Abstract
Study Objectives:
To determine the effect of continuous positive airway pressure (CPAP) treatment on health-related quality of life (HRQoL) in adults with coronary artery disease (CAD) and nonsleepy obstructive sleep apnea (OSA).
Methods:
This was a secondary outcome analysis of the RICCADSA trial, conducted in Sweden between 2005 and 2013. Adults with CAD, nonsleepy OSA (apnea-hypopnea index [AHI] ≥ 15 events/h; Epworth Sleepiness Scale [ESS] score < 10) and complete Short-Form (SF)-36 questionnaires at baseline and after 12 months were included. Patients were randomized to CPAP (n = 102) or no CPAP (n = 104). The primary outcome was the between-group difference in absolute change in the SF-36 components. Within-group changes as well as variables associated with absolute change in the domains in the entire population were also tested.
Results:
Mean SF-36 scores were similar at baseline, ranging from 44.9 ± 9.6 to 92.2 ± 15.8 in various domains, and between-group changes from baseline were not statistically significant at 1 year. There was a significant increase in Role physical, Vitality, Role emotional, Mental health and Mental Component Summary (MCS), and a decrease in Bodily pain and General health scores in the CPAP group. The change in Physical Component Summary (PCS) was determined by female sex (beta coefficient −0.19, 95% confidence interval [CI] −7.25 to −0.98, P = .010), baseline AHI (beta coefficient −0.19, 95% CI −0.21 to −0.03, P = .009), CPAP use (h/night) (beta coefficient −0.16, 95% CI −0.93 to −0.06, P = .028), and acute myocardial infarction at baseline (beta coefficient 0.18, 95% CI 0.59 to 5.19, P = .014). Determinants of the change in MCS from baseline were change in the ESS score (beta coefficient −0.14, 95% CI −0.87 to −0.01, P = .054) and change in the Zung Self-rated Depression Scale scores (beta coefficient −0.33, 95% CI −0.58 to −0.24, P < .001).
Conclusions:
Assignment to CPAP treatment compared to no CPAP had no significant effect on HRQoL as measured by the SF-36 in adults with CAD and nonsleepy OSA. Although several components of the SF-36 scores were improved within the CPAP group, CPAP use was associated with a decrease in PCS. The improvement in MCS was determined by the improvement in daytime sleepiness and depressive mood.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Identifier: NCT00519597
Citation:
Wallström S, Balcan B, Thunström E, Wolf A, Peker Y. CPAP and health-related quality of life in adults with coronary artery disease and nonsleepy obstructive sleep apnea in the RICCADSA trial. J Clin Sleep Med. 2019;15(9):1311–1320.
Keywords: cardiovascular disease, health-related quality of life, SF-36, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Health-related quality of life is impaired in patients with coronary artery disease as well as in those with obstructive sleep apnea (OSA) with daytime sleepiness. Although treatment with continuous positive airway pressure (CPAP) improves health-related quality of life in patients with sleepy OSA phenotype, its effect in nonsleepy phenotype is uncertain.
Study Impact: This study showed that assignment to CPAP treatment compared to no CPAP had no significant effect on health-related quality of life as measured by the Short-Form (SF)-36 questionnaires in nonsleepy patients with OSA. Although several components of the SF-36 scores were improved within the CPAP group, CPAP use (hours/night/all nights) was associated with a decrease in physical component summary. The improvement in mental component summary was determined by improvement in the daytime sleepiness and depressive mood.
BACKGROUND
Coronary artery disease (CAD) is a common health problem in Western countries and has a poor prognosis and a high risk of mortality.1 Obstructive sleep apnea (OSA) is a common concurrent condition in patients with CAD,2 causing episodic hypoxemia and nocturnal sympathetic nervous system activation,3 elevated blood pressure,4 and markers of oxidative stress, inflammation, and hypercoagulation.5,6 OSA affects daily life with sleep deprivation, which may result in decreased energy, impaired cognition, altered mood,7 and increased risk of traffic accidents.8 These daytime consequences are often more important to patients than the nocturnal events that they may not be aware of.9 Patients with OSA and excessive daytime sleepiness (EDS) have reduced health-related quality of life (HRQoL) compared to the general population.10 Several studies have found that symptoms of OSA, especially EDS, have higher negative correlation with HRQoL than disease severity.11,12 Continuous positive airway pressure (CPAP), which eliminates obstructive apneas and hypopneas, is the first-line treatment for OSA.13 CPAP has, in observational sleep clinic cohort studies, been associated with decreased disease-specific mortality and complications,14,15 as well as reduced daytime sleepiness and increased HRQoL in randomized controlled trials (RCTs).13,16,17 CPAP has been shown to be most beneficial if used for at least 4 hours per night 70% of the nights, which equals 2.8 hours per night of adjusted use.18 There are, however, controversies regarding cutoff values for CPAP concordance,16,17 and conflicting evidence regarding the benefit of CPAP in patients with nonsleepy OSA phenotype, especially in clinical cohorts with cardiovascular disease.19–22
The Randomized Intervention with CPAP in CAD and OSA (RICCADSA) trial was designed to address the effect of CPAP on the composite of repeat revascularization, myocardial infarction, stroke, and cardiovascular mortality in patients with CAD and nonsleepy OSA.23 The aim of the current study was to investigate the 12-month effect of CPAP on HRQoL in patients with CAD and nonsleepy OSA.
