Abstract
Accurately evaluating response in the treatment of high-grade gliomas presents considerable challenges. This review looks at the advancements made in response criteria while critically outlining remaining weaknesses, and directs our vision toward promising endpoints to come. The 2010 guidelines from the Response Assessment in Neuro-Oncology (RANO) working group have enhanced interpretation of clinical trials involving novel treatments for high-grade glioma. Yet, while the criteria are considered clinically applicable to high-grade glioma trials, as well as reasonably accurate and reproducible, RANO lacks sufficient detail for consistent implementation in certain aspects and leaves some issues from the original Macdonald guidelines unresolved. To provide the most accurate assessment of response to therapeutic intervention currently possible, it is essential that trial oncologists and radiologists not only have a solid understanding of RANO guidelines, but also proper insight into the inherent limitations of the criteria. With the expectation of improved data collection as a standard, the author anticipates that the next high-grade glioma response criteria updates will incorporate advanced MRI methods and quantitative tumor volume measurements, availing a more accurate interpretation of response in the future.
Keywords: clinical trials, high grade glioma, MRI, RANO criteria
High-grade gliomas are the most common malignant, primary intracranial neoplasms found in adults1 and include WHO grade III anaplastic glioma (comprised of anaplastic astrocytoma, mixed anaplastic oligoastrocytoma, and anaplastic oligodendroglioma) and WHO grade IV glioblastoma multiforme (GBM). Despite recent advances in chemoradiation therapy and the development of antiangiogenic agents for recurrent tumors, the prognosis for patients with most types of high-grade gliomas is still very poor. Patients diagnosed with anaplastic astrocytoma have a median survival of 14 to 36 months, while those with GBM have a median survival of only 9 to 15 months.2–4
In addition to the gold standard of overall survival, high-grade glioma trials frequently measure imaging-related endpoints, primarily progression-free survival and objective radiographic response rate.5–7 Endpoints based on imaging, which involve the evaluation and measurement of tumor, can shorten trial lengths and reduce drug development costs.8 They are also suitable for crossover study designs.9 To standardize the assessment and reporting of results, objective evaluation of measurable and nonmeasurable disease relies on the use of 4 categories to describe response: complete response (CR), partial response (PR), stable disease (SD [referred to as “no change” originally]), and progressive disease (PD). These categories were introduced as the essential measurement of systemic cancer treatment response in the WHO criteria.10
In 1990, Macdonald et al published the first standard for high-grade gloima, taking their cue from WHO by measuring the maximal cross-sectional diameters of enhancing tumor, and recording response using the WHO categories. The publication was pivotal in introducing uniform criteria to assess post-therapeutic response of high-grade glioma using computed tomography (CT).11 The Macdonald criteria were later adapted to include gadolinium (Gd)-contrast-enhanced MRI in addition to incorporating corticosteroid dose and clinical status into the response-grading scheme.
Gd contrast enhancement is nonspecific (for instance, it is not useful in differentiating recurrent high-grade glioma from radiation necrosis). The enhancement mainly captures the leakage of contrast across a disrupted blood-brain barrier. As a result, increased enhancement after chemoradiation is not a consistently reliable indication of actual progression.12 In addition, efficacy measurements of novel treatments, such as antiangiogenic agents,13 also emphasize the need to assess nonenhancing components of tumors. Limitations of the Macdonald criteria include addressing only the contrast-enhancing component of tumor and inadequate instruction for measuring the enhancing lesion in the wall of cystic or surgical cavities, for handling irregularly shaped tumors, and for assessing multifocal tumors.14–16 Over time, the Macdonald criteria have become associated with these significant limitations and with high inter-reader variability.
In 2010, the Response Assessment in Neuro-Oncology (RANO) working group improved the guidelines for determining high-grade glioma response to therapy considerably.17 The RANO criteria were promptly adopted for many high-grade glioma trials, largely superseding the Macdonald criteria. Nevertheless, some aspects of RANO lack the detail needed for consistent implementation. Explicitly, these include shortcomings in assessing pseudoprogression,18 the absence of measurement guidelines for nonenhancing lesion,19 and the hindrance of applying two-dimensional (2D) measurements to complex three-dimensional (3D) lesions that commonly surround postoperative cavities.20 Particularly in multicenter trials, the resulting discordance in interpretation and outright misinterpretation of disease progression can lead to high inter-reader variability and reduced study power. Due to these factors, trial neuro-oncologists and neuroradiologists must not only comprehend the essentials of RANO criteria, they must also comprehend the predictable operational challenges.
The essential RANO guidelines
The RANO guidelines improved the Macdonald criteria in three major areas: (i) by standardizing imaging definitions, including measureable and nonmeasurable lesions and specifying the size and number of enhancing T1 lesions; (ii) by interpreting pseudoprogression and pseudoresponse; and (iii) by expanding radiographic response to account for changes in T2 and FLAIR signal abnormality, which is especially pertinent in assessing the effect of antiangiogenic therapies.
