Abstract
Background
The survival trends and the patterns of clinical practice pertaining to radiation therapy and surgical resection for WHO grade I, II, and III astrocytoma patients remain poorly characterized.
Methods
Using the Surveillance, Epidemiology and End Results (SEER) database, we identified 2497 grade I, 4113 grade II, and 2755 grade III astrocytomas during the period of 1999–2010. Time-trend analyses were performed for overall survival, radiation treatment (RT), and the extent of surgical resection (EOR).
Results
While overall survival of grade I astrocytoma patients remained unchanged during the study period, we observed improved overall survival for grade II and III astrocytoma patients (Tarone-Ware P < .05). The median survival increased from 44 to 57 months and from 15 to 24 months for grade II and III astrocytoma patients, respectively. The differences in survival remained significant after adjusting for pertinent variables including age, ethnicity, marital status, sex, tumor size, tumor location, EOR, and RT status. The pattern of clinical practice in terms of EOR for grade II and III astrocytoma patients did not change significantly during this study period. However, there was decreased RT utilization as treatment for grade II astrocytoma patients after 2005.
Conclusion
Results from the SEER database indicate that there were improvements in the overall survival of grade II and III astrocytoma patients over the past decade. Analysis of the clinical practice patterns identified potential opportunities for impacting the clinical course of these patients.
Keywords: population-based SEER database; practice pattern; survival; WHO grade I, II, III astrocytomas
Glioma refers to tumors derived from neoplastic transformation of glial cells and constitutes the most common form of primary brain cancer.1–3 “Glia” is a Greek word meaning “glue.” The word is meant to describe the non-neuronal cells in the nervous system that are found interspersed among neurons, “gluing” the distinct neurons into a cohesive system. Central nervous system (CNS) glial cells consist of distinct histologic cellular subtypes. In the adult CNS, the three types of glial cells that give rise to tumors include astrocytes, oligodendrocytes, and ependymal cells. The tumors derived from these glial cells differ in terms of the mechanism of pathogenesis as well as clinical behavior. Of these glioma types, tumors derived from astrocytes or their precursors are called astrocytomas and are most common.
Astrocytic tumors are classified histologically based on World Health Organization (WHO) criteria.4 Grade I tumors are biologically benign and complete surgical excision is typically curative. Grade II astrocytomas are characterized by hypercellularity with diffuse infiltration into the surrounding cerebral parenchyma. Complete surgical excision of grade II tumors cannot generally be achieved. The median survival for patients afflicted with grade II astrocytomas ranges from 5 to 8 years.5 Grade III or IV astrocytomas are considered malignant. In addition to hypercellularity, grade III astrocytomas, also known as anaplastic astrocytomas, exhibit nuclear atypia and increased mitotic figures. The median survival for patients with grade III tumors is ∼3 years.6 Grade IV astrocytomas, or glioblastomas, are characterized by histologic findings of angiogenesis and necrosis. Grade IV tumors are extremely aggressive and are associated with a median survival of 12 to 18 months.7
Radiation, chemotherapy, and surgical resection play critical roles in the management of grade I, II, and III astrocytoma patients. Surgery remains a primary treatment modality for all three cancer types. Because gross total resection is typically curative for grade I astrocytoma, these patients are rarely treated with radiation and chemotherapy.8 For grade II and III astrocytomas, there is accumulating evidence based on institutional experiences that the extent of resection influences overall survival.6,9–11 However, controversy remains in this matter as the thesis has not been definitively demonstrated through randomized control studies.12 In terms of chemotherapy and radiation therapy, oncologists typically stratify the risk profile of grade II astrocytoma patients based on clinical variables to determine whether chemotherapy and radiation therapy would be appropriate. Of note, there is no formal consensus in terms of standard of treatment for grade II astrocytoma patients.13,14 Similarly, there is relatively little data specifically addressing the standard of care for grade III astrocytoma patients. However, these patients are routinely treated with radiation and chemotherapy.15
Analysis of the historical trends of patient survival represents a valuable means of assessing progress in clinical outcome.16 Correlating these trends to the changing patterns of clinical practices further identifies needs for future improvement. While a great deal of effort has been focused on glioblastoma in this regard,17–20 few studies have been devoted to lower grade astrocytomas. The goal of this study is to assess changes in overall survival pattern of patients afflicted with grade I, II, and III astrocytomas using the Surveillance, Epidemiology, and End Results (SEER) registry.21 We used the SEER registry because the data set is broadly representative of the oncology care provided to the U.S. population, including patients treated at both academic and nonacademic centers.22 Furthermore, the registry offers access to data related to surgical resection and radiation treatment. Finally, given the rarity of low-grade gliomas, the SEER registry affords analysis on a scale that cannot be matched by any single institutional experience.
Materials and Methods
Data and Study Population
The Surveillance, Epidemiology, and End Results (SEER) Program was established by the National Cancer Institute (NCI) to collect cancer incidence and survival data from 18 population-based cancer registries that cover ∼28% of the total U.S. population (SEER Research Data 1973–2010). We used the data set released in April 2013 that was based on November 2012 submissions downloaded as an ASCII text file.23
This study included patients who were diagnosed between 1999 and 2010 with WHO grade I through IV intracranial astrocytomas as the only cancer diagnosis. The following International Classification of Disease for Oncology, 3rd Edition (ICD-O-3) histology codes were used: 9421 (pilocytic astrocytoma, WHO grade I), 9400, 9410, 9411, 9420 (diffuse astrocytoma, WHO grade II), 9401 (anaplastic astrocytoma, WHO grade III), 9440-9442 (glioblastoma, WHO grade IV), and ICD-O-3 topologic site codes C71.0-C71.9. These codes were described in Table 1 of the Central Brain Tumor Registry of the United States (CBTRUS) Statistical Report.24 Patients were excluded from the study if the surgical status was coded as unknown (n = 626, 1.8% of our study population) or if the histology was coded as unconfirmed (n = 2075, 6% of our study population). Notably, the excluded patients were older than patients who remained in the analysis (P < .05), suggesting that presumptive diagnosis without tissue biopsy is more likely in the elderly population.16 After these exclusions, we identified a total of 2497 grade I, 4113 grade II, 2755 grade III, and 21 962 grade IV glioma cases.