METHODS
Study Design and Oversight
The current study was a secondary outcome analysis of the RICCADSA trial, which was a single-center (two sites), prospective, open, randomized, parallel, interventional, superiority trial of CPAP in patients with CAD and OSA, with its rationale including the ethical concerns for patients not receiving treatment as well as the methodological details that have been previously published.23–25 The study protocol was approved by the Regional Ethical Review Board in Gothenburg (approval no. 207-05; 09.13.2005; amendment T744-10; 11.26.2010; amendment T512-11; 06.16.2011), and all patients provided written informed consent. The trial was registered with the national researchweb.org (FoU i Sverige – Research and development in Sweden; No. VGSKAS-4731; 04.29.2005) and with ClinicalTrials.gov (NCT 00519597).
Study Participants and Settings
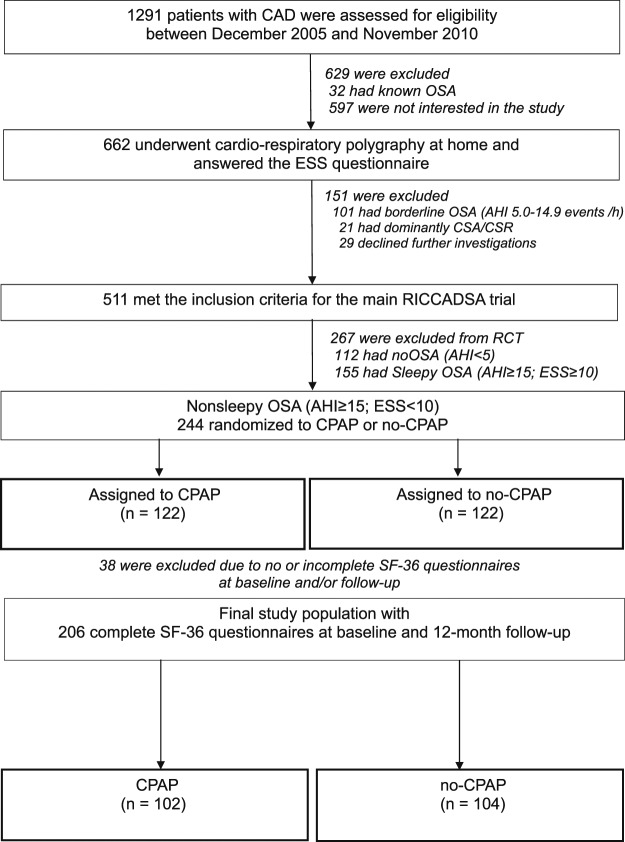
Patients were recruited from Skaraborg County, West Sweden between December 2005 and November 2010 with follow-up completed in May 2013. Inclusion criteria for the RCT arm were (1) angiography-verified CAD; (2) had undergone percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) in the previous 6 months; (3) had an apnea-hypopnea index (AHI) ≥ 15 events/h on the baseline cardiorespiratory polygraphy (CRPG); and (4) Epworth Sleepiness Scale (ESS) score < 10. Exclusion criteria for the RCT arm were (1) an already known OSA diagnosis; (2) an AHI 5.0–14.9 events/h; and (3) predominantly central apneas with Cheyne-Stokes respiration. Patients with CAD and sleepy OSA phenotype (AHI ≥ 15 events/h; ESS score ≥ 10) and patients with no OSA (AHI < 5 events/h) were followed in the observational arm (Figure 1).
Figure 1. Flow of study participants.
AHI = apnea-hypopnea index, CAD = coronary artery disease, CPAP = continuous positive airway pressure, CSA-CSR = central sleep apnea-Cheyne Stokes respiration, ESS = Epworth Sleepiness Scale, OSA = obstructive sleep apnea, RICCADSA = Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnea, SF-36 = Short Form-36 Health Survey.
