Abstract
Study Objectives:
Adenotonsillectomy (AT) is the treatment of choice for obstructive sleep apnea (OSA) in children with adenotonsillar hypertrophy. Severe OSA, identified by the apnea-hypopnea index (AHI), is a risk factor for surgical complications and AHI thresholds are used by surgeons to decide elective postoperative hospital admissions. The objective of this study was to identify the prevalence of surgical complications of AT in children with severe OSA and determine their association with specific parameters of polysomnography (PSG).
Methods:
Retrospective evaluation of respiratory and nonrespiratory complications in children undergoing AT for severe OSA was performed. Events were then compared to several individual PSG indices. PSG indices included classic parameters such as AHI, and obstructive apnea indexes (OAI) as well as gas exchange parameters including the oxygen desaturation index (ODI), lowest oxyhemoglobin saturation (lowest SpO2), peak end-tidal CO2 (peak ETCO2), the percentage of the total sleep time (%TST) with ETCO2 > 50 mmHg (%TST ETCO2 > 50 mmHg) and oxygen saturation < 90% (%TST O2 < 90%).
Results:
A total of 158 children were identified with severe OSA. Major respiratory complications occurred in 21.5% and were only associated with the ODI (P = .014), lowest SpO2 (P = .001) and %TST O2 < 90% (P < .001). Minor respiratory complications occurred in 19.6% and these were not associated with any PSG parameters. Major nonrespiratory complications occurred in 4.4% and also were not associated with any PSG parameters; however, minor nonrespiratory complications occurring in 37.3%, and were associated with %TST O2 < 90% (P < 0.001).
Conclusions:
PSG measures of gas exchange are strongly associated with postoperative complications of AT and are better suited for postoperative planning than classic indices such as AHI.
Citation:
Molero-Ramirez H, Tamae Kakazu M, Baroody F, Bhattacharjee R. Polysomnography parameters assessing gas exchange best predict postoperative respiratory complications following adenotonsillectomy in children with severe OSA. J Clin Sleep Med. 2019;15(9):1251–1259.
Keywords: adenotonsillectomy, obstructive sleep apnea, pediatrics, polysomnography
BRIEF SUMMARY
Current Knowledge/Study Rational: Adenotonsillectomy is the treatment of choice for children with obstructive sleep apnea related to enlarged adenoids and tonsils. Although severe obstructive sleep apnea as defined by the apnea-hypopnea index is associated with surgical risk, few studies have examined the strength of individual polysomnography parameters to predict specific surgical complications in children.
Study Impact: We found that surgical complications, particularly major respiratory complications, were only associated with polysomnography parameters assessing gas exchange rather than classic parameters such as apnea-hypopnea index. This emphasizes that precise evaluation of polysomnography should focus on measures of gas exchange to help identify children at risk for surgical complications following adenotonsillectomy.
INTRODUCTION
Obstructive sleep apnea (OSA) in children, a variant of sleep-disordered breathing, presents typically with snoring and is characterized by prolonged periods of increased upper airway resistance, and intermittent partial to complete obstruction of the upper airway during sleep. Consequentially, OSA disrupts normal ventilation leading to episodic oxyhemoglobin desaturation, hypercapnia, frequent arousals during sleep, and subsequent sleep fragmentation.1,2 The reported prevalence of OSA in children varies between 1% to 4%,3 with an even higher reported prevalence in obese children of up to 13% to 59%.4 The timeliness of effective treatment of OSA in children is particularly relevant because OSA is associated with numerous comorbidities including neurocognitive and behavioral disturbances,5–7 nocturnal enuresis,8,9 cardiovascular disturbances such as systemic and pulmonary hypertension,10,11 endothelial dysfunction,12,13 and finally somatic growth failure.14 Unsurprisingly, untreated OSA in children is associated with substantial health care costs.15
The primary etiology of OSA in children is related to hypertrophy of adenotonsillar tissue. Recent published guidelines have affirmed that adenotonsillectomy (AT) remains the treatment of choice in children with OSA and enlarged tonsils.16 Almost 500,000 procedures are performed each year, and the prevalence has increased mostly because of the increased prevalence and awareness of pediatric OSA.17 Further, recent trends have revealed that the primary indication for AT is for the treatment of OSA rather than recurrent adenotonsillar infections as was observed in the past.18
AT has been shown to be efficacious in treating pediatric OSA, but a large population of children will be found to have residual OSA following AT, particularly if their underlying OSA is very severe.19,20 The presence of severe OSA as defined by the apnea-hypopnea index (AHI) also constitutes a risk factor for surgical complications associated with AT including postoperative hypoxemia, need for supplemental oxygen, and prolonged hospital course.21–25 Although variations exist in published guidelines regarding which children require admission to monitor for postoperative respiratory complications, there is a consensus that severe OSA constitutes a major risk following AT. However, and adding to the confusion, the threshold for defining severe OSA as described by the AHI also varies. Accordingly, a preoperative AHI > 24 events/h, as suggested by the American Academy of Pediatrics16 or a preoperative AHI > 10 events/h as suggested by the American Academy of Otolaryngology – Head and Neck Surgery26 are cutoff criteria recommended for inpatient postoperative observation.
