CITATION
Javaheri S, Gay PC. To die, to sleep – to sleep, perchance to dream…without hypertension: dreams of the visionary Christian Guilleminault revisited. J Clin Sleep Med. 2019;15(9):1189–1190.
In this issue of the Journal of Clinical Sleep Medicine, Budhiraja and colleagues1 used the American Academy of Sleep Medicine (AASM) definition of hypopneas2 and reported a strong association with the presence of hypertension using data from 6,131 participants in the Sleep Heart Health Study (SHHS).3 Hypopnea was defined as 30% reduction in airflow associated with ≥ 3% desaturation and/or an arousal. The current Centers for Medicare and Medicaid Services (CMS) criterion is a reduction of ≥ 4% with no consideration for an arousal component.
This is the first systematic report incorporating these criteria and applying them to a large population. The study should encourage CMS and other third-party payers to allow hypopneas with arousals alone to be considered a valid disordered breathing event as arousals are associated with a crucial number of health care outcomes, including hypertension.
Table 1 depicts some of the adverse consequences of obstructive sleep apnea (OSA) attributed solely to arousals. Arousals are important biological consequences of OSA mediating excessive daytime sleepiness (EDS),4–7 and many patients with EDS have hypopneas with cortical arousals but without significant desaturation. Aside from impairment of quality of life, EDS is a major public hazard, and perhaps many who are denied treatment, may have been subsequently involved in traffic/occupational accidents because of suboptimal attention and vigilance.
Table 1.
Adverse consequences of chronic sleep fragmentation and arousals.
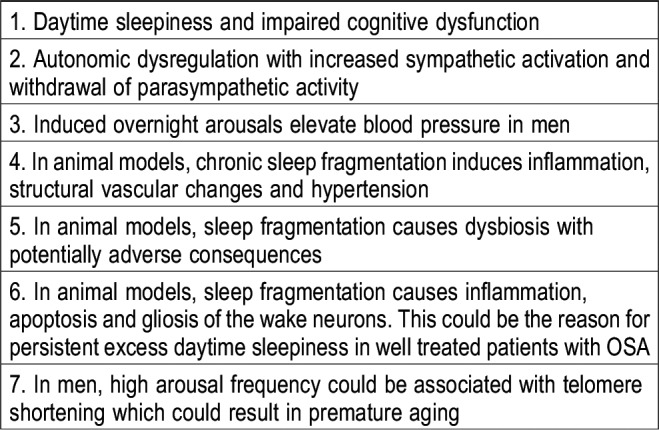
Consistent with the current report,1 polysomnographic studies in men without OSA show that induced arousals are associated with increased blood pressure8,9 (autonomic arousal). With repetitive cycles of apnea and associated arousals heightened diurnal sympathetic activity and hypertension could prevail. In the Cleveland Family Study involving 394 participants, after adjusting for age, sex, race, and body habitus, the odds of hypertension increased approximately 20% per 5-unit increase in arousal index. The association of hypertension with oxygen saturation was weaker.10 Animal studies are confirmatory.11–13 Arousals are associated with sympathetic activation and withdrawal of parasympathetic activity with consequent elevated blood pressure and long-term sleep fragmentation induces morphologic vessel changes with elastic fiber disruption leading to hypertension.14
Animal studies also show chronic sleep fragmentation and arousals impose pathological consequences on wake neurons with potential permanent damage,15 which may account for persistent EDS, in adequately treated patients with OSA.16 Chronic sleep fragmentation increases plasma IL-6, recruits inflammatory cells and activates nicotinamide adenine dinucleotide phosphate oxidase 2 contributing to increased proliferation and differentiation of adipocyte progenitors and eventually obesity.17 Evidence is accumulating that sleep fragmentation is associated with gut dysbiosis leading to a number of adverse consequences attributed to OSA such as hypertension, insulin resistance18 and EDS.
Lastly, in the exploratory analysis of the longitudinal Multi-Ethnic Study of Atherosclerosis involving 672 participants, higher arousal index was associated with greater decline in leukocyte telomere length which could accelerate biological aging.19
Based on the aforementioned animal and human data, we believe that using arousals alone to define hypopnea is a necessary way to capture the full picture of OSA and its consequences. The best means to promote appropriate treatment for all patients with OSA is to advocate for change in current reimbursement policies. Nearly 20 years ago, the AASM and the National Association for the Medical Direction of Respiratory Care brought the SHHS data to the attention of CMS and were able to provide recognition of hypopneas in OSA. This changed reimbursement policy for CMS and other private insurers guaranteeing treatment for OSA. It is time to return to the table and lobby for needed treatment of the “OSA arousal syndrome.” Of course the late Christian Guilleminault realized the clinical importance of treating arousals alone in sleep patients and astutely brought this forever to the attention of the sleep community back in 1993 as the upper airway resistance syndrome.20
We congratulate Budhiraja and colleagues for incorporating arousals in this systematic study involving 6,131 participants. Their study, however, leaves us with 4 important questions, some could be answered from reanalysis of their data:
-
1.
