Abstract
Metastasis means the dissemination of the cancer cells from one organ to another which is not directly connected to the primary site. Metastasis has a crucial role in the prognosis of cancer patients. A few theories, different types of cell and several molecular pathways have been proposed to explain the mechanism of metastasis. In this work, the related articles in the limited period of time, 2000–mid -2018 were reviewed, through search in PubMed, Google Scholar and Scopus database. The articles published in the last two decades related to the biology of cancer metastasis were selected and the most important factors were discussed. Metastasis is critical factor to predict survival in patients with advanced cancer and prognosis determines the treatment plan. Many different cell types and various signaling pathways control the metastatic process. Metastasis is a multistep process. Many signaling pathways and molecules are involved in metastasis. Increasing knowledge about the mechanism of metastasis can help in finding the promising targets of cancer therapy.
Key Words: Biomarkers, Circulating cells, Exosomes, Extracellular matrix, Metastasis, MicroRNAs, Neoplasms, Stem cells
Introduction
Metastasis means the dissemination of the cancer cells from one organ to another which is not directly connected to the primary site. Metastasis has a crucial role in the prognosis of cancer patients. In the first stage of metastasis, cancer cells detach from the primary tumor and disseminate in the tissue. Then, cancer cells enter the vascular or lymphatic channels (1-3). The survival of the circulating tumor cells (CTCs) inside the lymphatic or blood channels is critical for metastasis to establish a micro-metastasis. Finally, cancer cells extravasate through the vessel wall and proliferate at the secondary site. For each step, a lot of signaling pathways are involved. A few theories have been proposed to explain the mechanism of metastasis. (a) The “organ selection concept” suggests that the growth factors establish a successful metastasis in the metastatic site (4, 5). (b) The “adhesion theory” proposes that the tissue specific adhesion molecules which are expressed on endothelial cells of recepient organs anchor the migrating cancer cells, therefore, provide a premetastatic niche. (c) The “chemo-attraction theory” explains that the cancer cells express chemokine receptors. (d) Paget in 1889 first reported the theory of “seed” for metastatic tumor cells and of “soil” for the secondary site. According to this concept, the organ distribution is determined by the site and histopathological type of the primary tumor. (e) Recently, premetastatic niche has been indicated to explain metastasis. This concept proposes that prior to co-localization; the primary tumor induces the microenvironment of secondary site by CTCs. In the next step a metastatic niche is generated to support disseminated tumor cells (DTCs) and localize them to develop a metastasis. (f) The last one is a new theory that describes a bidirectional relationship between the primary and secondary sites. According to this theory, the surviving cancer cells in the metastatic tumor can return to the primary site to promote the primary tumor progression (6, 7).
Metastasis to a specific organ is determined by some factors such as circulation patterns. Efficient and direct blood flow can explain the probability of metastasis to the specific organs like hepatic metastasis in patients with colon cancer which receive direct blood flow from the primary site (8). The other factor is vascular permeability which significantly promotes extravasation at the metastatic site (9). At the present time, understanding of molecular mechanisms of metastasis remains incomplete. This review focuses on recent advancement in molecular pathways of metastasis. Many molecular pathways and signals are involved in cancer metastasis, but only some of them were discussed in this review article.
Materials and Methods
In this work, the related articles in the limited period of time, 2000–mid -2018 were reviewed, through search in PubMed, Google Scholar and Scopus database. The articles published in the last two decades related to the biology of cancer metastasis were selected and the most important factors were discussed.
Results
Cancer stem cells
In the last decades, cancer stem cells (CSCs) also known as tumor initiating cells (TICs) have been identified to explain tumor growth, tumor recurrence and metastasis and tumor chemo-resistance (10). It has been suggested that cancer tissue contains a small subset of tumor cells involving cancer development. These cells are not only able to reproduce the original phenotype but also capable of self-renewal (11-13). Self-renewal is crucial to maintain the cell stemness, which is important in tumor cell proliferation and differentiation. Asymmetric cell division of CSCs helps the cells to have a low proliferation rate with long-lived properties. These characters enhance the widely spread of CSCs and resistant to cytotoxic conditions. Chemo-resistance is the major issue of treatment failure of cancers resulting in cancer recurrence and is also associated with acquisition of more aggressive form of cancer cells and/or metastatic type (14). Besides, CSCs explain intra-tumoral heterogeneity (15). CSCs may have a great impact on metastasis as they migrate and attach to a new location (16). Inactivated apoptosis signaling pathways help CSCs to survive, possess self-renewal properties and elude cytotoxicity of most anticancer therapies (17). Regarding the origin of CSCs, there are two theories. One suggests that CSCs originate from normal stem cells or progenitor cells. The other theory indicates that CSCs originate from normal cells which acquire stem cell-like properties via epithelial-mesenchymal transition (EMT) process. In squamous cell carcinoma (SCC), CSCs have two different phenotypes. One phenotype is similar to normal epithelial stem cells related to tissue growth and the other is similar to the migrating CSCs acquiring EMT-associated genes (18). Because of the similarities between physiological stem cells and CSCs, experimental cancer stem cell research faces a lot of difficulties. To overcome these difficulties, cancer stem cell markers are needed but the markers are not specific to cancer stem cells and are found also on normal stem cells. A variety of markers has been suggested to identify CSCs; however, the clinical significance of these markers is not clear. Nonetheless, previous studies have indicated that CSC surface markers are considered as molecular targeted therapies for different types of cancer (19). A majority of cell surface proteins and a number of intracellular proteins have also been postulated to function as CSC markers. Immunohistochemistry of primary tumors is proposed as a useful technique to identify CSC markers. The problem arises in the identification of CSC markers involved in metastasis and in therapeutic resistance due to the difficulty of obtaining metastatic or recurrent biopsies. It is necessary to combine markers to get very high specificity which is tumor type–dependent; however, the use of multiple markers significantly decreases the numbers of cells isolated from cancer patients making the diagnosis more difficult (20). Several biomarkers have been identified for CSCs. Among them CD44, CD133, and ALDH1 are the most widely studied. Some epithelial cells and macrophages express the CD44 variant (CD44v) isoforms at various stages of development. In normal tissues, CD44 serves to activate lymphocytes, release of cytokines and regulate hyaluronic metabolism. Hence, it involves in wound healing, and keratinocyte proliferation. Besides, CD44 makes non-metastatic cell lines to become more metastatic (20). The degradation of extracellular matrix (ECM) is regulated by matrix metalloproteinases (MMPs) (21). Emerging literature reveals the association of CD44 with MMP-9 in mouse and human tumor cells to enhance the invasion of cancer cells (22). Localization of MMP-9 on the surface of keratinocytes depends on its interaction with CD44 which activates transforming growth factor-β (TGF-β), and is essential for the promotion of tumor invasion and angiogenesis (23). Previous published work has shown that CD44 and MMP-9 co-localize in tumor cells at the invasive front (24). CD44 is also a marker associated with EMT and CSCs in oral cancer and promotes cancer cell aggressiveness by targeting extracellular signal-regulated kinases 1, 2 (ERK1/2) (25). CD44 may control bone metastasis in hematopoietic cancers and solid tumors. The interaction between CD44 and hyaluronan acid may facilitate the entrance of CSCs into bone marrow and their colonization (26).