In this study, we explore the prevalence of postoperative respiratory and nonrespiratory complications of AT in children with severe OSA (AHI > 10 events/h). We hypothesize that PSG measures of gas exchange including the oxygen desaturation index (ODI), the lowest SpO2 or oxygen saturation nadir, the TST with an observed oxygen saturation below 90%, the peak maximal end tidal carbon dioxide measured during sleep (mmHg), and the percent of TST with an observed end tidal carbon dioxide (ETCO2) greater than 50 mmHg are more strongly associated with postoperative respiratory and nonrespiratory complications than classic measures such as the AHI. In this context, we examined the strength of the association of the AHI to predict postoperative respiratory and nonrespiratory complications in children with severe OSA following AT.
METHODS
We performed a retrospective chart review of all children with a diagnosis of severe OSA as determined by in-laboratory PSG and who underwent AT at Comer Children’s Hospital, a tertiary care hospital, from February 2009 to December 2012. AT was performed by the same surgeon. The study was approved by the Institutional Review Board of the University of Chicago (IRB15-1447).
Only children undergoing AT with severe OSA were included. Severe OSA was defined by an obstructive apnea-hypopnea index (OAHI) > 10 events/h during total sleep time (TST). Because central apneas are included in the AHI, we elected to use the OAHI, which includes obstructive hypopneas, obstructive apneas, and mixed apneas but excludes central apnea events. We opted to use an OAHI > 10 events/h because this was the criteria used by our otolaryngology department based on published guidelines.26 AT was performed within 6 weeks of PSG. Children with central sleep apnea (central apnea index > 5 events/h) were excluded from this study. In addition, children with craniofacial syndromes, Down syndrome, and neuromuscular and other congenital disorders were excluded. Among children who met inclusion criteria, a retrospective chart review of demographic and clinical variables examining specific postoperative respiratory complications was obtained using the anesthesiology records (immediate postoperative or perioperative) and the electronic medical record (postoperative period). The electronic medical record was then examined to see the postoperative disposition (discharged home, admitted to the ward, or admitted to the pediatric intensive care unit [PICU]), and used to evaluate postoperative adverse events during their admission, including oxyhemoglobin desaturation.
Polysomnography
Pediatric sleep studies were done at Comer Children’s Hospital or at the University of Chicago Sleep Laboratory and were performed by the same group of registered sleep technicians with vast experience in performing pediatric sleep studies. Children underwent full-night in-laboratory PSG using Nihon Koden PSG equipment and software (Tokyo, Japan). Children were studied for up to 12 hours in a quiet, darkened room maintained at an ambient temperature of 24°C in the presence of one of their parents. No drugs were used to induce sleep. The following parameters were measured: chest and abdominal wall movement by inductance plethysmography, heart rate by electrocardiography, and air flow was monitored with a side-stream end-tidal capnograph (Nihon Koden) that also provides breath-by-breath assessment of end-tidal carbon dioxide levels, a nasal pressure cannula, and an oronasal thermistor. Arterial pulse oxygen saturation (SpO2) was assessed by pulse oximetry (Nellcor N 100, Nellcor Inc, Hayward, California), with simultaneous recording of the pulse waveform. The bilateral electrooculogram, eight channels of electroencephalogram (two frontal, two occipital, two temporal, and two central leads), chin and anterior tibial electromyograms, and analog output from a body position sensor were also monitored. PSG was scored by board registered technologists and interpreted by board-certified sleep medicine physicians according to The AASM Manual for the Scoring of Sleep and Related Events: Rules, Terminology and Technical Specifications.27
We reviewed all PSG tests for the following parameters: AHI (total number of apneas and hypopneas per hour of TST); OAHI (total number of obstructive apneas, mixed apneas, and obstructive hypopneas per hour of TST); obstructive apnea index (OAI; total number of obstructive apneas per hour of TST); and central apnea index (total number of central apneas per hour of TST). The oxygen desaturation index (ODI) was defined as the number of desaturation events ≥ 3% per hour of TST; the lowest SpO2 or oxygen saturation nadir was the lowest observed oxygen saturation during sleep; and the %TST O2 < 90% was the percentage of TST with an observed oxygen saturation below 90%. The peak ETCO2 was the observed maximal end tidal carbon dioxide measured during sleep (mmHg), and the %TST ETCO2 > 50 mmHg was the percent of TST with an observed ETCO2 that was greater than 50 mmHg. Finally, PSG was also characterized by examining the sleep efficiency (the ratio of the total amount of time spent asleep to the total recording time), the spontaneous arousal index, (number of spontaneous arousals per hour of TST), and the respiratory arousal index, (number of respiratory related arousals per hour of TST). The PSG was reviewed by board-certified pediatric sleep medicine physicians.
