Abstract
Primary ovarian insufficiency (POI) is defined by the loss or dysfunction of ovarian follicles associated with amenorrhea before the age of 40. Symptoms include hot flashes, sleep disturbances, and depression, as well as reduced fertility and increased long-term risk of cardiovascular disease. POI occurs in ∼1% to 2% of women, although the etiology of most cases remains unexplained. Approximately 10% to 20% of POI cases are due to mutations in a single gene or a chromosomal abnormality, which has provided considerable molecular insight into the biological underpinnings of POI. Many of the genes for which mutations have been associated with POI, either isolated or syndromic cases, function within mitochondria, including MRPS22, POLG, TWNK, LARS2, HARS2, AARS2, CLPP, and LRPPRC. Collectively, these genes play roles in mitochondrial DNA replication, gene expression, and protein synthesis and degradation. Although mutations in these genes clearly implicate mitochondrial dysfunction in rare cases of POI, data are scant as to whether these genes in particular, and mitochondrial dysfunction in general, contribute to most POI cases that lack a known etiology. Further studies are needed to better elucidate the contribution of mitochondria to POI and determine whether there is a common molecular defect in mitochondrial function that distinguishes mitochondria-related genes that when mutated cause POI vs those that do not. Nonetheless, the clear implication of mitochondrial dysfunction in POI suggests that manipulation of mitochondrial function represents an important therapeutic target for the treatment or prevention of POI.
Primary ovarian insufficiency (POI), which is also commonly referred to as premature ovarian failure, is defined by the loss or dysfunction of ovarian follicles associated with amenorrhea before the age of 40 (1). POI is a major cause of female infertility, with a prevalence between 1% and 2%. It can occur spontaneously or be the result of medical interventions such as removal of the ovaries, chemotherapy, or radiation treatment. Symptoms of POI are similar to menopause and commonly include hot flashes, sleep disturbances, vaginal dryness, and night sweats (2). Other less commonly reported symptoms include decreased energy, dry eyes, hair loss, urinary incontinence, cold intolerance, cognitive changes, depression, and joint pain, among others (3). Longer-term consequences associated with the premature decrease in estrogen levels include increased risk of cardiovascular disease and decreased bone mineral density (4, 5). Symptom severity can be correlated with ovarian function, as POI encompasses a range of decreased ovarian functions from individuals with partial function who retain fertility to those who completely lack ovarian function and are infertile. Treatment will vary depending on the symptoms, but it can include hormone replacement therapy, fertility management, and psychosocial support, as well as annual screenings of thyroid and adrenal function (2).
In cases of POI that are not induced by chemotherapy or radiation, the etiology is determined for only ∼20% of all cases (6). Approximately 5% of all POI cases are of autoimmune origin, and 15% of cases have a clear genetic origin, either in the form of a chromosomal abnormality or a mutation in an individual gene (7, 8). Among the chromosomal abnormalities that are frequent causes of POI are a number of X chromosome defects, including Turner syndrome, triple X syndrome, and fragile X syndrome. A number of monogenic disorders resulting in POI have also been identified, with variants in >50 genes having been associated with POI (8). The genes that have been implicated in POI fall within a number of pathways that are critical for ovarian development and function, including DNA repair, meiosis, germ cell recruitment, and steroidogenesis. Additionally, mutations in many genes involved in mitochondrial function have been identified as causes of POI. Thus, this review summarizes the role of mitochondria in oocytes as well as discusses the genetic causes of POI related to mitochondrial dysfunction.
Mitochondrial Composition and Function
Mitochondria are maternally inherited double-membrane organelles that are best known for their role as the powerhouses of a cell, based on their generation of ATP via the process of oxidative phosphorylation (OXPHOS). The mitochondrial genome is ∼16.7 kb and encodes 13 proteins that function within the OXPHOS pathway, as well as 22 tRNAs and 2 rRNAs. However, estimates of the complete human mitochondrial proteome suggest the presence of at least 1500 proteins, and thus the vast majority of proteins localized to mitochondria are encoded by the nuclear genome (9). The proteomic complexity of mitochondria enables them to perform many critical cellular functions beyond energy production such as macromolecule biogenesis (i.e., protein and nucleic acids), lipid synthesis, regulation of cell death, calcium handling/homeostasis, generation of reactive oxygen species (ROS), and antioxidant protection (10).
OXPHOS is the process by which nutrients are oxidized by a series of five multisubunit protein complexes (complex I to complex V) within the mitochondrial inner membrane, ultimately resulting in the conversion of ADP to ATP. During the process of ATP generation, mitochondria also release ROS in the form of superoxide. Although typically thought of as a byproduct of OXPHOS that can damage cells, ROS possess antimicrobial properties, act as signaling molecules, and regulate autophagy (11). Nonetheless, excessive or mislocalized ROS can have deleterious effects on the cell and can induce oxidative damage to mitochondrial DNA (mtDNA), the latter of which may be particularly sensitive to damage due to the relative absence of DNA repair enzymes in mitochondria (12). ROS are eliminated in the mitochondria by three superoxide dismutases that convert superoxide to hydrogen peroxide, which can then be further converted to water by peroxiredoxins and glutathione peroxidases.
Mitochondria are not static organelles, rather their numbers are tightly regulated by balancing the processes of mitochondrial biogenesis and clearance (13). Mitochondrial clearance can be mediated by the process of mitophagy, which describes the degradation and recycling of mitochondria via autophagy. Triggers for mitophagy include mild increases in ROS (14) and the inability to maintain an electrochemical gradient that can result from the accumulation of mtDNA damage (15). Mitochondrial biogenesis is then used to repopulate the cellular mitochondrial population with more functional mitochondria. The process of mitochondrial biogenesis involves both fusion and fission events, and it is regulated by a cascade of transcription and translation factors, both nuclear and mitochondrial encoded, which serve to generate the materials necessary for organelle duplication (16). Mitochondrial fusion describes the merging of the outer and then inner membranes of two previously separate mitochondria, whereas mitochondrial fission is the process by which a single mitochondrion divides into two or more independent mitochondria. Mitochondrial fusion, fission, and mitophagy collectively serve not only an important quality control function, but can link changes in mitochondrial abundance to cellular energy demands (17). For example, nutrient deprivation prevents mitochondrial fission, which together with other molecular and structural changes to the mitochondria serves to increase ATP synthesis capacity, whereas thermogenesis or AMP-activated protein kinase activation serves to increase fission (18–20). Nutrient excess inhibits fusion, resulting in an increase in fragmentation, which can cause mitochondrial dysfunction and increase ROS production (21). OXPHOS activity is positively correlated with fusion, although the causal nature of this relationship remains unclear (22). Mitophagy can be triggered by energy stress via activation of AMP-activated protein kinase, hypoxia, and glucose deprivation (22). Collectively, the regulation of mitochondrial dynamics ensures that these organelles can help the cell respond and adapt to a variety of cellular states to match the bioenergetic and other mitochondrial functions with the cellular energy needs.
Mitochondrial Function in Ovarian Somatic Cells
Beyond the role of mitochondria in OXPHOS, ROS homeostasis, apoptosis, and thermogenesis, mitochondria have an important role in steroidogenesis. Steroidogenesis occurs in the cumulus granulosa and theca cells, which surround the oocyte, according to the two-cell, two-gonadotropin model. The first step in steroidogenesis, which is also the rate-limiting step, involves the transport of cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane by the steroid acute regulatory protein (STAR) and other accessory proteins (23). Once inside the mitochondria, cholesterol is metabolized to pregnenolone by cytochrome P450 family 11 subfamily A member 1 (CYP11A1, also known as P450SCC), which is then further metabolized in the endoplasmic reticulum to form the final steroid products, including estradiol and progesterone (24, 25).
Mitochondrial Function in Oocytes
Oogenesis, the process by which a haploid oocyte is formed within the ovary, originates from primordial germ cells that arise in the extraembryonic mesoderm, which then migrate to the genital ridge where they proliferate by mitosis and transform to oogonia (26). Following the arrest of mitosis, human germ cells initiate meiosis in the fetal ovary at 11 to 12 weeks of gestation and become primary oocytes (27). Oocytes enter meiosis in the prophase stage, during which time homologous chromosomes undergo synapsis and recombination. Subsequent to the crossing over of genetic material during recombination, oocytes progress to the diplotene stage, which is defined by a protracted arrest. During this resting state, oocytes become surrounded by pregranulosa cells to form primordial follicles, although most oocytes undergo apoptosis during this process and do not survive to form follicles (28). Oocyte survival appears in part to depend on the transfer of organelles, including Golgi and mitochondria, from nurse cells connected by intercellular bridges that are associated with the formation of a Balbiani body in the oocyte, a structure that is densely packed with Golgi, endoplasmic reticulum, and mitochondria (29, 30). The Balbiani body is a transient formation and disappears in late-stage oocytes. A subset of primordial follicles are further induced via intra-oocyte and extra-oocyte factors involving the phosphoinositide 3-kinase and mammalian target of rapamycin pathways to transition to primary follicles (31). The primary follicles then further develop to form secondary follicles, which are surrounded by at least two layers of granulosa cells and an outermost layer of theca cells. The theca cells contain many mitochondria with vesicular cristae (32). Further development preceding ovulation includes the formation of a cavity called the antrum and the selection of dominant follicles that will eventually ovulate. Reinitiation of meiosis in the dominant follicles can follow a surge of endogenous LH during puberty, but these follicles can also remain in the dormant state from the time of their formation during fetal development until menopause, a period of ∼50 years (33).
There is a tremendous increase in the number of mitochondria per cell that occurs during oogenesis, from the 10 to 100 mitochondria found in primordial germ cells to the >100,000 mitochondria found in a mature preovulatory oocyte (34). The small number of mitochondria in primordial germ cells contributes to the establishment of an inheritance bottleneck, in which rapid shifts in the inheritance of mitochondrial variants can occur between generations based on the small number of mitochondria that form the basis of the rapid expansion (35). Coupled with a dramatic reduction in mtDNA in primordial germ cells, there exists a strong selective pressure, including influence from the nuclear genome, to eliminate deleterious mitochondrial variants that are frequently present at low levels (36–38).