Cardiorespiratory Polygraphy
The portable CRPG was performed with the Embletta Portable Digital System device (Embla, Broomfield, Colorado, USA). As described in detail previously,23–25 the CRPG system included a nasal pressure detector, two respiratory belts for thoracoabdominal movements and body position, and finger pulse oximetry for detecting heart rate and oxyhemoglobin saturation (SpO2). An apnea was defined as at least 90% cessation of airflow; hypopnea was defined as having at least 50% of reduction in thoracoabdominal movement and/or in nasal pressure amplitude for at least 10 seconds regardless of reduction in SpO2, or events with a clear reduction in thoracoabdominal movement and/or in the nasal pressure amplitude for at least 10 seconds with an at least 4% reduction in SpO2.26
Randomization and Intervention
In total, 1,259 consecutive patients were eligible for the main RICCADSA trial and 662 (52.7%) agreed to participate. The design and primary outcomes of the trial have been reported elsewhere.24 Diagnostic CRPG was performed at home after an average of 63 days following mechanical revascularization (median 59, interquartile range 42–78). Patients fulfilling the inclusion criteria for the RCT arm (n = 244), or the observational arm (n = 267) underwent baseline investigations on average 35 days (median 30, interquartile range 20–45) after home sleep recordings.
For the RCT arm, the patients were randomized to CPAP or no CPAP. The 1:1 randomization was scheduled with a block size of eight patients (four CPAP, four control patients) stratified by sex and revascularization type (PCI/CABG). All patients allocated to CPAP were fitted with an automatic CPAP device (S8 or S9; ResMed, San Diego, CA) by trained staff. For the current study 38 patients had to be excluded because of no SF-36 data at either baseline or the 1-year follow up. The remaining 206 patients were included in the study (Figure 1).
Study Measurements and Data Collection
Data regarding HRQoL were collected with the Short Form-36 Health Survey (SF-36), which measures eight different domains (Physical functioning, Role physical, Bodily pain, General health, Vitality, Social functioning, Role emotional, Mental health) and two summary scores (Physical Component Summary [PCS], Mental Component Summary [MCS]).27,28 The SF-36 is a generic instrument and assesses health applicable to everyone’s functional status and well-being referring to the past 4 weeks at the time of the survey. Domain and summary component scores range from 0 to 100; higher scores correspond to better health status or well-being. The SF-36 is one of the most widely used patient-reported outcome measurements and allows comparisons between patients with different disease as well as general populations.28,29 The Swedish translation of SF-36 has been validated and found reliable.29
All participants were requested to complete the Zung Self-rating Depression Scale (SDS) questionnaire, at baseline, and at follow-ups. The Zung SDS is a widely accepted questionnaire that provides both a total score and a categorical rate of depression.30 In summary, 20 items are included, and the rating scale is scored from 1 to 4 points, resulting in a real total score from 20 to 80. The real score is multiplied by 1.25, resulting in a total range of 25 to 100. A higher SDS score represents more depression. The details of the questionnaire and the scoring manual as well as effect of CPAP on depressive mood assessed by the Zung SDS questionnaire in the RCT and observational arms of the RICCADSA cohort have recently been published.31,32
For discriminating sleepiness, the ESS33,34 was used. The ESS is an eight-item patient-reported outcomes measure, wherein the patients assess the likelihood that they would experience daytime sleepiness in eight everyday life situations. Answers range from 0 = would never doze off to 3 = high chance of dozing off, and a total score is obtained by a sum score of all eight answers. The instrument is a reliable and valid measure to discriminate between people with nonsleepy and sleepy OSA in sleep clinic cohorts.
Questionnaires for the current study were collected at inclusion and at 1-year follow-up. Data regarding background variables were obtained from patients’ medical records and, when necessary, from the Swedish Hospital Discharge Register and the Swedish National Cause of Death Registry.
Adherence to CPAP
Participants randomized to CPAP brought their devices to the clinic at each scheduled follow-up visit, and CPAP hours per night and CPAP days per period were downloaded from the device, and CPAP use was calculated by dividing total use by number of days during the follow up period. All technical adjustments were done according to the clinical routines by the sleep medicine unit staff.
Outcomes and the Sample Size
The main outcome of the current protocol, which was one of the secondary endpoints of the RICCADSA trial, was the between-group difference in absolute change in SF-36 domains compared from baseline. The sample size estimation for the RICCADSA trial was based on the estimates for the primary endpoints, and no specific power estimate was done for the current protocol.
Statistical Analysis
Data are shown as mean ± standard deviation or standard error of the mean for continuous variables, and categorical variables are represented as numbers (percentages). An independent sampled t test was used for between-group differences in means, and the chi-square test (or when appropriate, Fisher exact test) was used to compare categorical variables. Within-group changes were evaluated with paired t test. Post hoc power of the significant results was computed by using G*Power 3.1 online free software based on the sample size, two-tailed test, alpha level, and observed effect size (Cohen d).35 Likewise, a linear regression analysis was used to determine variables associated with absolute change in scores of the different domains of the SF-36, in which a stepwise backward model was used (variables with P > .10 removed). The following variables were entered into the model: age, sex, body mass index, AHI, hypertension, diabetes mellitus, acute myocardial infarction (AMI) at baseline, left ventricular ejection fraction, intervention type (PCI versus CABG), CPAP use (mean CPAP hours per night adjusted for proportion of days CPAP was used over the follow-up period), absolute change in ESS scores as well as in Zung SDS scores between baseline and 1-year follow-up. In order to address a possible collinearity among the independent variables included in the regression analysis, Pearson correlation as well as Variance Inflation Factor (VIF) was tested, and a VIF value > 5 was considered to be positive for collinearity. All statistical tests were two-sided, a value of P < .05 was considered significant, and beta-coefficients with 95% confidence intervals were calculated. Statistical analysis was performed using SPSS 22 (SPSS Inc., Chicago, IL).