Study Outcomes: Postoperative Complications
Respiratory complications were classified according to the time of their occurrence: at time of anesthesia induction (the intraoperative period), during the recovery period following surgery (the immediate postoperative period or perioperative), and, finally, during hospitalization if the child was admitted (the postoperative period). Complications were defined as difficult intubation (more than one attempt), breath holding, supraglottic obstruction, documented hypoxemia, need for supplemental oxygen, need for oral or nasal airway, reintubation during the postoperative period, and need for noninvasive ventilation. Patients could be admitted to either the medical floor or the PICU, or be discharged home. If available in the chart, the reasons for admission and any postoperative complications during this period were carefully recorded. Also, when available, documentation of the application of perioperative opiates following AT, during the recovery period, was examined. Intraoperative and immediately perioperative complications were obtained through examination of the anesthesia and nursing charting. Complications occurring during inpatient hospitalization including in the PICU were obtained by examination of the electronic medical record. Analysis was performed on each individual complication and complications were grouped based on major and minor respiratory and nonrespiratory complications. Minor respiratory complications included documented oxyhemoglobin desaturation event during the immediate postoperative period, need for supplemental oxygen, admission to the ward, or desaturation event during the admission. Major respiratory complications included a need for an oral or nasal airway, reintubation, need for noninvasive ventilation, or admission to PICU. Major and minor nonrespiratory complications included admission for intravenous fluids and pain control to the PICU or the ward determined by the surgeon based on the severity of symptoms and need for overnight monitoring.
Statistics
Analyses of PSG variables were sought in order to determine their ability to predict specific postoperative complications. Analysis of variance and coefficient of correlation were used to determine predictive value for postoperative complications of specific PSG parameters of interest. Fisher exact test was used to characterize statistical differences among distributions. Multivariate linear regression modeling of several postsurgical complications was conducted using all PSG parameters of interest, and also included the child’s age, body mass index (BMI) z-score, and post-AT opiate administration. IBM SPSS Statistics for Macintosh, Version 21.0. (IBM Corporation, Armonk, New York) was used for statistical analysis. Data are presented as means ± standard deviation (SD). Epi Info 7 was used to determine BMI z-scores; Centers for Disease Control 2000 Growth References were used for all children older than 2 years, and World Health Organization Child Growth Standards were used for all children younger than 2 years. Because this is a retrospective review, no sample size calculations were indicated.
RESULTS
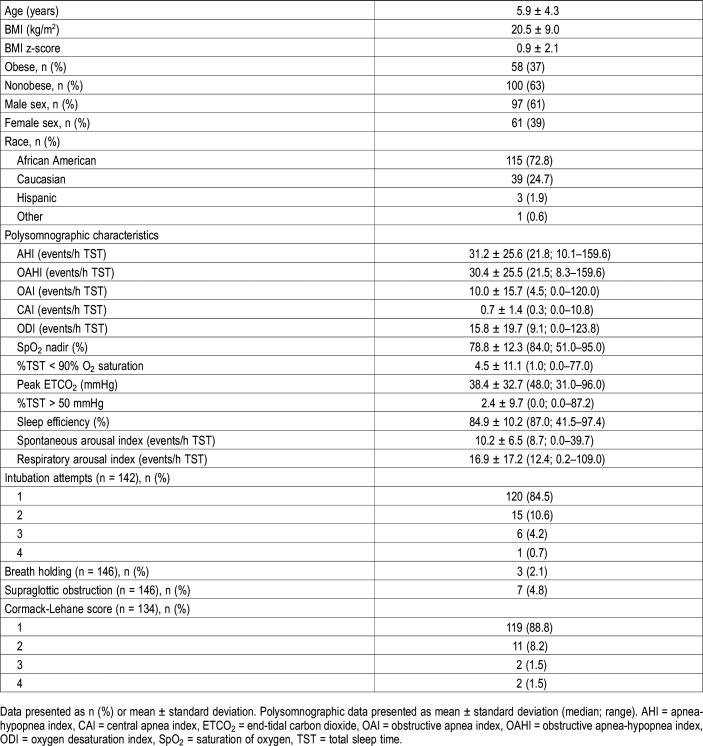
Of 378 patients who underwent adenotonsillectomy during the study review period, 158 children were identified to have severe OSA as defined by an OAHI > 10 events/h during TST. There were 61 females (39%). A demographic summary of the study cohort is presented in Table 1. Obesity, as defined as a BMI z-score greater than 1.65, was observed in 58 children (37%). Most of the children were African American (n = 115 or 73%).
Table 1.
Baseline summary (n = 158).