Although the numbers of mitochondria rapidly increase during oogenesis, they maintain a state of relatively low activity until the blastocyst stage. Mitochondria found within oocytes have a morphology that is more rounded than the typical rod-shaped morphology found in somatic cells, and there are fewer cristae found in the inner membrane (39). Oocyte mitochondria also have a relatively lower rate of oxygen consumption, together suggesting that mitochondria within the oocyte remain in a state of relatively low activity. It has been hypothesized that the large numbers of mitochondria functioning at minimal levels are sufficient to provide just the right amount of energy to the oocyte while minimizing the frequency of mtDNA mutations and the production of ROS (40). This activity is nonetheless critical for oocyte maturation because glycolysis is limited during oogenesis, creating a heavier reliance on ATP generated by OXPHOS. In addition to the energy generated by oocyte mitochondria, energy transfer from the surrounding granulosa and cumulus cells, in the form of extracellular pyruvate and glutamine, also contributes toward the energetic requirements of oocyte maturation and early embryogenesis (41). The relatively large numbers of “quiet” mitochondria during oogenesis further suggests that other functions of mitochondria beyond ATP production may be important at this stage, including the production of the reduced form of nicotinamide adenine dinucleotide phosphate and tricarboxylic acid (TCA) cycle intermediates, which may be used to decrease oxidative stress and provide substrates for other biosynthetic pathways (42).
In addition to dramatic changes in the numbers of mitochondria during oogenesis, the subcellular localization of these organelle is remodeled to suit the changing energy demands of the cell during maturation. The most significant shift in subcellular localization occurs when >40% of mitochondria accumulate to surround the forming meiosis I spindle, remaining with the spindle during its movement from the oocyte center to the periphery (43). This association of mitochondria with the developing spindle is dependent on their trafficking along microtubules in a dynein-dependent process. Following the first meiotic division, there is an asymmetric distribution of mitochondria among the daughter cells favoring the oocyte over the polar body, which is of particular importance given the paucity of mitochondrial biogenesis from this stage of oogenesis through implantation (44). Mitochondria again associate with the meiosis II (MII) spindle during its formation; however, following the completion of the MII spindle formation and arrest at the MII stage, the mitochondria no longer completely encase the spindle, but do remain somewhat enriched in the immediate vicinity (44).
Mutations in Mitochondrial Proteins in POI
Mitochondrial disease represents a group of clinically heterogeneous disorders that can typically be grouped together based on their common feature of having a primary defect in OXPHOS (45). The prevalence of mitochondrial disease in children is ∼6 of 100,000 and in adults is ∼23 of 100,000 (46, 47). Mitochondrial diseases can be caused by mutations in either mtDNA or nuclear-encoded genes, with mutations in >350 genes having been reported as a cause (48). The clinical presentations are varied in their onset, inheritance pattern, and features, but they are often found in organs with high energy demands, including the brain, skeletal muscle, and heart. Frequent features include hypertrophic cardiomyopathy, heart conduction defects, myopathy, sensorineural deafness, cerebellar ataxia, epilepsy, and peripheral neuropathy, among others (49). Nonsyndromic presentations of mitochondrial disease include mutations in the 12S rRNA that cause nonsyndromic deafness and certain individuals with Leber optic atrophy, which is caused by mutations in multiple genes, that present with visual loss as the only clinical feature (50, 51). This brings up one of the more perplexing features of mitochondrial disorders, which is that despite the fact that mitochondria are critical for the function of every cell in the body, their clinical presentation can often be organ or cell specific. Additionally, not all patients with mitochondrial disease have OXPHOS defects, as evidenced by patients with TCA cycle defects due to mutations in aconitase 2 (ACO2) who present with infantile cerebellar-retinal degeneration. ACO2, which catalyzes the conversion of citrate to isocitrate within the TCA cycle, is not consistently associated with defects in OXPHOS in patient-derived tissue samples, but rather is characterized by abnormal TCA metabolite levels and mtDNA depletion (52–54).
In addition to the frequently observed defects in the heart, brain, and muscle, the presentation of mitochondrial dysfunction often presents with a range of endocrine features, including diabetes mellitus, GH deficiency, hypogonadism, thyroid disease, and ovarian dysfunction, among others (55, 56). Ovarian dysfunction can be seen as part of a complex syndromic presentation, but it also includes disorders where it is the only clinical finding. Mitochondrial mutations that lead to ovarian dysfunction have been linked to mtDNA, mitochondrial RNA, and mitochondrial protein synthesis, with functions in multiple distinct biological pathways (57). Much of what is known about the pathophysiology underlying ovarian dysfunction has been learned from the identification of disease genes identified in rare monogenic disorders (58). Below is a discussion of eight genes that when mutated give rise to either isolated POI or POI as part of a syndrome (Table 1), as well as three additional genes that have recently been described in a single patient or family, suggesting a potential link between those genes and POI as well.
Table 1.
Mitochondria-Related Genes in Which Mutations Can Cause POI
| Gene | Inheritance | Clinical Presentation | Gene Function | Molecular Function in Mitochondria |
| POLG | AR, AD | Progressive external ophthalmoplegia | DNA polymerase | mtDNA replication and maintenance |
| Mitochondrial DNA depletion syndrome | ||||
| Mitochondrial recessive ataxia syndrome | ||||
| TWNK | AR | Perrault syndrome | mtDNA helicase | mtDNA replication and proofreading |
| Mitochondrial DNA depletion syndrome | ||||
| Progressive external ophthalmoplegia | ||||
| LARS2 | AR | Perrault syndrome | Leucine tRNA | mRNA translation |
| HARS2 | AR | Perrault syndrome | Histidine tRNA | mRNA translation |
| AARS2 | AR | Ovarioleukodystrophy | Alanine tRNA | mRNA translation |
| Combined OXPHOS deficiency | ||||
| CLPP | AR | Perrault syndrome | Protease | Protein degradation |
| LRPPRC | AR | Leigh syndrome | RNA binding protein | Gene expression |
| MRPS22 | AR | Isolated POI | Ribosomal subunit | mRNA translation |
| Combined OXPHOS deficiency |
Abbreviations: AD, autosomal dominant; AR, autosomal recessive.
Rare Monogenic Mitochondrial Disorders Associated With POI
DNA polymerase γ, catalytic subunit
DNA polymerase γ, catalytic subunit (POLG) is the catalytic subunit of the mitochondrial DNA polymerase that is responsible for replication of the mitochondrial genome (59). Mutations in POLG cause depletion of the mtDNA and/or the accumulation of deletions in the mtDNA and are the most commonly inherited form of monogenic mitochondrial disease (60). Mutations in POLG cause a spectrum of disorders, including myocerebrohepatopathy spectrum, Alpers–Huttenlocher syndrome, and progressive external ophthalmoplegia, among others, but they lack a clear cut genotype–phenotype correlation between the >300 described pathogenic mutations and the clinical presentation (61). Among the disparate clinical features associated with POLG mutations are epilepsy, ataxia, parkinsonism, hearing loss, cataracts, as well as POI (60, 62). Although the clinical presentation of POLG mutations can vary, POI has typically been described as part of a syndrome including the previously mentioned neurologic features, and not in isolation (55). However, a recent case report described identifying the first case of nonsyndromic ovarian dysfunction associated with a mutation in POLG (p.R964C), although caution must be taken in interpreting this study until additional cases are identified (63). Finally, although candidate gene–based studies have previously failed to find evidence of POLG mutations as a common cause of POI (64, 65), a recent meta–genome-wide association study analysis identified an association between a single-nucleotide polymorphism located near POLG (rs1054875) and the age at natural menopause, suggesting that variation in POLG may contribute to the more common polygenic forms of POI (66).
Twinkle mtDNA helicase
Twinkle mtDNA helicase (TWNK; also known as C10ORF2) encodes a mitochondrial helicase that together with the above-mentioned POLG, the DNA polymerase γ accessory subunit POLG2, and the mitochondrial single-stranded DNA-binding protein SSBP1 form the core factors required for mtDNA replication (67, 68). Similar to POLG, mutations in TWNK can cause a spectrum of genetic disorders, including progressive external ophthalmoplegia, mtDNA depletion syndrome 7 (hepatocerebral type), and Perrault syndrome, with clinical presentations that can contain POI in association with other neurologic findings. For example, 2 unrelated families each had 2 individuals present with ataxia, neuropathy, hyporeflexia, progressive hearing loss, and ovarian dysgenesis, with affected individuals in both families found to be compound heterozygous for missense mutations in TWNK (69). Given the functional overlap between POLG and TWNK in mtDNA replication, it is perhaps not surprising that mutations in both genes can cause a similar constellation of clinical features, including POI, and together clearly implicates the importance of mtDNA replication in ovarian development. However, thus far mutations in the other core components of mtDNA replication, POLG2 and SSBP1, have not been associated with ovarian dysfunction, although mutations have been observed in POLG2 that, similar to POLG, cause progressive external ophthalmoplegia (70).
Leucyl-tRNA synthetase 2, mitochondrial and histidyl-tRNA synthetase 2, mitochondrial
Histidyl-tRNA synthetase 2, mitochondrial (HARS2) and leucyl-tRNA synthetase 2, mitochondrial (LARS2) both encode aminoacyl-tRNA synthetases (aaRSs) that are used for the translation of mitochondrial-encoded genes. Mitochondrial aaRSs can be categorized into two major classification groups, class I and class II, based on their structural motifs, with LARS2 encoding a class I aaRS based on the presence of a Rossmann fold that serves as an ATP biding region and HARS2 encoding a class II aaRS based on three structural motifs referred to as 1, 2, and 3 (71). Mutations in both HARS2 and LARS2 are associated with Perrault syndrome, which describes a rare recessively inherited condition composed of sensorineural hearing loss in both males and females and ovarian dysfunction, including ovarian dysgenesis and primary amenorrhea, and can include neurologic features including ataxia, learning disability, and neuropathy (72–74). Testicular dysfunction has not been observed in males with the same mutations that cause ovarian dysfunction in females (72). However, the number of deaf males with Perrault syndrome mutations whose sperm function has been clinically evaluated is small, and a mouse model of Perrault syndrome due to complete Clpp deficiency (discussed below) is associated with infertility in both males and females (75, 76). Among the 19 mitochondrial aaRSs, it remains unclear why mutations in these two particular genes result in hearing loss and ovarian dysfunction, whereas mutations in other mitochondrial aaRSs result in multiorgan systemic disorders (77). However, although most disease-causing mutations in aaRSs occur at residues that are not evolutionarily conserved, mutations in both HARS2 and LARS2 have been described at conserved positions, suggesting that the mutations in these genes may similarly affect the canonical functions of these proteins (78).