RESULTS
Patient Population and Baseline Characteristics
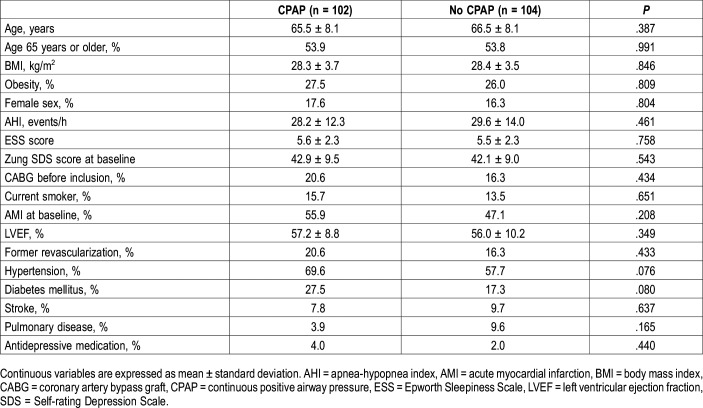
As shown in Figure 1, we included 206 patients in the study, allocated to two groups: (1) adults with CAD and nonsleepy OSA on CPAP (n = 102) and (2) adults with CAD and nonsleepy OSA without CPAP (n = 104). There were no significant differences regarding demographic or baseline medical characteristics between the groups (Table 1). Most patients were male, age 65 years or older, and the mean ESS score was low (5.6 versus 5.5). In both groups, approximately half of all patients had an AMI at baseline before the sleep study (56% versus 47%). Hypertension and diabetes mellitus were more common in the group with CPAP (70% versus 58% and 28% versus 17%, respectively).
Table 1.
Baseline characteristics of the entire study population with coronary artery disease and nonsleepy obstructive sleep apnea (n = 206).
CPAP Adherence
In the CPAP group, 30 (29.4%) stopped using the device within 12 months. In the no CPAP group, three patients (2.9%) had crossed over to the CPAP arm within the follow-up period. Among 72 participants remaining on CPAP, 55 (76.4%) were using the device at least 3 h/night for all nights, 46 (63.9%) at least 4 h/night for all nights, and 34 (47.2%) at least 5 h/night for all nights, respectively. Average CPAP use was 4.5 ± 2.3 h/night for all nights.
Outcomes
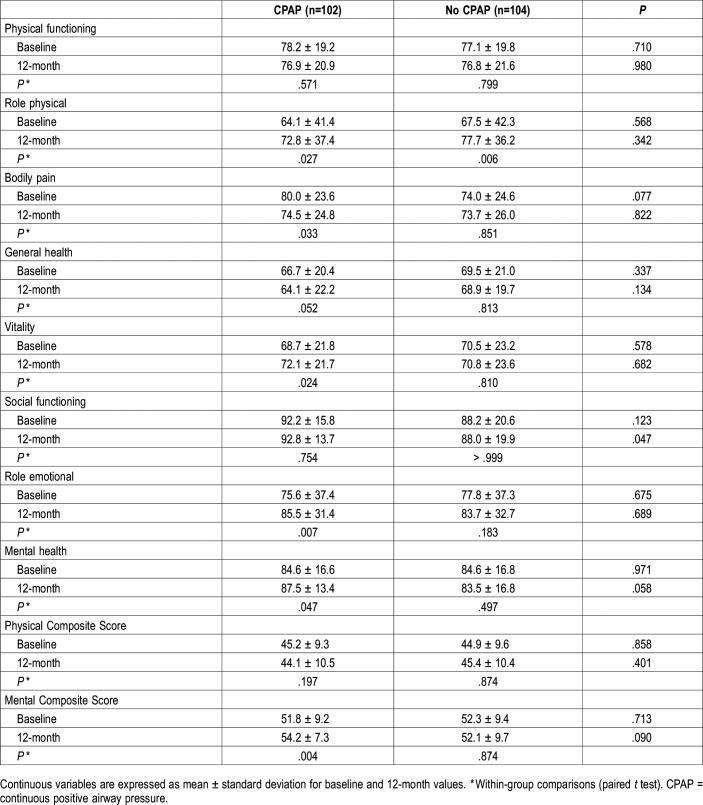
The analysis showed no significant differences between the groups in any of the SF-36 domains or summary scores at baseline in the entire study population (Table 2; Figure 2A). Regarding the within-group changes, the CPAP arm had significantly higher scores in four of eight dimensions (Role physical, Vitality, Role emotional and Mental health) as well as in the MCS scores, whereas there was a decrease in Bodily pain and General health scores compared to baseline. The no CPAP group showed significant increase only in the Role physical domain (Table 2; Figure 2A). Post hoc power of the test, given the sample size, two-tailed test, alpha level, and observed effect size, was 36% for PCS and 89% for MCS in the CPAP group.