CD133, a cell surface glycoprotein, is another CSCs marker (27). CD133-positive cells exist in both primary and metastatic tumors; however, CD133-positive cells are more numerous in metastatic sites. Several lines of evidence indicate that elevated expression of CD133 is correlated with increased migration, stemness and tumorigenicity level due to induction of EMT (28). CD133 also contributes to metastatic process in several cancers such as colon cancer, and pancreatic cancer (28, 29). CD133+ stem-like cells promote invasion ability and tumorigenesis compared with CD133- cells (30). CD133+ cells enhance activity of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway compared with CD133- cells (28). Importantly, in head and neck squamous cell carcinoma (HNSCC), CD44+ CSCs express higher CD133 levels than CD44- cells (31).
Aldehyde dehydrogenase 1 (ALDH1) is also a biomarker of CSCs in cancers and correlates with the migration of cancer cells and poor prognosis (32). ALDH1 is also involved in cell differentiation, metastasis, detoxification and drug resistance through the oxidation of intracellular aldehydes (33).
CD24, a small cell surface protein, is expressed in different cancers such as breast, ovarian, prostate and bladder cancers. As it contributes to cell adhesion and metastasis, it might be a marker in cancer prognosis and diagnosis (34). CD24 cell membrane and intracellular expression in pancreas cancer inhibits the cancer cell invasion and metastasis (35). The other CSCs markers are CD26, CD29, CD166 (36). EMT phenomenon also contributes to the tumorigenicity of CSCs and metastasis.
EMT and cancer progression
Epithelial- mesenchymal transition (EMT) was first coined by Elizabeth Hey in 1980s to describe epithelial mesenchymal phenotype changes in chick embryo. EMT and its reverse mesenchymal-epithelial transition (MET) are characteristics of cellular plasticity during embryogenesis and tumor metastasis (37). EMT is characterized by the decreased expression levels of E-cadherin and β-catenin and elevated expression levels of vimentin, fibronectin and N-cadherin (11). In cancers, EMT is a master process by which cancer cells lose their epithelial characteristics to acquire mesenchymal-like properties. Tumor cell migration is a pre-requisite for the metastatic process; hence, EMT is the most critical step to initiate metastasis including metastasis to lymph nodes (38). During EMT phenomenon, cancer cells lose their cell-to-cell junctions and cellular polarity via multiple signaling pathways which increase the motilities and invasive phenotype of them (39). MMPs mediate the cleavage of E-cadherin to increase the tumor cell motility and invasion (40). In addition, EMT has a key role in drug resistance. For example, a previous study has detected high levels of vimentin in adriamycin and vinblastine resistant breast cancer cell lines (41). EMT phenomenon promotes CSCs motility, cancer cell invasion, tumor metastasis and recurrence and drug resistance. Expression of stem cell like markers and formation of tumor spheres by CSCs are enhanced by EMT process. CSCs acquire mesenchymal features by undergoing EMT phenomenon. By doing this, CSCs become resistant to anti-cancer therapies; hence, they can survive and cause cancer recurrence. Besides, CSCs invade to the adjacent stromal tissues, enter the vascular channels, and finally reach the distant organs. In the target organs, CSCs cause MET phenomenon which results in the acquisition of epithelial characteristics. In addition, MET phenomenon increases the cell-to-cell attachment, cancer cells proliferation and differentiation to form metastatic lesions (42). Taken together, EMT induces CSC properties as well as metastatic activities. On the other hand, EMT and CSCs collaborate in invasion capacity of invasive front zone; therefore, targeting the EMT/CSC phenotype can be a therapeutic therapy for the treatment of metastasis and tumor recurrence (43).
The invasive front area of cancers
Invasion is the initial step in the metastatic process. The mode of tumor invasion is an important prognostic factor in the cases of presence of lymph node metastasis. Both EMT and MET are essential for cancer progress, as EMT of primary tumor cells is a necessity for motility and invasiveness and MET is important for the final stage of metastasis when the extravasated cancer cells revert to epithelial cells and proliferate as a secondary tumor in the metastatic site (11-13). The interface between the growing primary tumor and the tissue stroma is called” the invasive front”. The invasive front zone is composed of stromal cells such as fibroblasts, myofibroblasts, myeloid progenitor cells, immune cells including tumor-associated macrophages (TAMs) and many blood vessels (44). Signals from these cells promote the cancer cell invasion. At the invasive front, TAMs promote EMT, invasion and cancer stem cell (CSC)-like properties via transforming growth factor beta (TGF-β) (45). Activation of Wnt/β-catenin pathway induces cell motility and invasion. Wnt/β-catenin and RAS-extracellular signal-regulated kinase (ERK) pathways are essential for formation of CSCs and metastasis (46). High level expression of CSCs markers such as CD44 has been detected at invasive front in OSCC and mucoepidermoid carcinoma (MEC) (11, 13). Besides, high expression levels of vimentin, another EMT marker, and vascular endothelial cadherin (VE-cadherin), a tumor angiogenic and progression marker, have been shown at invasive front in OSCC and MEC (12, 13).