Preoperative PSG revealed a mean ± SD OAHI of 30.4 ± 25.5 events/h during TST (AHI of 31.2 ± 25.6 events/h during TST) with a oxygen saturation nadir of 78.8% ± 12.3%. A summary of additional pertinent PSG indices is also presented in Table 1. During the induction of anesthesia for the AT surgical procedure, in data available from anesthesia records (n = 134), most of the children had a Cormack-Lehane score of 1 (n = 119/134 or 89%) signifying a straightforward view of the glottis through laryngoscopy at time of intubation (Table 1). As expected, in most of the transcribed anesthesia documents (n = 120/142 or 85%), intubation was successful on the first attempt (Table 1). The %TST ETCO2 > 50 mmHg emerged as the only PSG parameter that was significantly associated with a higher number of intubation attempts (P < .001) (Table S1 in the supplemental material). Breath holding and supraglottic obstruction were rare events during anesthesia induction and these were not associated with any PSG parameter.
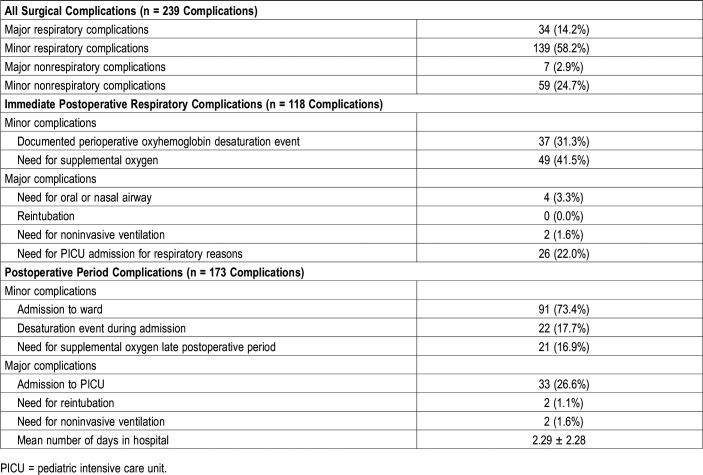
We identified 118 complications during the immediate postoperative period or perioperative period and 173 complications during the postoperative period when patients were admitted (Table 2). Many patients were identified to have greater than one complication during either period. For purpose of simplicity, we grouped complications as major or minor respiratory complications and major or minor nonrespiratory complications during either period. Upon grouping both periods, 239 distinct major or minor respiratory and major or minor nonrespiratory complications occurred with minor respiratory complications consisting of the largest number at 139 or 58.2% of observed complications (Table 2).
Table 2.
Summary of postoperative complications.
In the perioperative period following AT, in data available (n = 152 patients) from anesthesia records, minor respiratory complications including oxyhemoglobin desaturation (n = 37/152 or 24%) and the need for supplemental oxygen (n = 49/152 or 32%) were frequent events (Table 2). Major respiratory complications were relatively infrequent.
In the postoperative period, 124 of 158 children were admitted to hospital (78.5%). The mean ± SD duration of admission days was 2.29 ± 2.28 days. The reasons for admission were related to respiratory complications, pain management, or oral intolerance with need for intravenous hydration. Postoperative complications during this period were only available from 124 inpatient patient records. Again, minor complications were frequently observed, whereas major complications were rare events (Table 2).
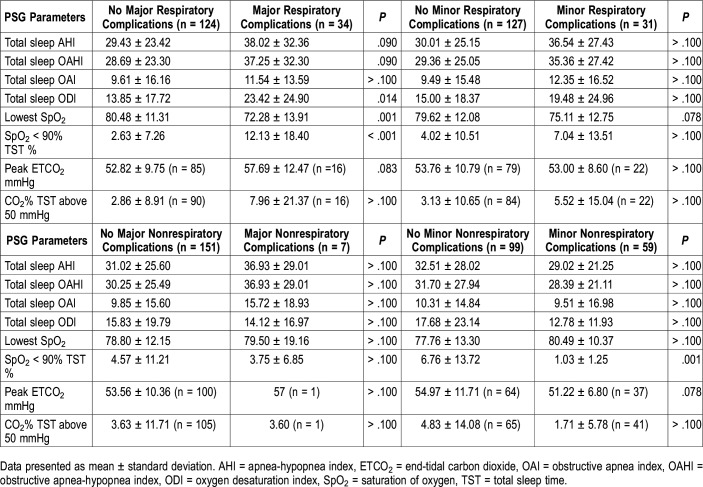
We subsequently analyzed individual patients in whom either a respiratory and/or nonrespiratory complication developed (Table 3). Major respiratory complications occurred in 34 patients (21.5%), whereas only minor respiratory complications occurred in 31 of the 158 patients (19.6%) in our cohort. Major nonrespiratory complications occurred in 7 patients (4.4%) whereas only minor nonrespiratory complications occurred in 59 (37.3%).
Table 3.
Association of polysomnography parameters and patients with perioperative and/or postoperative complications.