Table 2.
Baseline and 12-month health-related quality of life status in patients with coronary artery disease and nonsleepy obstructive sleep apnea.
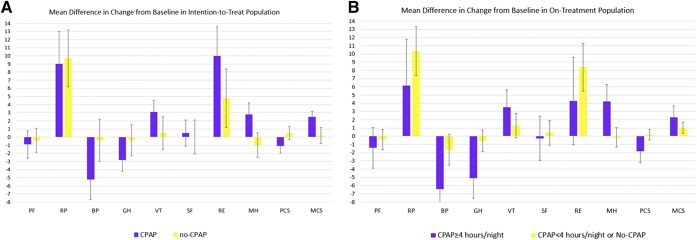
Figure 2. Mean differences in change from baseline with standard error of means in subdomains of the SF-36 questionnaires in intention-to-treat population.
(A) Differences between treatment groups. (B) Difference between CPAP adherence. BP = Bodily pain, CPAP = continuous positive airway pressure, GH = General health, MCS = Mental Composite Score, MH = Mental health, PCS = Physical Composite Score, PF = Physical functioning, RE = Role emotional, RF = Role physical, SF = Social functioning, SF-36 = Short Form-36 Health Survey, VT = Vitality.
In a secondary analysis, the magnitude of changes from baseline did not change significantly among the subgroup of patients using the device ≥ 4 h/night compared with patients with less adherence or no CPAP (Figure 2B).
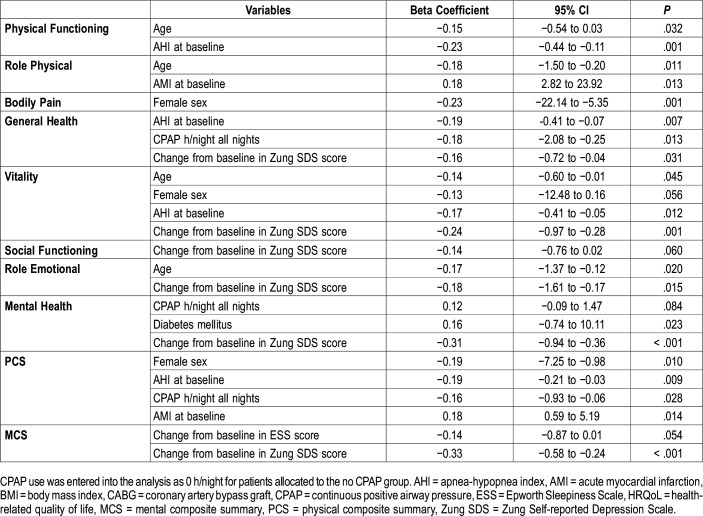
When addressing the determinants of the change in the SF-36 domains in the whole group in linear regression analysis, CPAP use, baseline AHI and change in the Zung SDS score were negatively correlated with an increase in the General health score at the 12-month follow-up (Table 3). The variables that were negatively correlated with an increase in the PCS in the multivariate model were female sex, AHI at baseline, and CPAP use whereas AMI at baseline before the sleep study showed a positive relationship. There was a significant but weak positive relationship between CPAP use and Mental health scores (r = .19; P = .006) but not with ESS in this nonsleepy CAD cohort. No collinearity was found between the independent continuous variables (all VIF values ranging between 1.00–1.06). Increase in MCS was determined by improvement in the ESS and the Zung SDS scores (Table 3).
Table 3.
Variables associated with change in the health-related quality of life component scores at 12-month follow-up in a multivariate linear regression analysis in the whole population.
DISCUSSION
The current study demonstrated that 12 months of CPAP treatment, compared to no CPAP, had no significant effect on HRQoL in this cohort with CAD and nonsleepy OSA, whereas there were improvements within the CPAP group. CPAP use (h/night all nights) was weakly correlated with a decline in the PCS score and increase in the MCS score. Other correlates of decline in the PCS score were AHI at baseline and female sex. The increase in the MCS was determined by the improvement in the ESS and the Zung SDS scores.