Role of ECM in cancer progression and its remodeling
ECM is composed of collagens, elastin, fibronectin, laminins, proteoglycans/glycosaminoglycans and some other glycoproteins and is a non-cellular three-dimensional macromolecular network of tissues. The components bind to each other and to the cell surface receptors to form a network. This network helps the cells to reside in all tissues and organs. These components make up the interstitial matrix and basement membrane (BM). Basement membrane is a specialized ECM which separates the epithelium or endothelium from stroma. Basement membrane is less porous and more compact than the interstitial matrix and is composed of type IV collagen, fibronectin, laminins and some linker proteins such as nidogen and entacin. Interestitial matrix is rich in fibrillar collagen, proteoglycans and some other glycoproteins such as tenacin C and fibronectin. Under pathological conditions, the biomechanical properties of ECM can change which have a great impact on the cell migration. Additionally, ECM is a dynamic environment which is constantly remodeled in different tissues at developmental stages; embryonic and postnatal. Lastly, cell-ECM interactions allow cells and tissues to adapt with the environment (47). Many matrix-degrading enzymes continuously rebuild ECM during normal and pathological conditions. ECM has a critical role in the maintenance of cell and tissue structure and function and organ morphogenesis. Therefore, the deregulation of composition results in several pathological conditions (48). Remodeling of ECM including basement membrane is one of the master switches for cancer invasion, neo-angiogenesis and metastasis (49). Moreover, ECM has a leading role in maintaining of stem cell properties and controls the stem cell differentiation. Stem cells are located in a specific microenvironment, called niche and play a critical role in the regeneration and maintenance of tissues (50). Normal niches are composed of ECM components, endothelial cells and perivascular cells or their progenitors as well as fibroblasts, immune cells, cytokines and growth factors (51). Thus, ECM functions as a reservoir for growth factors by making them unavailable, insoluble and non-functioning. Collagen, fibronectin, and proteoglycans, for example, bind to fibroblast growth factors (FGFs), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), transforming growth factor beta (TGF-β) and bone morphogenetic proteins (BMPs) (50). The lines of evidence indicate that the mediators of cell-ECM interactions such as integrin receptors provide transducing signals and physical links with the cytoskeleton from ECM to the cell protein modification processes including proliferation, migration and survival (52). CSCs need nutrients and signals from the surrounding microenvironment, also called CSCs niche, to achieve a balance between differentiation and self-renewal. In cancers, niche is a specialized local microenvironment including cancer-associated fibroblasts (CAFs), immune cells, non-CSC cancer cells, blood and lymphatic vessels, ECM, growth factors and cytokines. The expression and secretion of cytokines and growth factors regulate the cell-cell interactions between CSCs and stroma (12, 53). CSCs niche enhances CSCs differentiation and inhibits apoptosis. Moreover, niche may promote the accumulation of gene mutations by stem cells which lead to their malignant transformation into cancer cells (51). ECM promotes tumor progression by providing proliferative signals and disseminating tumor cells (54). Cancer cells release chemo-attractants from distant tissues; thus, interactions between tumor cells and stroma promote cancer development and metastasis (9). In the early stage of cancer metastasis, cancer cells degrade the epithelial basement membrane and come into contact with tumor stroma. Tumor cells invade the local stroma either in clusters/sheets (collective model) or as a single cell and enter the blood vessels (44). MMPs, the proteolytic enzymes, are necessary to degrade ECM (21). For example, in retinoblastoma, MMP2 and MMP9 degrade type IV collagen to facilitate invasion. MMP9 also promotes angiogenesis in cancers (55). The over-expression of MMP9 and MMP2 has been observed in head and neck squamous cell carcinomas patients presenting with lymph node involvement (56, 57). Bone metastasis is a frequent event in different cancers. Some factors which release growth factors in ECM promote bone degradation which release growth factors in ECM. Bone destruction enhances metastatic outgrowth (49). Metastasis to the liver is another predicting factor. Collagen IV is the main component of ECM within liver. Binding of collagen-IV to integrins (in particular integrin-α2) has been implicated within hepatic metastasis. Collagen-IV rescues the cancer cells from anoikis within the liver (58).
Anoikis apoptotic process
Anoikis is a Greek word and means loss of “home” or “homelessness”. It is a particular apoptotic process in response to loss of cell adhesion to ECM. Separation of a normal epithelial cell from its ECM results in anoikis. There are two apoptotic pathways; the mitochondrial (intrinsic) pathway and cell death receptor (extrinsic) pathway (59). Anoikis is essential to maintain tissue homeostasis by eliminating displaced endothelial / epithelial cells and therefore, preventing those cells from inappropriate seeding. Resistance to anoikis is a hallmark of EMT phenomenon and is a pre-requisite for metastasis (52). Also, resistance to anoikis increases the number of circulating tumor cells (CTCs) to facilitate recurrence and metastasis (60). A previous study has revealed that the heavily glycosylated mucin protein (MUC1) which over-express in all types of epithelial cancer cells can prevent the initiation of anoikis in response to loss of cell adhesion (61). Besides, activation of insulin receptor or insulin-like growth factor receptors has a key role in cancer cell resistance to anoikis. The exact role of p53 in anoikis has not been demonstrated; moreover, a protective role of the p53-via induction of ECM and expression of integrin gene has been shown. Another factor related to anoikis is E-cadherin. Knockdown of E-cadherin enhances cancer cell resistance to anoikis which results in EMT phenomenon. Both the TGF-β and Wnt pathways induce EMT which drives anoikis resistance (62). In contrary, Bcl-2 inhibitor of transcription 1 (Bit1) promotes apoptosis. After loss of cell attachment, Bit1 is secreted to the cytosol and interacts with the transcriptional regulator amino terminal enhancer of split (AES) to induce a caspase-independent form of apoptosis. Additionally, Bit1 inhibits EMT which has a great impact on tumor progression and aggressiveness; thus, it is considered as a tumor suppressor. Down–regulation of Bit1 in cancers such as lung adenocarcinoma enhances anoikis anchorage-independent growth and resistance, which results in tumorigenicity and metastasis (63). Platelets induce anoikis resistance, and promote metastasis via their association with extravasating tumor cells or detached. Cancer cells activate platelets via secretion of some factors such as adenosine 5′-diphosphate (ADP) which in turn leads to release the pro-angiogenic and pro-tumorigenic factors (64). These findings provide new insight into the molecular mechanism of anoikis regulation in cancer progression and metastasis.
Pre-metastatic niche
In 2005, Kaplan and colleagues first introduced the concept of pre-metastatic niche. They demonstrated that bone marrow-derived hematopoietic progenitor cells can express vascular endothelial growth factor receptor 1 (VEGFR1) which accumulates in the pre-metastatic lung tissue before tumor cell arrival (65). The environment at the distant target organ called pre- metastatic niche and is induced by factors derived from the primary site. A pre-metastatic niche is a supportive environment for cancer growth in the host tissue before spread of cancer cells. The formation of the pre-metastatic niche is a critical step towards metastasis (66). Host microenvironment is modified by primary tumor via secretion of some factors such as tumor cell-secreted factors and exosomes, host cell (fibroblast) alternations and non-resident cell (haematopoietic progenitor cells) recruitment (67). The pre-metastatic niche contains activated fibroblasts which secrete high levels of ECM proteins like type I collagen (68). In addition, tumor cells-host ECM interactions result in increasing capillary permeability and clot formation which promote metastasis (67, 69). In other words, for successful metastasis, tumor cells not only have to get specific genetic properties but also need to prepare the local microenvironment at the distant recipient organs. Metastatic cancers have preferences for specific target organs and specific microenvironments. For instance, metastatic breast cancer cells often migrate to the pre-metastatic niches located at the lungs, liver, brain, bone, and lymph nodes. These findings suggest that each tissue has specific characteristics that promote tumor cell homing, adhesion, and growth (7, 70). Hence, the distribution of distant metastases to particular organs is not a random process (metastatic organotropism)(69).