Table 1.
Demographic and Clinical Characteristic of WHO grade I-IV Astrocytoma Cases, SEER 1999–2010
| Grade I | Grade II | Grade III | Grade IV | Total | |
|---|---|---|---|---|---|
| Pilocytic | Diffuse | Anaplastic | Glioblastoma | ||
| Number of Patients, No. (% of Total) | 2497 (7.97) | 4113 (13.13) | 2755 (29.89) | 21 962 (70.11) | 31 327 (100) |
| Age, median (IQR), years | 12 (5–20) | 44 (29–59) | 50 (35–64) | 61 (52–71) | 58 (44–69) |
| Age Category, years, No. (%) | n = 2497 | n = 4113 | n = 2775 | n = 21 962 | n = 31 327 |
| Age <18 | 1739 (69.64) | 528 (12.84) | 177 (6.42) | 255 (1.16) | 2699 (8.62) |
| Age 18–44 | 596 (23.87) | 1591 (38.68) | 929 (33.72) | 2256 (10.27) | 5372 (17.15) |
| Age 45–59 | 113 (<5) | 974 (23.68) | 745 (27.04) | 7179 (32.69) | 9011 (28.76) |
| Age 60–74 | 41 (<2) | 673 (16.36) | 593 (21.52) | 8520 (38.79) | 9827 (31.37) |
| Age ≥75 | <10 (<1) | 347 (8.44) | 311 (11.29) | 3752 (17.08) | 4418 (14.1) |
| Race, No. (%) | n = 2497 | n = 4113 | n = 2775 | n = 21 962 | n = 31 327 |
| White | 1623 (65) | 3008 (73.13) | 2097 (76.12) | 17 665 (80.43) | 24 393 (77.87) |
| Black | 207 (8.29) | 287 (6.98) | 180 (6.53) | 1213 (5.52) | 1887 (6.02) |
| Asian/Pacific Islander | 118 (4.73) | 189 (4.6) | 138 (5.01) | 845 (3.85) | 1290 (4.12) |
| Hispanic | 493 (19.74) | 576 (14) | 325 (11.8) | 2137 (9.73) | 3531 (11.27) |
| American Indian/Alaskan Native | 18 (0.72) | 28 (0.68) | <10 (<1) | 66 (0.3) | 119 (0.38) |
| Other/Unknown, Non-Hispanic | 38 (1.52) | 25 (0.61) | <10 (<1) | 36 (0.16) | 107 (0.34) |
| Marital Status, No. (%) | n = 2460 | n = 4000 | n = 2669 | n = 21,311 | n = 30 440 |
| Single | 2125 (86.38) | 1379 (34.48) | 697 (26.11) | 3008 (14.11) | 7209 (23.68) |
| Married | 288 (11.71) | 2094 (52.35) | 1581 (59.24) | 14 165 (66.47) | 18 128 (59.55) |
| Separated, Divorced, Widowed | 47 (1.91) | 527 (13.18) | 391 (14.65) | 4,138 (19.42) | 5,103 (16.76) |
| Sex, No. (%) | n = 2497 | n = 4113 | n = 2775 | n = 21 962 | n = 31 327 |
| Male | 1263 (50.58) | 2354 (57.23) | 1533 (55.64) | 12 905 (58.76) | 18 055 (57.63) |
| Female | 1234 (49.42) | 1759 (42.77) | 1222 (44.36) | 9057 (41.24) | 13 272 (42.37) |
| Tumor Size, No. (%) | n = 1725 | n = 2436 | n = 1744 | n = 16 847 | n = 22 752 |
| <5 cm | 1158 (67.13) | 1568 (64.37) | 1107 (63.47) | 9630 (57.16) | 13 463 (59.17) |
| 5–7 cm | 471 (27.3) | 620 (25.45) | 429 (24.6) | 5728 (34) | 7248 (31.86) |
| >7 cm | 96 (5.57) | 248 (10.18) | 208 (11.93) | 1489 (8.84) | 2041 (8.97) |
| Tumor Site, No. (%) | n = 2497 | n = 4113 | n = 2775 | n = 21 962 | n = 31 327 |
| Frontal Lobe | 117 (4.69) | 1179 (28.67) | 861 (31.25) | 5840 (26.59) | 7997 (25.53) |
| Temporal Lobe | 134 (5.37) | 821 (19.96) | 531 (19.27) | 5377 (24.48) | 6863 (21.91) |
| Parietal Lobe | 65 (2.6) | 450 (10.94) | 323 (11.72) | 3691 (16.81) | 4529 (14.46) |
| Occipital Lobe | 38 (1.52) | 79 (1.92) | 61 (2.21) | 952 (4.33) | 1130 (3.61) |
| Brainstem | 305 (12.21) | 197 (4.79) | 88 (3.19) | 133 (0.61) | 723 (2.31) |
| Overlapping Lesion of Brain | 76 (3.04) | 579 (14.08) | 405 (14.7) | 3713 (16.91) | 4773 (15.24) |
| Cerebrum | 217 (8.69) | 330 (8.02) | 272 (9.87) | 851 (3.87) | 1670 (5.33) |
| Brain, NOS | 397 (15.9) | 262 (6.37) | 136 (4.94) | 1154 (5.25) | 1949 (6.22) |
| Ventricle, NOS | 181 (7.25) | 74 (1.8) | 32 (1.16) | 95 (0.43) | 382 (1.22) |
| Cerebellum, NOS | 967 (38.73) | 142 (3.45) | 46 (1.67) | 156 (0.71) | 1311 (4.18) |
| Year of Diagnosis, No. (%) | n = 2497 | n = 4113 | n = 2775 | n = 21 962 | n = 31 327 |
| 1999 | 96 (3.84) | 163 (3.96) | 103 (3.74) | 873 (3.98) | 1235 (3.94) |
| 2000 | 218 (8.73) | 391 (9.51) | 254 (9.22) | 1728 (7.87) | 2591 (8.27) |
| 2001 | 221 (8.85) | 341 (8.29) | 227 (8.24) | 1708 (7.78) | 2497 (7.97) |
| 2002 | 224 (8.97) | 366 (8.9) | 240 (8.71) | 1776 (8.09) | 2606 (8.32) |
| 2003 | 225 (9.01) | 361 (8.78) | 250 (9.07) | 1861 (8.47) | 2697 (8.61) |