To our knowledge, this is one of the first studies investigating the effect of CPAP on HRQoL as measured by the SF-36 in adults with CAD and nonsleepy OSA. As extensively summarized in a recent meta-analysis of effects of positive airway pressure treatment on several outcomes in patients with OSA (American Academy of Sleep Medicine Task Force report),36 previous studies on HRQoL outcomes were mainly focused on the sleep clinic cohorts, more specifically on patients with EDS. Only a few of those studies addressed the question in adults with CAD: In the SAVE trial, comprising 2,410 patients with CAD and cerebrovascular disease, CPAP treatment was associated with an improvement in mean PCS (from 45.4 ± 7.7 to 46.9 ± 8.0 at the end of the study), and in mean MCS (from 52.6 ± 8.6 to 53.6 ± 8.0).22 Given the minor magnitude of the change, the clinical significance of this finding in the SAVE trial could be regarded as minimal. Moreover, patients with mild sleepiness (ESS 10–15) were also included (approximately 20% of the entire population) in that trial, leaving the confounding effect of the reduction in ESS on change in HRQoL unanswered. Another study addressed the effect of nocturnal supplemental oxygen and CPAP on HRQoL compared with healthy lifestyle education in patients with OSA with CAD or at least three risk factors for CAD37 and showed a significant increase in MCS (from 48.9 ± 11.0 to 52.6 ± 10.0) in the CPAP group after 12 weeks. Though we found significant improvements in Mental health and MCS scores within the CPAP group, this change was determined by the decline in the ESS and Zung SDS scores in the entire population. This is in concordance with numerous studies that have shown the benefits of CPAP on HRQoL in patients with primarily sleepy OSA.36–39 Of note, our entire study population were “nonsleepy,” based on the cutoff level of 10 in the ESS score. This indicates that lowering of ESS even under the suggested cutoff levels might be important in order to improve mental health in cardiac populations.
However, mixed results regarding the physical components of SF-36 were observed in the current study. Although no significant between-group difference in change in PCS score was observed, both the CPAP and no CPAP arms showed an increase in the Role physical domain scores, and this was the only domain showing an increase within the no CPAP group. The multivariate analysis showed that the increase in the Role physical score was determined by age and AMI at baseline, so there was no relationship with CPAP allocation, and this increase might be a reflection of a natural improvement after revascularization in a cardiac population with AMI at baseline. Interestingly, there were significant declines in the Bodily pain and General health scores in the CPAP arm. One explanation for Bodily pain might be the fact that the CPAP group had higher scores at the baseline, and the levels decreased to similar levels among those in the no CPAP group at 12-month follow-up. Moreover, these changes seemed to be sex dependent in the multivariate analysis. Regarding the General health component, the magnitude of the decline was small, and was determined by CPAP use as well as AHI at baseline and increase in the Zung SDS score. On the one hand, it might also be argued that prescription of CPAP device to an asymptomatic patient increases illness perception. On the other hand, a patient with increased illness perception might be more prone to use the CPAP device with expectation of medical improvement. Of note, almost one-third of the patients in the CPAP arm stopped using the device within 12 months, and of those who continued using the device, the average use was 4.5 h/night. Studies have shown that the thresholds for mean duration of overnight CPAP use in achieving improvement were 4 hours for ESS, 6 hours for Multiple Sleep Latency Test, and 7.5 hours for Functional Outcome of Sleep Questionnaire scores.40 Given that those studies were conducted in sleep clinic cohorts in patients with symptomatic OSA, maximal treatment effect was not possible to realize in the current CAD population with nonsleepy OSA. Thus, the lack of significant between-group differences in results might have been related to the lack of adequate use.
