Abstract
Mankind has always been fascinated with nature and have heavily explored natural products since the ancient times. Evolution of diseases led to research on synthetic structure, specificity and activity-guided treatment. To combat threats of new developing diseases and the deleterious side effects posed by modern therapy, researchers have once again looked back towards natural resources. Although plants have been the main source of natural drugs, lower fungi are being recently paid attention to. Among them, mushrooms have emerged as an under-explored yet immensely rich resource, especially for bioactive terpenoids. A lot of research is going on around the world with mushroom-derived terpenoids especially their medicinal properties, some of which have even been used in pre- and post-clinical studies. From the literatures that are available, it was found that mushroom terpenoids have activity against a wide range of diseases. In this review, we have summarized different mushroom-derived terpenoids and their therapeutic properties.
Keywords: Diseases, Medicinal mushroom, Natural product, Secondary metabolites
Introduction
Natural products have always played a huge role in human therapeutics since time immemorial. Plants have been the richest source of bioactive components due to their unique and diverse biosynthetic capability (Raskin and Ripoll 2004). The discovery of aspirin in 1897, which was a derivative of salicylic acid, a major component of herbal therapies and penicillin in 1928, which added microbes as a source of therapeutic drugs, revolutionised medicinal research in the ensuing decades. Recently, due to the ever-evolving nature of the diseases, research for therapeutics led to more structure, target-specificity and activity-guided synthetic approach towards medicine (Schmidt et al. 2008). Although this approach seemed apparently beneficial, the targeted synthetic drugs often affected non-targeted parts of the physiological system, directly or indirectly, giving rise to several detrimental side effects some of which also gave rise to newer symptoms and newer diseases. Researchers are thus resorting again to natural sources for drugs.
Exploration of natural sources for remedying modern diseases have led to several reports of bioactive compounds. One of the most important compounds have been bioactive secondary metabolites, especially terpenoids. Terpenoids are organic compounds composed of linked isoprene units, classified as monoterpenes, diterpenes, sesquiterpenes etc. depending on the number of carbon atoms, produced through biosynthetic pathways. Terpenoids have been reported to be therapeutically extremely versatile, with effectiveness against several diseases like microbial (Souza et al. 2011), viral (Lin et al. 2015), neurodegenerative (Yoo and Park 2012) as well as cancer (He et al. 2009; Rabi and Bishayee 2009; Nwodo et al. 2016) and many more.
The therapeutic terpenoids that are available in the market like paclitaxel (Weaver 2014), eleutherobin (Long et al. 1998), sarcodictyin A (Nakao et al. 2003), artemisinin (Krishna et al. 2008), excoecariatoxin (Vidal et al. 2011) etc. have been mostly derived from plants. Another immensely important source of bioactive terpenoids has come to the limelight in the last two decades which are higher basidiomycetes or mushrooms. Even though research on mushroom-derived terpenoids was going on discreetly around the world, it drew attention during the beginning of the twenty first century. There has been a huge surge in exploring mushrooms for therapeutic terpenoids after 2010, which is evident from the frequent scientific papers reporting isolation of new terpenoids. Some of the mushroom terpenoids and their medicinal properties are summarized in Table 1.
Table 1.
Mushroom-derived terpenoinds and their therapeutic potential
| Mushroom | Terpenoid | Activity | References |
|---|---|---|---|
| Ganoderma lucidum | Ganoderic acid | Cytotoxicity against hepatoma cells in vitro | Min et al. (2000) |
| Antihistamine releasing activity in rat mast cells | |||
| Inhibitory activity against angiotensin converting enzyme | |||
| Hepatoprotective activity | |||
| Inhibitory effect on farnesyl protein transferase | |||
| Ganoderic acid C and its derivatives | Inhibit the biosynthesis of cholesterol | Komoda et al. (1989) | |
| Ganoderic acid F | Atherosclerosis protection | Morigiwa et al. (1986) | |
| Triterpenoids | Antioxidative and free radical effects | Rathee et al. (2012) | |
| Ganoderiol, ganodermanontriol, and ganoderic acid | Antiviral activity | El-Mekkawy et al. (1998) | |
| Ganoderma pfeifferi | Lucialdehyde D, ganoderone A and ganoderone C | Antifungal, antibacterial, and antiviral | Niedermeyer et al. (2005) |
| Inonotus obliquus | Trametenolic acid, ergosterol peroxide, 3b-hydroxy-8, 24-dien-21-al, ergosterol and inotodiol | Anti-inflammatory and anticancer activities | Ma et al. (2013) |
| Merulius tremellosus | Sesquiterpenedialdehyde (Merulidial 1) | Antifungal activity | Giannetti and Steglich (1978) |
| Marasmius alliaceus | Sesquiterpenes | Anticancer activity | Anke et al. (1981) |
| Lentinellus omphalodes | Lentinellic acid | Antibacterial activity and inhibit protein synthesis in Ehrlich ascetic carcinomas | Rahi and Malik (2016) |
| Cyathus africanus | Cyathanediterpenes (cyathins D, H, neosarcodonin, cyathatriol, and 11-O-acetylcyathatriol) | Anti-inflammatory properties | Han et al. (2013) |
| Antrodia cinnamomea. | Antcin A | Anti-inflammatory effects | Zhang et al. (2019) |
| Antcin B, methylantcinate B | Anticancer activity | ||
| Antcin H | Hepatoprotective activity | ||
| Dehydroeburicoic acid | Antidiabeticactivity | ||
| Eburicoic acid | Antihypertensive activities | ||
| Sesquiterpene lactone antrocin | Anticancer activity | ||
| Antcin K | Anticancer activity | Ganesan et al. (2019) | |
| Antrolone | Anti-inflammatory | ||
| Inonotus obliquus | Inotolactones (A-C), 6β-hydroxy-trans-dihydroconfertifolin, inotodiol, 3β,22-dihydroxyanosta-7,9(11),24-triene, 3β-hydroxycinnamolide, and 17-hydroxy-ent-atisan-19-oic acid | Antihyperglycemic | Ying et al. (2014) |
Three million species of fungi have been predicted to exist in the world (Hawksworth 2012; Blackwell 2011) among which 140,000 species are predicted to be mushrooms. Only 14,000 mushrooms have been described till date, amounting to 10% of the total mushroom species (Miles and Chang 2004). Current research is unveiling newer and newer terpenoids from mushrooms, many of which have therapeutic potential. However, numerically, there still is a whole ocean yet to be explored in search for medicinal terpenoids from this group. We have thus made an effort to summarize the mushroom-derived terpenoids reported to have therapeutic significance and their therapeutic properties, which might help the researchers in this field to assess and uncover new therapies for human diseases.
Antimicrobial activity
Due to the rapid evolution of bacterial species acquiring resistance against antibiotics, the search for natural compounds with antibacterial properties is rapidly increasing. In recent research, a number of mushroom extracts have been analysed for potent antibacterial compounds (Gazzani et al. 2011; Harikrishnan et al. 2012). Many of the secondary metabolites in mushrooms act as antibiotics and are effective against bacteria and fungi (Gilardoni et al. 2007).
Coprinus sp. has been reported to exhibit activity against multi-drug-resistant Gram-positive bacteria with Coprinol, a cuparane-type terpenoid (Johansson et al. 2001). Antimicrobial activity of wild edible mushrooms has been widely reported (Lai et al. 2012; Giri et al. 2012). Pleuromutilin is a tricyclic diterpenoid isolated from Clitopilus passeckerianus from which retapamulin, an antibiotic has been derived (Nagabushan 2010; Paukner and Riedl 2017). Infections of Staphylococcus aureus are becoming dangerous due to the absence of antibiotics for the rapidly evolving bacteria. Sesquiterpenoids from Ganoderma praelongum have been reported to show potent inhibitory effect (Ameri et al. 2011). Mycobacterium tuberculosis, the causal agent of tuberculosis, is responsible for a large number of deaths worldwide. Nambinone A-D and 1-epinambinone B isolated from Neonothopanus nambi have been shown to be effective antitubercular drugs (Duru and Cayan 2015). A lanostane triterpenoid, astraodoric acid A from Astraeus pteridis and astraodoric acid B from Astraeus odoratus have been found to possess antibacterial properties (Stanikunaite et al. 2008; Arpha et al. 2012). Lanostane triterpenoids ganorbiformins A–G, isolated from Ganoderma orbiforme has also been reported to exhibit antimycobacterial activity (Isaka et al. 2013). Hirsutane-type sequiterpenoids from Stereum hirsutum have been shown to possess antimicrobial activity (Ma et al. 2014). Mushroom extracts from Fomitopsis rosea, F. pinicola, Jahnoporus hirtus, and Albatrellus flettii with lanostane triterpenoids are effective against Enterococcus species which cause clinical conditions such as urinary tract infections, meningitis, diverticulitis (Liu et al. 2010; Fisher and Phillips 2009). Arnamial and a number of other sesquiterpene aryl esters have been reported from Armillaria sp. to show antimicrobial properties (Misiek and Hoffmeister 2012). Sesquiterpenoid sudasterpurenol A and udalactaranes A and B from Phlebia uda have been reported to inhibit fungal spore germination in Fusarium graminearum (Schuffler et al. 2012). A sesquiterpene dialdehyde named Merulidial 1 having high antifungal activity has been reported from Merulius tremellosus. Some antimicrobial terpenoids are shown in Fig. 1.
Fig. 1.
