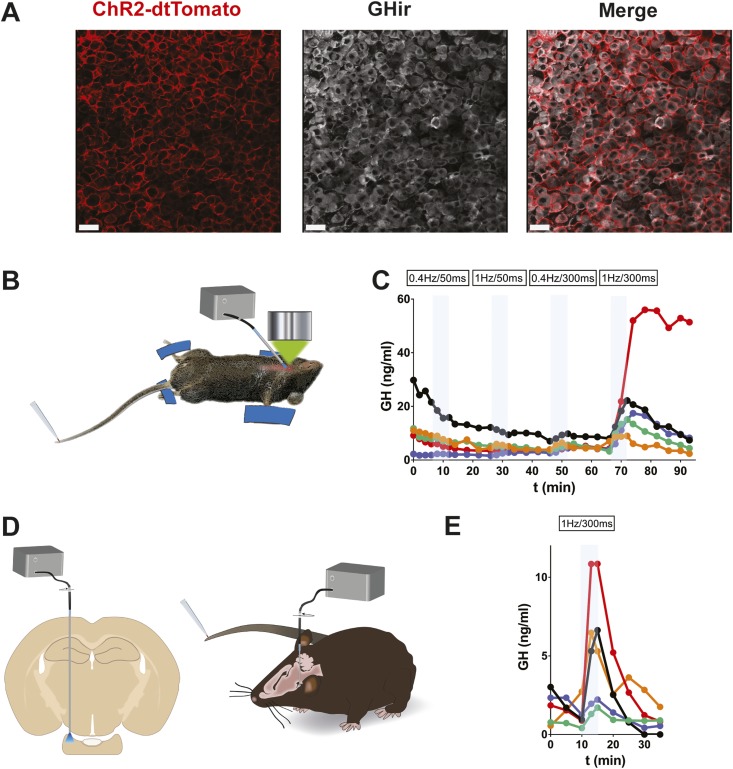
Optogenetic manipulation of pituitary hormone pulsatility in awake mice
The ability to implant lenses and optical devices into the pituitary of awake mice also enables control of the secretory activity of pituitary cells. For this, we used a Cre-lox strategy by crossing GH-Cre and R26-fl-fl-ChR2-dtTomato mice, resulting in expression of ChR2 specifically in somatotrophs (GH-ChR2) (Fig. 5A). To determine which blue laser illumination pattern was efficient at triggering hormone output from somatotrophs, we used the ventral imaging approach in anesthetized mice to stimulate the pituitary cells with a 400-µm-diameter fiber optic positioned close to the pituitary surface (Fig. 5B) and measured GH in blood samples collected from the tail (Fig. 5C). The requirement for a 1-Hz stimulation for 300 ms to elicit a robust output of GH agrees with simulations of the generation of sustained trains of calcium spikes based on from in vivo calcium spike kinetics (13). Application of this pattern of laser light triggered GH pulses in awake GH-ChR2 mice chronically implanted with an optical fiber, which was located above the dorsal side of the pituitary (Fig. 5D and 5E).
Figure 5.
Optogenetic stimulation of GH pulses in vivo. (A) Colabeling of dtTomato and GH in the pituitary from a GH-Cre mouse injected with Cre-activated AAV5-GAG-ChR2-dtTomato particles. (B) Laser light illumination of the ventral pituitary side in terminally anesthetized mice subjected to tail-tip blood sampling (3 µL every 3 min). (C) In experimental conditions as in (B), trains of blue laser light pulses (300-ms pulses at 1 Hz) triggered GH pulses (n = 5 male mice). (D) Laser light illumination of the pituitary in the awake mouse in which tail-tip blood sampling was carried out. (E) GH pulses triggered by a train of laser light pulses (300-ms pulses at 1 Hz) in GH-Cre mice injected with Cre-selective AAV5-GAG-ChR2-dtTomato particles (n = 5 male mice). Data can be viewed in materials saved to an online repository (13).