Alanyl-tRNA synthetase 2, mitochondrial
Alanyl-tRNA synthetase 2, mitochondrial (AARS2), similar to HARS2 and LARS, is a mitochondrial aaRS; however, mutations in AARS2 do not cause Perrault syndrome, but instead are associated with recessively inherited ovarioleukodystrophy syndrome in females (79–83). Mutations in AARS2 have also been linked to infantile mitochondrial cardiomyopathy and primary pulmonary hypoplasia resulting in early childhood lethality (84, 85). The ovarioleukodystrophy syndrome consists of neurologic features stemming from neurologic deterioration, including ataxia, spasticity, tremor, and cognitive decline, with ages of onset ranging from childhood to adulthood, in the absence of cardiomyopathy. Ovarian failure occurs in the 20s or 30s and has been reported with both primary amenorrhea or secondary amenorrhea. AARS2 mutations that lead to infantile cardiomyopathy reduce protein stability with minimal effects on aminoacylation, whereas the molecular basis underlying the ovarioleukodystrophy syndrome remains unknown (86). However, it has been hypothesized that the ovarioleukodystrophy is associated with a reduction in aminoacylation efficiency, although this remains to be experimentally validated (87).
Caseinolytic mitochondrial matrix peptidase proteolytic subunit
Caseinolytic mitochondrial matrix peptidase proteolytic subunit (CLPP) is an ATP-dependent protease that is localized to the mitochondrial matrix where, together with caseinolytic mitochondrial matrix peptidase chaperone subunit (CLPX), it is part of the quality control system that degrades oxidized and denatured proteins (88). A number of CLPP substrates for degradation have been identified and include proteins involved in electron transport, metabolic processes, the TCA cycle, and mitochondrial mRNA, among others (89–91). Consistent with the identified substrates, CLPP deficiency in experimental model systems leads to reduced mitochondrial respiration, increased ROS, and impaired mitochondrial protein synthesis associated with defects in assembly of the mitochondrial ribosome (89, 92). Mutations in CLPP cause recessively inherited Perrault syndrome type 3 associated with ovarian dysfunction, hearing loss, and neurologic defects such as lower limb spasticity, epilepsy, microcephaly, and learning difficulties (93–96). Functional characterization of the identified mutations in CLPP resulted in a variety of functional defects, including decreased peptidase activity, inhibition of CLPX binding, prevention of oligomerization, and enhanced peptidase activity, suggesting that despite the clustering of most CLPP mutations near the CLPX-docking site or the active site of the peptidase, there may not be a common defect in the molecular function of CLPP (97). A mouse knockout of Clpp has also been generated, which similarly demonstrated hearing loss and infertility due to the failure of ovarian follicular differentiation in females, as well as growth retardation, decreased activity, impaired survival, and disruption of spermatogenesis in males, providing a model to better study the cellular and molecular defects associated with CLPP deficiency (76).
Leucine-rich pentatricopeptide repeat containing
Leucine-rich pentatricopeptide repeat containing (LRPPRC) is an RNA-binding protein that plays a critical role in regulating mitochondrial gene expression. LRPPRC together with the interacting protein SRA stem-loop interacting RNA binding protein (SLIRP) directly bind to and coat nearly all mitochondrial mRNAs, thereby regulating their structure to improve their translational fidelity and increase their stability (98, 99). Although SLIRP and LRPPRC both depend on the other for stability, they also have unique functions within their complex, with only LRPPRC being required for polyadenylation of mitochondrial mRNAs, whereas SLIRP functions to target mRNAs to the mitochondrial ribosome (100). Nuclear functions have also been ascribed to LRPPRC, including as a cofactor for eukaryotic translation initiation factor 4E (eIF4e) that regulates nuclear export and translation, and a component of the PGC1α transcription regulation complex that controls the expression of key metabolic genes such as phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase catalytic subunit (G6PC) in addition to genes involved in mitochondrial biogenesis (101, 102).
Mutations in LRPPRC cause a rare type of Leigh syndrome referred to as French-Canadian, because most patients originate from the Saguenay-Lac-Saint-Jean region of Québec. A p.A354V founder mutation is present in this region at a relatively high allele frequency, resulting in an incidence of ∼1 of 2000 births (103–106). Leigh syndrome, French-Canadian variety, has an autosomal recessive inheritance pattern with clinical features that include developmental delay, failure to thrive, characteristic facial features, and hypotonia. Patients are at high risk of death due to neurologic or acidotic crises that can be instigated by triggers, including infection, stress, diet, illness, or exercise. Although life expectancy is typically <5 years, patients can survive to adulthood (107). Female patients who survive until at least adolescence, in addition to the neurologic features, present with POI, including elevated FSH, small ovaries that lack follicles, and primary amenorrhea (106). Although LRPPRC clearly has a significant effect on expression of mtDNA-encoded genes, caution must be taken when interpreting this as conclusive support for the role of mitochondrial dysfunction in POI, given that LRPPRC also regulates nuclear gene expression.
Mitochondrial ribosomal protein S22
Mitochondrial ribosomal protein S22 (MRPS22) is a component of the 28S small subunit of the mitochondrial ribosome that enables the translation of mtDNA-encoded polypeptides (108). MRPS22 is also required for maintaining the stability of the mitochondrial ribosomal complex (109). Mutations in MRPS22 have been identified to cause autosomal recessive inheritance of POI (110, 111). Unlike the syndromes mentioned above, mutations in MRPS22 caused isolated POI in the absence of other neurologic or other syndromic features. Surprisingly, MRPS22 deficiency did not cause defects in OXPHOS, suggesting that the etiology of POI was related to the nonbioenergetic functions of mitochondria. In Drosophila, germ cell–specific deficiency of the MRPS22 ortholog resulted in infertility, suggesting a cell-autonomous role for MRPS22 in germ cell development (110). Mutations in MRPS22, which are presumably more deleterious to protein function, have been identified that impair OXPHOS activity and lead to systemic disease, including cardiomyopathy, hypotonia, brain abnormalities, and in certain instances lethality (112–115). Thus, variants in MRPS22 are associated with a phenotypic spectrum of disorders ranging from isolated POI to infantile mitochondrial lethality (110).
Mitochondria-Related Candidate Genes for POI Identified in Single Families or Probands
Mitochondrial ribosomal protein S7, protein only RNase P catalytic subunit, and required for meiotic nuclear division 1 homolog
Caution must be exercised when interpreting mutations that have thus far been identified in only a single family, as the sheer number of potentially deleterious variants present in all individuals makes it difficult to identify definitively the true causal variants. Nonetheless, the initial identification and reporting of these mutations may facilitate other investigators who encounter mutations in those genes and is thus worthwhile. As such, mutations have been reported in protein-only RNase P catalytic subunit (PRORP) as a novel cause of Perrault syndrome (116). A single consanguineous family was identified with three individuals presenting with sensorineural hearing loss and POI that were each homozygous for a missense variant in PRORP (p.A485V). Functional studies in patient-derived fibroblasts supported the presence of mitochondrial dysfunction as evidenced by impaired processing of mitochondrial RNA transcripts and an accompanying decrease in protein levels of multiple OXPHOS components (116). Similarly, another consanguineous family was identified with two female siblings who presented with congenital sensorineural deafness and lactic acidemia. One sibling had a significantly more severe presentation, including failure to thrive, hepatomegaly, and hypoglycemia, and ultimately died at 14 years of age. The sibling has hypoglycemia in early childhood that resolved, mild learning difficulties, and was found at age 16 to have POI (117). Both siblings were homozygous for a mutation in the mitochondrial ribosomal protein S7 (MRPS7) at a highly conserved amino acid residue (p.M184V), and functional studies in patient-derived fibroblasts identified OXPHOS defects consistent with a mitochondrial disorder (117). Another study identified a homozygous missense mutation in required for meiotic nuclear division 1 homolog (RMND1; p.N238S) that had previously been reported as a disease causing mutation associated with neurologic, muscle, and kidney defects (118, 119). However, the proband in this study carrying the RMND1 variant had the characteristic features of Perrault syndrome, including POI that was observed at 10 years of age, as well as kidney and growth defects (118). Mutations in RMND1 have previously been associated with defects in mitochondrial mRNA translation (120, 121). Although these studies each describe a strong candidate gene for POI, with the studies for MRPS7 and PRORP in particular elegantly combining both genetic and functional data, caution must be taken until additional patients are identified to more concretely establish the genotype–phenotype correlations.
Mitochondrial Dysfunction Associated With Common POI
Rare monogenic forms of POI have been instrumental into opening a window into the key genes and pathways that are important for ovarian function (Fig. 1) (122). However, with ∼1% of women affected by POI, it is not a rare disorder, and even cumulatively, the rare monogenic causes of POI fail to account for most cases. Nonetheless, that so many genes involved in mitochondrial function, and mitochondrial mRNA translation in particular, have been implicated as causes of rare syndromic or isolated POI suggests that this organelle may be involved in the more common and genetically and environmentally complex presentations of POI that account for most cases. Toward this end, pilot studies of POI patients with no known etiology have identified an increased frequency of mutations in the mitochondrially encoded ATP synthase 6 gene (MT-ATP6) and the mitochondrially encoded cytochrome c oxidase I gene (MT-CO1); however, in both cases the number of patients screened was small (n = 24 cases and n = 63 cases, respectively) (123, 124). The need for caution in interpreting small studies for a common disease is evident, as the conclusions linking MT-ATP6 variants to POI have been called into question, based on potential ancestry differences between the case and control populations analyzed in that study (125). In addition to genetic mutations in mitochondria, elevated levels of ROS and decreased levels of OXPHOS activity and ATP production in oocytes have also been correlated with POI, although again these studies are limited in size (123, 126–128). Interestingly, treatment of mice with coenzyme Q10, a product of the TCA cycle that controls multiple aspects of mitochondrial biology, including transcription and succinate dehydrogenase activity, prevented the onset of POI in both genetic- and aging-based mouse models of POI (129). Although these preliminary studies are intriguing, future studies with increased power and further studies in model organisms will be needed to determine whether mitochondrial dysfunction is a common contributing factor to the onset of POI.