Standardization of imaging definitions
Accurate assessment of change in tumor burden is the very basis of imaging endpoints in clinical trials for predicting therapeutic effect on clinical outcome or survival. While using the same MRI scanner across visits is highly preferred for accurate interpretation of change, using the same magnet strength is absolutely mandatory.17 Consistency in imaging parameters at each time point acquisition, e.g. Gd dose, is also essential for accurate interpretation. Although measurements may be recorded in any viewing plane, the axial plane is most typical; and again, consistency is a prerequisite for accuracy.
Perhaps the most valuable component of RANO is the clarification on evaluating contrast-enhancing measureable and nonmeasurable disease, including the number of lesions. RANO defines measurable lesions as those bi-dimensionally contrast-enhancing lesions with clearly defined margins that have 2 perpendicular diameters, each at least 10 mm in diameter. These requirements deter likely variation in measuring smaller lesions (resulting from slice selection and volume averages). Furthermore, a slice thickness of ≤5 mm (without gap) is recommended. In the event that thicker slices are acquired, the size of a measurable lesion at baseline should be 2 times the slice thickness. When there are multiple measurable lesions, a minimum of the 2 largest lesions should be measured, with a maximum of 5 measured lesions.
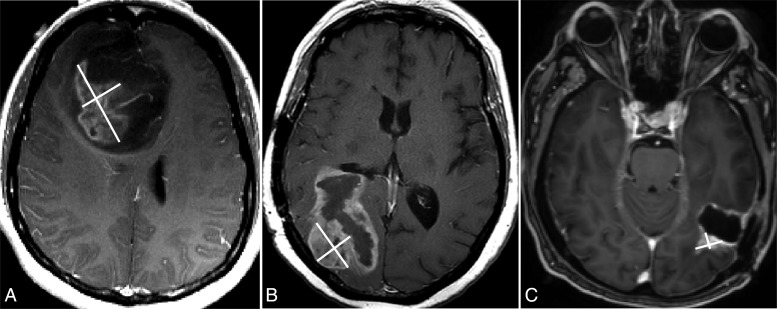
Although enhancing tumor frequently rims all or part of the periphery of a cyst or surgical cavity, in general, only a nodular component ≥10 mm in diameter can be considered measureable in RANO; the cystic or surgical cavity should not be measured in determining response (Fig. 1). Lesions are defined as nonmeasureable when either or both maximal perpendicular diameters are <10 mm (e.g. 11 × 9 mm or 9 × 9 mm). The nonmeasurable definition also applies to masses with poorly defined margins, predominantly cystic or necrotic lesions, and leptomeningeal tumors. Nonenhancing lesions seen on FLAIR or T2 imaging that decisively represent tumor should also be considered nonmeasureable (Fig. 2). Since patients with remaining nonmeasurable tumor can only achieve SD as a best radiographic outcome, measurable disease is customarily required for patient study eligibility when response rate is the primary endpoint of the trial. Conversely, when determination of progression is the primary interest, such as for progression-free survival or duration of tumor control, measurable disease is not a criterion for enrollment.
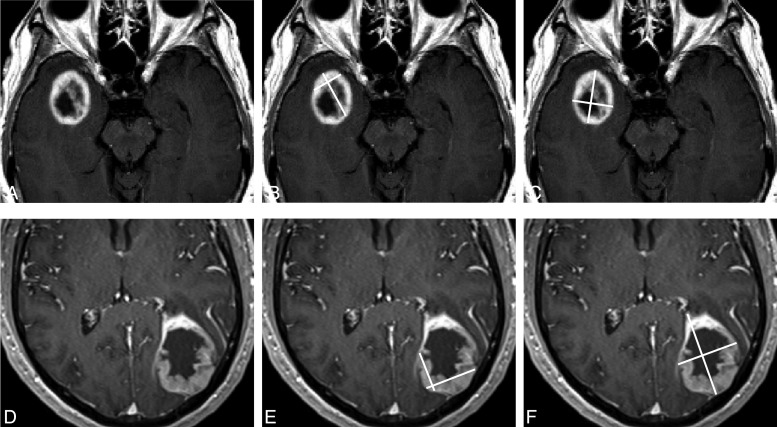
Fig. 1.
Measureable lesion selection and measurements using RANO. Only the enhancing and nodular component, measuring ≥10 mm in diameter bi-dimensionally, should be considered measureable (A and B). The cystic component or surgical cavity should not be measured (C) unless the nodular component measures ≥10 mm in diameter (note: the nodular enhancement has 1.9 × 0.9 cm in the diameters).