Discussion
By adapting stereotaxic approaches to access the ventral side of the brain, we successfully applied a wide range of tools and techniques for imaging and manipulating specific cell activities in the pituitary gland of awake mice. These technical developments now allow the study of the function of this gland and its intimate relationship with the brain in health and disease at a level hitherto unachievable in awake animal models. Analysis of dynamic pituitary function over periods of days to months in animals with intact interactions between multiple organs will provide important insights into a range of conditions with dysregulated physiological function, which may occur at different levels within an axis. For example, it is unclear to what extent altered pituitary, hypothalamic, or ovarian function contributes to dysregulated LH secretion, which is a hallmark of polycystic ovarian syndrome, the most common endocrine pathology in reproductive aged females [prevalence of 7% to 15% in premenopausal women (36)].
Live imaging with multicellular resolution in GRIN lens‒implanted awake mice is well suited to real-time studies of cell signals, as illustrated here with calcium signals that are essential for hormone exocytosis (8), and can be used to monitor cell-cell communication within the variety of intermingled cell networks wiring the gland (14). Because multicellular signal events can be directly combined with frequent blood microsamples and high-sensitive hormone ELISA (17), online monitoring of “stimulus-secretion” coupling (37, 38) is now achievable at the organ (pituitary) level in awake animals, avoiding the well-described blunting of hypothalamic inputs by anesthetics (12). In addition, these studies will be augmented by combining laser light control of cell functions with monitoring of cell activity within the same field of view of the GRIN lens, which is now possible given the efficiency of optogenetic tools to control pituitary cell networks.
Online monitoring and manipulation of in vivo stimulus-secretion coupling is now readily applicable to answer long-standing questions concerning pituitary gland integration of both brain and peripheral signals for the generation of pulsatile hormonal output. For example, it is now clear that dynamic pulses of corticotroph ACTH output is generated by a combination of both hypothalamic (corticotropin-releasing factor and vasopressin) inputs and negative cortisol feedback (39). Future use of miniature imaging systems in GRIN lens‒implanted animals (3) will allow monitoring and manipulation of corticotroph cell activity that regulates the stress axis, with simultaneous modification of environmental conditions in freely moving mouse models and the study of behavioral effects. To date, such interrogation of the role of pituitary corticotrophs in the stress axis has been restricted to simpler animal models, such as larval zebrafish (40), which lack delivery of hypophysiotropic input via a portal blood system and thus may differ from humans in important aspects (8). An ability to manipulate pituitary cell output via optogenetic stimulation and/or inhibition will also allow dissection of the role of specific patterns of pituitary hormone output [e.g., the sexually dimorphic GH-dependent regulation of liver gene expression (41)]. Male and female GH secretion patterns can now be optogenetically triggered irrespective of animal sex.
A remarkable feature of this suite of tools is their capacity to allow long-term pituitary imaging and manipulation in awake animals. With the restriction of studying adult animals, both short-lived cell events (as discussed previously) and slowly evolving remodeling of the tissue, as in angiogenesis and expansion/shrinkage of a cell population, can now be examined over weeks to months in individual animals, which act as their own controls (42). This will be especially relevant for visualizing and studying online potential repopulation of the pituitary with stem cells/progenitors (43–45) (e.g., fluorescent cells locally injected in immune-suppressed mice), which have the potential to restore cell populations in the hypoplastic pituitary. It will also be possible to explore the function of either sick or healthy tissue zones within one pituitary by local injection of tumor cells or a virus encoding a CRISPR-driven gene mutation in Cas9-expressing mice (46, 47).
In summary, the ability to image at multiple time scales and manipulate the pituitary gland enables the interrogation of its function in awake mammalian models and the study of how it delivers highly ordered hormone pulses essential for controlling body functions such as reproduction, growth, stress, and metabolism. Because endocrine cells can be photo-painted in situ (10), longitudinal in vivo studies will give access to the history of cells (48) and how they interact with neighbors in their native environment (9, 49). Single-cell multiomics, an approach that includes transcriptomics, epigenomics, and proteomics (50), would then be applicable to individual pituitary cells, which have been monitored for days to months in awake mouse models. Together with these newly developed single cell‒level techniques, application of our cellular in vivo imaging and manipulation toolkit to longitudinal studies of awake animal models will provide a unique ability to explore the origin and development of pituitary hormone defects.