Many patients with CAD and OSA do not experience daytime sleepiness, and it has been suggested that patients with nonsleepy OSA have poor CPAP concordance because they do not experience self-reported benefits from therapy.34,41 In order to achieve optimal benefit, it might be important to investigate how CPAP works in the life of each individual patient through follow-up visits. It is important to listen to each patient’s narrative in order to find out how the CPAP works for them in their daily life and, if necessary, how the use of CPAP can be adapted to suit the patient. Even if noninvasive, CPAP treatment affects routines and becomes a reminder of an illness. Partnership is therefore pivotal to increase the chance of optimizing treatment outcome as well as concordance. This can be achieved through a more person-centered care approach. In person-centered care, the illness narrative, which include symptoms and side effects, lays a foundation for planning the care as a partnership between the patient and professionals.42,43
The current study has a number of limitations that need to be taken into consideration. First, the power estimate for the RCT population was conducted for the primary outcome (composite of repeat revascularization, myocardial infarction, stroke, and cardiovascular mortality), and not for the secondary outcomes addressed in the current study. However, post hoc calculations showed high power (89%) for MCS whereas the power was low (36%) for PCS in the CPAP group. Of note, there is yet no consensus regarding how much change in SF-36 domains should be considered as minimum clinically important difference (MCID) in adults with CAD and concomitant OSA. Though three points of change in PCS and MCS were suggested as MCID, respectively, according to Ware et al,44 the studies summarized in the recent meta-analysis36 failed to show such a significant effect of CPAP treatment. In the current study, we found an average of 2.5 points of increase in the MCS score among the patients with CAD treated with CPAP who had nonsleepy OSA. Second, randomization was not stratified by comorbidities, which have led to a higher proportion of patients with AMI, diabetes mellitus, and hypertension in the CPAP group. However, this limitation was minimized by including these factors in the model for statistical adjustments. Third, no sham CPAP was used in the study, which might influence the assessment of HRQoL. However, there is no true sham CPAP or other appropriate placebo for CPAP in a long-term trial in patients with cardiovascular disease. As previously argued, it is also possible that sham CPAP consisting of a mask attached to tubing, but without pressure application, would worsen sleep disturbance and act as a “negative placebo.”45 Fourth, CPAP usage data were downloaded at each follow-up period for the main trial, that is, after 3 months, 6 months, 12 months, and yearly up to 6 years, so we did not save the metrics separately for the month prior to completing the questionnaire. Thus, the participant’s CPAP use during the last month prior to the 12-month period would possibly better reflect the relationship between CPAP usage and HRQoL. Fifth, our results are limited to the SF-36 survey, and it is possible that another HRQoL questionnaire may have produced different results. Finally, the age distribution of the cohort is not typical for the OSA population, and thus, our results are not generalizable to younger sleep clinic cohorts with symptomatic OSA. Notwithstanding the limitations, the secondary outcome analysis of the current study gives important insight into how HRQoL is affected by CPAP in patients with CAD and nonsleepy OSA.
CONCLUSIONS
To conclude, assignment to CPAP treatment, compared to no CPAP, had no significant impact on HRQoL as measured by the SF-36 in adults with CAD and nonsleepy OSA. Although several components of the SF-36 scores were improved within the CPAP group, CPAP use was associated with a decrease in PCS. In the whole population, other determinants of decrease in PCS were female sex and baseline AHI, whereas the increase in MCS scores was determined by decrease in the scores of daytime sleepiness and depressive mood.
DISCLOSURE STATEMENT
All authors approved this manuscript in its final form. This study was supported by grants from the Swedish Research Council (521-2011-537 and 521-2013-3439); the Swedish Heart-Lung Foundation (20080592, 20090708 and 20100664); the “Agreement concerning research and education of doctors” of Västra Götalandsregionen (ALFGBG-11538 and ALFGBG-150801), research fund at Skaraborg Hospital (VGSKAS-4731, VGSKAS-5908, VGSKAS-9134, VGSKAS-14781, VGSKAS-40271 and VGSKAS-116431); Skaraborg Research and Development Council (VGFOUSKB-46371); the Heart Foundation of Kärnsjukhuset; ResMed Foundation; and ResMed Ltd. ResMed Sweden provided some of the sleep recording devices and technical support. None of the funders had any direct influence on the design of the study, the analysis of the data, the data collection, drafting of the manuscript, or the decision to publish. SW, AW, and BB report no conflicts of interest. ET received consultant fees from ResMed and Pfizer. YP received institutional grants from ResMed for the current work, and consultant fees from BresoTec, and lecture fees from ResMed and Philips-Respironics outside the current work.
ACKNOWLEDGEMENTS
Author contributions: Conception and design: YP. Analysis and interpretation: SW, ET, BB, AW, YP. Drafting the manuscript for important intellectual content: SW, ET, BB, AW, YP.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AMI
acute myocardial infarction
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CI
confidence interval
- CPAP
continuous positive airway pressure
- CRPG
cardiorespiratory polygraphy
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- HRQoL
health/related quality of life
- MCS
Mental Component Summary
- OSA
obstructive sleep apnea
- PCS
Physical Component Summary
- PCI
percutaneous coronary intervention
- RCT
randomized controlled trial
- RICCADSA
Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea
- SDS
Self-rating Depression Scale
- SF-36
Short Form-36 Health Survey
REFERENCES
- 1.Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedner J, Franklin KA, Peker Y. Coronary Artery Disease and Sleep Apnea. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 5th ed. St Louis, MO: Elsevier Saunders; 2011:1393–1399. [Google Scholar]