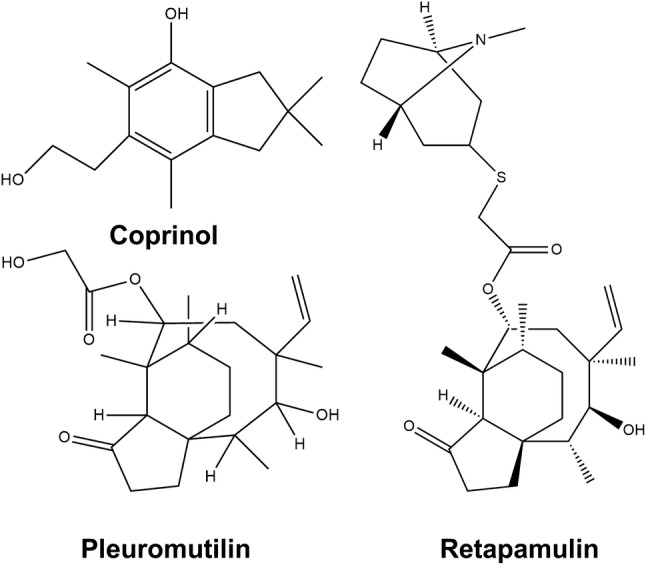
Some antimicrobial terpenoids from mushrooms
Antiviral activity
The search for antiviral drugs is a tedious task for three reasons: virus uses the host cells replicative machinery for propagation, shows structural variability and increased resistance to drugs, and it is difficult to design drugs which will not harm the normal host cells (De Clercq and Field 2006). Around 40 semisynthetic drugs derived from plant metabolites that are under clinical trials for their effectivity against viruses (DeChristopher et al. 2012). However, natural products which demonstrate structural diversity and pharmaceutical activities are being considered for antiviral drug designing with particular emphasis on the metabolites from basidiomycetes (De Clercq and Field 2006).
Both crude extracts and isolated compounds from mushrooms exert their antiviral effect by inhibiting viral enzymes, synthesis of viral nucleic acid or viral infection of mammalian cells. The triterpenoids from Ganoderma pfeifferi as well as other Ganoderma sp. such as ganodermadiol, lucidadiol and applanoxidic acid G, depict in vitro antiviral activity against influenza virus type A and ganodermadiol is also active against herpes simplex virus type 1. Eleven mushroom species including Daedaleopsis confragosa, Datronia mollis, Ischnoderma benzoinum, Laricifomes officinalis, Lenzites betulina, Trametes gibbosa and T. versicolor have been reported to produce effective antiviral compounds (Kabanov et al. 2011; Teplyakova et al. 2012). Agrocybe salicacola is known to produce agrocybone which has been identified as a novel illudane–illudane bis-sesquiterpene and reported to exhibit weak antiviral activity against respiratory syncytial virus (RSV). Some terpenoids with antiviral activity are illustrated in Fig. 2.
Fig. 2.
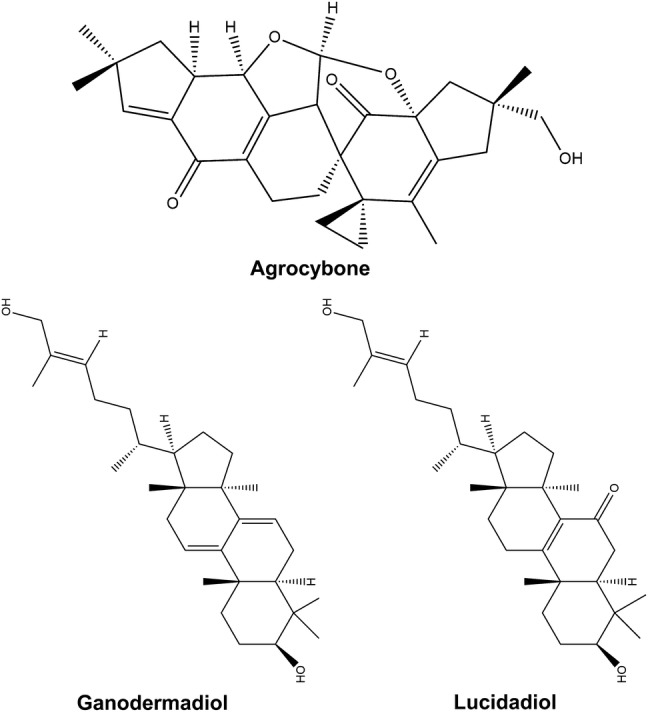
Terpenoids with antiviral activity
The deadliest viral disease affecting the population worldwide is AIDS (acquired immune-deficiency syndrome) caused by infection of HIV-1 (human immunodeficiency virus type I) for which no cure has been found till date. However, research to combat HIV-1 with natural products is ongoing and several bioactive compounds from mushrooms, specifically the low molecular weight triterpenoids, pose to be potential antiviral candidates (Asres et al. 2005).
Colossolactones which are lanostane triterpenes isolated from Ganoderma colossum have been shown to be active against HIV-1 with a few variants such as colossolactone V, colossolactone G, and schisanlactone A (El Dine et al. 2008). Ganoderic acid GS-2, 20-hydroxylucidenic acid N, 20(21)-dehydrolucidenicacid N and andganoderiol F are lanostane triterpenoids from Ganoderma sinense which are effective against HIV-1 protease under in vitro examinations (Sato et al. 2009). Several anti-HIV-1 protease compounds have also been identified from Ganoderma lucidum.
Antiparasitic activity of terpenoids
Malaria is one of the most devastating parasitic infections and not many therapeutic agents are yet available with antimalarial activities (Anthony et al. 2012; Kulangara et al. 2012). The bioactive compounds from mushrooms have been analysed for their antiplasmodial activities and six lanostane terpenoids have been identified from Ganoderma lucidum with in vitro antiplasmodial potency (Adams et al. 2010). Sterostrein A, one of the five terpenoids from Stereum ostrea show antimalarial activity against P. falciparum (Isaka et al. 2012). A novel poisonous mushroom Neonothopanus nambi (Marasmiaceae) has six sesquiterpenes; aristolane, a dimeric sesquiterpene and aurisin A, which act as antiparasitic agents (Kanokmedhakul et al. 2012). Aurisin A and aurisin K are effective against Plasmodium falciparum and Mycobacterium tuberculosis (Kanokmedhakul et al. 2012). Several sesquiterpenoids and triterpenoids from mushrooms have been analysed for their antiplasmodial activities.
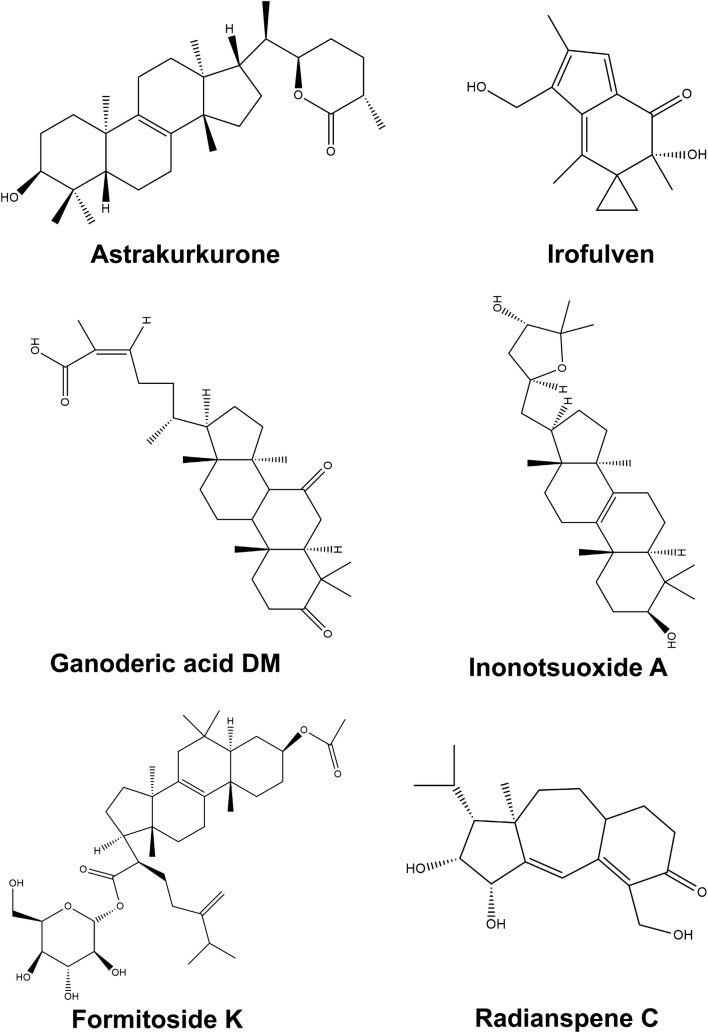
Leishmaniasis is another parasitic disease which affects around 350 million people worldwide, having spread to more than 90 countries in the tropical and sub-tropical regions (World Health Organization 2015) and ranks as the ninth largest disease affecting population (Alvar et al. 2012). The incidences of visceral leishmaniasis (VL) ranges from affecting 200,000–400,000 individuals every year and is endemic to the Indian subcontinent and regions of Africa. Pentavalent antimonials form the first line of defence against different forms of Leishmania, however, its use has been restricted taking into consideration its efficacy and development of resistance against a particular drug to the constant evolution of the pathogen (World Health Organization 2015). Drugs which formed the first line of oral treatment, one of them being miltefosine, had been declined due to its inefficacy, relapse of symptoms and gastrointestinal toxicity (Sundar et al. 2014; Pandey et al. 2009). Other drugs such as paramomycin show systemic hepato-toxicity despite high cure rates (Sundar et al. 2014). The WHO Advisory Panel for Leishmaniasis Control had suggested the use of liposomal amphotericin B for the elimination of VL from India (World Health Organization 2010). The identification of immunomodulatory substances from basiodiomycetes provides an alternative to produce therapeutic medication for the prevention and treatment of Leishmania. However, current research in this area has remained limited to only a few mushroom species. Isolation of a novel compound, 5-heptadeca-8 = Z,11 = Z,16-trienylresorcinol, from a polypore mushroom, Merulius incarnates (Corticiaceae), was the first report of such substance which inhibited the growth of the parasite in vitro with no toxicity on Vero cells (Jin and Zjawiony 2006). Two terpenoids, hypnophilin and panepoxydone, isolated from Lentinus strigosus (Polyporaceae, a basidiomycete), inhibited the growth of Leishmania amazonensis (amastigote-like) (Souza-Fagundes et al. 2010). Agaricus blazei extract showed its activity against L. amazonensis, L. chagasi, and L. major in vitro and against Leishmania amazonensis in vivo (Valadares et al. 2011). Extracts of Astreus hygrometricus showed differential antileishmanial effect against L. donovani promastigotes and intracellular amastigotes in vitro (Mallick et al. 2014). The isolation and structural elucidation of a novel triterpene Astrakurkurone showed its effectivity against promastigotes (Lai et al. 2012). Continuation of this work by another group and search for antileishmanial compounds in other mushrooms identified Grifola frondosa ethanolic extracts to be effective against promastigotes (Sultana et al. 2018). Chemical characterization of the semi-purified fraction showed that it is a mixture of isomers of phthalic acid.