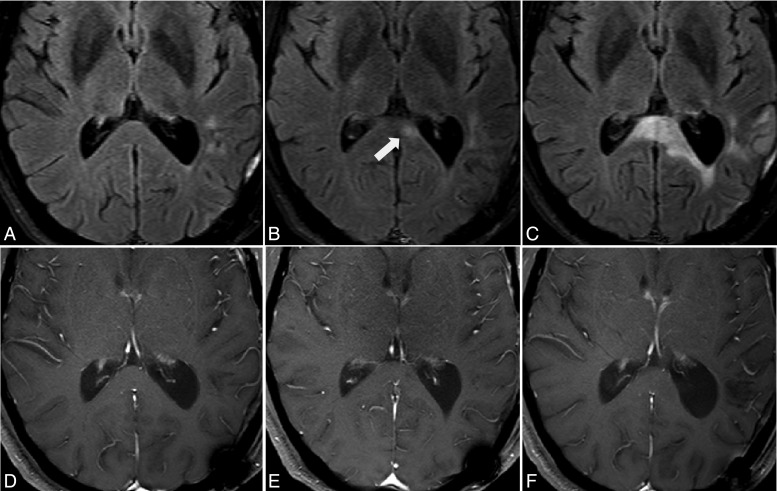
Fig. 2.
Nonmeasureable lesions are those <10 mm in diameter either uni- or bi-dimensionally measured (A). Lesions (arrows) with margins that are not clearly defined and that blend in with brain tissue (B and C), a cystic lesion (D), leptomeningeal dissemination (E, arrow), and nonenhancing lesions on FLAIR and T2 imaging (F) should also be characterized as nonmeasureable lesions.
The surgical goal is typically to remove the enhancing portion of the tumor; however, nonspecific enhancement frequently develops in the wall of the surgical cavity within 48 to 72 hours after a surgical intervention and can remain for weeks thereafter.21,22 To avoid interpreting postoperative changes as residual enhancing disease, RANO recommends that unenhanced and enhanced baseline MRI scans should be obtained within 24 to 48 hours after surgery and no later than 72 hours following surgery. Unenhanced T1-weighted images are helpful in distinguishing residual disease from postoperative blood products that appear as hyperintense areas (Supplementary Material, Fig. S1). In addition to the immediate postoperative pre- and post-Gd-enhanced T1-weighted images, a diffusion-weighted imaging (DWI) sequence, also acquired immediately following surgery, is particularly useful in distinguishing areas of infarction from residual tumor along the surgical cavity (Supplementary Material, Fig. S2). Notably in 2006, Ulmer et al found that cerebral infarcts demonstrated enhancement in 43% of cases on follow-up MRI scans and could mimic tumor progression.23
Definition of radiographic response
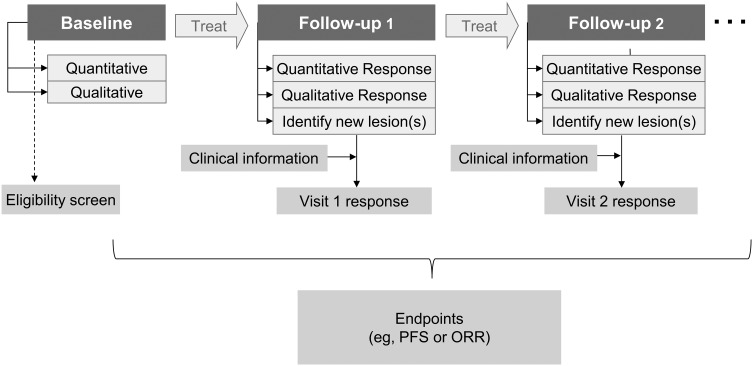
Fig. 3 illustrates a typical tumor-response evaluation paradigm. When initiating assessment for objective response or progression, the baseline tumor burden must be estimated on screening scans to compare subsequent measurements. One or more lesions that qualify as measurable and that lend themselves to reproducible and repeated measurement should be selected as targets, from the largest to the smallest in the case of multiple lesions. The sum of the products of diameters (SPD) for all target lesions should be calculated and reported as the baseline measurement (Supplementary Material, Fig. S3). Any other lesions should be identified as nontarget lesions, and should also be documented at baseline.
Fig. 3.
The paradigm of evaluating tumor response. PFS, progression-free survival; ORR, objective response rate.
At each subsequent visit, the lesions identified as measureable at baseline must be continuously monitored and measured using the same methods (the quantitative evaluation of target lesions). Also at each follow-up visit, the reviewer evaluates nontarget lesions qualitatively, identifies any new lesions, and monitors corticosteroid dosage. The RANO criteria also incorporate clinical factors in support of MRI interpretation as follows: To achieve a rating of response or stability (CR, PR, SD), RANO requires improvement or stability of neurological symptoms. In the absence of a confirmatory scan at least 4 weeks later, CR or PR should only be considered SD. Furthermore, patients who have nonmeasurable enhancing disease that significantly increases in size (>5 mm increase in maximal diameter or ≥25% increase in SPD) should be considered to have progressed. If there is any doubt as to whether a lesion has progressed, the patient may continue on treatment until subsequent evaluation proves PD. In such a case, the date of PD should reflect the time point that first prompted suspicion. Moreover, RANO guidelines define clinical deterioration as follows: change in the Karnofsky Performance Status (KPS) from ≥90 to ≤70, or at least a decline of 20 points from 80, or lasting at least 7 days at ≤50; or Eastern Cooperative Oncology Group and WHO performance scores from ≤1 to 2, or from 2 to 3 unless the neurologic deterioration is attributable to comorbid events or changes in corticosteroid dose.