| 2004 | 207 (8.29) | 367 (8.92) | 241 (8.75) | 1967 (8.96) | 2782 (8.88) |
| 2005 | 193 (7.73) | 343 (8.34) | 246 (8.93) | 1934 (8.81) | 2716 (8.67) |
| 2006 | 208 (8.33) | 355 (8.63) | 242 (8.78) | 1877 (8.55) | 2682 (8.56) |
| 2007 | 217 (8.69) | 359 (8.73) | 225 (8.17) | 2026 (9.23) | 2827 (9.02) |
| 2008 | 228 (9.13) | 384 (9.34) | 229 (8.31) | 2063 (9.39) | 2904 (9.27) |
| 2009 | 219 (8.77) | 355 (8.63) | 245 (8.89) | 2041 (9.29) | 2860 (9.13) |
| 2010 | 241 (9.65) | 328 (7.97) | 253 (9.18) | 2108 (9.6) | 2930 (9.35) |
| Radiotherapy, No. (%) | n = 2462 | n = 4003 | n = 2698 | n = 21 483 | n = 30 646 |
| No | 2266 (92.04) | 1894 (47.31) | 611 (22.65) | 5353 (24.92) | 10 124 (33.04) |
| Yes | 196 (7.96) | 2109 (52.69) | 2087 (77.35) | 16 130 (75.08) | 20 522 (66.96) |
| Surgery, No. (%) | n = 2497 | n = 4113 | n = 2775 | n = 21 962 | n = 31 327 |
| Gross Total Resection | 1161 (46.5) | 916 (22.27) | 537 (19.49) | 6615 (30.12) | 9229 (29.46) |
| Partial Resection | 553 (22.15) | 904 (21.98) | 624 (22.65) | 6170 (28.09) | 8251 (26.34) |
| Local Excision/Biopsy | 589 (23.59) | 806 (19.6) | 503 (18.26) | 4459 (20.3) | 6357 (20.29) |
| No Surgery | 194 (7.77) | 1487 (36.15) | 1091 (39.6) | 4718 (21.48) | 7490 (23.91) |
| Overall Mortality, No. (%) | n = 2497 | n = 4113 | n = 2775 | n = 21 962 | n = 31 327 |
| Living | 2351 (94.15) | 2008 (48.82) | 898 (32.6) | 3108 (14.15) | 8365 (26.7) |
| Deceased | 146 (5.85) | 2105 (51.18) | 1857 (67.4) | 18 854 (85.85) | 22 962 (73.3) |
Abbreviations: SEER, Surveillance, Epidemiology, and End Results; IQR, Interquartile range; NOS, Not otherwise specified.
Covariates and Extent of Resection
Using published methodology,17,20 patients were grouped into 4 equal time periods for comparison: 1999–2001, 2002–2004, 2005–2007, and 2008–2010. Survival time was defined as the number of months from diagnosis to the date of death due to any cause or the date of last known follow-up. Demographic variables in the statistical analysis included age (<18, 18–44, 45–59, 60–74, or >75 years); race/ethnicity (white, black, Asian/Pacific Islander, Hispanic, American Indian/Alaskan native, or other/unknown); marital status (single, married, or [separated, divorced, or windowed]); and sex (male or female). Clinical variables included tumor size (<5 cm, 5–7 cm or >7 cm), tumor location (based on ICD-O-3 topologic site codes C71.0-C71.9), radiotherapy status (treatment or no treatment), and surgical treatment received (no surgery, subtotal resection, or gross total resection).
With regard to surgical treatment, we used the following surgery codes from the SEER registry: no surgery (code 00), local excision/biopsy (code 20), partial resection (code 21, 40), or gross total resection (code 30, 55). It is important to note that the exact definition of surgical codes has been slightly modified with each edition of the SEER Program Coding and Staging Manual (1998–2003, 2004–2006, 2007–2009, 2010-present) but remained roughly consistent. The current definition for surgical codes can be found in the SEER Program Coding and Staging Manual 2013 released on February 28, 2013 under Appendix C: Surgical Codes for Brain.25 Historical definitions can also be found on the SEER website.26 We combined local excision/biopsy (code 20) with partial resection (code 21, 40) into one category of subtotal resection in the extended multivariate Cox proportional hazards analysis because the extent of resection achieved between the two categories is ambiguous. Furthermore, a separate analysis showed that the two categories exhibited similar survival curves that were distinct from those of no surgery and gross total resection.
Information regarding the use of chemotherapy, performance status, local control, and specific radiotherapy technique (such as fractionation, dose, and beam energy) is not provided in the SEER database and therefore could not be included in this study.