Apoptosis has been demonstrated as a distinct cell death mechanism which is employed for the removal of deleterious cells. The generation of reactive oxygen species (ROS) from mitochondria serve as signals for inducing apoptosis (Halliwell and Gutteridge 1990). The presence of a single mitochondria in Leishmania makes it an ideal drug target. The extracts of G. frondosa induce oxidative stress and caspase independent apoptosis which was confirmed by morphological alteration and increased proportion of cells in the sub G0/G1 phase (Sultana et al. 2018). The extract from G. frondosa was found to be more effective than the activity of miltefosin, paramomycin and amphotericin as indicated from increased NO production and production of pro-inflammatory cytokines such as IL-12, IL-1β and TNF-α (Sultana et al. 2018). Figure 3 illustrates a few antiparasitic terpenoids from mushrooms.
Fig. 3.
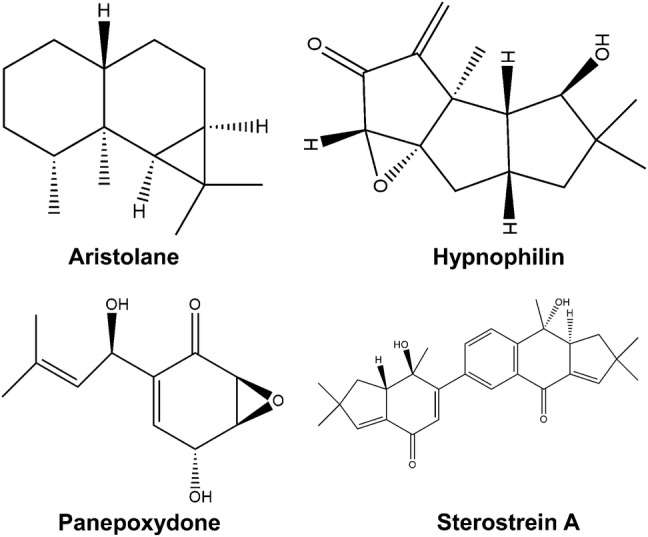
A few mushroom-derived terpenoids with activity against parasites
The effects of Astrakurkurone as antileishmanial product have been associated with the selective production of ROS which target disruption of parasite mitochondria and induce apoptosis (Mallick et al. 2015). More specifically, Astrakurkurone was found to alter the protein and lipid composition of promastigotes and result in exposure of phosphatidylserine to the outer leaflet of plasma membrane which is an event that marks the late stages of apoptosis (Mallick et al. 2015, 2016). The increase in ROS was also correlated with depletion of glutathione levels, increase in lipid peroxidation, and rise in intracellular calcium which linked these cellular changes to apoptosis of Leishmanial cells. These reports point to the fact that further research could refine the antileishmania leads indentified in Astrakurkurone. Its potency has been demonstrated by its ROS mediated, mitochondria-dependent and caspase-independent apoptosis in L. donovani promastigotes which demonstrates the effectivity of a novel triterpene from an Indian mushroom species.
Antioxidant activity
Stress often leads to the production of reactive oxygen species which cause oxidation of cell membrane and organ damage due to the activity of free radicals. It is a common phenomenon under aging and disease conditions of cancer, diabetes, cardiovascular disease, neurodegenerative disorders, bacterial and fungal infections, inflammations etc. (Thetsrimuang et al. 2011; Alfadda and Sallam 2012). These free radicals stabilize by interacting with DNA, lipids and proteins leading to oxidative damage and affecting metabolism and cellular processes. Antioxidants counter the effect of free radicals and terminate such reactions (Halliwell 2012). The identification of natural products with antioxidant and therapeutic potential for delaying the aging process as well as in controlling oxidative damage is an important field of research.
For many years now, wild mushrooms have been analysed for their antioxidant properties and a number of reports have been made (Dasgupta et al. 2014, 2015; Acharya et al. 2017). Sesquiterpenoids from Stereum hirsutum have been isolated which show mild antioxidant activity analysed from DPPH assay (Ma et al. 2014). Sesquiterpenes such as 2, 5-cuparadiene-1, 4-dione (Fig. 4), enokipodin B and enokipodin D (Fig. 4) from Flammulina velutipes has been reported to show antioxidant activity in DPPH scavenging assays (Wang et al. 2012). Extracts of Ganoderma lucidum have been reported to show potential antioxidant activity in DPPH assays and potency in reducing ferric ions to ferrous ions. The isolation and identification of terpenoids for their antioxidant properties remains open for future research.
Fig. 4.
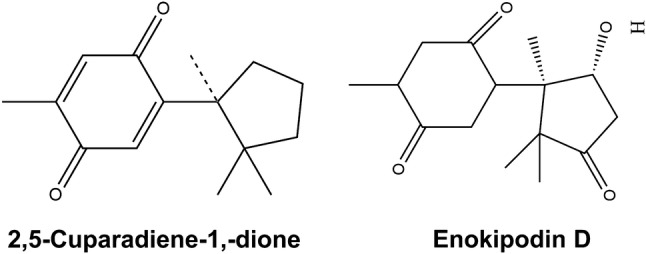
Two antioxidant terpenoids reported from mushroom source
Anti-inflammatory activity
The largest group of anti-inflammatory constituents in mushrooms is composed of terpenes which have been isolated from a wide variety of strains, some of which are presented in Fig. 5. Ethyl acetate extracts of Cyathus africanus produced five novel cyathane diterpenes, identified as cyathins D, H together with three diterpenes neosarcodonin, cyathatriol, and 11-O-acetylcyathatriol (Han et al. 2013). Neosarcodonin, Cyathins D-H 3 and 5, as well as and 11-O-acetylcyathatriol, showed potent inhibition activity against NO production in lipopolysaccharide-mouse monocyte activated macrophage and inhibition of inflammation.
Fig. 5.
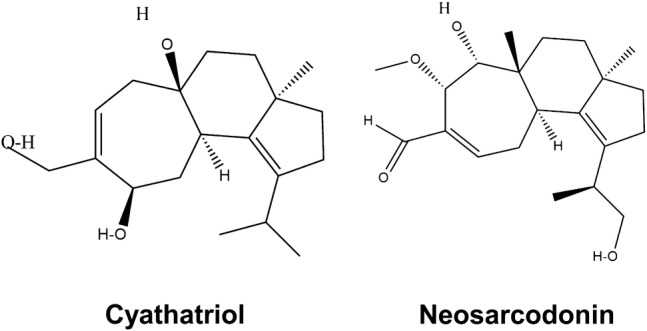
Two mushroom-derived terpenoids with anti-inflammatory activity
Three diterpenes from C. hookeri such as cyathin, (12R)-11a, 14a-epoxy-13a, 14b, 15-trihydroxycyath-3-ene, and erinacine I have been reported, each of which show anti-inflammatory properties (Xu et al. 2013). They also act by inhibiting NO production in mouse monocyte–macrophages. Triterpenes have also been reported to possess anti-inflammatory properties (Dudhgaonkar et al. 2009).
Extracts of Ganoderma lucidum rich in triterpenes are effective in lipopolysaccharide- (LPS-) stimulated macrophages. These bioactive terpenes suppress the secretion of inflammatory cytokine tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), as well as the inflammatory mediators nitric oxide (NO) and prostaglandin E2 (PGE2), from LPS-stimulated murine RAW 264.7 cells. These terpenes also downregulate LPS-dependent expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) in RAW 264.7 cells. Inhibition of transcription factor NF-κB forms the basis for the anti-inflammatory properties of these triterpenes. They further downregulate the expression of AP-1 subunit of c-Jun and inhibit LPS-dependent AP-1-DNA binding activity which also downregulates the MAPK activity. Lanostane-type triterpenoids from Ganoderma lucidum have also been shown to possess anti-inflammatory properties. Nine lucidenic acids and four ganoderic acids have been isolated from G. lucidum, which have been shown to inhibit 12-O-tetradecanoylphorbol-13-acetate induced inflammation in experiments with mice (Akihisa et al. 2005).
Terpenoids active against neurodegenerative diseases
Alzheimer’s is the most common form of dementia that strikes people above the age of 65 (Salmon 2012). It is a progressive neurological disorder which occurs due to the accumulation of insoluble plaques around the neural cells and fibrillar deposits of hyperphosphorylated tau proteins resulting in decrease in neurotransmitter signalling and death of cells (Duyckaerts et al. 2009). Lack of treatment for Alzheimer’s calls for the need to identify novel compounds from natural sources (Pasinetti 2012; Young 2013).