Acknowledgments
We thank Margarita Arango (IGF, Montpellier, France) for helpful comments and suggestions about AAV experiments; Jerome Lecoq (Allen Institute) for advice about the use of GRIN lenses, Danielle Carmignac (National Institute for Medical Research–Medical Research Council, London, UK) for helpful suggestions about AAV injections; Yan Chastagnier (IGF, Montpellier, France) for help and advice about image analysis; and Muriel Asari for her schematic rendition of technical setups. Antibodies and recombinant mouse growth hormone and prolactin were supplied by Dr. A.F. Parlow and the NIDDK–NHPP (Torrance, CA). We also thank all members of the Montpellier core facilities IPAM and BioNanoNMRI for their unconditional support and thoughtful comments during the course of this work.
Financial Support: The authors were supported by grants from the Biotechnology and Biological Sciences Research Council, United Kingdom (BB/N007026/1) (to P.L.T.); the US Department of Veterans Affairs, Office of Research and Development Merit Award BX001114, and National Institutes of Health Grant No. R01DK088133 (to R.D.K); Junta de Andalucía (CTS-1406, BIO-0139), Instituto de Salud Carlos III (PI16/00264) (to R.M.L.); ANR-CONACyT 273513, Estancia Sabática apoyada con el Programa PASPA-DGAPA Universidad Nacional Autónoma de México (T.F.C.); the Agence Nationale de la Recherche (ANR 12 BSV1 0032-01, ANR-15-CE14-0012-01), France-Bioimaging (INBS10-GaL/AR-11/12), Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Université de Montpellier, and Fondation pour la Recherche Médicale (DEQ20150331732) (to P.M.). O.H. was supported by a fellowship from Fondation pour la Recherche Médicale (FDT20160435494).
Glossary
Abbreviations:
- AAV
adeno-associated virus
- CAG
cytomegalovirus β-actin β-globin
- GRIN
gradient-index
- NIDDK-NHPP
National Institute of Diabetes and Digestive and Kidney Diseases–National Hormone and Pituitary Program
Additional Information
Current Affiliation: O. Hoa’s current affiliation is the Center for Interdisciplinary Research in Biology, Collège de France, Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, PSL, Université Paris-Sciences-et-Lettres, 7523 Paris, France.
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80(3):751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deisseroth K, Schnitzer MJ. Engineering approaches to illuminating brain structure and dynamics. Neuron. 2013;80(3):568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, Murthy VN, Dulac C.. Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell. 2017;171(5):1176–1190.e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ecker JR, Geschwind DH, Kriegstein AR, Ngai J, Osten P, Polioudakis D, Regev A, Sestan N, Wickersham IR, Zeng H. The BRAIN Initiative Cell Census Consortium: lessons learned toward generating a comprehensive brain cell atlas. Neuron. 2017;96(3):542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jorgenson LA, Newsome WT, Anderson DJ, Bargmann CI, Brown EN, Deisseroth K, Donoghue JP, Hudson KL, Ling GS, MacLeish PR, Marder E, Normann RA, Sanes JR, Schnitzer MJ, Sejnowski TJ, Tank DW, Tsien RY, Ugurbil K, Wingfield JC. The BRAIN initiative: developing technology to catalyse neuroscience discovery. Philos Trans R Soc Lond B Biol Sci. 2015;370(1668):20140164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Südhof TC. Molecular neuroscience in the 21st century: a personal perspective. Neuron. 2017;96(3):536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12(8):452–466. [DOI] [PubMed] [Google Scholar]