CR: complete disappearance of all enhancing lesions for at least 4 weeks; no new lesions; stable or improved nonenhancing (T2/FLAIR) tumor lesion; off corticosteroids or on physiologic replacement dose only; and stable or improved clinically.
PR: ≥50% decrease in SPD of all target lesions compared with baseline measurement for at least 4 weeks; no progression of nonmeasurable disease; no new lesions; stable or improved nonenhancing (T2/FLAIR) tumor lesion without higher dose of corticosteroids compared with baseline scan; stable or reduced corticosteroid dose, and stable or improved clinically.
PD: ≥25% increase in SPD of all target lesions compared with the smallest tumor measurement (nadir) at either baseline or best response; significant increase in nonenhancing (T2/FLAIR) tumor lesion without lower dose of corticosteroids; any new lesion; clear progression of nonmeasurable disease; or clear clinical deterioration due to tumor, not due to decrease in corticosteroid dose.
SD: not qualify as CR, PD, or PR; stable nonenhancing (T2/FLAIR) tumor lesion without higher dose of corticosteroids compared with baseline scan and clinically stable status.
Pseudoprogression and pseudoresponse
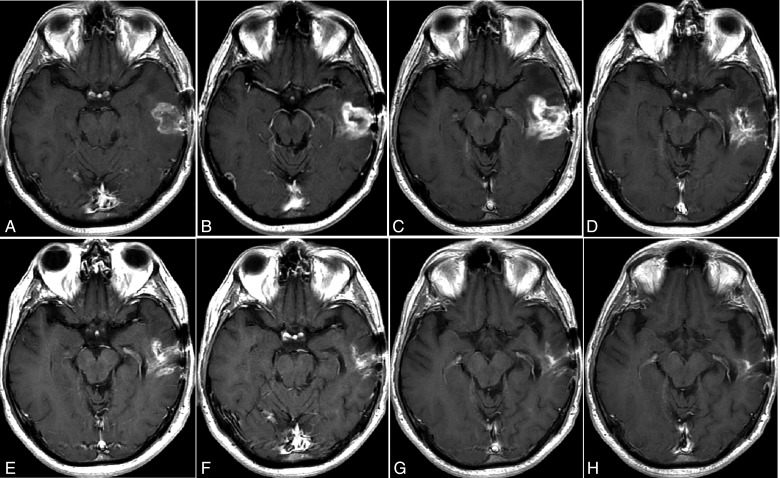
Pseudoprogression is defined as new enhancement or an increase in the size of a contrast-enhancing tumor that shows subsequent improvement or stabilization without further treatment, and thereby refutes true PD. The phenomenon generally occurs after completion of radiochemotherapy or radiotherapy, and frequently in patients with a methylated methyl-guanine methyl transferase (MGMT) gene promoter.24 Such patients typically have a longer median overall survival than those without MGMT promoter methylation.25,26 Pseudoprogression results primarily from the effects of irradiation, which can include an inflammatory component; edema; and abnormal vessel permeability, although the pathophysiology of pseudoprogression is not entirely clear.27,28 The incidence is considerable, with recent reports estimating pseudoprogression in 31% to 48% of GBM patients.18,24 Since pseudoprogression is most prevalent within the first 12 weeks following radiotherapy (Fig. 4), RANO suggests that true PD can only be determined when there is pathologic confirmation of PD or if the majority of new enhancement is outside of the radiation field (i.e. beyond the 80% isodose line). Patients for whom pseudoprogression cannot be differentiated from true PD should not be permitted to enroll in trials that evaluate tumor recurrence. If pseudoprogression is suspected, therapy should continue as long as the patient remains clinically stable.
Fig. 4.
A pseudoprogression case after chemoradiotherapy. An axial, Gd-contrast-enhanced T1-weighted image before chemoradiotherapy (A); 4 and 12 weeks after radiotherapy and concomitant temozolomide showing increased enhancement (B and C), raising the possibility of progressive disease; after additional 18, 28, 43, 50, 64 weeks of treatment (D–H) with adjuvant temozolomide after radiotherapy, showing a steady decrease in the Gd-enhancing lesion –typical pseudoprogression.
Pseudoresponse on the other hand, is a rapid decrease in contrast-enhancing tumor without true antitumor effect. This occurs after the administration of biologic therapies that target vascular endothelial growth factor (VEGF) pathways, such as bevacizumab (anti-VEGF antibody) and cediranib (VEGF receptor tyrosine kinase inhibitor). Such agents alter the permeability of the blood-brain barrier in such a way that simulates improvement of enhancing tumor on MRI. Over time however, worsening disease is typically noted by changes on T2-weighted and FLAIR images, which suggest progressive infiltrative disease – even in the absence of postcontrast enhancement on T1-weighted images (Fig. 5). As a result, radiologic responses in studies with antiangiogenic agents should be interpreted with caution. Consequently, RANO criteria suggest that radiologic responses persist for at least 4 weeks before they are considered true response.