- 3.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia–revisited–the bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Phillips CL, McEwen BJ, Morel-Kopp MC, et al. Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea: a randomised, placebo-controlled crossover study. Thorax. 2012;67(7):639–644. doi: 10.1136/thoraxjnl-2011-200874. [DOI] [PubMed] [Google Scholar]
- 7.Soldatos CR, Paparrigopoulos TJ. Sleep physiology and pathology: pertinence to psychiatry. Int Rev Psychiatry. 2005;17(4):213–228. doi: 10.1080/09540260500104565. [DOI] [PubMed] [Google Scholar]
- 8.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27(3):453–458. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 9.Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118(2):372–379. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- 10.Björnsdottir E, Janson C, Gislason T, et al. Insomnia in untreated sleep apnea patients compared to controls. J Sleep Res. 2012;21(2):131–138. doi: 10.1111/j.1365-2869.2011.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldwin CM, Griffith KA, Nieto FJ, O’Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24(1):96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 12.Silva GE, An MW, Goodwin JL, et al. Longitudinal evaluation of sleep-disordered breathing and sleep symptoms with change in quality of life: the Sleep Heart Health Study (SHHS) Sleep. 2009;32(8):1049–1057. doi: 10.1093/sleep/32.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53(5):341–345. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 15.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 16.Peker Y. Growing research evidence for continuous positive airway pressure treatment for sleepy patients with milder obstructive sleep apnea. Am J Respir Crit Care Med. 2012;186(7):583–584. doi: 10.1164/rccm.201208-1400ED. [DOI] [PubMed] [Google Scholar]
- 17.Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186(7):677–683. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65(9):829–832. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 19.Barbé F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001;134(11):1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 20.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27(6):1229–1235. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 21.Durán-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 22.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 23.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 24.Peker Y, Glantz H, Thunstrom E, Kallryd A, Herlitz J, Ejdeback J. Rationale and design of the randomized intervention with CPAP in coronary artery disease and sleep apnoea–RICCADSA trial. Scand Cardiovasc J. 2009;43(1):24–31. doi: 10.1080/14017430802276106. [DOI] [PubMed] [Google Scholar]
- 25.Glantz H, Thunstrom E, Herlitz J, et al. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann Am Thorac Soc. 2013;10(4):350–356. doi: 10.1513/AnnalsATS.201211-106OC. [DOI] [PubMed] [Google Scholar]
- 26.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine task force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 27.Ware JE., Jr SF-36 health survey update. Spine. 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Kosinski M, Dewey JE, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric Inc.; 2000; [Google Scholar]
- 29.Sullivan M, Karlsson J, Ware JE., Jr The Swedish SF-36 Health Survey–I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41(10):1349–1358. doi: 10.1016/0277-9536(95)00125-q. [DOI] [PubMed] [Google Scholar]
- 30.Zung WWA. A self-rating depression scale. Arch Gen Psychiatry. 1965;12(1):63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 31.Balcan B, Thunström E, Strollo PJ, Jr, Peker Y. Continuous positive airway pressure treatment and depression in adults with coronary artery disease and nonsleepy obstructive sleep apnea. A secondary analysis of the RICCADSA trial. Ann Am Thorac Soc. 2019;16(1):62–70. doi: 10.1513/AnnalsATS.201803-174OC. [DOI] [PubMed] [Google Scholar]
- 32.Balcan B, Thunström E, Strollo PJ, Peker Y. Determinants of depressive mood in coronary artery disease patients with obstructive sleep apnea and response to continuous positive airway pressure treatment in non‐sleepy and sleepy phenotypes in the RICCADSA cohort. J Sleep Res. 2018;28(4):e12818. doi: 10.1111/jsr.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Luyster FS, Strollo PJ, Jr, Thunstrom E, Peker Y. Long-term use of continuous positive airway pressure therapy in coronary artery disease patients with nonsleepy obstructive sleep apnea. Clin Cardiol. 2017;40(12):1297–1302. doi: 10.1002/clc.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ:: L. Erlbaum Associates; 1988; [Google Scholar]
- 36.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15(02):301–334. doi: 10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis EF, Wang R, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: a Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59–67. doi: 10.1016/j.ahj.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batool-Anwar S, Goodwin JL, Kushida CA, et al. Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA) J Sleep Res. 2016;25(6):731–738. doi: 10.1111/jsr.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jing J, Huang T, Cui W, Shen H. Effect on quality of life of continuous positive airway pressure in patients with obstructive sleep apnea syndrome: a meta-analysis. Lung. 2008;186(3):131–144. doi: 10.1007/s00408-008-9079-5. [DOI] [PubMed] [Google Scholar]
- 40.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51(5):434–451. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Wallström S, Ekman I. Person-centred care in clinical assessment. Eur J Cardiovasc Nurs. 2018;17(7):576–579. doi: 10.1177/1474515118758139. [DOI] [PubMed] [Google Scholar]
- 43.Coulter A, Entwistle VA, Eccles A, Ryan S, Shepperd S, Perera R. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev. 2015:Cd010523. doi: 10.1002/14651858.CD010523.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ware JE. User’s Manual for the SF-36v2 Health Survey. Lincoln, RI: Quality Metric; 2007; [Google Scholar]
- 45.Leung RS, Tkacova R, Bradley TD. Obstructive sleep apnoea. Lancet. 1999;354(9185):1212–1213. doi: 10.1016/s0140-6736(05)75424-6. [DOI] [PubMed] [Google Scholar]