Angiogenesis in cancer
In 1971, Folkman first proposed a theory regarding tumor angiogenesis. According to his hypothesis, a tumor produces its own new blood supply from existing blood vessels. Angiogenesis has an essential role in tumor growth and metastasis. Accumulating lines of evidence have indicated that some cancer blood vessels are not lined by endothelial cells (71). Maniotis et al. first found that the aggressive melanoma cells form vascular-like channels which function as tumor blood vessels to supply nutrition. This phenomenon was called “vasculogenic mimicry” (VM) (72). VM formation enhances tumor growth and cancer metastasis (71). In the early stage of VM formation, the vascular channels are lined by tumor cells and VM is the main source of blood supply. Over time, endothelial cells differentiate and proliferate to contribute to vessel formation. In this stage, the vessels are lined by both tumor cells and endothelial cells, called” mosaic pattern”. Lastly, endothelial lined vessels replace VM and mosaic vessels (73). VM formation demonstrates the ability of tumor cells to differentiate into endothelial-like cells to form blood channels independent of the endothelial cells. The transformed endothelial-like cells produce matrix proteins such as collagens IV and VI, laminin, proteoglycans and heparan sulfate which help to form tubular networks. Histologically, VM channels are lined by tumor cells with a basement membrane in the external wall where there is not any endothelial cells on the inner wall (11, 12, 74).
The plasticity and heterogeneity of cancer cells promote the acquisition of endothelial cell markers such as vascular endothelial growth factor receptor 2 (VEGFR2), CD31 and VE-Cadherin (CD144). Hypoxia is an important factor for VM formation which up-regulates VE-cadherin expression (75). Angiogenesis is a master switch in the development of metastasis not only because of supplying the nutrition but also because of providing some pathways for cancer cells which travel to other organs to form new tumors. Production of VEGF is the main signaling pathway for angiogenesis (76, 77). Tumor cells, reactive stromal cells and infiltrating inflammatory cells are the sources of VEGF (78). Besides, over-expression of hypoxia-induced factor-1 α (HIF-1α) induces EMT and metastasis in cancers such as head and neck cancer. Co-expression of HIF-1α, Snail and Twist is associated with metastasis and poor prognosis in human head and neck cancers (79). The Tie receptors 1 and 2 (Tie1/2) have essential roles in embryonic angiogenesis as well as tumorigenesis. Elevated expression of Tie1 has been detected in different types of cancer and has a negative correlation with clinical outcome. Besides, Tie1-positive cells show cancer stemness properties (80). Recent findings indicate that CSCs initiate tumor neovascularization. CSCs can differentiate to endothelial cells and vascular smooth muscle-like cells. In addition, CSCs form VM channels. For instance, a previous published work on glioma demonstrated that glioma stem cells expressed both neoplastic and endothelial markers and VM positive tumor tissues showed elevated expression levels of some genes associated with CSCs (81). Under hypoxic conditions, CSCs express hypoxia-inducible factors (HIFs) which are controled by TGF-β. HIFs are the primary factors for promoting angiogenesis through induction of VEGF. Both endothelial cells and CSCs produce VEGF. VEGF-A can recruit monocytes and macrophages (51). In addition to ECM and angiogenesis, several different cell types are involved in cancer metastasis.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent mesenchymal stromal cells which first described by Friedenstein et al. as fibroblast-like cells in the bone marrow (82). Some other tissues like placenta, and adipose tissue also contain MSCs (83). MSCs allow a cellular population to generate diverse cell types and can be characterized by specific cell surface markers. More than 95% of the cell population expresses CD105, CD73, CD44 and CD90 (84, 85). Due to proliferation and differentiation potential of MSCs, they are novel opportunities for some clinical applications, such as cell therapy, cancer gene therapy, treatment of graft versus host disease and regenerative medicine. Besides, MSCs are nearly unidentifiable by immune system which helps them to migrate through the circulation. In addition, because of low immunogenicity of MSCs they are novel therapeutic approaches even without HLA matching (86). The unique characteristic of MSCs is the ability to migrate to sites of inflammation, tissue injury and cancerous tissues (87). MSCs also suppress immune response via inhibition of T-cell proliferation, dendritic cell maturation and natural killer (NK)/B-cell activation (88). Cancer cells provoke a chronic inflammatory response within the tumor microenvironment via producing inflammatory chemokines and growth factors. Some of chemokines associated with angiogenesis and tumor progression are epidermal growth factor (EGF), fibroblast growth factor (FGF), granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM-SCF),vascular endothelial growth factor-A (VEGF-A), platelet-derived growth factor (PDGF), angiopoietin-1, urokinase-type plasminogen activator (uPA), IL-6, IL-8 and TGF-β1 (86). Within the tumor microenvironment, MSCs have this ability to differentiate into cancer associated fibroblasts (CAFs) to support tumor progression (89). MSCs participate in several crucial steps of invasion and metastasis, such as EMT phenomenon (90). Cancers contain a number of factors for activating and recruiting of MSCs. In turn, MSCs modulate biological properties of tumor cells directly by EMT phenomenon. Migration of MSCs toward the primary and metastatic tumor microenvironments has been indicated in some cancer types such as skin cancer and lung cancer (91). In cancer microenvironment, MSCs also induce angiogenesis and resistance to drugs (92). In general, MSCs enhance cancer cell proliferation, angiogenesis and metastasis.
Cancer- associated fibroblasts
Cancer-associated fibroblasts (CAFs) are spindle shaped cells which morphologically look like myofibroblasts and are one of the most abundant cell types in the stroma (93). A previous study has indicated that bone marrow derived stromal cells and MSCs are the major sources of CAFs (94). Accumulated documents reported a cross talk between cancer cells and CAFs. In several cancers, the presence of CAFs in the stroma is associated with poor prognosis and increased risk of metastasis (95). CAFs promote tumor proliferation, invasion, and metastasis through producing several factors including cytokines such as uPA and growth factors which cleaves MMPs to induce ECM degradation and to promote angiogenesis and EMT (93). CAFs are involved in tumor cell proliferation via different mechanisms, for instance, in gastric cancer; CAFs target PTEN through the up-regulation of microRNA106b (96). Besides, CAFs improve the ability of cancer cells to invade and metastasize via EMT phenomenon (94) and the secretion of angiogenic factors such as VEGF and angiopoietin. On the other hand, CAFs promote the infiltration of immune cells in cancer tissues by producing inflammatory mediators such as chemokine (97). CD44 is expressed in CAFs and enhances the interactions between cancer cells and CAFs which may suggest the contribution of CD44 in tumorigenicity, stemness and drug resistance (11, 13, 98). CAFs are mainly located at the tumor periphery (93). CSC-like cells make-up a heterogeneous population of cells surrounded by myofibroblast-like cells. It is hypothesized that CSCs might be the source of the CAFs and support tumor maintenance and survival. In turn, CAFs support CSC self-renewal (99). Furthermore, in prostate cancer, cancer-associated fibroblasts (CAFs) induce EMT via the secretion of MMPs (51).