- 8. Le Tissier P, Campos P, Lafont C, Romanò N, Hodson DJ, Mollard P. An updated view of hypothalamic-vascular-pituitary unit function and plasticity. Nat Rev Endocrinol. 2017;13(5):257–267. [DOI] [PubMed] [Google Scholar]
- 9. Hodson DJ, Schaeffer M, Romanò N, Fontanaud P, Lafont C, Birkenstock J, Molino F, Christian H, Lockey J, Carmignac D, Fernandez-Fuente M, Le Tissier P, Mollard P. Existence of long-lasting experience-dependent plasticity in endocrine cell networks. Nat Commun. 2012;3(1):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, Ferrer J, Piemonti L, Marchetti P, Bugliani M, Bosco D, Berishvili E, Duncanson P, Watkinson M, Broichhagen J, Trauner D, Rutter GA, Hodson DJ. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab. 2016;24(3):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanchez-Cardenas C, Fontanaud P, He Z, Lafont C, Meunier AC, Schaeffer M, Carmignac D, Molino F, Coutry N, Bonnefont X, Gouty-Colomer LA, Gavois E, Hodson DJ, Le Tissier P, Robinson IC, Mollard P. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc Natl Acad Sci USA. 2010;107(50):21878–21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lafont C, Desarménien MG, Cassou M, Molino F, Lecoq J, Hodson D, Lacampagne A, Mennessier G, El Yandouzi T, Carmignac D, Fontanaud P, Christian H, Coutry N, Fernandez-Fuente M, Charpak S, Le Tissier P, Robinson IC, Mollard P. Cellular in vivo imaging reveals coordinated regulation of pituitary microcirculation and GH cell network function. Proc Natl Acad Sci USA. 2010;107(9):4465–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoa O, Lafont C, Fontanaud P, Guillou A, Kemkem Y, Kineman RD, Luque RM, Fiordelisio Coll T, Le Tissier P, Mollard P. Data from: Imaging and manipulating pituitary function in the awake mouse. Dryad 2019. Accessed 26 August 2019. 10.5061/dryad.63v6b83. [DOI] [PMC free article] [PubMed]
- 14. Budry L, Lafont C, El Yandouzi T, Chauvet N, Conéjero G, Drouin J, Mollard P. Related pituitary cell lineages develop into interdigitated 3D cell networks. Proc Natl Acad Sci USA. 2011;108(30):12515–12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Featherstone K, Hey K, Momiji H, McNamara AV, Patist AL, Woodburn J, Spiller DG, Christian HC, McNeilly AS, Mullins JJ, Finkenstädt BF, Rand DA, White MR, Davis JR. Spatially coordinated dynamic gene transcription in living pituitary tissue. eLife. 2016;5:e08494 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luque RM, Amargo G, Ishii S, Lobe C, Franks R, Kiyokawa H, Kineman RD. Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology. 2007;148(5):1946–1953. [DOI] [PubMed] [Google Scholar]
- 17. Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, Parlow AF, Veldhuis JD, Waters MJ, Chen C. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152(8):3165–3171. [DOI] [PubMed] [Google Scholar]
- 18. Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou Y, Wang VX, Tang CY, Olsen O, Dulac C, Osten P, Tessier-Lavigne M. Mapping of brain activity by automated volume analysis of immediate early genes. Cell. 2016;165(7):1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RRID:AB_393754, https://scicrunch.org/resolver/AB_393754.
- 20.RRID:AB_2756840, https://scicrunch.org/resolver/AB_2756840.
- 21.RRID:AB_221570, https://scicrunch.org/resolver/AB_221570.
- 22.RRID:AB_2340695, https://scicrunch.org/resolver/AB_2340695.
- 23.RRID:AB_2340461, https://scicrunch.org/resolver/AB_2340461.
- 24.RRID:AB_141708, https://scicrunch.org/resolver/AB_141708.
- 25.RRID:AB_2665511, https://scicrunch.org/resolver/AB_2665511.
- 26.RRID:AB_2756841, https://scicrunch.org/resolver/AB_2756841.