Statistical Analysis
All analyses were conducted using Stata version 11.2,27 and the level of statistical significance was set at P < .05. Kaplan-Meier method was used to generate 2- and 10-year survival curves for grade I-IV gliomas across 4 equal time periods between 1999 and 2010. Statistical significance was determined using the Tarone-Ware test across survival functions.16 We also calculated the percentage of subjects alive at 2 years with 95% confidence interval (CI) and median survival with 95% CI. Next, we conducted an extended multivariate Cox proportional hazard analysis adjusting for demographic and clinical covariates mentioned above to obtain hazard ratios (HR) and 95% CI for death. Lastly, we used multivariate logistic regression analysis adjusting for the same demographic and clinical covariates to obtain odds ratios (OR) of receiving gross total resection and receiving radiotherapy in four time periods for grade II and III astrocytoma.
Results
Patient and Clinical Characteristics
Patient characteristics are summarized in Table 1. Out of a total of 31 327 patients, there were 2497 (7.97%) grade I pilocytic astrocytoma, 4113 (13.13%) grade II diffuse astrocytoma, 2755 (29.89%) grade III anaplastic astrocytoma, and 21 962 (70.11%) grade IV glioblastoma cases. The median age at diagnosis (interquartile range) was 12 years (5–20 years) for grade I, 44 years (29–59 years) for grade II, 50 years (35–64 years) for grade III, and 61 years (52–71 years) for grade IV gliomas. Overall mortality due to all causes increased with increasing histologic grade; there was 5.86% mortality for grade I, 51.18% for grade II, 67.4% for grade III, and 85.85% for grade IV astrocytoma. The most common sites for grade I tumors were the cerebellum and brainstem, whereas grade II-IV tumors were most commonly found in the frontal lobe and temporal lobe. The epidemiology exhibited by this study sample is consistent with existing literature.5,24
Time Trend Analysis of Survival
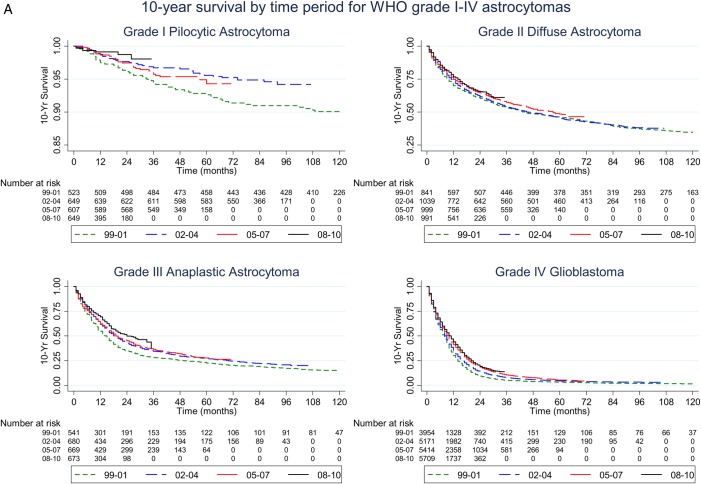
To examine the survival trends we had adapted the published convention17,20 of dividing the study period into 4 equal intervals (1999–2001, 2002–2004, 2005–2007, and 2008–2010). Kaplan-Meier plots of these time periods are shown in Fig. 1A. Consistent with prior studies,17–20 the median survival for patients with grade IV glioblastoma showed a modest increase over the past decade. Importantly, we observed similar improvements for patients with grade II and grade III astrocytomas with the Tarone-Ware test (all P < .05, Table 2). Median survival for patients with grade II astrocytoma increased from 44 months in 1999–2001 to 57 months in 2005–2007. For the 2008–2010 period, grade II astrocytoma did not reach the 50% survivor function. Median survival for patients with grade III astrocytoma increased from 15 months in 1999–2001 to 24 months in 2008–2010 period. Because the 10-year survival for patients with grade I pilocytic astrocytoma was 89.69% in our analysis, 50% survivor function was not reached in any of the time periods.
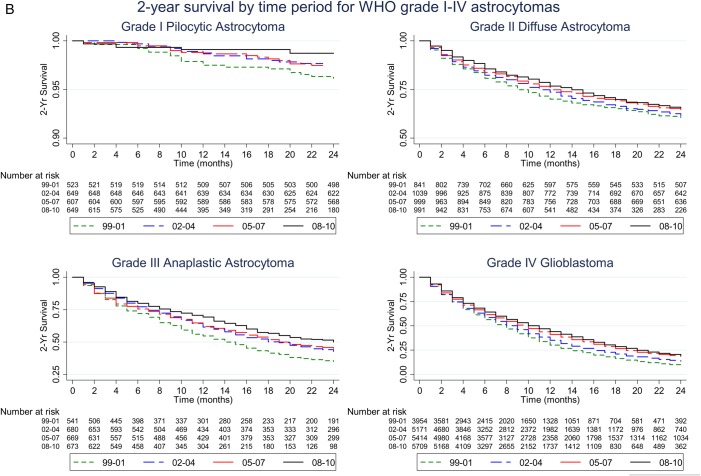
Fig. 1.
(A) Kaplan-Meier plots of 10-year survival by time period for patients with WHO grade I-IV astrocytomas. Note the survival rate (y-axis) ranges from 0.85 to 1 for grade I pilocytic astrocytoma, from 0.25 to 1 for grade II diffuse astrocytoma, and from 0 to 1 for grade III anaplastic astrocytoma and grade IV glioblastoma. (B) Kaplan-Meier plots of 2-year survival by time period for patients with WHO grade I-IV astrocytomas. Note the survival rate (y-axis) ranges from 0.9 to 1 for grade I pilocytic astrocytoma, from 0.5 to 1 for grade II diffuse astrocytoma, from 0.25 to 1 for grade III anaplastic astrocytoma, and from 0 to 1 for grade IV glioblastoma.