Fig. 5.
Progressive disease suggested by changes on FLAIR images. Serial MR imaging after left parietal lobe GBM resection for a patient treated with anti-VEGF therapy and temozolomide chemotherapy. Small, high-intensity spots are seen on FLAIR imaging (A) at cycle 2; a new high-intensity area (arrow) is found in the left splenium of the corpus callosum on FLAIR imaging (B) at cycle 4 and significantly worsens on FLAIR imaging (C) at cycle 6; No enhancement is detected on Gd-enhanced T1-weighted images (D–F).
The challenges of the RANO criteria and future needs
The RANO criteria are an evolving set of guidelines and are widely considered to be clinically applicable and reasonably reproducible, i.e. accurate for use in interpreting high-grade glioma treatment results. However, a number of aspects still need to be fully addressed in future updates. This is especially true in relation to early phases of clinical trials where study sponsors look to conserve every bit of statistical power. The standardization of image acquisition protocols across multicenter high-grade glioma trials is of foremost importance. RANO should include recommendations on consistent use of field strength, sequence parameters, and contrast agent dose and timing to reduce unnecessary variability.
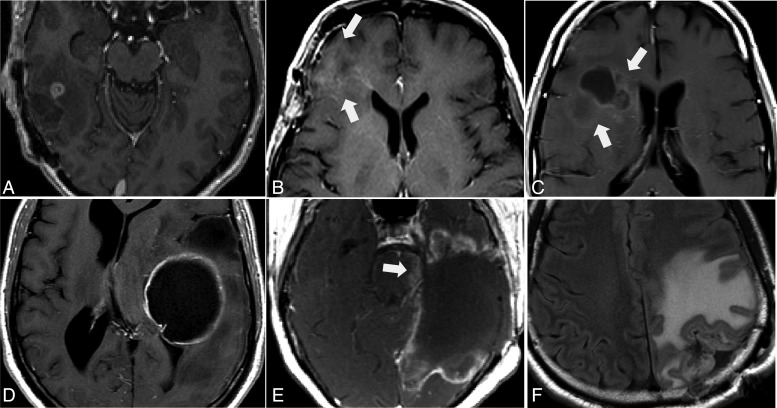
As GBMs often demonstrate heterogeneous enhancement with areas of cystic degeneration and necrosis, guidance in the task of quantifying primary cystic GBMs should also be addressed more clearly. Of particular concern is the method for determining whether the enhancing wall is thick enough or the cystic component small enough to be included in measurements (Fig. 6). The challenge is even greater when evaluating those patients who have undergone recent tumor resection. This factor also represents a source of variability between readers that underlines the need for consensus and further clarification in an updated publication.
Fig. 6.
Measurement of tumor with less predominant cystic components (A) or with irregular, enhancing thick wall (D) is a particularly difficult challenge. Different measurements, such as measurements of enhancing portions (B and E) or the entire lesion (C and F), can be a source of variability even among highly experienced RANO readers.
Also of notable concern is the need for guidance in the area of discerning PR from SD when previously measurable lesions reduce to a size that is no longer measurable. While some instances of reduction are clearly interpretable (e.g. a 1.5 × 1.5-cm enhancing lesion becomes a 0.8 × 0.8-cm enhancing nodule), other instances are more ambiguous and beg clarification (e.g. a 1.1 × 1.1-cm enhancing measurable lesion becomes a 0.8 × 0.8-cm, thin-wall cystic lesion that is too difficult to measure, or a 5 × 5-cm clearly enhancing lesion becomes only faintly enhancing and likely <5 cm in any dimension, yet not clearly distinguishable).
Although further studies are needed to validate such approaches, ongoing investigation is revealing methods that may reduce errors in evaluating enhancement and improve accuracy in response assessment,29 e.g. incorporating contrast enhancement with T1 subtraction. In fact, reports from the Jumpstarting Brain Tumor Drug Development Coalition workshop, conducted in close collaboration with the FDA in early 2014, predicted that future revisions may include T1 subtraction maps, in addition to volumetric imaging and other advanced techniques such as perfusion and diffusion MRI—again, contingent upon validation.30
Certainly, the rapid increase of novel immunotherapies ushers in new challenges that also require careful consideration of revised response criteria. As immunotherapies capitalize on a patient's own immune system to counter disease, often the body reacts with the appearance of new lesions or transient increases in existing lesions before reduction or resolution of disease. This phenomenon has lead to the development of immune-related response criteria for melanoma.31 Similarly, the growing number of immunotherapy trials for high-grade gliomas are also showing these complex radiographic effects, which also pose significant challenges in differentiating immune-related pseudoprogression from true progression.32 Incorporating the necessary considerations into the RANO criteria (ideas currently referred to as iRANO) should improve response assessment and clarify clinical guidelines for patients undergoing immunotherapy trials for high-grade gliomas.