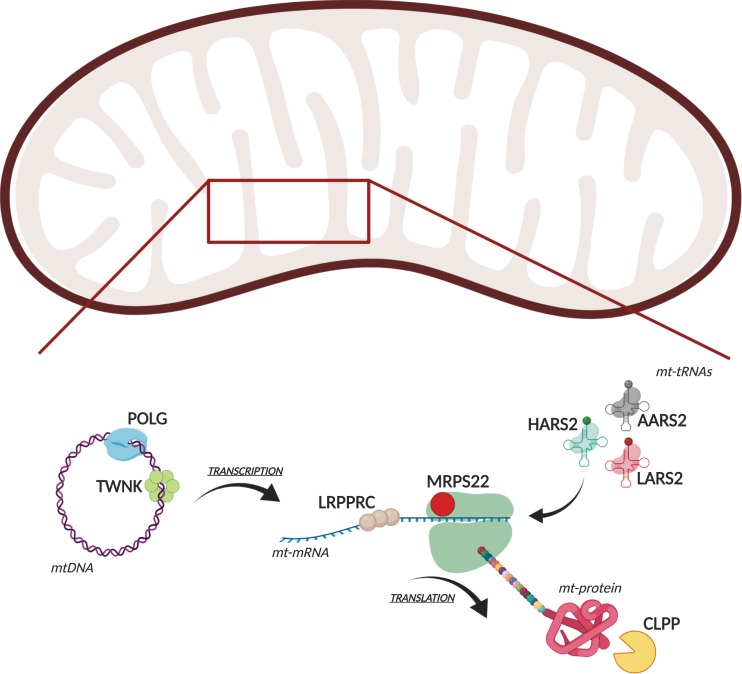
Figure 1.
Molecular functions of mitochondria-related proteins implicated in POI. A cartoon depicts the various molecular functions in mitochondria of the proteins in which mutations have been identified that can cause POI.
Summary and Future Directions
It is clear from studies of rare monogenic disorders that functional mitochondria are necessary to prevent ovarian dysfunction and infertility. However, larger and better powered studies are necessary to access the impact and causal role of dysfunctional mitochondria in most cases of POI. Nonetheless, we have learned a tremendous amount about the genetics and pathophysiology of POI by studying the rare disorders in cases where a disease-causing mutation has been identified (Fig. 1). These studies, which have identified mutations in genes including MRPS22, HARS2, LARS2, and AARS2, suggest that one putative therapeutic target for treating or preventing POI will be to improve mitochondrial mRNA translation. Proof-of-principle experiments have shown that supplementation with l-cysteine is able to improve OXPHOS activity in cell lines from a patient with a genetic defect that impairs mitochondrial mRNA translation (130). In other cellular studies, overexpression of mitochondrial tRNAs were able to correct function defects in mRNA translation and cellular respiration (131–134). Treatment with acetyl-l-carnitine has also long been known to have beneficial effects on mitochondrial function in vivo, including improved efficiency of mitochondrial mRNA translation, and has been proposed as a treatment of a variety of mitochondrial disorders (135). Treatment with acetyl-l-carnitine improves in vitro maturation of oocytes and reduces the number of abnormal mitochondria, although it is unclear what molecular mechanism underlies these improvements and whether these finding will translate to in vivo treatment or prevention of POI (136–138). These studies have shown that it is possible to manipulate the mitochondrial mRNA translational machinery, although it remains to be seen whether this can be accomplished on a therapeutic basis and what, if any, clinical benefits would be seen.
The discoveries that mutations in the mitochondria-related genes described above and in Table 1 can cause POI were largely made possible by advances in DNA sequencing technologies that greatly reduced the cost of whole-exome sequencing (WES) (139). The initial studies linking mutations in 8 of the 11 genes to POI used WES in the process of identifying the causal variant. However, given the rarity of mutations in these genes as a cause of POI, the current guidelines for diagnosing and treating nonsyndromic POI do not recommend testing for mutations in these genes or other monogenic causes of POI (140). Nonetheless, this may change as WES analyses continue to become more integrated into routine clinical practice. There is mounting evidence from numerous clinical settings demonstrating benefits in terms of both cost reductions and improved clinical care by incorporating WES early into the diagnostic process (141–146). However, to make this a clinical reality for POI, more accurate predictions of whether variants are likely to be pathogenic will be required. Fortunately, progress can be made toward this end with limited sample sizes. The studies utilizing WES discussed above were each based on analyses of individuals with POI from just one to three families, illustrating the progress that can result from even small-scale studies.
Despite all that we have learned, many pressing questions remain unanswered. (i) Why do mutations in the genes described above cause POI, whereas mutations in many more related mitochondrial genes do not? (ii) Why do mutations in genes such as MRPS22 cause a spectrum of disorders, ranging from isolated POI to multiorgan systemic disease, and can we predict the genotype–phenotype correlations? (iii) Why does mitochondrial dysfunction preferentially affect the ovary? (iv) Is mitochondria-associated POI due to cell-autonomous defects in the oocyte, or rather defects in ovarian somatic cells or other systemic changes? (v) Is ovarian dysfunction in rare monogenic cases of POI strictly due to impaired cellular respiration or are there nonbioenergetic defects that underlie the disorder? Answers to these questions will provide insight into the underlying pathophysiology of POI and may identify new therapeutic targets to improve the diagnosis and treatment of POI.
Acknowledgments
Financial Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK112846 (to D.A.B.).
Glossary
Abbreviations:
- aaRS
aminoacyl-tRNA synthetase
- AARS2
alanyl-tRNA synthetase 2, mitochondrial
- CLPP
caseinolytic mitochondrial matrix peptidase proteolytic subunit
- CLPX
caseinolytic mitochondrial matrix peptidase chaperone subunit
- HARS2
histidyl-tRNA synthetase 2, mitochondrial
- LRPPRC
leucine-rich pentatricopeptide repeat containing
- MII
meiosis II
- MRPS22
mitochondrial ribosomal protein S22
- mtDNA
mitochondrial DNA
- OXPHOS
oxidative phosphorylation
- POI
primary ovarian insufficiency
- POLG
DNA polymerase γ, catalytic subunit
- PRORP
protein-only RNase P catalytic subunit
- ROS
reactive oxygen species
- TCA
tricarboxylic acid
- TWNK
twinkle mtDNA helicase
- WES
whole-exome sequencing
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Santoro NF, Cooper AR, eds. Primary Ovarian Insufficiency. Cham, Switzerland: Springer International Publishing; 2016. 10.1007/978-3-319-22491-6 [DOI] [Google Scholar]
- 2. Cox L, Liu JH. Primary ovarian insufficiency: an update. Int J Womens Health. 2014;6:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allshouse AA, Semple AL, Santoro NF. Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause. 2015;22(2):166–174. [DOI] [PubMed] [Google Scholar]
- 4. Christ JP, Gunning MN, Palla G, Eijkemans MJC, Lambalk CB, Laven JS, Fauser BC. Estrogen deprivation and cardiovascular disease risk in primary ovarian insufficiency. Fertil Steril. 2018;109(4):594–600.e1. [DOI] [PubMed] [Google Scholar]
- 5. Podfigurna-Stopa A, Czyzyk A, Grymowicz M, Smolarczyk R, Katulski K, Czajkowski K, Meczekalski B. Premature ovarian insufficiency: the context of long-term effects. J Endocrinol Invest. 2016;39(9):983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossetti R, Ferrari I, Bonomi M, Persani L. Genetics of primary ovarian insufficiency. Clin Genet. 2017;91(2):183–198. [DOI] [PubMed] [Google Scholar]
- 7. Silva CA, Yamakami LY, Aikawa NE, Araujo DB, Carvalho JF, Bonfá E. Autoimmune primary ovarian insufficiency. Autoimmun Rev. 2014;13(4–5):427–430. [DOI] [PubMed] [Google Scholar]
- 8. Tucker EJ, Jaillard S, Sinclair AH. Genetics and genomics of primary ovarian insufficiency. In: Leung PC, Qiao J, eds. Human Reproductive and Prenatal Genetics. San Diego, CA: Elsevier; 2019:427–445. 10.1016/B978-0-12-813570-9.00019-X [DOI] [Google Scholar]
- 9. Pfanner N, Warscheid B, Wiedemann N. Mitochondrial proteins: from biogenesis to functional networks. Nat Rev Mol Cell Biol. 2019;20(5):267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion. 2016;30:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79(1):683–706. [DOI] [PubMed] [Google Scholar]
- 13. Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2017;284(2):183–195. [DOI] [PubMed] [Google Scholar]
- 14. Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta. 2012;1823(12):2297–2310. [DOI] [PubMed] [Google Scholar]
- 15. Kulikov AV, Luchkina EA, Gogvadze V, Zhivotovsky B. Mitophagy: link to cancer development and therapy. Biochem Biophys Res Commun. 2017;482(3):432–439. [DOI] [PubMed] [Google Scholar]
- 16. Tandler B, Hoppel CL, Mears JA. Morphological pathways of mitochondrial division. Antioxidants. 2018;7(2):E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17(4):491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA. 2011;108(25):10190–10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wikstrom JD, Mahdaviani K, Liesa M, Sereda SB, Si Y, Las G, Twig G, Petrovic N, Zingaretti C, Graham A, Cinti S, Corkey BE, Cannon B, Nedergaard J, Shirihai OS. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J. 2014;33(5):418–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Losón OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351(6270):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects β-cells from nutrient-induced apoptosis. Diabetes. 2009;58(10):2303–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212(4):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63(1):193–213. [DOI] [PubMed] [Google Scholar]
- 24. Chien Y, Rosal K, Chung BC. Function of CYP11A1 in the mitochondria. Mol Cell Endocrinol. 2017;441:55–61. [DOI] [PubMed] [Google Scholar]
- 25. Takae S, Suzuki N. Ovarian endocrinology. In: Chian RC, Nargund G, Huang JYJ, eds. Development of In Vitro Maturation for Human Oocytes. Cham, Switzerland: Springer International Publishing; 2017:3–35. [Google Scholar]
- 26. Sánchez F, Smitz J. Molecular control of oogenesis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012;1822(12):1896–1912. [DOI] [PubMed] [Google Scholar]