Are arousals by themselves without including any desaturation metrics associated with observed hypertension or, is it the interaction of both arousals and desaturation events that leads to hypertension?
-
2.
Using a minimum of 3% de facto includes ≥ 4% desaturation. Is the association with hypertension and desaturation driven by 3%, or 4% and beyond?
-
3.
We suggest the authors report if arousals were associated with EDS, and if in the subset with EDS, blood pressure alterations were most significant.
-
4.
Are there any other cardiometabolic consequences of OSA beyond hypertension that could be driven by lowering desaturation threshold to minimum of 3%?
We conclude that OSA is associated with a number of cardiometabolic consequences and must be accurately diagnosed and appropriately treated.21
DISCLOSURE STATEMENT
Shahrokh Javaheri reports that one of the authors of the article on which this article comments is his daughter. Peter Gay reports no conflicts of interest.
REFERENCES
- 1.Budhiraja R, Javaheri S, Parthasarathy S, Berry RA, Quan SF. The association between obstructive sleep apnea characterized by a minimum 3 percent oxygen desaturation or arousal hypopnea definition and hypertension. J Clin Sleep Med. 2019;15(9):1261–1270. doi: 10.5664/jcsm.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhotra RK, Kirsch DB, Kristo DA, et al. Polysomnography for obstructive sleep apnea should include arousal-based scoring: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2018;14(7):1245–1247. doi: 10.5664/jcsm.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 4.Koch H, Schneider LD, Finn LA, et al. Breathing disturbances without hypoxia are associated with objective sleepiness in sleep apnea. Sleep. 2017;40(11) doi: 10.1093/sleep/zsx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roehrs T, Zorick F, Wittig R, Conway W, Roth T. Predictors of objective level of daytime sleepiness in patients with sleep-related breathing disorders. Chest. 1989;95(6):1202–1206. doi: 10.1378/chest.95.6.1202. [DOI] [PubMed] [Google Scholar]
- 6.Stepanski EJ. The effect of sleep fragmentation on daytime function. Sleep. 2002;25(3):268–276. doi: 10.1093/sleep/25.3.268. [DOI] [PubMed] [Google Scholar]
- 7.Colt HG, Haas H, Rich GB. Hypoxemia vs sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea. Chest. 1991;100(6):1542–1548. doi: 10.1378/chest.100.6.1542. [DOI] [PubMed] [Google Scholar]
- 8.Davies RJ, Belt PJ, Roberts SJ, Ali NJ, Stradling JR. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993;74(3):1123–1130. doi: 10.1152/jappl.1993.74.3.1123. [DOI] [PubMed] [Google Scholar]
- 9.O’Driscoll DM, Kostikas K, Simonds AK, Morrell MJ. Occlusion of the upper airway does not augment the cardiovascular response to arousal from sleep in humans. J Appl Physiol. 2005;98(4):1349–1355. doi: 10.1152/japplphysiol.00706.2004. [DOI] [PubMed] [Google Scholar]
- 10.Sulit L, Storfer-Isser A, Kirchner HL, et al. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29(6):777–783. doi: 10.1093/sleep/29.6.777. [DOI] [PubMed] [Google Scholar]
- 11.Schneider H, Schaub CD, Chen CA, et al. Effects of arousal and sleep state on systemic and pulmonary hemodynamics in obstructive apnea. J Appl Physiol. 2000;88(3):1084–1092. doi: 10.1152/jappl.2000.88.3.1084. [DOI] [PubMed] [Google Scholar]
- 12.Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol. 1995;79(1):151–162. doi: 10.1152/jappl.1995.79.1.151. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Panossian LA, Zhang J, et al. Effects of chronic sleep fragmentation on wake-active neurons and the hypercapnic arousal response. Sleep. 2014;37(1):51–64. doi: 10.5665/sleep.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carreras A, Zhang SX, Peris E, et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep. 2014;37(11):1817–1824. doi: 10.5665/sleep.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Fenik P, Zhan G, et al. Degeneration in arousal neurons in chronic sleep disruption modeling sleep apnea. Front Neurol. 2015;6:109. doi: 10.3389/fneur.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalyfa A, Wang Y, Zhang SX, et al. Sleep fragmentation in mice induces nicotinamide adenine dinucleotide phosphate oxidase 2-dependent mobilization, proliferation, and differentiation of adipocyte progenitors in visceral white adipose tissue. Sleep. 2014;37(5):999–1009. doi: 10.5665/sleep.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farré N, Ramon Farré R, Gozal D. Sleep apnea morbidity a consequence of microbial-immune cross-talk? Chest. 2018;154(4):754–759. doi: 10.1016/j.chest.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Carroll JE, Irwin MR, Seeman TE, et al. Obstructive sleep apnea, nighttime arousals, and leukocyte telomere length: the Multi-Ethnic Study of Atherosclerosis. Sleep. 2019;42(7) doi: 10.1093/sleep/zsz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of daytime sleepiness: the upper airway resistance syndrome. Chest. 1993;104(3):781–787. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 21.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]