PSG parameters that were significantly associated with patients sustaining major respiratory complications were total sleep ODI (P = .014), lowest SpO2 (P = .001), and %TST O2 < 90% (P < .001) (Table 3). PSG parameters were not predictive of patients sustaining only minor respiratory complications. Regarding nonrespiratory postoperative complications, only %TST O2 < 90% (P = .001) was associated with patients sustaining only minor nonrespiratory complications (Table 3).
Following the analysis of each individual perioperative and postoperative complication (Table S1), desaturation during recovery was significantly associated with the ODI (P = .019), the lowest SpO2 (P < .001), the %TST O2 < 90% (P < .001), and the %TST ETCO2 > 50 mmHg (P = .002). The need for supplemental oxygen was significantly associated with the %TST O2 < 90% (P = .038) and the %TST ETCO2 > 50 mmHg (P = .007) (Table S1). Neither of these events were associated with the standard parameters of PSG, namely the AHI, OAHI, or OAI.
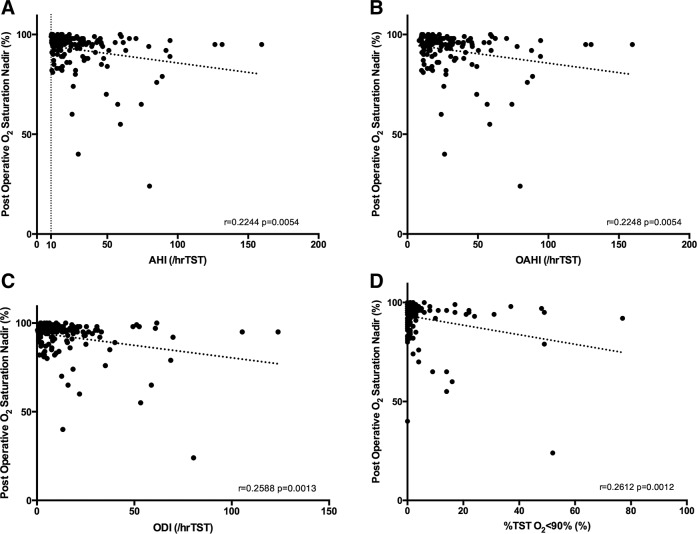
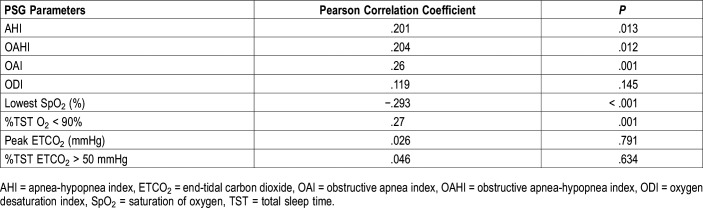
In addition, we evaluated the oxygen saturation monitoring from the anesthesia record during the perioperative period and observed statistically significant, albeit weak, correlations between the perioperative oxygen saturation nadir and the AHI and the OAHI (r = .22, P = .0054 and r = .22, P = .0054, respectively) (Figure 1). However, a stronger correlation was seen with the PSG gas exchange parameters: ODI (r = .26, P = .0013) and the %TST O2 < 90% (r = .26, P = .0012) and the perioperative oxygen saturation nadir (Figure 1).
Figure 1. Correlation of immediate postoperative oxygen saturation nadir and polysomnography parameters.
(A) Correlation with apnea-hypopnea index. (B) Correlation with obstructive apnea-hypopnea index. (C) Correlation with oxygen desaturation index. (D) Correlation with percent total sleep time with oxygen saturation less than 90%. AHI = apnea-hypopnea index, OAHI = obstructive apnea-hypopnea index, ODI = oxygen desaturation index, TST = total sleep time.
Most parameters of interest, except for the OAI and the peak ETCO2, were significantly associated with a desaturation during the admission; however, only the lowest SpO2 (P = .003), the %TST O2 < 90% (P < .001) and the %TST ETCO2 > 50 mmHg (P = .001) were significantly associated with requiring sustained supplemental oxygen support (Table S1). Of children requiring an admission to the PICU, only the ODI (P = .012), the lowest SpO2 (P < .001), and finally the %TST O2 < 90% (P < .001) were associated with a PICU admission.
Prior to admission, it was noted that 24 of 124 children (19%) had received opiates during the perioperative observation period. Children in whom desaturation occurred during their admission were more likely to have received immediate postoperative opiates, 8 of the 22 children who desaturated received opiates compared to 16 of 102 children in whom desaturation did not occur who received opiates (P = .037). Perioperative opiates did not, however, predict the need for supplemental oxygen during their admission (6 of 21 children requiring supplemental oxygen receive opiates compared to 18 of 103 children not requiring supplemental oxygen received opiates, P = .239).