- 27. Gondos B, Westergaard L, Byskov AG. Initiation of oogenesis in the human fetal ovary: ultrastructural and squash preparation study. Am J Obstet Gynecol. 1986;155(1):189–195. [DOI] [PubMed] [Google Scholar]
- 28. Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234(2):339–351. [DOI] [PubMed] [Google Scholar]
- 29. Pepling ME, Wilhelm JE, O’Hara AL, Gephardt GW, Spradling AC. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci USA. 2007;104(1):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lei L, Spradling AC. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science. 2016;352(6281):95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian folliculogenesis. In: Piprek RP, ed. Molecular Mechanisms of Cell Differentiation in Gonad Development. Vol. 58. Cham, Switzerland: Springer International Publishing; 2016:167–190. [DOI] [PubMed] [Google Scholar]
- 32. Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37(7):1344–1349. [DOI] [PubMed] [Google Scholar]
- 33. Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21(2):96–103. [DOI] [PubMed] [Google Scholar]
- 34. Poulton J, Marchington DR. Segregation of mitochondrial DNA (mtDNA) in human oocytes and in animal models of mtDNA disease: clinical implications. Reproduction. 2002;123(6):751–755. [DOI] [PubMed] [Google Scholar]
- 35. Cao L, Shitara H, Horii T, Nagao Y, Imai H, Abe K, Hara T, Hayashi J, Yonekawa H. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat Genet. 2007;39(3):386–390. [DOI] [PubMed] [Google Scholar]
- 36. Chen D, Clark AT. Mitochondrial DNA selection in human germ cells. Nat Cell Biol. 2018;20(2):118–120. [DOI] [PubMed] [Google Scholar]
- 37. Floros VI, Pyle A, Dietmann S, Wei W, Tang WCW, Irie N, Payne B, Capalbo A, Noli L, Coxhead J, Hudson G, Crosier M, Strahl H, Khalaf Y, Saitou M, Ilic D, Surani MA, Chinnery PF. Segregation of mitochondrial DNA heteroplasmy through a developmental genetic bottleneck in human embryos [published correction appears in Nat Cell Biol.2018;20(8):991]. Nat Cell Biol. 2018;20(2):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei W, Tuna S, Keogh MJ, Smith KR, Aitman TJ, Beales PL, Bennett DL, Gale DP, Bitner-Glindzicz MA, Black GC, Brennan P, Elliott P, Flinter FA, Floto RA, Houlden H, Irving M, Koziell A, Maher ER, Markus HS, Morrell NW, Newman WG, Roberts I, Sayer JA, Smith KG, Taylor JC, Watkins H, Webster AR, Wilkie AO, Williamson C, Ashford S, Penkett CJ, Stirrups KE, Rendon A, Ouwehand WH, Bradley JR, Raymond FL, Caulfield M, Turro E, Chinnery PF; NIHR BioResource—Rare Diseases; 100,000 Genomes Project—Rare Diseases Pilot. Germline selection shapes human mitochondrial DNA diversity. Science. 2019;364(6442):eaau6520. [DOI] [PubMed] [Google Scholar]
- 39. de Paula WB, Lucas CH, Agip AN, Vizcay-Barrena G, Allen JF. Energy, ageing, fidelity and sex: oocyte mitochondrial DNA as a protected genetic template. Philos Trans R Soc Lond B Biol Sci. 2013;368(1622):20120263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leese HJ, Guerif F, Allgar V, Brison DR, Lundin K, Sturmey RG. Biological optimization, the Goldilocks principle, and how much is lagom in the preimplantation embryo. Mol Reprod Dev. 2016;83(9):748–754. [DOI] [PubMed] [Google Scholar]
- 41. Collado-Fernandez E, Picton HM, Dumollard R. Metabolism throughout follicle and oocyte development in mammals. Int J Dev Biol. 2012;56(10–12):799–808. [DOI] [PubMed] [Google Scholar]
- 42. Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20(3):346–353. [DOI] [PubMed] [Google Scholar]
- 43. Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, Novara PV, Fadini R. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21(4):427–454. [DOI] [PubMed] [Google Scholar]
- 44. Dalton CM, Carroll J. Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte. J Cell Sci. 2013;126(Pt 13):2955–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lightowlers RN, Taylor RW, Turnbull DM. Mutations causing mitochondrial disease: What is new and what challenges remain? Science. 2015;349(6255):1494–1499. [DOI] [PubMed] [Google Scholar]
- 46. Gorman GS, Schaefer AM, Ng Y, Gomez N, Blakely EL, Alston CL, Feeney C, Horvath R, Yu-Wai-Man P, Chinnery PF, Taylor RW, Turnbull DM, McFarland R. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol. 2015;77(5):753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skladal D, Halliday J, Thorburn DR. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain. 2003;126(Pt 8):1905–1912. [DOI] [PubMed] [Google Scholar]
- 48. Rahman J, Rahman S. Mitochondrial medicine in the omics era. Lancet. 2018;391(10139):2560–2574. [DOI] [PubMed] [Google Scholar]
- 49. Alston CL, Rocha MC, Lax NZ, Turnbull DM, Taylor RW. The genetics and pathology of mitochondrial disease. J Pathol. 2017;241(2):236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prezant TR, Agapian JV, Bohlman MC, Bu X, Öztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, Shohat M, Fischel-Ghodsian N. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4(3):289–294. [DOI] [PubMed] [Google Scholar]
- 51. Wallace DC, Lott MT. Leber hereditary optic neuropathy: exemplar of an mtDNA disease. In: Singh H, Sheu SS, eds. Pharmacology of Mitochondria. Vol. 240. Cham, Switzerland: Springer International Publishing; 2017:339–376. [DOI] [PubMed] [Google Scholar]
- 52. Abela L, Spiegel R, Crowther LM, Klein A, Steindl K, Papuc SM, Joset P, Zehavi Y, Rauch A, Plecko B, Simmons TL. Plasma metabolomics reveals a diagnostic metabolic fingerprint for mitochondrial aconitase (ACO2) deficiency. PLoS One. 2017;12(5):e0176363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sadat R, Barca E, Masand R, Donti TR, Naini A, De Vivo DC, DiMauro S, Hanchard NA, Graham BH. Functional cellular analyses reveal energy metabolism defect and mitochondrial DNA depletion in a case of mitochondrial aconitase deficiency. Mol Genet Metab. 2016;118(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spiegel R, Pines O, Ta-Shma A, Burak E, Shaag A, Halvardson J, Edvardson S, Mahajna M, Zenvirt S, Saada A, Shalev S, Feuk L, Elpeleg O. Infantile cerebellar-retinal degeneration associated with a mutation in mitochondrial aconitase, ACO2. Am J Hum Genet. 2012;90(3):518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chow J, Rahman J, Achermann JC, Dattani MT, Rahman S. Mitochondrial disease and endocrine dysfunction. Nat Rev Endocrinol. 2017;13(2):92–104. [DOI] [PubMed] [Google Scholar]
- 56. Al-Gadi IS, Haas RH, Falk MJ, Goldstein A, McCormack SE. Endocrine disorders in primary mitochondrial disease. J Endocr Soc. 2018;2(4):361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Demain LA, Conway GS, Newman WG. Genetics of mitochondrial dysfunction and infertility. Clin Genet. 2017;91(2):199–207. [DOI] [PubMed] [Google Scholar]
- 58. Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N Engl J Med. 2012;366(12):1132–1141. [DOI] [PubMed] [Google Scholar]
- 59. Ropp PA, Copeland WC. Cloning and characterization of the human mitochondrial DNA polymerase, DNA polymerase gamma. Genomics. 1996;36(3):449–458. [DOI] [PubMed] [Google Scholar]
- 60. Rahman S, Copeland WC. POLG-related disorders and their neurological manifestations. Nat Rev Neurol. 2019;15(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Longley MJ, Graziewicz MA, Bienstock RJ, Copeland WC. Consequences of mutations in human DNA polymerase gamma. Gene. 2005;354:125–131. [DOI] [PubMed] [Google Scholar]
- 62. Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalainen A. Parkinsonism, premature menopause, and mitochondrial DNA polymerase γ mutations: clinical and molecular genetic study. Lancet. 2004;364(9437):875–882. [DOI] [PubMed] [Google Scholar]
- 63. Chen B, Li L, Wang J, Zhou Y, Zhu J, Li T, Pan H, Liu B, Cao Y, Wang B. Identification of the first homozygous POLG mutation causing non-syndromic ovarian dysfunction. Climacteric. 2018;21(5):467–471. [DOI] [PubMed] [Google Scholar]
- 64. Tong ZB, Sullivan SD, Lawless LM, Vanderhoof V, Zachman K, Nelson LM. Five mutations of mitochondrial DNA polymerase-gamma (POLG) are not a prevalent etiology for spontaneous 46,XX primary ovarian insufficiency. Fertil Steril. 2010;94(7):2932–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Duncan AJ, Knight JA, Costello H, Conway GS, Rahman S. POLG mutations and age at menopause. Hum Reprod. 2012;27(7):2243–2244. [DOI] [PubMed] [Google Scholar]