Metastasis initiating cells, circulating tumor cells, and Circulating tumor microemboli
Metastasis-initiating cells (MICs) are cancer cells with the ability of seeding in the secondary organs. The tumor-initiating cells (TICs) are the primary tumor counterparts of MICs and both increase the cancer cell plasticity and stemness. However, MICs need to get additional capabilities which enable them to survive the function and metastatic cascade as TICs in distant target organ microenvironment (100). MICs might represent a subpopulation of CSCs. MICs might be early- stage disseminating CSCs or might obtain from late-stage disseminating CSC clones (101). Metastasis results from the successful circulation of primary cancer cells into a distant organ; hence, it is logical to expect to find MICs among circulating tumor cells (CTCs) as well as disseminated tumor cells (DTCs) in the metastatic niche (102). CTCs are a cell population with heterogeneity in number, size, and clustering status as well as the dissemination pathways. CTCs are at a very low concentration especially in early tumor stages and are associated with poor prognosis. Detection of CTCs prior and during therapy has a great impact on cancer therapy outcome (92). CTCs infiltrate the circulation by the acquisition of EMT characteristics and survive within the circulation (103). However, not all CTCs and DTCs are capable of forming micro- or macro-metastasis, as most of the cells persist within the metastatic tissue but do not survive the oxygen tension changes and shear stresses (20). In the circulatory system, CTCs form cell clusters or circulating tumor microemboli (CTM). CTM is composed of 2 to 50 cells. In some cases, it is made up of more than 100 cells. CTM has a high capacity of metastasis compared to single cell. Furthermore, the contribution of CTM in metastatic process has been reported. Notably, discovering CTM, even a single one in the cancer patient blood significantly correlates with worse prognosis and decreased disease- free survival rate. In addition to cancer cells, CTMs contain some other cell types such as platelets, endothelial cells, fibroblasts, leukocytes and pericytes. Due to rapid entrapment in small capillaries, CTMs have a short half-life. Nevertheless, it has been shown that CTMs containing less than 20 cells form a single file pattern to traverse small blood channels. A previous study on melanoma indicated that cancer cells produce VEGF-A which forms micronodules of cancer cells. In the next phase, VEGF-A promotes vascular dilation to facilitate the intravasation of micronodules. A growing body of evidence indicates that dissemination of cancer cells is an early event in cancer development. Although, to get metastatic ability, they need further evolution at the metastatic site. EMT phenomenon might be a key mechanism which induces mesenchymal phenotypes of the cells within a CTM to promote metastasis. Higher expression level of plakoglobin, a cell-cell adhesion molecule, has been demonstrated in CTM compared to single CTC. Recent publications have reported that the cells within CTMs are negative for proliferation marker Ki67 which may indicate that these cells are chemo-resistant and may reflect the necessity of new drug development (104).
The role of platelets in the survival of CTCs
Some cancer cells secrete platelet-activating mediators such as thrombin , ADP and thromboxane A2 (TXA2) (105). Metastatic cancer cells induce the aggregation of platelets, in turn, platelets contribute to survival of CTCs in the blood stream (106). Autotaxin (ATX) an enzyme released by platelets, enhances cancer cell proliferation, angiogenesis and metastasis (107). Serotonin, another factor released by platelets, activates angiogenesis by enhancing the proliferation of endothelial cells (108). After activation of platelet, its granules release TGF-β1 which promotes EMT phenomenon. Besides, the increased number of platelets is associated with chemo-resistance and poor prognosis (109, 110). Platelets coat CTCs to protect them from shear forces; however, most CTCs die in circulation due to shear stress and/or anoikis (111). Additionally, tumor cells bind to the platelet adhesive proteins such as fibronectin and von Willebrand factor via integrins to form tumor emboli. In the next phase, tumor emboli can anchor to the luminal side of endothelial cells to get a greater chance of entrapment in small vessels which give more time to tumor cells to extravasate. CTCs may reach to the metastatic site even before the appearance of clinical features; thus, the presence of CTCs is associated with a poor prognosis (112).
Tumor immune network
Inflammatory response is another characteristic of cancer. Different immune cells have different impacts on tumors. Some immune cells act as promotor and some as antitumor. Besides, they play crucial roles in therapeutic resistance (113). Different types of immune cell are involved in cancers including macrophages, dendritic cells, mast cells, B cells, effector T cells (T-helper cells and cytotoxic T cells) and natural killer (NK) cells (114). Chronically activated leukocytes produce some mitogenic growth factors. For example, EGF, TGF-β, tumor necrosis factor alpha (TNF-α), fibroblast growth factor, interleukins (ILs) and chemokines. Additionally, granulocytes, monocytes, macrophages and mast cells secrete proteolytic enzymes to modify the structure and function of ECM. Moreover, in chronic inflammatory responses, leukocytes enhance angiogenesis and migration of cancer cells (115).
Tumor-associated macrophages (TAMs) might be the most abundant inflammatory cells which mainly aggregate within the tumor tissues rather than in peri-tumoral tissues. The number, phenotype and distribution pattern of TAMs influence patient outcomes (116). Interactions between cancer cells and macrophages play a critical role in regulation of ECM and immune surveillance of cancer cells. Previous studies have revealed that macrophages are heterogeneous cells which may explain the plasticity of these cells. High plasticity helps them to adapt to complex environments such as cancer (117). They are divided into M1 and M2 macrophages. They have paradoxical properties, for example, M1 type macrophages kill pathogens and enhance the activation of cytotoxic T lymphocytes (CTLs). Thus, they are correlated to more favourable prognosis and function as anti-tumor. In contrast, M2 type macrophages stimulate a CD4+ and regulatory T-cell response to promote angiogenesis and tissue remodeling. M2 type macrophages activate secretion of interleukin 6 (IL-6), TGF-β and interleukin 10 (IL-10), and VEGF to enhance tumor growth which are associated with a worse prognosis (114). M1 macrophages express type-1 cell-attracting chemokines such as C-X-C motif chemokine 10 (CXCL10) and Chemokine (C-X-C motif) ligand 9 (CXCL9) but M2 macrophages express the Chemokine (C-C motif) ligand 17 (CCL17), C-C motif chemokine 22 (CCL22) and Chemokine (C-C motif) ligand 24 (CCL24) (118). In glioblastoma, CSCs produce cytokines via activation of the STAT3 pathway to recruit and polarize macrophages to become M2-like cells, in turn, M2- like cells serve as a CSC niche to enhance CSC growth (119).