- 27.RRID:AB_2756856, https://scicrunch.org/resolver/AB_2756856.
- 28.RRID:AB_2756855, https://scicrunch.org/resolver/AB_2756855.
- 29.RRID:AB_2209751, https://scicrunch.org/resolver/AB_2209751.
- 30.RRID:AB_2340472, https://scicrunch.org/resolver/AB_2340472.
- 31.RRID:AB_2313568, https://scicrunch.org/resolver/AB_2313568.
- 32. Riley DJ, Nikitin AY, Lee WH. Adenovirus-mediated retinoblastoma gene therapy suppresses spontaneous pituitary melanotroph tumors in Rb+/− mice. Nat Med. 1996;2(12):1316–1321. [DOI] [PubMed] [Google Scholar]
- 33. Walls GV, Lemos MC, Javid M, Bazan-Peregrino M, Jeyabalan J, Reed AA, Harding B, Tyler DJ, Stuckey DJ, Piret S, Christie PT, Ansorge O, Clarke K, Seymour L, Thakker RV. MEN1 gene replacement therapy reduces proliferation rates in a mouse model of pituitary adenomas. Cancer Res. 2012;72(19):5060–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, Pincus SM, Robinson IC, Mollard P. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci USA. 2005;102(46):16880–16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Stener-Victorin E, Legro RS, Dunaif A; Reproductive Medicine Network. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations [published correction appears in Nat Commun. 2016;7:10762].Nat Commun. 2015;6(1):7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79(21):6712–6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas P, Surprenant A, Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990;5(5):723–733. [DOI] [PubMed] [Google Scholar]
- 39. Walker JJ, Spiga F, Waite E, Zhao Z, Kershaw Y, Terry JR, Lightman SL. The origin of glucocorticoid hormone oscillations. PLoS Biol. 2012;10(6):e1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Marco RJ, Thiemann T, Groneberg AH, Herget U, Ryu S. Optogenetically enhanced pituitary corticotroph cell activity post-stress onset causes rapid organizing effects on behaviour. Nat Commun. 2016;7(1):12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20(11):2613–2629. [DOI] [PubMed] [Google Scholar]
- 42. Pilz GA, Bottes S, Betizeau M, Jörg DJ, Carta S, Simons BD, Helmchen F, Jessberger S. Live imaging of neurogenesis in the adult mouse hippocampus. Science. 2018;359(6376):658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet C, Mollard P, Jacques TS, Le Tissier P, Dattani MT, Pevny LH, Martinez-Barbera JP. Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13(4):433–445. [DOI] [PubMed] [Google Scholar]
- 44. Pérez Millán MI, Brinkmeier ML, Mortensen AH, Camper SA. PROP1 triggers epithelial-mesenchymal transition-like process in pituitary stem cells. eLife. 2016;5:e14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rizzoti K, Akiyama H, Lovell-Badge R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell. 2013;13(4):419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33(1):102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. VanDusen NJ, Guo Y, Gu W, Pu WT.. CASAAV: a CRISPR-based platform for rapid dissection of gene function in vivo. Curr Protoc Mol Biol. 2017;120:31.11.1–31.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh SP, Janjuha S, Hartmann T, Kayisoglu Ö, Konantz J, Birke S, Murawala P, Alfar EA, Murata K, Eugster A, Tsuji N, Morrissey ER, Brand M, Ninov N. Different developmental histories of beta-cells generate functional and proliferative heterogeneity during islet growth. Nat Commun. 2017;8(1):664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van der Meulen T, Mawla AM, DiGruccio MR, Adams MW, Nies V, Dolleman S, Liu S, Ackermann AM, Caceres E, Hunter AE, Kaestner KH, Donaldson CJ, Huising MO.. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab. 2017;25(4):911–926.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Macaulay IC, Ponting CP, Voet T. Single-cell multiomics: multiple measurements from single cells. Trends Genet. 2017;33(2):155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.