Table 2.
Log-rank and Tarone-Ware Tests for Survivor Functions by Time Period for WHO Grade I-IV Astrocytoma
| Grade I Pilocytic Astrocytoma | Grade II Diffuse Astrocytoma | Grade III Anaplastic Astrocytoma | Grade IV Glioblastoma | |
|---|---|---|---|---|
| Tarone-Ware P value | .0369 | .0205 | <.0001 | <.0001 |
Patients diagnosed in different time periods had different lengths of follow-up. To correct for this, we examined the 2-year survival for all 4 time periods as this was the length of follow-up for the most recent time period 2008–2010. Kaplan-Meier plots of survival 2 years after diagnosis are shown in Fig. 1B. Over the study period, 2-year survival [95% CI] increased from 9.48% [8.58–10.42] (1999–2001) to 19.36% [7.93–20.83] (2008–2010) for grade IV glioblastoma patients. The 2-year survival for grade III astrocytoma patients increased from 35% [30.99–39.03] (1999–2001) to 49.96% [44.80–54.9] (2008–2010), and the 2-year survival for grade II astrocytoma patients increased from 60.54% [57.14–63.76] (1999–2001) to 65.62% [61.87–69.1] (2008–2010). In contrast, the 2-year survival for grade I astrocytoma patients remained fairly constant during the study period at 96.15% [94.1–97.5] (1999–2001), 97.68% [96.17–98.59] (2002–2004), 97.47% [95.84–98.47] (2005–2007) and 98.73% [97.02–99.46] (2008–2010).
Multivariate Adjusted Hazard Ratio of Death Analysis
To determine whether the improvements in survival persisted after adjusting for demographic (age, race/ethnicity, marital status, sex) and clinical (tumor size, tumor site, radiotherapy, and surgical treatment) variables, we derived adjusted HRs for death using an extended multivariate Cox proportional hazards model (Table 3). The HR demonstrated a downward trend for grade II, III, and IV gliomas, reflecting improved survival throughout the decade after adjusting for the variables described above. Compared with the HR for patients diagnosed during 1999–2001, there were statistically significant decreases in the subsequent 3 periods. For grade IV glioblastoma, the HR [95% CI] successively decreased from 1.00 (1999–2001) to 0.84 [0.8–0.89] (2002–2004) to 0.75 [0.71–0.97] (2005–2007) to 0.67 [0.63–0.71] (2008–10). For grade III anaplastic astrocytomas, there was a significant drop in HR around 2005, with HR decreased from 1.00 and 0.95 [0.8–1.13] (1999–2001 and 2002–2004, respectively) to 0.69 [0.58–0.83] and 0.65 [0.53–0.8] (2005–2007 and 2008–2010, respectively). Similarly, for grade II astrocytomas, there was a drop in HR around 2005, with HR decreasing from 1.00 and 0.99 [0.84–1.18] (1999–2001 and 2002–2004, respectively) to 0.85 [0.71–1.01] and 0.75 [0.62–0.93] (2005–2007 and 2008–2010, respectively). In contrast, no significant change in the HR of grade I pilocytic astrocytomas was observed during the study period. However, the interpretation of this finding should be caveated with the extremely low number of patients who died from this disease.
Table 3.
Multivariate-Adjusted HR* and 95% CI for Death† by Time Period for WHO Grade I-IV Astrocytomas
| Grade I |
Grade II |
Grade III |
Grade IV |
|||||
|---|---|---|---|---|---|---|---|---|
| Pilocytic Astrocytoma |
Diffuse Astrocytoma |
Anaplastic Astrocytoma |
Glioblastoma |
|||||
| Adjusted HR | P Value | Adjusted HR | P Value | Adjusted HR | P Value | Adjusted HR | P Value | |
| 1999–2001 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 2002–2004 | 0.71 (0.38–1.34) | .29 | 0.99 (0.84–1.18) | .95 | 0.95 (0.8–1.13) | .55 | 0.84 (0.8–0.89) | <.0001 |
| 2005–2007 | 0.88 (0.47–1.67) | .70 | 0.85 (0.71–1.01) | .07 | 0.69 (0.58–0.83) | <.0001 | 0.75 (0.71–0.79) | <.0001 |
| 2008–2010 | 0.57 (0.22–1.47) | .25 | 0.76 (0.62–0.93) | .01 | 0.65 (0.53–0.8) | <.0001 | 0.67 (0.63–0.71) | <.0001 |
*Adjusted for age, race/ethnicity, marital status, sex, tumor size, tumor site, radiotherapy and surgical treatment.
†Derived from Extended Cox Proportional Hazard Model.
Abbreviations: HR, hazard ratio; CI, confidence interval.
Multivariate Adjusted Odds Ratio of Radiotherapy and Surgical Treatment Analysis
To determine whether the improved survival in grade II and III astrocytoma patients could be attributed to increased extent of resection or utilization of radiotherapy (RT), we calculated the OR of patients who underwent gross total resection or RT during the study period using a multivariate logistic regression model adjusting for demographic and clinical variables described above (Tables 4 and 5). For grade II astrocytomas, there were no consistent changes in the OR of patients undergoing gross total resection. In contrast, there was a statistically significant decline in RT utilization in the 2005–2007 and 2008–2010 periods (OR of 0.68 and 0.76, respectively) relative to the 1999–2001 and 2002–2004 periods (OR of 1.00 and 1.14, respectively). For grade III astrocytoma patients, there were no significant changes in OR of receiving gross total resection or RT during the study period.
Table 4.