RANO's use of conventional 2D measurements (carried over from Macdonald and WHO) has proven largely inadequate for accurately characterizing the growth of certain complex geometric shapes not uncommon in high-grade glioma. A recent study concluded that to properly evaluate small gliomas (<20 mm in diameter) exact replication of MRI scanning conditions was required; furthermore, when slice thickness was ≥3 mm (regardless of head positioning), bi-dimensional measurements were plainly inadequate in evaluating tumor growth rates.33
In recent years, studies have demonstrated that 3D volume segmentation proved more reliable than 2D for tumor measurement while reducing variability.20,33,34 Three-dimensional tumor measurement has been found to be predictive of survival and progression-free survival in recurrent high-grade glioma using Gd-enhanced T1-weighted images,35 T1 subtraction map,29 and DWI.36 In fact, for determining tumor volume of postoperative recurrence, software based semautomated tumor volume estimations were more effective than manual measurements, rated as more reliable, more reproducible, and faster.37 Another study demonstrated that in terms of quantifying residual and recurrent glioblastomas alike, not only was semi-automatic volumetric segmentation faster than the manual technique, it was also more reliable and reproducible compared with the one-dimensional and 2D measurements.38
Of course, a term such as “semiautomated algorithms” broadly describes a class of software with varying degrees of automation; consequently, semiautomatic volumetric methods must be clarified and standardized to be incorporated into the high-grade glioma response criteria recommendations. Nevertheless, the volumetric approach is superior in circumventing areas of necrosis, cystic cavities, and surgical cavities, and also estimates tumor size more accurately than conventional 2D methods.38,39 The high-grade glioma case in Figure 6D is a perfect example in which applying volumetric measurement more accurately estimates tumor volume.
In terms of grading the involvement of a nonenhancing lesion on FLAIR and T2 imaging, no standard thresholds for the determination of PR or PD have been established to date. The resulting gap in the standard guidance can lead to a high discordance rate in radiographic interpretation between observers. Although variations in FLAIR and T2 images can mean greater difficultly in measurement than enhancing tumor, this factor alone does not justify excluding measurements of nonenhancing tumor in all situations.40 While there is concern over the lack of signal specificity (e.g. edema versus tumor), T2/FLAIR images benefit interpretation by distinguishing decreased vasogenic edema from vascular normalization. Furthermore, reduced T2/FLAIR signal is also associated with decreased morbidity, improved clinical status, and reduced steroid dosing, regardless of whether a patient is experiencing a pseudoresponse or true response to antiangiogenic agents.41–43 Therefore, the ability to quantify unequivocal tumor progression of nonenhancing FLAIR/T2 lesions is justified and guidelines for measurement should be addressed.
Furthermore, since many anaplastic gliomas do not enhance, eliminating the measurement of nonenhancing tumor thwarts the assessment of drug response in grade III tumor trials.19 Follow-up of T2 and FLAIR imaging with DWI can justly provide a superior means of discerning the presence of tumor.44 Likewise, adding DWI to the immediate postoperative MRI scan can act as an independent predictor for 6-month progression-free survival,45 a common primary endpoint in phase II trials, which represents clinically meaningful benefit in the rapidly progressing GBM.46 The RANO paper on low-grade glioma47 has proposed using FLAIR and T2 imaging as key tumor response evaluation sequences. Similar evaluation should be included for the next RANO update in high-grade glioma, particularly for nonenhancing grade III tumor.
Although the consideration of pseudoprogression was introduced in the first RANO publication, pseudoprogression continues to pose particular challenges for patient management and for efficacy assessment in high-grade glioma trials. When wrongly interpreted as treatment failure, pseudoprogression can affect clinical therapeutic decision, possibly resulting in premature discontinuation of effective adjuvant therapy. Conversely, failure to exclude patients with pseudoprogression from studies will result in falsely high objective radiographic response rate. In addition, eliminating patients from drug trials who progress within 3 months of the completion of radiation therapy may exclude the most malignant tumors, resulting in selection bias.19 For instance, although the RANO working group recommended that pseudoprogression should be considered inside the 80% isodose line of radiotherapy, precise radiation field information is rarely available to independent observers.
Pseudoprogression is not uncommon in the 3 to 6 month postradiation timeframe, and the prolonged effect of chemoradiation necrosis can manifest months to even years after treatment.12 In fact, a recent study reported that among 27 patients, almost 30% of pseudoprogression occurred more than 12 weeks after chemoradiotherapy.18 This raises doubts as to the value of the 12-week time limit implemented in the RANO criteria. Therefore, further guidelines are needed. In our central imaging review practice, pseudoprogression is assumed when growth of an existing lesion or appearance of a new lesion occurs within 12 weeks of completion of radiation therapy and the lesion later stabilizes or shrinks at continued follow-up imaging.