CD4+ T helper cells have a key role in the inflammatory processes in cancers via secretion of a set of cytokines. CD4+ T cell contribute to tumorigenesis through different mechanisms. For example, regulatory T cells (Tregs), an immunosuppressive subset of TH cells, prevent cytotoxic functions of CD8+ T cells to avoid rejection of tumor. TH cells also contribute to angiogenesis and recruitment of myeloid cells especially neutrophils. In breast cancer TH2 cells increase the risk of metastasis to sentinel lymph nodes by the expression of GATA binding protein 3 (GATA-3) (113).
Mast cells, another type of inflammatory cells, are present within the tumor and peri-tumoral microenvironment, termed tumor –associated mast cells (TAMCs) (120). The role of mast cells in tumor progression is still controversial. For example, a previous study has found that decreased number of mast cell in peri-tumoral stroma of deeply invasive melanoma is associated with poor prognosis; however, mast cell density is not a prognostic factor for superficially invasive melanoma. These results indicate that the role of mast cells in melanoma depends on the phase of tumor progression (120, 121). Another study has indicated that mast cells promote the growth of cancer in the initial stages not later stages of prostate cancer by producing MMP-9 in ECM (122). Mast cells produce several pro-angiogenic factors such as VEGF-A, -B, and FGF-2 as well as lymphangiogenic factors including VEGF-C and –D (120). In oral squamous cell carcinoma (OSCC) increased microvessel density is associated with increased mast cell density and is correlated to poor prognosis (123-125). Besides, mast cells induce EMT and stem cell features in human cancer via the synthesis of CXCL8 (IL-8), a member of the chemokine family (120).
Neutrophils also play a critical role in host inflammatory responses. Neutrophils regulate cancer development and metastatic processes. These cells can display either pro- or anti-tumor characteristics. Pro-tumor neutrophils enhance angiogenesis through producing pro-angiogenic factors. Endothelial cells recruit the immune cells such as neutrophils. Hence, activation of endothelial cells has a great impact on the regulation of metastasis. Besides, neutrophils have immunosuppressive properties and can limit anti-tumor immune responses. On the other hand, neutrophils have anti-tumor properties, such as ability to kill tumor cells. In addition, neutrophils stimulate anti-tumor responses leading to the activation of T cells and tumor rejection (66). Several lines of evidence point to the role of the neutrophils as the main population of cells in pre-metastatic niche. Neutrophils promote metastasis through extracellular matrix remodeling, angiogenesis, proliferation of cancer cells, promotion of invasion, and induction of inflammation at secondary sites. Granulocyte colony-stimulating factor (G-CSF) organizes neutrophils and simplifies their homing at distant target organs prior to the arrival of cancer cells (66). Besides, the aforementioned factors and different cell types some other factors such as exosomes, miRNAs and circRNAs are involved in cancer progression and metastasis.
Exosomes
Exosomes are small microvesicles (sized about 50–100 nm) obtain from cellular endocytosis (via multivesicular endosome pathway and reverse inward budding) and contain common proteins, such as membrane trafficking proteins (Rab proteins, ARF GTPases, and annexins), tetraspanins (CD9, CD63, and CD81) and cytoskeletal proteins. Besides, exosomes contain mRNAs, miRNAs and specific lipids encapsulated in a cholesterol-rich phospholipid membrane while playing critical roles in long-distance intercellular communications (126, 127). Exosomes are the smallest extracellular vesicles secreted by almost every cell type including mast cells, T lymphocytes, dendritic cells, platelets, epithelial cells, and neurons. They alter the functions of recipient cells by transferring bioactive molecules (127) and contribute to some interactions between endothelial cells, infiltrating immune cells, MSCs, and cancer cells. (128). Exosomes are released by cancer cells in the surrounding peripheral circulation and microenvironment. Exosomes derived from CAFs collaborate with cancer cells to optimize the tumor microenvironment (126). In tumor microenvironment, exosomes can modify recipient cell phenotypes through the exchange of genetic information (129). TAMs and MSCs also produce exosomes in various cell types to promote cancer cell growth and metastasis (127). Besides, the shedding of exosomes by cancer cells under normoxic and hypoxic conditions may promote EMT phenomenon, angiogenesis, and formation of pre-metastatic niches, metastases at distant tissues and organs, and treatment resistance (130). Exosomes have been reported to transfer the properties of chemo-resistance among different types of cancer cells and inhibits apoptosis (131). Exosomes also enhance the metastatic potential of primary tumors through MET phenomenon (132). Exosomes present in all body fluids such as urine, amniotic fluid, cerebrospinal fluid, plasma, synovial fluid and saliva (133).
MicroRNAs and circRNAs regulate cancers
MicroRNAs (miRNAs) are endogenous small non-coding RNAs. They can regulate gene expression in the post–transcriptional stage by interacting with the 3’ untranslated region (3’ UTR) of the target mRNA. MiRNAs play crucial roles in different cancers including proliferation, differentiation, apoptosis, survival, motility, invasion and metastasis, and morphogenesis. It has been shown that tmiRNAs can be used as novel molecular biomarkers for cancer diagnosis. Regarding the role of miRNAs in cancers, they are classified as two main groups; tumor suppressor miRNAs and oncogene miRNAs (oncomirs)(134). Altered miRNA expression depends on its role as a tumor suppressor or oncogene. Additionally, metastamir, a specialized family of miRNAs, has pro or anti-metastatic effects (134, 135). Different miRNAs have been investigated in a variety of cancers. For each miRNA, several functional roles and target genes have been indicated. Tables 1 and 2 summarize some of these results.
Table 1.