Using a stepwise multivariate linear regression model of all aforementioned PSG parameters with inclusion of additional variables of surgical age, BMI z-score, and administration of post-AT opiates, it was found that oxyhemoglobin desaturation during the child’s admission was only significantly associated with a higher %TST O2 < 90% (P < .001) followed by whether the child had received immediate post-AT opiates (P = .037). The need for supplemental oxygen was only associated with a higher %TST O2 < 90% (P < .001).
Finally, it was found that most PSG parameters were correlated with the number of days children were admitted to hospital (Table 4). Stepwise multivariate linear modeling revealed that the strongest factor associated with the number of hospital days was a higher %TST O2 < 90% (P < .001) followed by administration of perioperative opiates (P = .002), a younger surgical age (P = .013) and a higher OAI (P = .028).
Table 4.
Factors correlated to number of days admitted to hospital.
DISCUSSION
Prior to discussing the implications of the findings of our study, it is worthwhile to note that complications of AT done for OSA are relatively common. Therefore, in this context, it is unreasonable to deduce that AT is an entirely benign procedure in children. In a recently published meta-analysis of 1,254 studies, for which 23 articles met eligibility criteria, De Luca Canto and colleagues28 showed that respiratory compromise was encountered 9.4% of the time. Common respiratory complications included oxyhemoglobin desaturation, requirement for oxygen supplementation, supraglottic obstruction, and breath holding. In our study, in which we analyzed a cohort of children with severe OSA, we found that oxyhemoglobin desaturation occurred in 24% of children in the immediate postoperative period, and the frequency remained high in children who were admitted following surgery (17%). The need for supplemental oxygen was also higher in this cohort as 33% of children needing oxygen in the immediate postoperative period, and 17% of children requiring it during their admission. During the induction phase of anesthesia, supraglottic obstruction was seen in 4.8% of children. A need to stabilize the airway with an oral or nasal device immediately postoperatively was required in 5.3% of children. The higher prevalence of these respiratory complications in our cohort compared to the aforementioned meta-analysis is likely explained by the fact that our study cohort consisted of only children with severe OSA. The study by De Luca Canto and colleagues addressed whether OSA was associated with respiratory complications, and although only 4 of the 23 eligible articles were suitable to address this question, the analysis showed a 4.90 odds ratio of the development of postoperative respiratory complications following AT if children had PSG-documented OSA.
In agreement, the American Academy of Otolaryngology – Head and Neck Surgery Foundation had published guidelines recommending postoperative admission for all children with severe OSA with an AHI > 10 events/h during TST and/or lowest SpO2 < 80%.26 The American Academy of Pediatrics similarly published guidelines recommending postoperative admission for children with OSA; however, using a higher cutoff AHI (> 24 events/h during TST), lowest SpO2 < 80%, or if the peak ETCO2 was ≥ 60 mmHg.16 For these purposes, in our study, we chose to examine the complications of AT in children with only severe OSA (OAHI > 10 events/h during TST); however, in review of patient records, it was apparent that not all children were admitted for postoperative monitoring (n = 124/158 or 78%) suggesting that in our practice, the recommendation to admit all children postoperatively with underlying severe OSA (as defined by AHI) was not always followed. It is important to mention that all children were monitored for 3 hours in the recovery room prior to discharge and would have not been sent home had there been any evidence of respiratory compromise.
Notwithstanding, the principal findings of our study imply that specific parameters of PSG other than the AHI are better able to predict postoperative complications following AT in children with severe OSA. We did not observe that any of the PSG parameters of interest were able to predict complications during the induction of anesthesia. In the immediate postoperative period, desaturation and the need for supplemental oxygen were commonly documented events (25% and 32%, respectively), and the need for an oral and nasal airway was relatively uncommon (5.3%). Neither of these events were predicted by the AHI, OAHI, or the OAI but rather, by parameters of gas exchange including the %TST O2 < 90%, the ODI, the %TST ETCO2 > 50 mmHg and the lowest SpO2. Complications seen among admitted children were also better predicted by parameters measuring gas exchange than the standard PSG parameters of AHI, OAHI, or OAI, and these findings were corroborated using a multivariate logistic regression model. Finally, the duration of stay in hospital was better predicted by the %TST O2 < 90% and the lowest SpO2 than the AHI.
Taken together, our findings support that PSG parameters assessing gas exchange were better able to predict complications associated with AT than classic indices of PSG. A recent study suggests that when OSA affects oxygenation, automated analysis of the oximeter signal can accurately make diagnosis in patients with habitual snoring at risk of OSA.29 This publication, which supports the importance of gas exchange parameters in predicting OSA, could support similar automated analysis of oximetry to predict patients at risk of postoperative complications Prior to drawing this conclusion, it is pertinent to summarize key limitations of our study. First, the retrospective study design limits the study findings and their generalizability; for example, the decision to admit or to intervene may be related to the surgeon and/or anesthesiologists’ bias of existing knowledge of severe OSA and an increased tendency toward intervention. There was indeed nonstandardized clinical decision making of whether to admit and where to admit. Also, due to the retrospective design, patients did not receive a standardized method of anesthesia given the different preferences of involved anesthesiologists. Thus, it is plausible that variations during the intraoperative period may have had an effect on study outcomes. However, all the cases involved only one surgeon, and this potentially may have contributed to more homogeneous surgical practices and even more importantly the decision criteria used to admit patients postoperatively. Notwithstanding, the lack of standardization of other clinical practices at our center can limit the generalizability of our findings.