Nevertheless, conventional MRI signs have limited utility in diagnosing pseudoprogression in patients with recently treated GBMs and worsening enhancing lesions.48 Although further study validation is required, advanced imaging techniques such as PET/CT, perfusion MRI, and MRS have shown intriguing potential in differentiating pseudoprogression from true progression.18,49–51 While the sensitivity and specificity of such methods are not yet perfected, these techniques are often used as standard high-grade glioma imaging protocol at many medical and academic centers. The best choice for differentiating pseudoprogression may be an optimum combination of multiple imaging methods, e.g. MRS combined with DWI.52 Indeed, the experts from RANO working group have themselves recently listed volumetric, perfusion MRI, MRS, DWI, and PET as auxiliary or secondary endpoints in clinical high-grade glioma trials.8 Since the ability to differentiate pseudoprogression from true PD can affect clinical decisions regarding whether to continue patients on their current therapies or begin alternative treatment, improvement of these standards and introduction of additional modalities is crucial.
Summary
RANO is essentially a 2D anatomical assessment of tumor burden that includes nonenhancing tumor into the determination of overall tumor burden. Especially in the setting of antiangiogenic therapies, measurements of response and progression according to RANO are the best surrogate imaging endpoints available for high-grade glioma multicenter trials at this time. Nonetheless, the adequate assessment of response and time-to-progression is an evolving matter in the high-grade glioma arena; and, while concerns regarding the use of change-in-tumor-size endpoints persist, advances in neuroimaging techniques continue. Not only is it essential that trial oncologists and radiologists have a solid understanding of the RANO guidelines, they must also have proper insight into their limitations in generating an accurate assessment of response to therapeutic intervention. The author anticipates that with sufficient data collection, the next high-grade glioma response criteria updates will incorporate the advanced MRI methods discussed within this review, including quantitative tumor volume measurements, which will improve accuracy in the future interpretation of response to treatments.
Disclosure
The author is an employee of the Medical Imaging division of ICON plc, a global CRO.
Supplementary Material
Acknowledgments
The author thanks Dr. David Raunig, Dr. James Conklin, and Dr. Gregory Goldmacher for their useful suggestions and encouragement. The author also thanks Elizabeth Robson, MS for her kind editorial support.
References
- 1. Dolecek TA, Propp JM, Stroup NE et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reardon DA, Zalutsky MR, Bigner DD. Antitenascin-C monoclonal antibody radioimmunotherapy for malignant glioma patients. Expert Rev Anticancer Ther. 2007;7(5):675–687. [DOI] [PubMed] [Google Scholar]
- 3. Scoccianti S, Magrini SM, Ricardi U et al. . Radiotherapy and temozolomide in anaplastic astrocytoma: a retrospective multicenter study by the Central Nervous System Study Group of AIRO (Italian Association of Radiation Oncology). Neuro Oncol. 2012;14(6):798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Bonis P, Lofrese G, Anile C et al. . Radioimmunotherapy for high-grade glioma. Immunotherapy. 2013;5(6):647–659. [DOI] [PubMed] [Google Scholar]
- 5. Wong ET, Hess KR, Gleason MJ et al. . Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. [DOI] [PubMed] [Google Scholar]
- 6. Lamborn KR, Yung WK, Chang SM et al. . Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen MH, Shen YL, Keegan P et al. . FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. [DOI] [PubMed] [Google Scholar]
- 8. Reardon DA, Galanis E, DeGroot JF et al. . Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol. 2011;13(3):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henson JW. Treatment of glioblastoma multiforme: a new standard. Arch Neurol. 2006;63(3):337–341. [DOI] [PubMed] [Google Scholar]
- 10. Miller AB, Hoogstraten B, Staquet M et al. . Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. [DOI] [PubMed] [Google Scholar]
- 11. Macdonald DR, Cascino TL, Schold SC Jr. et al. . Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 12. Clarke JL, Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009;9(3):241–246. [DOI] [PubMed] [Google Scholar]
- 13. Kreisl TN, Kim L, Moore K et al. . Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sorensen AG, Batchelor TT, Wen PY et al. . Response criteria for glioma. Nat Clin Pract. Oncol. 2008;5(11):634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vos MJ, Uitdehaag BM, Barkhof F et al. . Interobserver variability in the radiological assessment of response to chemotherapy in glioma. Neurology. 2003;60(5):826–830. [DOI] [PubMed] [Google Scholar]
- 16. van den Bent MJ, Vogelbaum MA, Wen PY et al. . End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's Criteria. J Clin Oncol. 2009;27(18):2905–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wen PY, Macdonald DR, Reardon DA et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 18. Nasseri M, Gahramanov S, Netto JP et al. . Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question. Neuro Oncol. 2014;16(8):1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pope WB, Hessel C. Response assessment in neuro-oncology criteria: implementation challenges in multicenter neuro-oncology trials. AJNR Am J Neuroradiol. 2011;32(5):794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanaly CW, Ding D, Mehta AI et al. . A novel method for volumetric MRI response assessment of enhancing brain tumors. PloS One. 2011;6(1):e16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henegar MM, Moran CJ, Silbergeld DL. Early postoperative magnetic resonance imaging following nonneoplastic cortical resection. J Neurosurg. 1996;84(2):174–179. [DOI] [PubMed] [Google Scholar]