- 66. Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik-Sullivan B, Esko T, Johnson AD, Elks CE, Franceschini N, He C, Altmaier E, Brody JA, Franke LL, Huffman JE, Keller MF, McArdle PF, Nutile T, Porcu E, Robino A, Rose LM, Schick UM, Smith JA, Teumer A, Traglia M, Vuckovic D, Yao J, Zhao W, Albrecht E, Amin N, Corre T, Hottenga JJ, Mangino M, Smith AV, Tanaka T, Abecasis GR, Andrulis IL, Anton-Culver H, Antoniou AC, Arndt V, Arnold AM, Barbieri C, Beckmann MW, Beeghly-Fadiel A, Benitez J, Bernstein L, Bielinski SJ, Blomqvist C, Boerwinkle E, Bogdanova NV, Bojesen SE, Bolla MK, Borresen-Dale AL, Boutin TS, Brauch H, Brenner H, Brüning T, Burwinkel B, Campbell A, Campbell H, Chanock SJ, Chapman JR, Chen YD, Chenevix-Trench G, Couch FJ, Coviello AD, Cox A, Czene K, Darabi H, De Vivo I, Demerath EW, Dennis J, Devilee P, Dörk T, dos-Santos-Silva I, Dunning AM, Eicher JD, Fasching PA, Faul JD, Figueroa J, Flesch-Janys D, Gandin I, Garcia ME, García-Closas M, Giles GG, Girotto GG, Goldberg MS, González-Neira A, Goodarzi MO, Grove ML, Gudbjartsson DF, Guénel P, Guo X, Haiman CA, Hall P, Hamann U, Henderson BE, Hocking LJ, Hofman A, Homuth G, Hooning MJ, Hopper JL, Hu FB, Huang J, Humphreys K, Hunter DJ, Jakubowska A, Jones SE, Kabisch M, Karasik D, Knight JA, Kolcic I, Kooperberg C, Kosma VM, Kriebel J, Kristensen V, Lambrechts D, Langenberg C, Li J, Li X, Lindström S, Liu Y, Luan J, Lubinski J, Mägi R, Mannermaa A, Manz J, Margolin S, Marten J, Martin NG, Masciullo C, Meindl A, Michailidou K, Mihailov E, Milani L, Milne RL, Müller-Nurasyid M, Nalls M, Neale BM, Nevanlinna H, Neven P, Newman AB, Nordestgaard BG, Olson JE, Padmanabhan S, Peterlongo P, Peters U, Petersmann A, Peto J, Pharoah PD, Pirastu NN, Pirie A, Pistis G, Polasek O, Porteous D, Psaty BM, Pylkäs K, Radice P, Raffel LJ, Rivadeneira F, Rudan I, Rudolph A, Ruggiero D, Sala CF, Sanna S, Sawyer EJ, Schlessinger D, Schmidt MK, Schmidt F, Schmutzler RK, Schoemaker MJ, Scott RA, Seynaeve CM, Simard J, Sorice R, Southey MC, Stöckl D, Strauch K, Swerdlow A, Taylor KD, Thorsteinsdottir U, Toland AE, Tomlinson I, Truong T, Tryggvadottir L, Turner ST, Vozzi D, Wang Q, Wellons M, Willemsen G, Wilson JF, Winqvist R, Wolffenbuttel BB, Wright AF, Yannoukakos D, Zemunik T, Zheng W, Zygmunt M, Bergmann S, Boomsma DI, Buring JE, Ferrucci L, Montgomery GW, Gudnason V, Spector TD, van Duijn CM, Alizadeh BZ, Ciullo M, Crisponi L, Easton DF, Gasparini PP, Gieger C, Harris TB, Hayward C, Kardia SL, Kraft P, McKnight B, Metspalu A, Morrison AC, Reiner AP, Ridker PM, Rotter JI, Toniolo D, Uitterlinden AG, Ulivi S, Völzke H, Wareham NJ, Weir DR, Yerges-Armstrong LM, Price AL, Stefansson K, Visser JA, Ong KK, Chang-Claude J, Murabito JM, Perry JR, Murray A; PRACTICAL Consortium; kConFab Investigators; AOCS Investigators; Generation Scotland; EPIC-InterAct Consortium; LifeLines Cohort Study. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47(11):1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Suomalainen A, Battersby BJ. Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol. 2018;19(2):77–92. [DOI] [PubMed] [Google Scholar]
- 68. Korhonen JA, Pham XH, Pellegrini M, Falkenberg M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 2004;23(12):2423–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Morino H, Pierce SB, Matsuda Y, Walsh T, Ohsawa R, Newby M, Hiraki-Kamon K, Kuramochi M, Lee MK, Klevit RE, Martin A, Maruyama H, King MC, Kawakami H. Mutations in Twinkle primase-helicase cause Perrault syndrome with neurologic features. Neurology. 2014;83(22):2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Longley MJ, Clark S, Yu Wai Man C, Hudson G, Durham SE, Taylor RW, Nightingale S, Turnbull DM, Copeland WC, Chinnery PF. Mutant POLG2 disrupts DNA polymerase γ subunits and causes progressive external ophthalmoplegia. Am J Hum Genet. 2006;78(6):1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Suzuki T, Nagao A, Suzuki T. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu Rev Genet. 2011;45(1):299–329. [DOI] [PubMed] [Google Scholar]
- 72. Pierce SB, Gersak K, Michaelson-Cohen R, Walsh T, Lee MK, Malach D, Klevit RE, King MC, Levy-Lahad E. Mutations in LARS2, encoding mitochondrial leucyl-tRNA synthetase, lead to premature ovarian failure and hearing loss in Perrault syndrome. Am J Hum Genet. 2013;92(4):614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pierce SB, Chisholm KM, Lynch ED, Lee MK, Walsh T, Opitz JM, Li W, Klevit RE, King MC. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc Natl Acad Sci USA. 2011;108(16):6543–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kosaki R, Horikawa R, Fujii E, Kosaki K. Biallelic mutations in LARS2 can cause Perrault syndrome type 2 with neurologic symptoms. Am J Med Genet A. 2018;176(2):404–408. [DOI] [PubMed] [Google Scholar]
- 75. Rehman AU, Friedman TB, Griffith AJ. Unresolved questions regarding human hereditary deafness. Oral Dis. 2017;23(5):551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gispert S, Parganlija D, Klinkenberg M, Dröse S, Wittig I, Mittelbronn M, Grzmil P, Koob S, Hamann A, Walter M, Büchel F, Adler T, Hrabé de Angelis M, Busch DH, Zell A, Reichert AS, Brandt U, Osiewacz HD, Jendrach M, Auburger G. Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors. Hum Mol Genet. 2013;22(24):4871–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. González-Serrano LE, Chihade JW, Sissler M. When a common biological role does not imply common disease outcomes: disparate pathology linked to human mitochondrial aminoacyl-tRNA synthetases. J Biol Chem. 2019;294(14):5309–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sissler M, González-Serrano LE, Westhof E. Recent advances in mitochondrial aminoacyl-tRNA synthetases and disease. Trends Mol Med. 2017;23(8):693–708. [DOI] [PubMed] [Google Scholar]
- 79. Dallabona C, Diodato D, Kevelam SH, Haack TB, Wong LJ, Salomons GS, Baruffini E, Melchionda L, Mariotti C, Strom TM, Meitinger T, Prokisch H, Chapman K, Colley A, Rocha H, Ounap K, Schiffmann R, Salsano E, Savoiardo M, Hamilton EM, Abbink TE, Wolf NI, Ferrero I, Lamperti C, Zeviani M, Vanderver A, Ghezzi D, van der Knaap MS. Novel (ovario) leukodystrophy related to AARS2 mutations. Neurology. 2014;82(23):2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lynch DS, Zhang WJ, Lakshmanan R, Kinsella JA, Uzun GA, Karbay M, Tüfekçioglu Z, Hanagasi H, Burke G, Foulds N, Hammans SR, Bhattacharjee A, Wilson H, Adams M, Walker M, Nicoll JA, Chataway J, Fox N, Davagnanam I, Phadke R, Houlden H. Analysis of mutations in AARS2 in a Series of CSF1R-negative patients with adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. JAMA Neurol. 2016;73(12):1433–1439. [DOI] [PubMed] [Google Scholar]
- 81. Taglia I, Di Donato I, Bianchi S, Cerase A, Monti L, Marconi R, Orrico A, Rufa A, Federico A, Dotti MT. AARS2-related ovarioleukodystrophy: clinical and neuroimaging features of three new cases. Acta Neurol Scand. 2018;138(4):278–283. [DOI] [PubMed] [Google Scholar]
- 82. Hamatani M, Jingami N, Tsurusaki Y, Shimada S, Shimojima K, Asada-Utsugi M, Yoshinaga K, Uemura N, Yamashita H, Uemura K, Takahashi R, Matsumoto N, Yamamoto T. The first Japanese case of leukodystrophy with ovarian failure arising from novel compound heterozygous AARS2 mutations. J Hum Genet. 2016;61(10):899–902. [DOI] [PubMed] [Google Scholar]
- 83. Lee JM, Yang HJ, Kwon JH, Kim WJ, Kim SY, Lee EM, Park JY, Weon YC, Park SH, Gwon BJ, Ryu JC, Lee ST, Kim HJ, Jeon B. Two Korean siblings with recently described ovarioleukodystrophy related to AARS2 mutations. Eur J Neurol. 2017;24(4):e21–e22. [DOI] [PubMed] [Google Scholar]
- 84. Kiraly-Borri C, Jevon G, Ji W, Jeffries L, Ricciardi J-L, Konstantino M, Ackerman KG, Lakhani SA. Siblings with lethal primary pulmonary hypoplasia and compound heterozygous variants in the AARS2 gene: further delineation of the phenotypic spectrum. Cold Spring Harb Mol Case Stud. 2019;5(3):a003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Götz A, Tyynismaa H, Euro L, Ellonen P, Hyötyläinen T, Ojala T, Hämäläinen RH, Tommiska J, Raivio T, Oresic M, Karikoski R, Tammela O, Simola KOJ, Paetau A, Tyni T, Suomalainen A. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am J Hum Genet. 2011;88(5):635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sommerville EW, Zhou X-L, Oláhová M, Jenkins J, Euro L, Konovalova S, Hilander T, Pyle A, He L, Habeebu S, Saunders C, Kelsey A, Morris AAM, McFarland R, Suomalainen A, Gorman GS, Wang ED, Thiffault I, Tyynismaa H, Taylor RW. Instability of the mitochondrial alanyl-tRNA synthetase underlies fatal infantile-onset cardiomyopathy. Hum Mol Genet. 2019;28(2):258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Euro L, Konovalova S, Asin-Cayuela J, Tulinius M, Griffin H, Horvath R, Taylor RW, Chinnery PF, Schara U, Thorburn DR, Suomalainen A, Chihade J, Tyynismaa H. Structural modeling of tissue-specific mitochondrial alanyl-tRNA synthetase (AARS2) defects predicts differential effects on aminoacylation. Front Genet. 2015;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fischer F, Hamann A, Osiewacz HD. Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci. 2012;37(7):284–292. [DOI] [PubMed] [Google Scholar]