The fruiting body of Antrodia camphorata produces labdane diterpenes which have been predicted to show neuroprotective effects in vitro. These terpenoids also prevented serum deprivation-induced PC12 cell apoptosis (Huang et al. 2005; Lu et al. 2008) and suppressed amyloid β-peptide (Aβ) accumulation which is the main component of the plaques.
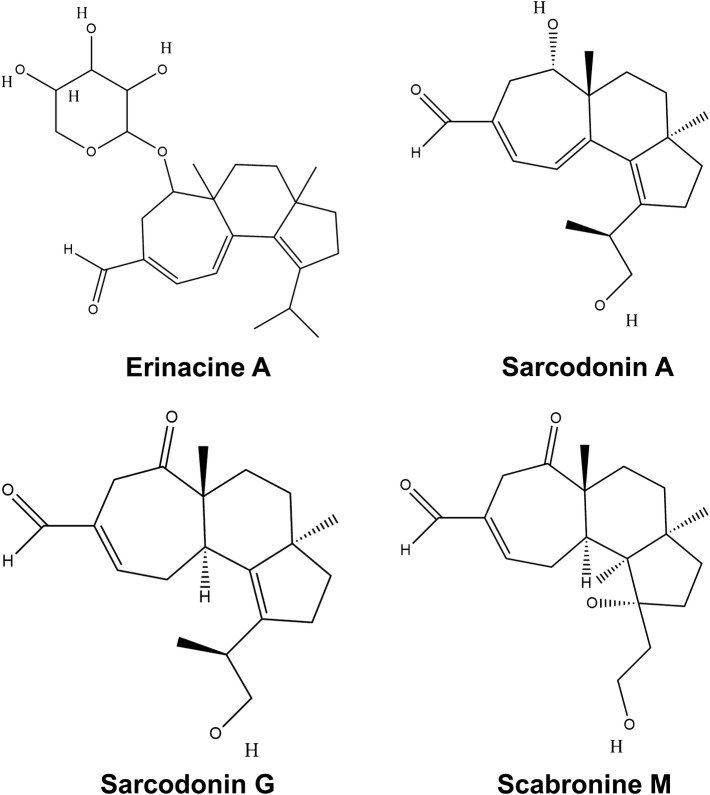
Extracts of Hericium erinaceus revealed terpenoids hericenones and erinacines which show the ability to cross the blood–brain barrier and are active neurotrophic factors particularly effective against neurodegenerative disorders (Moldavan et al. 2007; Ma et al. 2010; Kawagishi and Zhuang 2008). 3-hydroxyhericenone F has been reported to be active against ER stress dependent neuronal cell death (Ueda et al. 2008). Oral administration of erinacine A has been shown to significantly increase the level of nerve growth factor (NGF) in laboratory experiments with the rat’s locus coeruleus and hippocampus, but not in the cerebral cortex indicating its efficacy to combat neurological disorders. Cyathane diterpenoids from Sarcodon scabrosus produce scabronines and sarcodonins with sarcodonin G and A being most effective (Shi et al. 2011). In another report, scabronine M inhibited NGF-induced neurite outgrowth in PC12 cells probably by suppressing the phosphorylation of the receptor Trk A and the extracellular signal regulated kinases (ERK) (Liu et al. 2012). Cyrneines A and B are cyathane diterpenes from Sarcodon cyrneus which are active in neurite outgrowth in the PC12 cell and enhance neurite outgrowth in a Rac1-dependent mechanism (Marcotullio et al. 2006; Obara et al. 2007). Structures of a few mushroom-derived terpenoids are presented in Fig. 6.
Fig. 6.
Some antineurodegenerative terpenoids derived from mushrooms
Antitumor activities of terpenoids
Compounds which can counteract the growth of malignant tumors possess antitumor properties and many bioactive compounds having anticancer properties have been identified from mushrooms (de Silva et al. 2012; Petrova 2012). Extracts from Tricholoma giganteum have been shown to be effective against benzopyrene-induced lung cancer in mice (Chatterjee et al. 2016). Terpenoids have been particularly effective with potent antitumor properties. A few mushroom terpenoids with antitumor activity are illustrated in Fig. 7.
Fig. 7.
Terpenoids purified from mushrooms with antitumor activity
Ganoderma species show an accumulation of triterpenes which have been identified as anticancer agents (Paterson 2006; Cheng et al. 2010; de Silva et al. 2012; Wu et al. 2012). Cytotoxic effects of triterpenoids such as ganoderic acids, lucidimols, ganodermanondiol, ganoderiol F and ganodermanontriol on various cancer cells have been demonstrated (Chen and Chen 2003; Sliva 2003; Chang et al. 2006; Tang et al. 2006; Weng and Yen 2010). These have also been reported to inhibit human cervical cancer cells and have also been considered for the prevention of colitis-associated cancer (Cheng et al. 2010; Xu et al. 2010).
Extracts from Ganoderma lucidum which are enriched with triterpenoids inhibit the growth of hepatoma cells by suppressing protein kinase C and activating mitogen-activated protein kinases (Lin et al. 2003). Hepatoprotective activity of mushrooms have been widely analysed in recent studies (Acharya et al. 2012; Chatterjee et al. 2012). Ganoderic acid T acts via an intrinsic pathway and brings about mitochondrial dysfunction leading to apoptosis and prevents lung tumor cells (Tang et al. 2006). Semisynthetic modification of ganoderic acid T have been carried out to formulate effective anticancer agents (Liu et al. 2012). GA-Me, a ganoderic acid fraction has been tested on human colon cancer cells for its cytotoxicity which also acts via the mitochondria-dependent apoptotic pathway (Chen et al. 2008). Ganoderic acid DM, another triterpenoid isolated from G. lucidum has been reported to effectively inhibit cell proliferation and colony formation in MCF-7 human breast cancer cells by inducing cell cycle (G1) arrest and apoptosis in MCF-7 cells (Liu et al. 2012; Wu et al. 2012). In addition, fruiting bodies of a new strain of G. lucidum (YK-02) show the presence of lucidenic acids A, B, C, and N which are effective on hepatoma cells (Weng et al. 2007). Furthermore, a new ganoderic acid named 3α, 22β-diacetoxy-7α-hydroxy-5α-lanosta-8, 24E-dien-26-oic acid has been isolated from G. Lucidum with considerable cytotoxic activity (Li et al. 2013). Ganoderma lucidum AF (an antlered form of G. lucidum) accumulates a higher number of triterpenes than normal G. lucidum and has immunomodulatory and antitumor effects (Nonaka et al. 2008; Watanabe et al. 2011). The crude extract of G. zonatum contains lanostane-type triterpenoids, steroids and a benzene derivative (Kinge and Mih 2011). The lanostane triterpenoid, ganoderic acid Y showed moderate cytotoxicity against two human tumor cell lines, SMMC-7721 (liver cancer) and A549 (lung cancer).
A new sesquiterpene with a novel carbon skeleton, flammulinol A, new isolactarane sesquiterpene and six isolactarane-related norsesquiterpenes, flammulinolides A-G, as well as sterpuric acid, were isolated from Flammulina velutipes with Flammulinolide C showing cytotoxicity against HeLa cells (Wang et al. 2012). A range of bioactive sesquiterpenoids active against human cancer cell lines have been isolated from F. velutipes (Wang et al. 2012).
Fomitoside-K, a lanostane triterpene glycoside from the fruiting bodies of Fomitopsis nigra acts via the ROS-dependent mitochondrial apoptosis pathway and induced apoptosis of human oral squamous cell carcinomas (Bhattarai et al. 2012; Lee et al. 2012). Novel lanostane-type triterpenoids with potent anticancer effects have been reported from Inonotus obliquus. The structures of these triterpenoids inonotsuoxides A and B (Nakata et al. 2007), inonotsulides group A, B, and C (Taji et al. 2007), inonotsutriols group A, B, and C (Taji et al. 2008a), inonotsutriols D, and E (Tanaka et al. 2011), lanosta-8, 23E-diene-3β, 22R, 25-triol, lanosta-7:9, 23E-triene-3β,22R, 25-trioland 3β-hydroxylanosta-8, 24-dien-21-al (Taji et al. 2008b), from the I. obliquus sclerotia have been reported. They bring about caspase 3-dependent apoptosis and inhibit cell proliferation (Nomura et al. 2008).
Very recently, Dasgupta et al. (2019) reported a sesquiterpenoid from Astraeus hygrometricus, Astrakurkurone to induce mitochondria-mediated apoptosis in liver cancer, by modulating Bcl-2 family proteins. They have demonstrated that this sesuiterpenoid is effectively cytotoxic towards liver cancer in vitro and shown possible interaction of the drug with antiapoptotic Bcl-2 family proteins which facilitates apoptosis.
Some additional lanostane-type triterpenes such as spiroinonotsuoxodiol, inonotsudiol A and inonotsuoxodiol A have been discovered with moderate cytotoxic activity. Five lanostanes (24-triene-21-oic acid, 3β, 15α-dihydroxylanosta-7, 9, dehydroeburicoic acid, dehydrosulphurenic acid, 15α-acetyl-dehydrosulphurenic acid and sulphurenic acid and three ergostane type triterpenes (zhankuicacid A, methyl zhankuic acid A and zhankuic acid C) have been isolated from the fruiting bodies of A. camphorata, and exhibit in vitro cytotoxic effects analysed against human breast cancer cells (Yeh et al. 2009). Antrocin from A. camphorata has been reported to show the strongest antiproliferative effect against MDA-MB-231 and MCF-7 cells (Rao et al. 2011).