A Summary of some tumor suppressor miRNAs and their target genes
| miRNA | Cancer type (Reference No.) | Involved pathway | Target gene |
|---|---|---|---|
| miR-1 | Ovarian cancer (136) | Proliferation and invasion | c-Met |
| miR-7 | Lung cancer (137) | Tumor Growth | TTF-1 |
| miR-7 | Hepatocarcinoma (138) | Proliferation and invasion | KLF-4 |
| miR-9-3p | Nasopharynx carcinoma (139) | EMT | FN1,ITGB1 and ITGAV |
| miR-17-5p | Non-small cell lung cancer (140) | Proliferation and apoptosis | TGFβR2 |
| miR-29a/b/c | Glioma (141) | Invasion | CDC42 |
| miR-30 | Lung cancer (142) | EMT | Snail |
| miR-31 | Gastric cancer (143) | Metastasis | RhoA |
| miR-33a | Melanoma (144) | Angiogenesis | HIF-1α |
| miR-34a | Bladder cancer (145) | Proliferation and invasion | HNF4G |
| miR-99a | Cervical carcinoma (146) | Proliferation | TRIB2 |
| miR-100 | Esophageal carcinoma (147) | Tumor growth | CXCR7 |
| miR-107-5p | Non-small cell lung cancer (148) | Tumor growth | EGFR |
| miR-126 | Esophageal cancer (149) | Proliferation | VEGF-A |
| miR-141-3p | Prostate cancer (150) | Bone metastasis | NF-κB |
| miR-143 | Breast cancer (151) | Tumor growth | ERK5,MAP3K7 |
| miR-145-3p | Non-small cell lung cancer (152) | Migration and invasion | PDK1 |
| miR-148a | Renal cell carcinoma (153) | Proliferation and invasion | AKT2 |
| miR-195 | Laryngeal carcinoma (154) | Tumor growth and invasion | DCUN1D1 |
| miR-203 | Hepatocellular carcinoma (155) | Metastasis | RASAL2 |
| miR-211-5p | Thyroid cancer (156) | Proliferation and invasion | SOX 11 |
| miR-429 | Hepatocellular carcinoma (157) | Migration and invasion | CRKL |
| miR-551b | Gastric cancer (158) | EMT and metastasis | ERBB4 |
Table 2.
A summary of some Oncomirs and their target genes
| miRNA | Cancer type (Reference No.) | Involved in | Target gene |
| miR-10b | Laryngeal carcinoma (159) | EMT | E-Cadherin |
| miR-21 | Neuroblastoma (160) | Proliferation and apoptosis | PTEN and PDCD4 |
| miR-24 | Breast cancer (161) | Proliferation and invasion | SFRP4 and LATS2 |
| miR-93 | Hepatocellular carcinoma (162) | EMT and Metastasis | PDCD4 |
| miR-135b | Pancreatic cancer (163) | Metastasis | SFRP4 |
| miR-155 | Clear-cell renal-cell carcinoma (164) | Migration and invasion | FOXO3a |
| miR-181 | Non-small cell lung cancer (165) | Chemo-resistance | PI3K/AKT/mTOR |
| miR-191 | Cervical cancer (166) | Proliferation | CDK9, NOTCH2, and RPS6KA3 |
| miR-223 | Lung cancer (167) | Invasion | EPB41L3 |
MiRNAs are the major component of exosomes. Transportation of miRNAs by exosomes makes them resistant to degradation by extracellular ribonucleases. Therefore, miRNAs can be transported to different cell types or to the pre-metastatic niche which enable them to influence the gene expression of target cells. Accumulative evidence indicates that miRNAs may regulate CSCs functions and characteristics by controlling the self-renewal and differentiation of embryonic stem cells (168). MicroRNA alterations correlate to the initiation and progression of cancer cell proliferation or inhibition of tumorigenesis. For example, miR-155 is up-regulated in oral cancer and its dys-regulation is associated with the low level of cell division cycle 73 (CDC73), a tumor suppressor gene; therefore, it promotes cancer cell proliferation (169). A previous study on oral squamous cell carcinoma cells indicated that transfecting with miR-125b or miR-100 significantly decreased cell proliferation; however, co-transfection had a greater impact on proliferation than individual transfection (170). However, miR-551b, an oncogenic miRNA, promotes proliferation and invasion in ovarian cancer cells (171). MiRNAs also contribute to cancer development. Previous studies have indicated that dys-regulation of miRNAs plays a crucial role in the progression of oral precancerous lesion from dysplasia to OSCC. For instance, miR-31 negatively controls oral leukoplakia progression through the regulation of fibroblast growth factor 3 (FGF3) (134). In addition, in oral dysplasia, up-regulation of miR-21, miR-181b, and miR-345 is associated with lesion severity (172). The expression level of miR-126 is conversely related to VEGF-A protein in cancers such as oral cancer, esophageal cancer and gastric cancer (149, 173, 174). The miR-17-92 cluster is positively associated with angiogenesis in some cancers; however, individual components of the miR-17-92 cluster show the opposite effects on angiogenesis (134). Over-expression of miR-210 increases HIF-1α expression level and promotes EMT (175). Over-expression or sub-expression of miRNAs in cancers has the ability to activate or block the target mRNAs. Accumulating evidence shows that exosomes can fuse with the target cell membrane to carry miRNAs. For example, MSC-derived exosomes carry pro-angiogenesis miRNAs and transfer these miRNAs to endothelial cells to promote angiogenesis (176). miR-221 is highly expressed in exosomes secreted by MSCs (177). Finally, some miRNAs are involved in CAFs function. Among them, miR-31 and miR-214, are down-regulated and miR-155 is up-regulated in normal fibroblasts to convert the fibroblasts into a CAF-like phenotype (178).
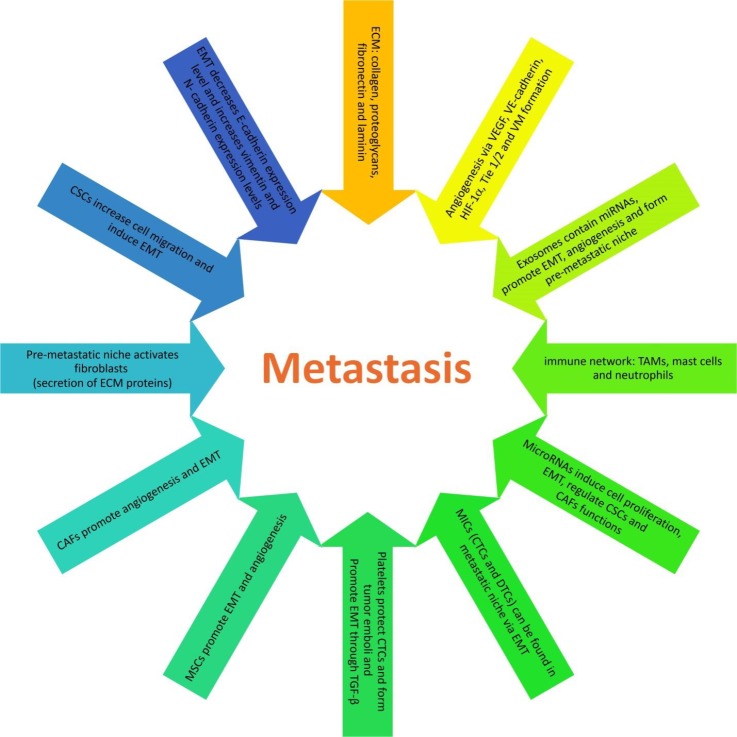
Recently, circular RNAs (circRNAs), 100 bp to 4 kb in size, have been demonstrated in different cancers. They are classified as three types: intronic type, exonic type and exonic-intronic type. Exonic circRNAs are mostly found in cell cytoplasm, but the other two types have been shown in cell nucleus. Recent studies indicate that circRNAs can block the binding of miRNAs to the 3’ UTR of a specific gene; hence, can regulate the gene expression (179). Circulating miRNAs may play a critical role in the diagnosis of various types of cancer. For example, elevated serum expression levels of miR-20a, miR-27a and miR-423-5p are recognized as biomarkers for gastric cancer (180). Figure 1 summarizes the specific signaling pathways that control metastasis.