- 89. Szczepanowska K, Maiti P, Kukat A, Hofsetz E, Nolte H, Senft K, Becker C, Ruzzenente B, Hornig-Do HT, Wibom R, Wiesner RJ, Krüger M, Trifunovic A. CLPP coordinates mitoribosomal assembly through the regulation of ERAL1 levels. EMBO J. 2016;35(23):2566–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cole A, Wang Z, Coyaud E, Voisin V, Gronda M, Jitkova Y, Mattson R, Hurren R, Babovic S, Maclean N, Restall I, Wang X, Jeyaraju DV, Sukhai MA, Prabha S, Bashir S, Ramakrishnan A, Leung E, Qia YH, Zhang N, Combes KR, Ketela T, Lin F, Houry WA, Aman A, Al-Awar R, Zheng W, Wienholds E, Xu CJ, Dick J, Wang JC, Moffat J, Minden MD, Eaves CJ, Bader GD, Hao Z, Kornblau SM, Raught B, Schimmer AD. Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2015;27(6):864–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fischer F, Langer JD, Osiewacz HD. Identification of potential mitochondrial CLPXP protease interactors and substrates suggests its central role in energy metabolism. Sci Rep. 2015;5(1):18375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Deepa SS, Bhaskaran S, Ranjit R, Qaisar R, Nair BC, Liu Y, Walsh ME, Fok WC, Van Remmen H. Down-regulation of the mitochondrial matrix peptidase ClpP in muscle cells causes mitochondrial dysfunction and decreases cell proliferation. Free Radic Biol Med. 2016;91:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lerat J, Jonard L, Loundon N, Christin-Maitre S, Lacombe D, Goizet C, Rouzier C, Van Maldergem L, Gherbi S, Garabedian EN, Bonnefont JP, Touraine P, Mosnier I, Munnich A, Denoyelle F, Marlin S. An application of NGS for molecular investigations in Perrault syndrome: study of 14 families and review of the literature. Hum Mutat. 2016;37(12):1354–1362. [DOI] [PubMed] [Google Scholar]
- 94. Dursun F, Mohamoud HSA, Karim N, Naeem M, Jelani M, Kırmızıbekmez H. A novel missense mutation in the CLPP gene causing Perrault Syndrome type 3 in a Turkish family. J Clin Res Pediatr Endocrinol. 2016;8(4):472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ahmed S, Jelani M, Alrayes N, Mohamoud HS, Almramhi MM, Anshasi W, Ahmed NA, Wang J, Nasir J, Al-Aama JY. Exome analysis identified a novel missense mutation in the CLPP gene in a consanguineous Saudi family expanding the clinical spectrum of Perrault syndrome type-3. J Neurol Sci. 2015;353(1–2):149–154. [DOI] [PubMed] [Google Scholar]
- 96. Jenkinson EM, Rehman AU, Walsh T, Clayton-Smith J, Lee K, Morell RJ, Drummond MC, Khan SN, Naeem MA, Rauf B, Billington N, Schultz JM, Urquhart JE, Lee MK, Berry A, Hanley NA, Mehta S, Cilliers D, Clayton PE, Kingston H, Smith MJ, Warner TT, Black GC, Trump D, Davis JR, Ahmad W, Leal SM, Riazuddin S, King MC, Friedman TB, Newman WG; University of Washington Center for Mendelian Genomics. Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am J Hum Genet. 2013;92(4):605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brodie EJ, Zhan H, Saiyed T, Truscott KN, Dougan DA. Perrault syndrome type 3 caused by diverse molecular defects in CLPP. Sci Rep. 2018;8(1):12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Siira SJ, Spåhr H, Shearwood AJ, Ruzzenente B, Larsson NG, Rackham O, Filipovska A. LRPPRC-mediated folding of the mitochondrial transcriptome. Nat Commun. 2017;8(1):1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ruzzenente B, Metodiev MD, Wredenberg A, Bratic A, Park CB, Cámara Y, Milenkovic D, Zickermann V, Wibom R, Hultenby K, Erdjument-Bromage H, Tempst P, Brandt U, Stewart JB, Gustafsson CM, Larsson NG. LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J. 2012;31(2):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lagouge M, Mourier A, Lee HJ, Spåhr H, Wai T, Kukat C, Silva Ramos E, Motori E, Busch JD, Siira S, Kremmer E, Filipovska A, Larsson NG; German Mouse Clinic Consortium. SLIRP regulates the rate of mitochondrial protein synthesis and protects LRPPRC from degradation. PLoS Genet. 2015;11(8):e1005423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Volpon L, Culjkovic-Kraljacic B, Sohn HS, Blanchet-Cohen A, Osborne MJ, Borden KL. A biochemical framework for eIF4E-dependent mRNA export and nuclear recycling of the export machinery. RNA. 2017;23(6):927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cooper MP, Qu L, Rohas LM, Lin J, Yang W, Erdjument-Bromage H, Tempst P, Spiegelman BM. Defects in energy homeostasis in Leigh syndrome French Canadian variant through PGC-1α/LRP130 complex. Genes Dev. 2006;20(21):2996–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Han VX, Tan TS, Wang FS, Tay SK. Novel LRPPRC mutation in a boy with mild Leigh syndrome, French–Canadian type outside of Québec. Child Neurol Open. 2017;4: 2329048X17737638. doi: 10.1177/2329048X17737638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Oláhová M, Hardy SA, Hall J, Yarham JW, Haack TB, Wilson WC, Alston CL, He L, Aznauryan E, Brown RM, Brown GK, Morris AAM, Mundy H, Broomfield A, Barbosa IA, Simpson MA, Deshpande C, Moeslinger D, Koch J, Stettner GM, Bonnen PE, Prokisch H, Lightowlers RN, McFarland R, Chrzanowska-Lightowlers ZM, Taylor RW. LRPPRC mutations cause early-onset multisystem mitochondrial disease outside of the French-Canadian population. Brain. 2015;138(Pt 12):3503–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mootha VK, Lepage P, Miller K, Bunkenborg J, Reich M, Hjerrild M, Delmonte T, Villeneuve A, Sladek R, Xu F, Mitchell GA, Morin C, Mann M, Hudson TJ, Robinson B, Rioux JD, Lander ES. Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proc Natl Acad Sci USA. 2003;100(2):605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ghaddhab C, Morin C, Brunel-Guitton C, Mitchell GA, Van Vliet G, Huot C. Premature ovarian failure in French Canadian Leigh syndrome. J Pediatr. 2017;184:227–229.e1. [DOI] [PubMed] [Google Scholar]
- 107. Debray FG, Morin C, Janvier A, Villeneuve J, Maranda B, Laframboise R, Lacroix J, Decarie JC, Robitaille Y, Lambert M, Robinson BH, Mitchell GA. LRPPRC mutations cause a phenotypically distinct form of Leigh syndrome with cytochrome c oxidase deficiency. J Med Genet. 2011;48(3):183–189. [DOI] [PubMed] [Google Scholar]
- 108. Amunts A, Brown A, Toots J, Scheres SH, Ramakrishnan V. The structure of the human mitochondrial ribosome. Science. 2015;348(6230):95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Emdadul Haque M, Grasso D, Miller C, Spremulli LL, Saada A. The effect of mutated mitochondrial ribosomal proteins S16 and S22 on the assembly of the small and large ribosomal subunits in human mitochondria. Mitochondrion. 2008;8(3):254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chen A, Tiosano D, Guran T, Baris HN, Bayram Y, Mory A, Shapiro-Kulnane L, Hodges CA, Akdemir ZC, Turan S, Jhangiani SN, van den Akker F, Hoppel CL, Salz HK, Lupski JR, Buchner DA. Mutations in the mitochondrial ribosomal protein MRPS22 lead to primary ovarian insufficiency. Hum Mol Genet. 2018;27(11):1913–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jolly A, Bayram Y, Turan S, Aycan Z, Tos T, Abali ZY, Hacihamdioglu B, Coban Akdemir ZH, Hijazi H, Bas S, Atay Z, Guran T, Abali S, Bas F, Darendeliler F, Colombo R, Barakat TS, Rinne T, White JJ, Yesil G, Gezdirici A, Gulec EY, Karaca E, Pehlivan D, Jhangiani SN, Muzny DM, Poyrazoglu S, Bereket A, Gibbs RA, Posey JE, Lupski JR. Exome sequencing of a primary ovarian insufficiency cohort reveals common molecular etiologies for a spectrum of disease. J Clin Endocrinol Metab. 2019;104(8):3049–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Saada A, Shaag A, Arnon S, Dolfin T, Miller C, Fuchs-Telem D, Lombes A, Elpeleg O. Antenatal mitochondrial disease caused by mitochondrial ribosomal protein (MRPS22) mutation. J Med Genet. 2007;44(12):784–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Smits P, Saada A, Wortmann SB, Heister AJ, Brink M, Pfundt R, Miller C, Haas D, Hantschmann R, Rodenburg RJ, Smeitink JAM, van den Heuvel LP. Mutation in mitochondrial ribosomal protein MRPS22 leads to Cornelia de Lange-like phenotype, brain abnormalities and hypertrophic cardiomyopathy. Eur J Hum Genet. 2011;19(4):394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Baertling F, Haack TB, Rodenburg RJ, Schaper J, Seibt A, Strom TM, Meitinger T, Mayatepek E, Hadzik B, Selcan G, Prokisch H, Distelmaier F. MRPS22 mutation causes fatal neonatal lactic acidosis with brain and heart abnormalities. Neurogenetics. 2015;16(3):237–240. [DOI] [PubMed] [Google Scholar]
- 115. Kılıç M, Oğuz K-K, Kılıç E, Yüksel D, Demirci H, Sağıroğlu MŞ, Yücel-Yılmaz D, Özgül RK. A patient with mitochondrial disorder due to a novel mutation in MRPS22. Metab Brain Dis. 2017;32(5):1389–1393. [DOI] [PubMed] [Google Scholar]
- 116. Hochberg I, Demain LA, Urquhart JE, Amberger A, Deutschmann AJ, Demetz S, Thompson K, O’Sullivan J, Belyantseva IA, Barzik M, Williams SG, Bhaskar SS, Jenkinson EM, AlSheqaih N, Blumenfeld Z, Yalonetsky S, Oerum S, Rossmanith W, Yue WW, Zschocke J, Taylor RW, Friedman TB, Munro KJ, O’Keefe RT, Newman WG. A homozygous variant in mitochondrial RNase P subunit PRORP is associated with Perrault syndrome characterized by hearing loss and primary ovarian insufficiency. bioRxiv. 2017:168252. [Google Scholar]
- 117. Menezes MJ, Guo Y, Zhang J, Riley LG, Cooper ST, Thorburn DR, Li J, Dong D, Li Z, Glessner J, Davis RL, Sue CM, Alexander SI, Arbuckle S, Kirwan P, Keating BJ, Xu X, Hakonarson H, Christodoulou J. Mutation in mitochondrial ribosomal protein S7 (MRPS7) causes congenital sensorineural deafness, progressive hepatic and renal failure and lactic acidemia. Hum Mol Genet. 2015;24(8):2297–2307. [DOI] [PubMed] [Google Scholar]
- 118. Demain LA, Antunes D, O’Sullivan J, Bhaskhar SS, O’Keefe RT, Newman WG. A known pathogenic variant in the essential mitochondrial translation gene RMND1 causes a Perrault-like syndrome with renal defects. Clin Genet. 2018;94(2):276–277. [DOI] [PubMed] [Google Scholar]