Many lanostane-type triterpene acids have been isolated from the epidermis of the sclerotia of Wolfiporia extensa (Poria cocos). Among these, the new derivative 25-methoxyporicoic acid A has been shown to inhibit skin tumor promotion (Akihisa et al. 2009). 13 new guanacastane-type diterpenoids, named radianspenes have been identified from the fermentation products of Coprinellus radians (Coprinus radians) of which radianspene C shows antitumor activity against MDA-MB-435 cells (Ou et al. 2012).
Irofulven also known as 6-hydroxymethylacylfulvene and MGI-114 is an effective semisynthetic anticancer agent. It is derived from illudin-S, a sesquiterpenoid isolated from mushroom Omphalotus illudens (McMorris et al. 1996; McMorris 1999; Schobert et al. 2011). Sesquiterpene synthases and illudin biosynthesis genes have been identified from the genome sequence of O. olearius as well. Irofulven shows activity in nano-molar range against human cancer cell lines, advanced melanoma (Pierson et al. 2002), renal cell carcinoma (Alexandre et al. 2007) and is a potential inhibitor of DNA synthesis which induces apoptosis in malignant cells (Kelner et al. 2008; Raymond et al. 2004). The precise molecular mechanism of this compound remains yet to be deciphered. However, irofulven has been reported to show antitumor activity in combination with other anticancer agents (Kelner et al. 2008), and antiangiogenic or chemotherapeutic drugs (Dings et al. 2008). Ongoing research promises to identify and isolate novel bioactive compounds from the rich source of Basidiomycota with research on therapeutic potential of these metabolites.
Conclusion
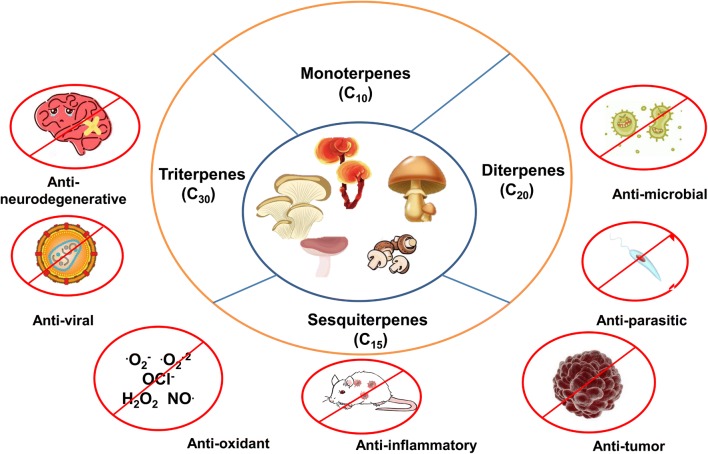
Almost 90% of therapeutic and semisynthetic drugs yet discovered have come from plants, bioactive constituents from mushrooms can add vast resources to the repertoire of modern day therapeutics. In recent times, mushrooms have slowly emerged as a rich resource for bioactive terpenoids. Terpenoids are among the most potent bioactive compounds in mushrooms with at least 5 monoterpenes, 70 sesquiterpenes, 44 diterpenes and 166 triterpenes having been discovered and analysed for their anticancer, antitumor, antimicrobial properties and effectivity in countering neurodegenerative diseases (Duru and Cayan 2015). Terpenoids exhibit a range of therapeutic and curative properties. As such, it has the potential for large-scale impact and can be safely called the penicillin of modern medicine. The plethora of terpenoids isolated from mushroom and their cytotoxic potential against cancer cells provides a path for enhanced drug designing based on natural products with the added advantage of minimum collateral damage of normal cells. Recent research has isolated terpenoids from mushrooms and identified their potential as pharmaceutical products providing a boon to the medicinal field (Khan and Tania 2012). The role of mushroom terpenoids in medicinal science is summarized in Fig. 8.
Fig. 8.
An outline of the therapeutic potential of mushroom-derived terpenoids
In the modern world, the ever-evolving drug-resistant pathogens, virus and cancer cells have become a major concern. Side effects that are posed by current therapies have complicated matters even more. Identification of natural compounds and understanding their mechanism of action is urgently needed (Yao et al. 2012). Majority of the recent research on the mushroom-derived bioactive compounds have been carried out in animal models and/or in vitro and have been suggested as possible adjuvants in therapy, only clinical trials with its improved methodologies can analyse and validate the effectiveness of these compounds (Roupas et al. 2012).
To meet that end it is still imperative to make the most of what we have and keep searching for bioactive terpenoids. With so many mushrooms including rare and endemic species yet to be explored, the answers to combating modern diseases may still be lying in the wilds.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Acharya K, Chatterjee S, Biswas G, et al. Hepatoprotective effect of a wild edible mushroom on carbon tetrachloride-induced hepatotoxicity in mice. Int J Pharm Pharm Sci. 2012;4(3):285–288. [Google Scholar]
- Acharya K, Das K, Paloi S, et al. Exploring a new edible mushroom Ramaria subalpina: chemical characterization and antioxidant activity. Pharmacogn J. 2017;9(1):30–34. [Google Scholar]
- Adams M, Christen M, Plitzko I, et al. Antiplasmodiallanostanes from the Ganoderma lucidum mushroom. J Nat Prod. 2010;73:897–900. doi: 10.1021/np100031c. [DOI] [PubMed] [Google Scholar]
- Akihisa T, Masaaki T, Motohiko U, et al. Oxygenated lanostane-type triterpenoids from the fungus Ganoderma lucidum. J Nat Prod. 2005;6(4):559–563. doi: 10.1021/np040230h. [DOI] [PubMed] [Google Scholar]
- Akihisa T, Uchiyama E, Kikuchi T, et al. Anti-tumor-promoting effects of 25-methoxyporicoic acid A and other triterpene acids from Poria cocos. J Nat Prod. 2009;72:1786–1792. doi: 10.1021/np9003239. [DOI] [PubMed] [Google Scholar]
- Alexandre J, Kahatt C, Bertheault-Cvitkovic F, et al. A phase I and pharmacokinetic study of irofulven and capecitabine administered every 2 weeks in patients with advanced solid tumors. Investig New Drugs. 2007;25:453–462. doi: 10.1007/s10637-007-9071-6. [DOI] [PubMed] [Google Scholar]
- Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;936486:14. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J, Velez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri A, Vaidya JG, Deokule SS. In vitro evaluation of anti-staphylococcal activity of Ganoderma lucidum, Ganoderma praelongum and Ganoderma resinaceum from Pune, India. Afr J Microbiol Res. 2011;5(3):328–333. [Google Scholar]
- Anke T, Watson WH, Giannetti BM, et al. Antibiotics from basidiomycetes XIII the alliacols A and B from Marasmius alliaceus. J Antibiot. 1981;34(10):1271–1277. doi: 10.7164/antibiotics.34.1271. [DOI] [PubMed] [Google Scholar]
- Anthony MP, Burrows JN, Duparc S, et al. The global pipeline of new medicines for the control and elimination of malaria. Malar J. 2012;11:316. doi: 10.1186/1475-2875-11-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpha K, Phosri C, Suwannasai N, et al. Astraodoric acids A-D: new lanostane triterpenes from edible mushroom Astraeus odoratus and their anti-Mycobacterium tuberculosis H37Ra and cytotoxic activity. J Agric Food Chem. 2012;60:9834–9841. doi: 10.1021/jf302433r. [DOI] [PubMed] [Google Scholar]
- Asres K, Seyoum A, Veeresham C, et al. Naturally derived anti-HIV agents. Phytother Res. 2005;19(7):557–581. doi: 10.1002/ptr.1629. [DOI] [PubMed] [Google Scholar]
- Bhattarai G, Lee YH, Lee NH, et al. Fomitoside-K from Fomitopsis nigra induces apoptosis of human oral squamous cell carcinomas (YD-10B) via mitochondrial signaling pathway. Biol Pharm Bull. 2012;35(10):1711–1719. doi: 10.1248/bpb.b12-00297. [DOI] [PubMed] [Google Scholar]
- Blackwell M. The fungi: 1, 2, 3…5.1 million species? Am J Bot. 2011;98(3):426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- Chang UM, Li CH, Lin L, et al. Ganoderiol F, a Ganoderma triterpene, induces senescence in hepatoma HepG2 cells. Life Sci. 2006;79:1129–1139. doi: 10.1016/j.lfs.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Datta R, Dey A, et al. In vivo hepatoprotective activity of ethanolic extract of Russula albonigra against carbon tetrachloride-induced hepatotoxicity in mice. Res J Pharm Tech. 2012;5(8):1034–1038. [Google Scholar]
- Chatterjee S, Chatterjee A, Chandra S, et al. Tricholoma giganteum ameliorates benzo[a]pyrene–induced lung cancer in mice. Int J Pharm Sci Rev Res. 2016;7(5):283–290. [Google Scholar]
- Chen DH, Chen WKD. Determination of ganoderic acids in triterpenoid constituents of Ganoderma tsugae. J Food Drug Anal. 2003;11:195–201. [Google Scholar]
- Chen NH, Liu JW, Zhong JJ. Ganoderic acid Me inhibits tumor invasion through down-regulating matrix metalloproteinases 2/9 gene expression. J Pharmacol Sci. 2008;108:212–216. doi: 10.1254/jphs.sc0080019. [DOI] [PubMed] [Google Scholar]
- Cheng CR, Yue QX, Wu ZY, et al. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochem. 2010;71:1579–1585. doi: 10.1016/j.phytochem.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Sherpa AR, Acharya K. Phytochemicals screening and antioxidant capacity of polyphenol rich fraction of Pleurotus flabellatus. J Chem Pharm Res. 2014;6(5):1059–1065. [Google Scholar]
- Dasgupta A, Dutta AK, Halder A, et al. Mycochemicals, phenolic profile and antioxidative activity of a wild edible mushroom from Eastern Himalaya. J Biol Act Prod Nat. 2015;5(6):373–382. [Google Scholar]