Multivariate-Adjusted OR* and 95% CI for Gross Total Resection† by Time Period for WHO grade II and III Astrocytomas
| Grade II |
Grade III |
|||
|---|---|---|---|---|
| Diffuse Astrocytoma |
Anaplastic Astrocytoma |
|||
| Adjusted OR | P Value | Adjusted OR | P Value | |
| 1999–2001 | 1.00 | Reference | 1.00 | Reference |
| 2002–2004 | 1.32 (0.98–1.78) | .073 | 1.27 (0.87–1.84) | .214 |
| 2005–2007 | 1.11 (0.83–1.5) | .475 | 1.15 (0.79–1.66) | .461 |
| 2008–2010 | 0.71 (0.52–0.96) | .028 | 0.83 (0.57–1.21) | .323 |
*Adjusted for age, race/ethnicity, marital status, sex, tumor size, tumor site, and radiotherapy.
†Derived from Logistic Regression Model.
Abbreviations: OR, odds ratio; CI, confidence interval.
Table 5.
Multivariate-Adjusted OR* and 95% CI for Radiotherapy† by Time Period for WHO Grade II and III Astrocytomas
| Grade II |
Grade III |
|||
|---|---|---|---|---|
| Diffuse Astrocytoma |
Anaplastic Astrocytoma |
|||
| Adjusted OR | P Value | Adjusted OR | P Value | |
| 1999–2001 | 1.00 | Reference | 1.00 | Reference |
| 2002–2004 | 1.14 (0.86–1.5) | .365 | 0.87 (0.59–1.28) | .465 |
| 2005–2007 | 0.68 (0.52–0.89) | .005 | 0.92 (0.63–1.35) | .669 |
| 2008–2010 | 0.76 (0.58–0.99) | .042 | 0.86 (0.59–1.25) | .428 |
*Adjusted for age, race/ethnicity, marital status, sex, tumor size, tumor site, and surgical treatment.
†Derived from Logistic Regression Model.
Abbreviations: OR, odds ratio; CI, confidence interval.
Survival Analysis in the Pre-Temozolomide and Post-Temozolomide Eras
Temozolomide (TMZ) treatment of grade II and III astrocytomas was frequently adopted after the landmark study in 2005 by Stupp et al published in the New England Journal of Medicine.28 In Table 6, we performed an analysis to examine whether the HRs for death in the post-TMZ era (2005–2010) were lower than those observed in the pre-TMZ era (1999–2004) after accounting for all pertinent demographic and clinical variables described above. No significant change in HR was observed for patients with grade I astrocytoma when comparing pre-TMZ and post-TMZ eras. In contrast, we observed a statistically significant decrease in the HR of death in the post-TMZ era for grade II, III, and IV astrocytomas. These correlative data supported the efficacy of TMZ in the treatment of grade II and III astrocytoma patients. However, we could not exclude the possibility that other advances in the care of neuro-oncology patients contributed to this effect (see discussion).
Table 6.
Multivariate-Adjusted HR* and 95% CI for Death† for Pre-TMZ and Post-TMZ eras for WHO Grade I-IV Astrocytomas
| Grade I |
Grade II |
Grade III |
Grade IV |
|||||
|---|---|---|---|---|---|---|---|---|
| Pilocytic Astrocytoma |
Diffuse Astrocytoma |
Anaplastic Astrocytoma |
Glioblastoma |
|||||
| Adjusted HR | P Value | Adjusted HR | P Value | Adjusted HR | P Value | Adjusted HR | P Value | |
| Pre-TMZ (1999–2004) | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Post-TMZ (2005–2010) | 0.92 (0.53–1.57) | .748 | 0.82 (0.72–0.93) | .002 | 0.70 (0.61–0.79) | <.0001 | 0.79 (0.76–0.82) | <.0001 |
*Adjusted for age, race/ethnicity, marital status, sex, tumor size, tumor site, radiotherapy, and surgical treatment.
†Derived from Extended Cox Proportional Hazard Model.
Abbreviations: HR, hazard ratio; CI, confidence interval.
Discussion
Our study is the first to analyze changes in the survival patterns of patients afflicted with grade I, II, and III astrocytomas as well as the prevailing clinical practice patterns in terms of treatment preference using the SEER registry. To date, this is the largest population-based study dedicated to the study of these histologically delineated glioma subtypes. Previous studies suggest improved survival for grade IV astrocytoma patients over time.17–20 We found a similar result in our study. On the other hand, survival patterns for lower grade astrocytomas remain poorly characterized. Our results indicate that over the past decade there has been an increase in the overall survival of patients afflicted with grade II and III astrocytomas. Importantly, this increase persists after adjusting for pertinent demographic and clinical variables including age, race/ethnicity, marital status, sex, tumor size, tumor location, extent of surgical resection, and RT status. Relative to patients diagnosed with grade II and III astrocytomas in the 1999–2001 period, the adjusted HR of death for patients diagnosed in 2008–2010 has decreased by 24% and 35%, respectively.
It is important to interpret these findings in the context of the shifting diagnostic landscape of glioma patients. During the study period, there was growing recognition that a diagnosis of oligodendroglioma conferred a more favorable prognosis than a diagnosis of purely astrocytic tumor of the same WHO grade.29 Moreover, oligodendrogliomas exhibit favorable response to select chemotherapy regimens,30–34 and the diagnosis qualifies the patient for treatment with these regimens. Consequently, there was an overall increase in the number of patients diagnosed with oligodendroglioma during the period of our study.35 One interpretation of this increase is that many patients who would have been diagnosed with astrocytoma were instead diagnosed with oligodendroglioma.36 If so, the diagnostic shift would deplete a population with favorable natural history from the diagnostic category of grade II astrocytomas. The increased median overall survival may be particularly notable in this context.