- 22. Sato N, Bronen RA, Sze G et al. . Postoperative changes in the brain: MR imaging findings in patients without neoplasms. Radiology. 1997;204(3):839–846. [DOI] [PubMed] [Google Scholar]
- 23. Ulmer S, Braga TA, Barker FG 2nd, Lev MH, Gonzalez RG, Henson JW et al. . Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology. 2006;67(9):1668–1670. [DOI] [PubMed] [Google Scholar]
- 24. Brandes AA, Franceschi E, Tosoni A et al. . MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 25. Hegi ME, Diserens AC, Gorlia T et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 26. Prados MD, Chang SM, Butowski N et al. . Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27(4):579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brandsma D, Stalpers L, Taal W et al. . Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 28. Hygino da Cruz LC Jr., Rodriguez I, Domingues RC et al. . Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Amn J Neuroradiol. 2011;32(11):1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ellingson BM, Kim HJ, Woodworth DC et al. . Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wen PY, Cloughesy TF, Ellingson BM et al. . Report of the Jumpstarting Brain Tumor Drug Development Coalition and FDA clinical trials neuroimaging endpoint workshop (January 30, 2014, Bethesda MD). Neuro Oncol. 2014;16(suppl 7):vii36–vii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolchok JD, Hoos A, O'Day S et al. . Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. [DOI] [PubMed] [Google Scholar]
- 32. Jackson CM, Lim M, Drake CG. Immunotherapy for brain cancer: recent progress and future promise. Clin Cancer Res. 2014;20:3651–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmitt P, Mandonnet E, Perdreau A et al. . Effects of slice thickness and head rotation when measuring glioma sizes on MRI: in support of volume segmentation versus two largest diameters methods. J Neurooncol. 2013;112(2):165–172. [DOI] [PubMed] [Google Scholar]
- 34. Sorensen AG, Patel S, Harmath C et al. . Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol. 2001;19(2):551–557. [DOI] [PubMed] [Google Scholar]
- 35. Dempsey MF, Condon BR, Hadley DM. Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D? AJNR Am J Neuroradiol. 2005;26(4):770–776. [PMC free article] [PubMed] [Google Scholar]
- 36. Hwang EJ, Cha Y, Lee AL et al. . Early response evaluation for recurrent high grade gliomas treated with bevacizumab: a volumetric analysis using diffusion-weighted imaging. J Neurooncol. 2013;112(3):427–435. [DOI] [PubMed] [Google Scholar]
- 37. Ertl-Wagner BB, Blume JD, Peck D et al. . Reliability of tumor volume estimation from MR images in patients with malignant glioma. Results from the American College of Radiology Imaging Network (ACRIN) 6662 Trial. Eur Radiol. 2009;19(3):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chow DS, Qi J, Guo X et al. . Semiautomated volumetric measurement on postcontrast MR imaging for analysis of recurrent and residual disease in glioblastoma multiforme. AJNR Am J Neuroradiol. 2014;35(3):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang RY, Rahman R, Hamdan A et al. . Recurrent glioblastoma: volumetric assessment and stratification of patient survival with early posttreatment magnetic resonance imaging in patients treated with bevacizumab. Cancer. 2013;119(19):3479–3488. [DOI] [PubMed] [Google Scholar]
- 40. Ellingson BM, Cloughesy TF, Lai A et al. . Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pope WB, Sayre J, Perlina A et al. . MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26(10):2466–2474. [PMC free article] [PubMed] [Google Scholar]
- 42. Batchelor TT, Sorensen AG, di Tomaso E et al. . AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–638. [DOI] [PubMed] [Google Scholar]
- 44. Gerstner ER, Frosch MP, Batchelor TT. Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J Clin Oncol. 2010;28(6):e91–e93. [DOI] [PubMed] [Google Scholar]
- 45. Furuta T, Nakada M, Ueda F et al. . Prognostic paradox: brain damage around the glioblastoma resection cavity. J Neurooncol. 2014;118(1):187–192. [DOI] [PubMed] [Google Scholar]
- 46. Ballman KV, Buckner JC, Brown PD et al. . The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van den Bent MJ, Wefel JS, Schiff D et al. . Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 48. Young RJ, Gupta A, Shah AD et al. . Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology. 2011;76(22):1918–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Terakawa Y, Tsuyuguchi N, Iwai Y et al. . Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy.J Nucl Med. 2008;49(5):694–699. [DOI] [PubMed] [Google Scholar]
- 50. Barajas RF Jr., Chang JS, Segal MR et al. . Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253(2):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith EA, Carlos RC, Junck LR et al. . Developing a clinical decision model: MR spectroscopy to differentiate between recurrent tumor and radiation change in patients with new contrast-enhancing lesions. AJR Am J Roentgenol. 2009;192(2):W45–W52. [DOI] [PubMed] [Google Scholar]
- 52. Zeng QS, Li CF, Liu H et al. . Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2007;68(1):151–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.