Our study is also limited by a small sample size. Future studies with larger sample sizes could be used to evaluate the influence of stage distribution, specifically rapid eye movement versus non-rapid eye movement sleep on PSG parameters related to OSA.
Administration of postoperative opiates may influence the frequency of complications following AT. For example, in our study we did see that this may have influenced the frequency of desaturation events, but did not influence the need for supplemental oxygen during the admission of these children. The administration of opiates in the immediate postoperative state was not standardized in this retrospective study. Nonetheless, subsequent studies should aim to standardize the criteria for administration and dosing of opiate therapy in these children in the immediate postoperative period, because children with OSA are known to be more sensitive to opiates in relation to possible respiratory depression.29
Finally, there was not a standardization of the administration of supplemental oxygen, nor was there adequate documentation of the duration of supplemental oxygen to include this measure as a study outcome.
In the only published prospective study design examining post-AT complications,30 Elden and colleagues carefully examined 329 children at a single tertiary center and similarly revealed that most major and minor respiratory complications were better predicted by parameters of gas exchange abnormalities rather than classical parameters such as the AHI. These investigators also showed that all respiratory complications were associated with African American race, despite most of their cohort being Caucasian. A predominant race group, in the case of a predominant African American population in our study cohort, can also be considered a study limitation because of narrowing external validity, although it should also be considered a strength to better illustrate the effect of the risk factor of race (African American) on the outcomes of management of OSA. Most of the children were African American, largely related to the demographic of patients in our hospital. Finally, the presence of African American race19 and severe OSA20 are risk factors of residual OSA following AT, and may explain the high proportion of respiratory complications following surgery.
Our study is also limited by presenting findings from a single site, similar to the aforementioned prospective study, thereby indicating the pressing need to examine complications of AT using a multicenter prospective study design. In the multicenter randomized control childhood adenotonsillectomy trial (CHAT), Konstantinopoulou and colleagues31 also evaluated operative complications related to AT and did not observe any significant associations with PSG parameters; however, given this cohort had mostly mild OSA, overall complication rates were relatively miniscule and a lack of statistical significance is not unexpected.
CONCLUSIONS
We demonstrate that postoperative respiratory complications of AT in children with severe OSA are better predicted by PSG parameters assessing gas exchange rather than classic indices such as the AHI. It is important to mention that the AHI provides equal weight to obstructive events that result in cortical arousal as it does to events that cause desaturations. As such, perhaps the shortcomings of the AHI are in its ability to distinguish children who are more hyperarousable following obstructive events compared to children who fail to arouse and are more likely to sustain gas exchange abnormalities. Plausibly, the reduced propensity to arousal in some children may in fact represent a loss of a protective reflex from prolonged obstructive breathing that may contribute to postoperative complications following anesthesia; it is likely that these children are better identified by PSG parameters of gas exchange rather than the AHI.
DISCLOSURE STATEMENT
This study was conducted at Comer Children’s Hospital at the University of Chicago, Chicago, Illinois. All authors have read and approved this manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AT
adenotonsillectomy
- OSA
obstructive sleep apnea
- PSG
polysomnography
- AHI
apnea-hypopnea index
- OAHI
obstructive apnea-hypopnea index
- OAI
obstructive apnea index
- CAI
central apnea index
- SpO2
oxygen saturation
- ETCO2
end-tidal carbon dioxide
- TST
total sleep time
- PICU
pediatric intensive care unit
REFERENCES
- 1.Tan HL, Gozal D, Kheirandish-Gozal L. Obstructive sleep apnea in children: a critical update. Nat Sci Sleep. 2013;5:109–123. doi: 10.2147/NSS.S51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tauman R, Gozal D. Obstructive sleep apnea syndrome in children. Expert Rev Respir Med. 2011;5(3):425–440. doi: 10.1586/ers.11.7. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thora Soc. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhulst SL, Van Gaal L, De Backer W, Desager K. The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents. Sleep Med Rev. 2008;12(5):339–346. doi: 10.1016/j.smrv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3):616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 6.Gozal D. Obstructive sleep apnea in children: implications for the developing central nervous system. Semin Pediatr Neurol. 