- Dasgupta A, Dey D, Ghosh D, et al. Astrakurkurone, a sesquiterpenoid from wild edible mushroom, targets liver cancer cells by modulating Bcl2 family proteins. IUBMB Life. 2019;71(7):992–1002. doi: 10.1002/iub.2047. [DOI] [PubMed] [Google Scholar]
- De Clercq E, Field HJ. Antiviral prodrugs—the development of successful prodrug strategies for antiviral chemotherapy. Br J Pharmacol. 2006;147:1–11. doi: 10.1038/sj.bjp.0706446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva DD, Rapior S, Fons F, et al. Medicinal mushrooms in supportive cancer therapies: anapproachto anti-cancer effects and putative mechanisms of action—a review. Fungal Divers. 2012;55:1–35. [Google Scholar]
- DeChristopher BA, Loy BA, Marsden MD, et al. Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat Chem. 2012;4:705–710. doi: 10.1038/nchem.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dings RPM, Laar ESV, Webber J, et al. Ovarian tumor growth regression using a combination of vascular targeting agents anginex or topomimetic 0118 and the chemotherapeutic irofulven. Cancer Lett. 2008;265:270–280. doi: 10.1016/j.canlet.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudhgaonkar S, Thyagarajan A, Sliva D. Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int Immuno-pharmacol. 2009;9(11):1272–1280. doi: 10.1016/j.intimp.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Duru EM, Cayan TG. Biologically active terpenoids from mushroom origin: a review. Rec Nat Prod. 2015;9(4):456–483. [Google Scholar]
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer’s disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- El Dine RS, El Halawany AM, Ma C-M, et al. Anti-HIV-1 protease activity of lanostane triterpenes from the Vietnamese mushroom Ganoderma colossum. J Nat Prod. 2008;71:102–1026. doi: 10.1021/np8001139. [DOI] [PubMed] [Google Scholar]
- El-Mekkawy S, Meselhy MR, Nakamura N, et al. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochem. 1998;49(6):1651–1657. doi: 10.1016/s0031-9422(98)00254-4. [DOI] [PubMed] [Google Scholar]
- Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiol. 2009;155:1749–1757. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- Ganesan N, Baskaran R, Velmurugan BK, et al. Antrodia ci-nnamomea-an updated minireview of its bioactive components and biological activity. J Food Biochem. 2019 doi: 10.1111/jfbc.12936. [DOI] [PubMed] [Google Scholar]
- Gazzani G, Daglia M, Papetti A. Food components with anti-caries activity. Curr Opin Biotechnol. 2011;23:153–159. doi: 10.1016/j.copbio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Giannetti B, Steglich W. Antibiotics from Basidiomycetes V Merulidial, a new antibiotic from the Basidiomycete Merulius tremellosus Fr. J Antibiot. 1978;31(8):737–741. doi: 10.7164/antibiotics.31.737. [DOI] [PubMed] [Google Scholar]
- Gilardoni G, Clericuzio M, Tosi S, et al. Antifungal acylcyclopentenediones from fruiting bodies of Hygrophorus chrysodon. J Nat Prod. 2007;70(1):137–139. doi: 10.1021/np060512c. [DOI] [PubMed] [Google Scholar]
- Giri S, Biswas G, Pradhan P, et al. Antimicrobial activities of basidiocarps ofwild edible mushrooms of West Bengal, India. Int J PharmTech Res. 2012;4(4):1554–1560. [Google Scholar]
- Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70(5):257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human diseases: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Han J, Chen Y, Bao L, et al. Anti-inflammatory and cytotoxic cyathane diterpenoids from the medicinal fungus Cyathus africanus. Fitoterapia. 2013;84:22–31. doi: 10.1016/j.fitote.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Harikrishnan R, Balasundaram C, Heo MS. Effect of Inonotus obliquus enriched diet on hematology, immune response, and disease protection in kelp grouper, Epinephelus bruneus against Vibrio harveyi. Aquaculture. 2012;344:48–53. [Google Scholar]
- Hawksworth DL. Global species numbers of fungi: are tropical studies and molecular approaches contributing to a more robust estimate? Biodivers Conserv. 2012;21(9):2425–2433. [Google Scholar]
- He MF, Liu L, Ge W, et al. Antiangiogenic activity of Tripterygium wilfordii and its terpenoids. J Ethnopharmacol. 2009;121(1):61–68. doi: 10.1016/j.jep.2008.09.033. [DOI] [PubMed] [Google Scholar]
- Huang NK, Cheng JY, Lai WL, et al. Antrodia camphorata prevents rat pheochromocytoma cells from serum deprivation induced apoptosis. FEMS Microbiol Lett. 2005;244(1):213–219. doi: 10.1016/j.femsle.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Isaka M, Urarat S, Malipan S, et al. Sterostreins F–O, illudalanes and norilludalanes from cultures of the Basidiomycete Stereumostrea BCC22955. Phytochem. 2012;79:116–120. doi: 10.1016/j.phytochem.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Isaka M, Chinthanom P, Kongthong S, et al. Lanostane triterpenes from cultures of the Basidiomycete Ganoderma orbiforme BCC 22324. Phytochem. 2013;87:133–139. doi: 10.1016/j.phytochem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Jin W, Zjawiony JK. 5-Alkylresorcinols from Merulius incarnatus. J Nat Prod. 2006;69:704–706. doi: 10.1021/np050520d. [DOI] [PubMed] [Google Scholar]
- Johansson M, Sterner O, Labischinski H, et al. Coprinol, a new antibiotic cuparane from a Coprinus species. Zeitschriftfür Natur Schung. 2001;C.56(1–2):31–34. doi: 10.1515/znc-2001-1-205. [DOI] [PubMed] [Google Scholar]
- Kabanov AS, Kosogova TA, Shishkina LN, et al. Study of antiviral activity of extracts obtained from basidial fungi against influenza viruses of different subtypes in experiments in vitro and in vivo. Zh Microbiol Epidemiol Immunobiol. 2011;1:40–43. [PubMed] [Google Scholar]
- Kanokmedhakul S, Lekphrom R, Kanokmedhakul K, et al. Cytotoxic sesquiterpenes from luminescent mushroom Neonothopanus nimbi. Tetrahedron. 2012;68:8261–8266. [Google Scholar]
- Kawagishi H, Zhuang C. Compounds for dementia from Hericium erinaceum. Drugs Futur. 2008;33(2):149. [Google Scholar]
- Kelner MJ, McMorris TC, Rojas RJ, et al. Synergy of irofulven in combination with other DNA damaging agents: synergistic interaction with altretamine, alkylating, and platinum-derived agents in the MV522 lung tumor model. Cancer Chem Pharm. 2008;63:19–26. doi: 10.1007/s00280-008-0703-0. [DOI] [PubMed] [Google Scholar]
- Khan MA, Tania M. Nutritional and medicinal importance of Pleurotus mushrooms: an overview. Food Rev Int. 2012;28(3):313–329. [Google Scholar]
- Kinge TR, Mih AM. Secondary metabolites ofoil palm isolates of Ganoderma zonatum Murill. from Cameroon and their cytotoxicity against five human tumor cell lines. Afr J Biotechnol. 2011;10(42):8440–8447. [Google Scholar]
- Komoda Y, Shimizu M, Sonoda Y, et al. Ganoderic acid and its derivatives as cholesterol synthesis inhibitors. Chem Pharm Bull. 1989;37:531–533. doi: 10.1248/cpb.37.531. [DOI] [PubMed] [Google Scholar]
- Krishna S, Bustamante L, Haynes RK, et al. Artemisinins: their growing importance in medicine. Trends Pharmacol Sci. 2008;29(10):520–527. doi: 10.1016/j.tips.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulangara C, Luedin S, Dietz O, et al. Cell biological characterization of the malaria vaccine candidate trophozoite exported protein 1. PLoS One. 2012;7(10):e46112. doi: 10.1371/journal.pone.0046112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TK, Biswas G, Chatterjee S, et al. Leishmanicidal and anticandidal activity of constituents of Indian edible mushroom Astraeus hygrometricus. Chem Biodivers. 2012;9:1517–1524. doi: 10.1002/cbdv.201100272. [DOI] [PubMed] [Google Scholar]
- Lee IK, Jung JY, Yeom JH, et al. Fomitoside K, a new lanostane triterpene glycoside from the fruiting body of Fomitopsis nigra. Mycobiol. 2012;40(1):76–78. doi: 10.5941/MYCO.2012.40.1.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YB, Liu RM, Zhong JJ. A new ganoderic acid from Ganodermalucidum mycelia and its stability. Fitoterapia. 2013;84:115–122. doi: 10.1016/j.fitote.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Lin SB, Li CH, Lee SS, et al. Triterpene-enriched extracts from Ganoderma lucidum inhibit growth of hepatoma cellsvia suppressing protein kinase C, activating mitogen-activated protein kinases and G2-phase cell cycle arrest. Life Sci. 2003;72:2381–2390. doi: 10.1016/s0024-3205(03)00124-3. [DOI] [PubMed] [Google Scholar]