- 119. Ng YS, Alston CL, Diodato D, Morris AA, Ulrick N, Kmoch S, Houštěk J, Martinelli D, Haghighi A, Atiq M, Gamero MA, Garcia-Martinez E, Kratochvílová H, Santra S, Brown RM, Brown GK, Ragge N, Monavari A, Pysden K, Ravn K, Casey JP, Khan A, Chakrapani A, Vassallo G, Simons C, McKeever K, O’Sullivan S, Childs AM, Østergaard E, Vanderver A, Goldstein A, Vogt J, Taylor RW, McFarland R. The clinical, biochemical and genetic features associated with RMND1-related mitochondrial disease. J Med Genet. 2016;53(11):768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Janer A, Antonicka H, Lalonde E, Nishimura T, Sasarman F, Brown GK, Brown RM, Majewski J, Shoubridge EA. An RMND1 mutation causes encephalopathy associated with multiple oxidative phosphorylation complex deficiencies and a mitochondrial translation defect. Am J Hum Genet. 2012;91(4):737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Garcia-Diaz B, Barros MH, Sanna-Cherchi S, Emmanuele V, Akman HO, Ferreiro-Barros CC, Horvath R, Tadesse S, El Gharaby N, DiMauro S, De Vivo DC, Shokr A, Hirano M, Quinzii CM. Infantile encephaloneuromyopathy and defective mitochondrial translation are due to a homozygous RMND1 mutation. Am J Hum Genet. 2012;91(4):729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Posey JE, O’Donnell-Luria AH, Chong JX, Harel T, Jhangiani SN, Coban Akdemir ZH, Buyske S, Pehlivan D, Carvalho CMB, Baxter S, Sobreira N, Liu P, Wu N, Rosenfeld JA, Kumar S, Avramopoulos D, White JJ, Doheny KF, Witmer PD, Boehm C, Sutton VR, Muzny DM, Boerwinkle E, Günel M, Nickerson DA, Mane S, MacArthur DG, Gibbs RA, Hamosh A, Lifton RP, Matise TC, Rehm HL, Gerstein M, Bamshad MJ, Valle D, Lupski JR; Centers for Mendelian Genomics. Insights into genetics, human biology and disease gleaned from family based genomic studies. Genet Med. 2019;21(4):798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Venkatesh S, Kumar M, Sharma A, Kriplani A, Ammini AC, Talwar P, Agarwal A, Dada R. Oxidative stress and ATPase6 mutation is associated with primary ovarian insufficiency. Arch Gynecol Obstet. 2010;282(3):313–318. [DOI] [PubMed] [Google Scholar]
- 124. Zhen X, Wu B, Wang J, Lu C, Gao H, Qiao J. Increased incidence of mitochondrial cytochrome c oxidase 1 gene mutations in patients with primary ovarian insufficiency. PLoS One. 2015;10(7):e0132610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Palanichamy MG, Zhang Y-P. Are ATPase6 polymorphisms associated with primary ovarian insufficiency? Arch Gynecol Obstet. 2011;283(3):671–672. [DOI] [PubMed] [Google Scholar]
- 126. Kumar M, Pathak D, Venkatesh S, Kriplani A, Ammini AC, Dada R. Chromosomal abnormalities & oxidative stress in women with premature ovarian failure (POF). Indian J Med Res. 2012;135(1):92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kumar M, Pathak D, Kriplani A, Ammini AC, Talwar P, Dada R. Nucleotide variations in mitochondrial DNA and supra-physiological ROS levels in cytogenetically normal cases of premature ovarian insufficiency. Arch Gynecol Obstet. 2010;282(6):695–705. [DOI] [PubMed] [Google Scholar]
- 128. Ding Y, Xia BH, Zhuo GC, Zhang CJ, Leng JH. Premature ovarian insufficiency may be associated with the mutations in mitochondrial tRNA genes. Endocr J. 2019;66(1):81–88. [DOI] [PubMed] [Google Scholar]
- 129. Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y, Alexis J, Meriano J, Sung H-K, Gasser DL, Moley KH, Hekimi S, Casper RF, Jurisicova A. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14(5):887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Boczonadi V, Smith PM, Pyle A, Gomez-Duran A, Schara U, Tulinius M, Chinnery PF, Horvath R. Altered 2-thiouridylation impairs mitochondrial translation in reversible infantile respiratory chain deficiency. Hum Mol Genet. 2013;22(22):4602–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wang G, Shimada E, Zhang J, Hong JS, Smith GM, Teitell MA, Koehler CM. Correcting human mitochondrial mutations with targeted RNA import. Proc Natl Acad Sci USA. 2012;109(13):4840–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Perli E, Giordano C, Pisano A, Montanari A, Campese AF, Reyes A, Ghezzi D, Nasca A, Tuppen HA, Orlandi M, Di Micco P, Poser E, Taylor RW, Colotti G, Francisci S, Morea V, Frontali L, Zeviani M, d’Amati G. The isolated carboxy-terminal domain of human mitochondrial leucyl-tRNA synthetase rescues the pathological phenotype of mitochondrial tRNA mutations in human cells. EMBO Mol Med. 2014;6(2):169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hornig-Do HT, Montanari A, Rozanska A, Tuppen HA, Almalki AA, Abg-Kamaludin DP, Frontali L, Francisci S, Lightowlers RN, Chrzanowska-Lightowlers ZM. Human mitochondrial leucyl tRNA synthetase can suppress non cognate pathogenic mt-tRNA mutations. EMBO Mol Med. 2014;6(2):183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhao X, Han J, Zhu L, Xiao Y, Wang C, Hong F, Jiang P, Guan M-X. Overexpression of human mitochondrial alanyl-tRNA synthetase suppresses biochemical defects of the mt-tRNAAla mutation in cybrids. Int J Biol Sci. 2018;14(11):1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gadaleta MN, Petruzzella V, Daddabbo L, Olivieri C, Fracasso F, Loguercio Polosa P, Cantatore P. Mitochondrial DNA transcription and translation in aged rat. Effect of acetyl-l-carnitine. Ann N Y Acad Sci. 1994;717(1):150–160. [DOI] [PubMed] [Google Scholar]
- 136. Liang LF, Qi ST, Xian YX, Huang L, Sun XF, Wang WH. Protective effect of antioxidants on the pre-maturation aging of mouse oocytes. Sci Rep. 2017;7(1):1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zare Z, Masteri Farahani R, Salehi M, Piryaei A, Ghaffari Novin M, Fadaei Fathabadi F, Mohammadi M, Dehghani-Mohammadabadi M. Effect of l-carnitine supplementation on maturation and early embryo development of immature mouse oocytes selected by brilliant cresyle blue staining. J Assist Reprod Genet. 2015;32(4):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yamada T, Imai H, Yamada M. Beneficial effects of acetyl-l-carnitine treatment during IVM on post-fertilization development of bovine oocytes in vitro. Reprod Fertil Dev. 2006;18(2):280. [Google Scholar]
- 139. Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, Shendure J. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12(11):745–755. [DOI] [PubMed] [Google Scholar]
- 140. Committee on Adolescent Health Care. Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193–197. [DOI] [PubMed] [Google Scholar]
- 141. Stark Z, Schofield D, Alam K, Wilson W, Mupfeki N, Macciocca I, Shrestha R, White SM, Gaff C. Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet Med. 2017;19(8):867–874. [DOI] [PubMed] [Google Scholar]
- 142. Monroe GR, Frederix GW, Savelberg SMC, de Vries TI, Duran KJ, van der Smagt JJ, Terhal PA, van Hasselt PM, Kroes HY, Verhoeven-Duif NM, Nijman IJ, Carbo EC, van Gassen KL, Knoers NV, Hövels AM, van Haelst MM, Visser G, van Haaften G. Effectiveness of whole-exome sequencing and costs of the traditional diagnostic trajectory in children with intellectual disability. Genet Med. 2016;18(9):949–956. [DOI] [PubMed] [Google Scholar]
- 143. Tan TY, Dillon OJ, Stark Z, Schofield D, Alam K, Shrestha R, Chong B, Phelan D, Brett GR, Creed E, Jarmolowicz A, Yap P, Walsh M, Downie L, Amor DJ, Savarirayan R, McGillivray G, Yeung A, Peters H, Robertson SJ, Robinson AJ, Macciocca I, Sadedin S, Bell K, Oshlack A, Georgeson P, Thorne N, Gaff C, White SM. Diagnostic impact and cost-effectiveness of whole-exome sequencing for ambulant children with suspected monogenic conditions. JAMA Pediatr. 2017;171(9):855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, Yeung A, Peters H, Mordaunt D, Cowie S, Amor DJ, Savarirayan R, McGillivray G, Downie L, Ekert PG, Theda C, James PA, Yaplito-Lee J, Ryan MM, Leventer RJ, Creed E, Macciocca I, Bell KM, Oshlack A, Sadedin S, Georgeson P, Anderson C, Thorne N, Gaff C, White SM; Melbourne Genomics Health Alliance. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18(11):1090–1096. [DOI] [PubMed] [Google Scholar]
- 145. Vissers LE, van Nimwegen KJ, Schieving JH, Kamsteeg EJ, Kleefstra T, Yntema HG, Pfundt R, van der Wilt GJ, Krabbenborg L, Brunner HG, van der Burg S, Grutters J, Veltman JA, Willemsen MA. A clinical utility study of exome sequencing versus conventional genetic testing in pediatric neurology. Genet Med. 2017;19(9):1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Stavropoulos DJ, Merico D, Jobling R, Bowdin S, Monfared N, Thiruvahindrapuram B, Nalpathamkalam T, Pellecchia G, Yuen RK, Szego MJ, Hayeems RZ, Shaul RZ, Brudno M, Girdea M, Frey B, Alipanahi B, Ahmed S, Babul-Hirji R, Porras RB, Carter MT, Chad L, Chaudhry A, Chitayat D, Doust SJ, Cytrynbaum C, Dupuis L, Ejaz R, Fishman L, Guerin A, Hashemi B, Helal M, Hewson S, Inbar-Feigenberg M, Kannu P, Karp N, Kim R, Kronick J, Liston E, MacDonald H, Mercimek-Mahmutoglu S, Mendoza-Londono R, Nasr E, Nimmo G, Parkinson N, Quercia N, Raiman J, Roifman M, Schulze A, Shugar A, Shuman C, Sinajon P, Siriwardena K, Weksberg R, Yoon G, Carew C, Erickson R, Leach RA, Klein R, Ray PN, Meyn MS, Scherer SW, Cohn RD, Marshall CR. Whole-genome sequencing expands diagnostic utility and improves clinical management in paediatric medicine. NPJ Genom Med. 2016;1(1):15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.