The pattern of clinical practice pertaining to RT utilization for the treatment of astrocytoma patients during the study period is of interest. Most grade III astrocytoma patients undergo RT.15 Our results indicate that the proportion of these patients receiving RT has not changed significantly over the past decade. In contrast, there is a notable decrease in the use of RT for grade II astrocytoma patients after 2005. This change in clinical practice is temporally associated with the publication of 2 major landmark studies. First, long-term outcomes from EORTC 22845, a randomized clinical trial comparing up-front RT with RT at the time of progression for patients with low-grade glioma, revealed that up-front RT does not affect overall survival.37 Second, the efficacy of TMZ against high-grade gliomas was reported in another landmark study.28 Since this report, there have been ongoing studies to explore TMZ-only treatment for grade II gliomas.38–42 It is likely that the combined effects of these studies influenced the decreased utilization of RT for patients afflicted with grade II astrocytomas. Validation of this thesis can facilitate our understanding of the influence of published literature on clinical practice patterns.
On the other hand, there is no clear trend in terms of the clinical practice pattern pertaining to extent of resection of grade II or III astrocytomas. Over the study period, there was mounting evidence from carefully designed and executed studies that suggested clinical benefit of a maximal surgical resection for grade II and III astrocytomas.43 The largest surgical series on the matter emerged from the French Glioma Network involving 1097 low-grade glioma patients. In this series, extent of resection and postsurgical residual volume remained independent prognostic factors in a multivariate model that accounted for pertinent clinical variables.44 This association has been reproducibly validated by multiple independent clinical studies.10,45,46 Similar results have been reported for anaplastic astrocytomas.6,11 Our results indicate that these published studies have not significantly affected the overall surgical practice for treatment of grade II and III astrocytomas patients in the U.S. As such, meaningful gains in clinical outcome may be achieved by improving the extent of resection for grade II and III astrocytoma patients.
Our results indicate that extent of resection and RT are unlikely to have contributed to the improved overall survival for grade II and III astrocytoma patients. Instead, we suggest the following contributing factors. First, our stratified survival analyses of grade II and III astrocytoma patients in the pre-TMZ and post-TMZ eras suggest that TMZ may be efficacious for these patient populations. However, this thesis awaits formal validation. Second, there has been a general improvement in the neuro-oncologic standard of care over the past decade,47 including recognition of the need for dedicated training of neuro-oncologists, the adaptation of multidisciplinary tumor boards, and dedicated brain cancer centers. Third, advances in neuroimaging tools, a better understanding of the natural history and prognostic factors for these diseases,48 and elucidation of the efficacy of RT37 afford opportunities for rational decisions in terms of therapeutic intervention. Finally, we cannot exclude the possibility of lead-time bias associated with increased utilization of MR imaging as workup for neurologic complaint.49 Such practice may afford early disease detection, thereby artificially inflating the overall survival estimate.
Our study design is subject to several limitations, including the veracity of the data contained in the SEER registry and the absence of key variables (such as the type of chemotherapy that the patients received, quality-of-life measures, and location of the tumor in relation to eloquent cortex). Despite these limitations, we have used the registry data set to the fullest extent by adjustment of all pertinent clinical variables available. Additionally, the study result is subject to distortion related to the shifting landscape in terms of the diagnosis of astrocytomas and oligodendrogliomas that took place during the study period.35 The observation of improved survival for astrocytoma patients despite an increasing number of patients diagnosed with oligodendroglioma, however, suggests that our findings are robust. Finally, we are unable to tease out the relative contribution of TMZ or lead-time bias to the overall increase in survival. The observed improvement in survival represents valuable information to clinical practitioners, patients, and patient advocacy groups. Moreover, analysis of the clinical practice patterns further identified potential opportunities for impacting the clinical course of patients afflicted with grade II and III astrocytomas.
In conclusion, results from the SEER database indicate improvements in the overall survival of grade II and III astrocytoma patients over the past decade. Analysis of corresponding changes in clinical practice patterns suggests opportunities for improvement in the surgical management of these patients as it pertains to extent of resection.
Funding
None declared.
Conflicts of interest statement. None declared.
References
- 1. Dolecek TA, Propp JM, Stroup NE et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14:Suppl 5:v1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartek J Jr., Ng K, Bartek J et al. Key concepts in glioblastoma therapy. J Neurol Neurosurg Psychiatry. 2012;83(7):753–760. [DOI] [PubMed] [Google Scholar]
- 3. Ng K, Kim R, Kesari S et al. Genomic profiling of glioblastoma: convergence of fundamental biologic tenets and novel insights. J Neurooncol. 2012;107(1):1–12. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Ohgaki H, Wiestler OD et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93–108. [DOI] [PubMed] [Google Scholar]
- 6. Keles GE, Chang EF, Lamborn KR et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006;105(1):34–40. [DOI] [PubMed] [Google Scholar]
- 7. Hegi ME, Diserens AC, Gorlia T et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 8. Karajannis M, Allen JC, Newcomb EW. Treatment of pediatric brain tumors. J Cell Physiol. 2008;217(3):584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGirt MJ, Chaichana KL, Attenello FJ et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700–707, author reply 707–708. [DOI] [PubMed] [Google Scholar]
- 10. Smith JS, Chang EF, Lamborn KR et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 11. Nomiya T, Nemoto K, Kumabe T et al. Prognostic significance of surgery and radiation therapy in cases of anaplastic astrocytoma: retrospective analysis of 170 cases. J Neurosurg. 2007;106(4):575–581. [DOI] [PubMed] [Google Scholar]
- 12. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764. discussion 264–756. [DOI] [PubMed] [Google Scholar]
- 13. Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J Neurosurg. 2011;115(5):948–965. [DOI] [PubMed] [Google Scholar]
- 14. Pedersen CL, Romner B. Current treatment of low grade astrocytoma: a review. Clin Neurol Neurosurg. 2013;115(1):1–8. [DOI] [PubMed] [Google Scholar]
- 15. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 16. Noorbakhsh A, Tang JA, Marcus LP et al. Gross-total resection outcomes in an elderly population with glioblastoma: a SEER-based analysis. J Neurosurg. 2014;120(1):31–39. [DOI] [PubMed] [Google Scholar]
- 17. Koshy M, Villano JL, Dolecek TA et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107(1):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson DR, O'Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. [DOI] [PubMed] [Google Scholar]
- 19. Johnson DR, Leeper HE, Uhm JH. Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: a population-based analysis. Cancer. 2013;119(19):3489–3495. [DOI] [PubMed] [Google Scholar]
- 20. Darefsky AS, King JT Jr., Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118(8):2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Surveillance, Epidemiology, and End Results (SEER) Program (http://www.seer.cancer.gov) Research Data (1973–2010), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. Published April 2013, based on the November 2012 submission. Downloaded as ASCII text data file on August 9, 2013.