- Lin LT, Chung CY, Hsu WC, et al. Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J Hepatol. 2015;62(3):541–548. doi: 10.1016/j.jhep.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Liu XT, Winkler AL, Schwan WR, Volk TJ, Rott M, et al. Antibacterial compounds from mushrooms II: lanostane triterpenoids and an ergostane steroid with activity against Bacillus cereus isolated from Fomitopsis pinicola. Planta Med. 2010;76(5):464–466. doi: 10.1055/s-0029-1186227. [DOI] [PubMed] [Google Scholar]
- Liu LY, Li ZH, Dong ZJ, et al. Two novel fomannosane-type sesquiterpenoids from the culture of the basidiomycete Agrocybe salicacola. Nat Prod Bioprospecting. 2012;2:130–132. [Google Scholar]
- Long BH, Carboni JM, Wasserman AJ, et al. Eleutherobin, a novel cytotoxic agent that induces tubulin polymerization, is similar to paclitaxel (Taxol®) Cancer Res. 1998;58(6):1111–1115. [PubMed] [Google Scholar]
- Lu MK, Cheng JJ, Lai WL, et al. Fermented Antrodia cinnamomea extract protects rat PC12 cells from serum deprivation-induced apoptosis: the role of the MAPK family. J Agric Food Chem. 2008;56(3):865–874. doi: 10.1021/jf072828b. [DOI] [PubMed] [Google Scholar]
- Ma BJ, Shen JW, Yu HY, Ruan Y, et al. Hericenones and erinacines: stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycol Int J Fungal Biol. 2010;1(2):92–98. [Google Scholar]
- Ma L, Chen H, Dong P, et al. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013;139(1–4):503–508. doi: 10.1016/j.foodchem.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Ma K, Ren J, Han J, et al. Ganoboninketals A-C, antiplasmodial 3,4-seco-27-norlanostane triterpenes from Ganoderma boninense Pat. J Nat Prod. 2014;77:1847–1852. doi: 10.1021/np5002863. [DOI] [PubMed] [Google Scholar]
- Mallick S, Dutta A, Dey S, et al. Selective inhibition of Leishmania donovani by active extracts of wild mushrooms used by the tribal population of India: an in vitro exploration for new leads against parasitic protozoans. Exp Parasitol. 2014;138:9–17. doi: 10.1016/j.exppara.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Mallick S, Dey S, Mandal S, et al. A novel triterpene from Astraeus hygrometricus induces reactive oxygen species leading to death in Leishmania donovani. Futur Microbiol. 2015;10:763–789. doi: 10.2217/fmb.14.149. [DOI] [PubMed] [Google Scholar]
- Mallick S, Dutta A, Chaudhuri A, et al. Successful therapy of murine visceral Leishmaniasis with astrakurkurone, a triterpene isolated from the mushroom Astraeus hygrometricus, involves the induction of protective cell-mediated immunity and TLR9. Antimicrob Agents Chemother. 2016;60(5):2696–2708. doi: 10.1128/AAC.01943-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotullio MC, Pagiotti R, Maltese F, et al. Neurite outgrowth activity of cyathane diterpenes from Sarcodon cyrneus, cyrneines A and B. Planta Med. 2006;72(9):819–823. doi: 10.1055/s-2006-946681. [DOI] [PubMed] [Google Scholar]
- McMorris TC. Discovery and development of sesquiterpenoid derived hydroxyl methylacylfulvene: a new anticancer drug. Bioorganic Med Chem. 1999;7:881–886. doi: 10.1016/s0968-0896(99)00016-4. [DOI] [PubMed] [Google Scholar]
- McMorris TC, Kelner MJ, Wang W, et al. (Hydroxymethyl) acylfulvene: an illudin derivative with superior antitumor properties. J Nat Prod. 1996;59:896–899. doi: 10.1021/np960450y. [DOI] [PubMed] [Google Scholar]
- Miles PG, Chang ST. Mushrooms: cultivation, nutritional value, medicinal effect, and environmental impact. Boca Raton: CRC Press; 2004. [Google Scholar]
- Min BS, Gao JJ, Nakamura N. Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against meth-A and LLC tumor cells. Chem Pharm Bull. 2000;48(7):1026–1033. doi: 10.1248/cpb.48.1026. [DOI] [PubMed] [Google Scholar]
- Misiek M, Hoffmeister D. Sesquiterpene aryl ester natural products in North American Armillaria species. Mycol Prog. 2012;11:7–15. [Google Scholar]
- Moldavan MG, Gryganski AP, Kolotushkina OV. Neurotropic and trophic action of lion’s mane mushroom Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) extracts on nerve cells in vitro. Int J Med Mushrooms. 2007;9(1):15–28. [Google Scholar]
- Morigiwa A, Kitabatake K, Fujimoto Y, et al. Angiotensin converting enzyme inhibitory triterpenes from Ganoderma lucidum. Chem Pharm Bull. 1986;34:3025–3028. doi: 10.1248/cpb.34.3025. [DOI] [PubMed] [Google Scholar]
- Nagabushan H. Retapamulin: a novel topical antibiotic. Indian J Dermatol Venereol Leprol. 2010;76(1):77–79. doi: 10.4103/0378-6323.58693. [DOI] [PubMed] [Google Scholar]
- Nakao Y, Yoshida S, Matsunaga S, et al. (Z)-sarcodictyin A, a new highly cytotoxic diterpenoid from the soft coral bellonella a lbiflora. J Nat Prod. 2003;66(4):524–527. doi: 10.1021/np0205452. [DOI] [PubMed] [Google Scholar]
- Nakata T, Yamada T, Taji S, et al. Structure determination of inonotsuoxides A and B and in vivo antitumor promoting activity of inotodiol from the sclerotia of Inonotus obliquus. Bioorganic Med Chem. 2007;15:257–264. doi: 10.1016/j.bmc.2006.09.064. [DOI] [PubMed] [Google Scholar]
- Niedermeyer TH, Lindequist U, Mentel R. Antiviral terpenoid constituents of Ganoderma pfeifferi. J Nat Prod. 2005;68(12):1728–1731. doi: 10.1021/np0501886. [DOI] [PubMed] [Google Scholar]
- Nomura M, Takahashi T, Uesugi A, et al. Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspase-3-dependent apoptosis. Anticancer Res. 2008;28(5A):2691–2696. [PubMed] [Google Scholar]
- Nonaka Y, Ishibashi H, Nakai M, et al. Effects of the antlered form of Ganoderma lucidum on tumor growth and metastasis in cyclophosphamide-treated mice. Biosci Biotechnol Biochem. 2008;72(6):1399–1408. doi: 10.1271/bbb.70607. [DOI] [PubMed] [Google Scholar]
- Nwodo JN, Ibezim A, Simoben CV, et al. Exploring cancer therapeutics with natural products from African medicinal plants, part II: alkaloids, terpenoids and flavonoids. Anticancer Agents Med Chem. 2016;16:108–127. doi: 10.2174/1871520615666150520143827. [DOI] [PubMed] [Google Scholar]
- Obara Y, Hoshino T, Marcotullio MC, et al. Novel cyathane diterpene, cyrneine A, induces neurite outgrowth in a Rac1-dependent mechanism in PC12 cells. Life Sci. 2007;80(18):1669–1677. doi: 10.1016/j.lfs.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Ou YX, Li YY, Qian XM, et al. Guanacastane-type diterpenoids from Coprinus radians. Phytochem. 2012;78:190–196. doi: 10.1016/j.phytochem.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Pandey BD, Pandey K, Kaneko O, et al. Relapse of visceral leishmaniasis after miltefosine treatment in a Nepalese patient. Am J Trop Med Hyg. 2009;80:580–582. [PubMed] [Google Scholar]
- Pasinetti GM. Novel role of red wine-derived polyphenols in the prevention of Alzheimer’s disease dementia and brain pathology: experimental approaches and clinical implications. Planta Med. 2012;78:1614–1619. doi: 10.1055/s-0032-1315377. [DOI] [PubMed] [Google Scholar]
- Paterson RRM. Ganoderma—A therapeutic fungal biofactory. Phytochem. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Paukner S, Riedl R. Pleuromutilins: potent drugs for resistant bugs—mode of action and resistance. Cold Spring Harb Perspect Med. 2017;7(1):a027110. doi: 10.1101/cshperspect.a027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova RD. New scientific approaches to cancer treatment: can medicinal mushrooms defeat the curse of the century? Int J Med Mushrooms. 2012;14(1):1–20. doi: 10.1615/intjmedmushr.v14.i1.10. [DOI] [PubMed] [Google Scholar]
- Pierson AS, Gibbs P, Richards J, et al. A phase II study of Irofulven (MGI 114) in patients with stage IV melanoma. Investig New Drugs. 2002;20:357–362. doi: 10.1023/a:1016261918256. [DOI] [PubMed] [Google Scholar]
- Rabi T, Bishayee A. Terpenoids and breast cancer chemoprevention. Breast Cancer Res Treat. 2009;115(2):223–239. doi: 10.1007/s10549-008-0118-y. [DOI] [PubMed] [Google Scholar]
- Rahi DK, Malik D. Diversity of mushrooms and their metabolites of nutraceutical and therapeutic significance. J Mycol. 2016;2016:7654123. [Google Scholar]
- Rao YK, Wu AT, Geethangili M, et al. Identification of antrocin from Antrodia camphorata as a selective and novel class of small molecule inhibitor of Akt/mTOR signalling in metastatic breast cancer MDA-MB-231 cells. Chem Res Toxicol. 2011;24(2):238–245. doi: 10.1021/tx100318m. [DOI] [PubMed] [Google Scholar]
- Raskin I, Ripoll C. Can an apple a day keep the doctor away? Curr Pharm Des. 2004;10(27):3419–3429. doi: 10.2174/1381612043383070. [DOI] [PubMed] [Google Scholar]
- Rathee S, Rathee D, Rathee D, et al. Mushrooms as therapeutic agents. Braz J Pharmacogn. 2012;22(2):459–474. [Google Scholar]