2008;15(2):100–106. doi: 10.1016/j.spen.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kheirandish L, Gozal D. Neurocognitive dysfunction in children with sleep disorders. Dev Sci. 2006;9(4):388–399. doi: 10.1111/j.1467-7687.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 8.Sans Capdevila O, Crabtree VM, Kheirandish-Gozal L, Gozal D. Increased morning brain natriuretic peptide levels in children with nocturnal enuresis and sleep-disordered breathing: a community-based study. Pediatrics. 2008;121(5):e1208–e1214. doi: 10.1542/peds.2007-2049. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos EI, Kaditis AG, Kostadima E, Gourgoulianis K. Resolution of nocturnal enuresis in snoring children after treatment with nasal budesonide. Urology. 2005;66(1):194. doi: 10.1016/j.urology.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Amin R, Somers VK, McConnell K, et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51(1):84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- 11.Tal A, Leiberman A, Margulis G, Sofer S. Ventricular dysfunction in children with obstructive sleep apnea: radionuclide assessment. Pediatr Pulmonol. 1988;4(3):139–143. doi: 10.1002/ppul.1950040304. [DOI] [PubMed] [Google Scholar]
- 12.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116(20):2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharjee R, Alotaibi WH, Dayyat E, Sans Capdevila O, Gozal D, Kheirandish-Gozal L. Obstructive sleep apnea exacerbates endothelial dysfunction in both obese and non-obese children. Sleep. 2009;32(Abstract Suppl):A63. [Google Scholar]
- 14.Bonuck K, Parikh S, Bassila M. Growth failure and sleep disordered breathing: a review of the literature. Int J Pediatr Otorhinolaryngol. 2006;70(5):769–778. doi: 10.1016/j.ijporl.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Tarasiuk A, Greenberg-Dotan S, Simon-Tuval T, et al. Elevated morbidity and health care use in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2007;175(1):55–61. doi: 10.1164/rccm.200604-577OC. [DOI] [PubMed] [Google Scholar]
- 16.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya N, Lin HW. Changes and consistencies in the epidemiology of pediatric adenotonsillar surgery, 1996-2006. Otolaryngol Head Neck Surg. 2010;143(5):680–684. doi: 10.1016/j.otohns.2010.06.918. [DOI] [PubMed] [Google Scholar]
- 18.Erickson BK, Larson DR, St Sauver JL, Meverden RA, Orvidas LJ. Changes in incidence and indications of tonsillectomy and adenotonsillectomy, 1970-2005. Otolaryngol Head Neck Surg. 2009;140:894–901. doi: 10.1016/j.otohns.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 19.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–683. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 21.McColley SA, April MM, Carroll JL, Naclerio RM, Loughlin GM. Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol.Head Neck Surg. 1992;118(9):940–943. doi: 10.1001/archotol.1992.01880090056017. [DOI] [PubMed] [Google Scholar]
- 22.Rosen GM, Muckle RP, Mahowald MW, Goding GS, Ullevig C. Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics. 1994;93:784–788. [PubMed] [Google Scholar]
- 23.Hill CA, Litvak A, Canapari C, et al. A pilot study to identify pre- and peri-operative risk factors for airway complications following adenotonsillectomy for treatment of severe pediatric OSA. Int J Pediatr Otorhinolaryngol. 2011;75(11):1385–1390. doi: 10.1016/j.ijporl.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Nixon GM, Kermack AS, McGregor CD, et al. Sleep and breathing on the first night after adenotonsillectomy for obstructive sleep apnea. Pediatr Pulmonol. 2005;39(4):332–338. doi: 10.1002/ppul.20195. [DOI] [PubMed] [Google Scholar]
- 25.Jaryszak EM, Shah RK, Vanison CC, Lander L, Choi SS. Polysomnographic variables predictive of adverse respiratory events after pediatric adenotonsillectomy. Arch Otolaryngol Head Neck Surg. 2011;137(1):15–18. doi: 10.1001/archoto.2010.226. [DOI] [PubMed] [Google Scholar]
- 26.Roland PS, Rosenfeld RM, Brooks LJ, et al. Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1_suppl):S1–S15. doi: 10.1177/0194599811409837. [DOI] [PubMed] [Google Scholar]
- 27.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 28.De Luca Canto G, Panchêco-Pereira C, Aydinoz S, et al. Adenotonsillectomy complications: a meta-analysis. Pediatrics. 2015;136(4):425–428. doi: 10.1542/peds.2015-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown KA, Laferriere A, Lakheeram I, Moss IR. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology. 2006;105(4):665–669. doi: 10.1097/00000542-200610000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Thongyam A, Marcus CL, Lockman JL, et al. Predictors of perioperative complications in higher risk children after adenotonsillectomy for obstructive sleep apnea: a prospective study. Otolaryngol Head Neck Surg. 2014;151(6):1046–1054. doi: 10.1177/0194599814552059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konstantinopoulou S, Gallagher P, Elden L, et al. Complications of adenotonsillectomy for obstructive sleep apnea in school-aged children. Int J Pediatr Otorhinolaryngol. 2015;79(2):240–245. doi: 10.1016/j.ijporl.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.