- 22. Surveilance, Epidemiology, and End Results (SEER) Program Overview. SEER Program Web site. http://seer.cancer.gov/about/overview.html. Accessed on July 21, 2013.
- 23. SEER Data 1973–2010. SEER Program Web site. http://seer.cancer.gov/data. Accessed on July 21, 2013.
- 24. Ostrom QT, Gittleman H, Farah P et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. SEER Appendix C: Site Specific Coding Modules. SEER Program Web site http://seer.cancer.gov/manuals/2013/appendixc.html. Accessed on July 21, 2013.
- 26. SEER Historical Staging and Coding Manuals. SEER Program Web site http://seer.cancer.gov/tools/codingmanuals/historical.html. Accessed on July 21, 2013.
- 27. StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP. [Google Scholar]
- 28. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 29. van den Bent MJ. Anaplastic oligodendroglioma and oligoastrocytoma. Neurol Clin. 2007;25(4):1089–1109, ix-x. [DOI] [PubMed] [Google Scholar]
- 30. Cairncross G, Macdonald D, Ludwin S et al. Chemotherapy for anaplastic oligodendroglioma. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994;12(10):2013–2021. [DOI] [PubMed] [Google Scholar]
- 31. Cairncross JG, Ueki K, Zlatescu MC et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. [DOI] [PubMed] [Google Scholar]
- 32. Gorlia T, Delattre JY, Brandes AA et al. New clinical, pathological and molecular prognostic models and calculators in patients with locally diagnosed anaplastic oligodendroglioma or oligoastrocytoma. A prognostic factor analysis of European Organisation for Research and Treatment of Cancer Brain Tumour Group Study 26951. Eur J Cancer. 2013;49(16):3477–3485. [DOI] [PubMed] [Google Scholar]
- 33. van den Bent MJ, Kros JM, Heimans JJ et al. Response rate and prognostic factors of recurrent oligodendroglioma treated with procarbazine, CCNU, and vincristine chemotherapy. Dutch Neuro-oncology Group. Neurology. 1998;51(4):1140–1145. [DOI] [PubMed] [Google Scholar]
- 34. van den Bent MJ, Looijenga LH, Langenberg K et al. Chromosomal anomalies in oligodendroglial tumors are correlated with clinical features. Cancer. 2003;97(5):1276–1284. [DOI] [PubMed] [Google Scholar]
- 35. Youland RS, Schomas DA, Brown PD et al. Changes in presentation, treatment, and outcomes of adult low-grade gliomas over the past fifty years. Neuro Oncol. 2013;15(8):1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973–2001. Cancer. 2006;106(6):1358–1363. [DOI] [PubMed] [Google Scholar]
- 37. van den Bent MJ, Afra D, de Witte O et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 38. Pace A, Vidiri A, Galie E et al. Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol. 2003;14(12):1722–1726. [DOI] [PubMed] [Google Scholar]
- 39. Quinn JA, Reardon DA, Friedman AH et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol. 2003;21(4):646–651. [DOI] [PubMed] [Google Scholar]
- 40. Kaloshi G, Benouaich-Amiel A, Diakite F et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68(21):1831–1836. [DOI] [PubMed] [Google Scholar]
- 41. Lashkari HP, Saso S, Moreno L et al. Using different schedules of Temozolomide to treat low grade gliomas: systematic review of their efficacy and toxicity. J Neurooncol. 2011;105(2):135–147. [DOI] [PubMed] [Google Scholar]
- 42. Chen CC, Motegi A, Hasegawa Y et al. Genetic analysis of ionizing radiation-induced mutagenesis in Saccharomyces cerevisiae reveals TransLesion Synthesis (TLS) independent of PCNA K164 SUMOylation and ubiquitination. DNA Repair (Amst). 2006;5(12):1475–1488. [DOI] [PubMed] [Google Scholar]
- 43. Hardesty DA, Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol. 2012;3:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Capelle L, Fontaine D, Mandonnet E et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013;118(6):1157–1168. [DOI] [PubMed] [Google Scholar]
- 45. van Veelen ML, Avezaat CJ, Kros JM et al. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatry. 1998;64(5):581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Claus EB, Horlacher A, Hsu L et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–1233. [DOI] [PubMed] [Google Scholar]
- 47. Weller M, Wick W. Neuro-oncology in 2013: improving outcome in newly diagnosed malignant glioma. Nat Rev Neurol. 2014;10(2):68–70. [DOI] [PubMed] [Google Scholar]
- 48. Van Meir EG, Hadjipanayis CG, Norden AD et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quaday KA, Salzman JG, Gordon BD. Magnetic resonance imaging and computed tomography utilization trends in an academic ED. Am J Emerg Med. 2014;32(6):524–528. [DOI] [PubMed] [Google Scholar]