- Raymond E, Kahatt C, Rigolet MH, et al. Characterization and multiparameter analysis of visual adverse events in irofulven single-agent phase I and II trials. Clin Cancer Res. 2004;10(22):7566–7574. doi: 10.1158/1078-0432.CCR-04-0869. [DOI] [PubMed] [Google Scholar]
- Roupas P, Keogh J, Noakes M, et al. The role of edible mushrooms in health: evaluation of the evidence. J Funct Foods. 2012;4:687–709. [Google Scholar]
- Salmon DP. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer’s disease. Curr Top Behav Neurosci. 2012;10:187–212. doi: 10.1007/7854_2011_171. [DOI] [PubMed] [Google Scholar]
- Sato N, Zhang Q, Ma C-M, et al. Anti-human immunodeficiency virus-1 protease activity of new lanostane- type triterpenoids from Ganoderma sinense. Chem Pharm Bull. 2009;57:1076–1080. doi: 10.1248/cpb.57.1076. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Ribnicky DM, Poulev A, et al. A natural history of botanical therapeutics. Metabolism. 2008;57:S3–S9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert R, Knauer S, Seibt S, et al. Anticancer active illudins: recent developments of a potent alkylating compound class. Curr Med Chem. 2011;18(6):790–807. doi: 10.2174/092986711794927766. [DOI] [PubMed] [Google Scholar]
- Schuffler A, Wollinsky B, Anke T, et al. Isolactarane and sterpurane sesquiterpenoids from the basidiomycete Phlebiauda. J Nat Prod. 2012;75(7):1405–1408. doi: 10.1021/np3000552. [DOI] [PubMed] [Google Scholar]
- Shi XW, Liu L, Gao JM, et al. Cyathane diterpenes from Chinese mushroom Sarcodon scabrosus and their neurite outgrowth promoting activity. Eur J Med Chem. 2011;46(7):3112–3117. doi: 10.1016/j.ejmech.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Sliva D. Ganoderma lucidum (Reishi) in cancer treatment. Integr Cancer Ther. 2003;2:358–364. doi: 10.1177/1534735403259066. [DOI] [PubMed] [Google Scholar]
- Souza AB, Martins CH, Souza MG, et al. Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf. against cariogenic bacteria. Phytother Res. 2011;25(2):215–220. doi: 10.1002/ptr.3244. [DOI] [PubMed] [Google Scholar]
- Souza-Fagundes EM, Cota BB, et al. In vitro activity of hypnophilin from Lentinus strigosus: a potential prototype for Chagas disease and leishmaniasis chemotherapy. Braz J Med Biol Res. 2010;43:1054–1061. doi: 10.1590/s0100-879x2010007500108. [DOI] [PubMed] [Google Scholar]
- Stanikunaite R, Radwan MM, Trappe JM, et al. Lanostane-type triterpenes from the mushroom Astraeus pteridis with antituberculosis activity. J Nat Prod. 2008;71:20772079. doi: 10.1021/np800577p. [DOI] [PubMed] [Google Scholar]
- Sultana SS, Ghosh J, Chakraborty S, et al. Selective in vitro inhibition of Leishmania donovani by a semi-purified fraction of wild mushroom Grifola frondosa. Exp Parasitol. 2018;192:73–84. doi: 10.1016/j.exppara.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Sundar S, Pandey K, Thakur CP, et al. Efficacy and safety of amphotericin B emulsion versus liposomal formulation in Indian patients with visceral leishmaniasis: a randomized, open-label study. PLoS Negl Trop Dis. 2014;8:e3169. doi: 10.1371/journal.pntd.0003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taji S, Yamada T, In Y, et al. Three new lanostane triterpenoids from Inonotus obliquus. Helv Chim Acta. 2007;90:2047–2057. [Google Scholar]
- Taji S, Yamada T, Tanaka R. Three new lanostane triterpenoids, inonotsutriols A, B and C from Inonotus obliquus. Helv Chim Acta. 2008;91:1513–1524. [Google Scholar]
- Taji S, Yamada T, Wada S, et al. Lanostane-type triterpenoids from the sclerotia of Inonotus obliquus possessing anti-tumor promoting activity. Eur J Med Chem. 2008;43(11):2373–2379. doi: 10.1016/j.ejmech.2008.01.037. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Toyoshima M, Yamada T. New lanostane triterpenoids, inonotsutriols D, and E from Inonotus obliquus. Phytochem Lett. 2011;4(3):328–332. [Google Scholar]
- Tang W, Liu JW, Zhao WM, et al. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006;80:205–211. doi: 10.1016/j.lfs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Teplyakova TV, Psurtseva NV, Kosogova TA, et al. Antiviral activity of polyporoid mushrooms (higher Basidiomycetes) from Altai Mountains (Russia) Int J Med Mushrooms. 2012;14(1):37–45. doi: 10.1615/intjmedmushr.v14.i1.40. [DOI] [PubMed] [Google Scholar]
- Thetsrimuang C, Khammuang S, Sarnthima R. Antioxidant activity of crude polysaccharides from edible fresh and dry mushroom fruiting bodies of Lentinus sp. strain RJ-2. Int J Pharmacol. 2011;7:58–65. [Google Scholar]
- Ueda K, Tsujimori M, Kodani S, et al. An endoplasmic reticulum (ER) stress-suppressive compound and its analogues from the mushroom Hericium erinaceum. Bioorganic Med Chem. 2008;16:9467–9470. doi: 10.1016/j.bmc.2008.09.044. [DOI] [PubMed] [Google Scholar]
- Valadares DG, Duarte MC, Oliveira JS, et al. Leishmanicidal activity of the Agaricus blazei Murill in different Leishmania species. Parasitol Int. 2011;60:357–363. doi: 10.1016/j.parint.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Vidal V, Pottera O, Louvel S, et al. Library-based discovery and characterization of daphnane diterpenes as potent and selective HIV inhibitors in Daphne gnidium. J Nat Prod. 2011;75(3):414–419. doi: 10.1021/np200855d. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bao L, Liu D, et al. Two new sesquiterpenes and six nor sesquiterpenes from the solid culture of the edible mushroom Flammulina velutipes. Tetrahedron. 2012;68:3012–3018. [Google Scholar]
- Watanabe K, Shuto T, Sato M, et al. Lucidenic acids-rich extract from antlered form of Ganoderma lucidum enhances TNFα induction in THP-1 monocytic cells possibly via its modulation of MAP kinases p38 and JNK. Biochem Biophys Res Commun. 2011;408(1):18–24. doi: 10.1016/j.bbrc.2011.03.108. [DOI] [PubMed] [Google Scholar]
- Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–2681. doi: 10.1091/mbc.E14-04-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng CJ, Yen GC. The in vitro and in vivo experimental evidences disclose the chemo-preventive effects of Ganoderma lucidum on cancer invasion and metastasis. Clin Exp Metastasis. 2010;27:361–369. doi: 10.1007/s10585-010-9334-z. [DOI] [PubMed] [Google Scholar]
- Weng CJ, Chau CF, Chen KD, et al. The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol Nutr Food Res. 2007;51(12):1472–1477. doi: 10.1002/mnfr.200700155. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniasis. Geneva: World Health Organ; 2010. [Google Scholar]
- World Health Organization . Leishmaniasis fact sheet 375. Geneva: World Health Organizatio; 2015. [Google Scholar]
- Wu GS, Lu JJ, Guo JJ, et al. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia. 2012;83(2):408–414. doi: 10.1016/j.fitote.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Xu K, Liang X, Gao F, et al. Anti-metastatic effect of ganoderic acid T in vitro through inhibition of cancer cell invasion. Process Biochem. 2010;45:1261–1267. [Google Scholar]
- Xu Z, Yan S, Bietal K. Isolation and identification of a new anti-inflammatory cyathane diterpenoid from the medicinal fungus Cyathus hookeri Berk. Fitoterapia. 2013;86(1):159–162. doi: 10.1016/j.fitote.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Yao X, Li G, Xu H. Chaotian Lü1 inhibition of the JAK-STAT3 signalling pathway by ganoderic acid A enhances chemo-sensitivity of HepG2 cells to Cisplatin. Planta Med. 2012;78:1740–1748. doi: 10.1055/s-0032-1315303. [DOI] [PubMed] [Google Scholar]
- Yeh CT, Rao YK, Yao CJ, et al. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29human colon cancer cells. Cancer Lett. 2009;285(1):73–79. doi: 10.1016/j.canlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Ying YM, Zhang LY, Zhang X, et al. Terpenoids with alpha-glucosidase inhibitory activity from the submerged culture of Inonotus obliquus. Phytochem. 2014;108:171–176. doi: 10.1016/j.phytochem.2014.09.022. [DOI] [PubMed] [Google Scholar]
- Yoo KY, Park SY. Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules. 2012;17(3):3524–3538. doi: 10.3390/molecules17033524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SN. Single treatments that have lasting effects: some thoughts on the antidepressant effects of ketamine and botulinum toxin and the anxiolytic effect of psilocybin. J Psychiatry Neurosci. 2013;38(2):78–83. doi: 10.1503/jpn.120128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BB, Guan YY, Hu PF, et al. Production of bioactive metabolites by submerged fermentation of the medicinal mushroom Antrodia cinnamomea: recent advances and future development. Crit Rev Biotech. 2019;39(4):541–554. doi: 10.1080/07388551.2019.1577798. [DOI] [PubMed] [Google Scholar]