Figure 1.
Metastasis is controlled by specific signaling pathways
Discussion
Metastasis means the dissemination of the cancer cells from one organ to another which is not directly connected to the primary site and has a crucial role in the prognosis of cancer patients. Metastasis is critical factor to predict survival in patients with advanced cancer and prognosis determines the treatment plan. Metastasis is the main cause of cancer-related death around the world. The field of molecular pathology and new diagnostic methods has rapidly grown in the last decade. According to the recent genetic and molecular findings, a successful metastasis requires both the accumulation of mutations and acquiring more invasive phenotypes and establishment of a pre-metastatic niche (67). Due to high death rate from cancer around the world, it is worth to find therapeutic strategies to eradicate the metastatic process. A variety of therapeutic approaches have been tested for the cancer treatment.
The presence of CSCs explains several biologic behaviors of cancers such as tumor growth, tumor recurrence and metastasis and tumor chemo-resistance. Inability to eradicate CSCs may be one of the most important reasons of therapy failure. Hence, CSC surface markers are the promising targets for cancer therapy. A growing body of studies demonstrates that the conjugation of an anti CSC surface marker to a cytotoxic drug is able to inhibit cancer cell growth (181). EMT phenomenon starts the initial metastatic step, local tumor cell invasion and intravasation into the blood channels. Besides, EMT has an essential role in drug resistance. Blocking the EMT- related signaling pathways controls tumor, growth and metastasis. Another important factor for metastasis is ECM which promotes tumor progression by providing several factors for dissemination of cancer cells (54) and pre-metastatic niche construction, a promising therapeutic target (67). Consequently, cancer cells release chemo-attractants from distant tissues to enhance metastasis (9). Different cell types such as MSCs, CAFs, and immune cells are novel therapeutic targets as well. MSCs have this ability to gather in the tumor microenvironment; then, MSC-secreted exosomes should be investigated for therapeutic applications (128). Angiogenesis is a crucial for cancer cell growth; therefore, angiogenic factors like VEGF are also promising therapeutic targets (77). CTCs express some surface markers such as EpCAM and different subtypes of cytokeratin (CK) (182). These markers can be used as minimally invasive biomarkers for early diagnosis, prognosis and monitoring of recurrence. Exosome also can be used as a biomarker. It is suggested that specific exosomal miRNAs serve as indicator of the metastatic process. For example, increased expression of serum exosomal miR-203 in cases of colorectal cancer indicates poor prognosis and liver metastasis. Exosomes are shed into biological fluids; hence, can be used as noninvasive tool for diagnosis and follow-up monitoring of cancer patients. CD9, CD63, CD81, tubulin, actin and CD82 are the common biomarkers of most exosomes (183). Moreover, tumor cell–derived exosomes express stem cell–like markers including CD44, CD133 and/or CD105 (131). Hence, exosomes are promising cancer targets. In addition to different intracellular miRNAs, several extracellular/circular miRNAs have been demonstrated outside the cells including body fluids. These miRNAs are considered as biomarkers for several pathophysiological conditions (184). In a metastatic carcinoma, the miRNA expression profile has been demonstrated to be different from a non-metastatic tumor. Therefore, interference with the expression level of miRNAs has an effect on cancer prognosis. These findings provide a therapeutic use for miRNAs (134). Besides, some circRNAs present in human body fluids. Therefore, they may newly found clinical biomarkers in cancers.
Conclusion
Metastasis is a multistep process. Many signaling pathways and molecules are involved in metastasis. Most of the involved factors can be easily detected due to the presence of reliable detection techniques. However, many other novel and valid techniques are needed to predict target genes and it is still a long a way before these progression can be used as routine clinical practice. Increasing knowledge about the mechanism of metastasis can help in finding the promising targets of cancer therapy (185, 186). Further investigation is necessary regarding the regulatory mechanisms of cancers.
Acknowledgment
The author would like to acknowledge the funding from Hamadan University of Medical Sciences, Hamadan, Iran.
References
- 1.Irani S. Pre-cancerous lesions in the oral and maxillofacial region: A literature review with special focus on etopathogenesis. Iran j pathol. 2016;11:303–322. [PMC free article] [PubMed] [Google Scholar]
- 2.Irani S, Bidari –Zerehpoush F, Sabeti S. Prevalence of pathological entities in neck masses: A study of 1208 consecutive cases. Avicenna J Dent Res. 2016;8:e25614. [Google Scholar]
- 3.Irani S, Moshref M, Lotfi A. Metastasis of a gastric adenocarcinoma to the mandible:A case report. Oral Oncol extra. 2004;40:85–87. [Google Scholar]
- 4.Irani S. Metastasis to the Jawbones: A review of 453 cases. J Int Soc Prev Community Dent. 2017;7:71–81. doi: 10.4103/jispcd.JISPCD_512_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irani S. Metastasis to the oral soft tissues: A review of 412 cases. J Int Soc Prev Community Dent. 2016;6:393–401. doi: 10.4103/2231-0762.192935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irani S. Metastasis to head and neck area: a 16-year retrospective study. Am J Otolaryngol. 2011;32:24–27. doi: 10.1016/j.amjoto.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Irani S. Distant metastasis from oral cancer: A review and molecular biologic aspects. J Int Soc Prev Community Dent. 2016;6:265–271. doi: 10.4103/2231-0762.186805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pein M, Oskarsson T. Microenvironment in metastasis: roadblocks and supportive niches. Am J Physiol Cell Physiol. 2015;309:C627–638. doi: 10.1152/ajpcell.00145.2015. [DOI] [PubMed] [Google Scholar]
- 9.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. J Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qureshi-Baig K, Ullmann P, Haan S, Letellier E. Tumor-initiating cells: a critical review of isolation approaches and new challenges in targeting strategies. Mol Cancer. 2017;16:40. doi: 10.1186/s12943-017-0602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irani S, Dehghan A. The expression and functional significance of vascular endothelial-cadherin, CD44, and vimentin in oral squamous cell carcinoma. J Int Soc Prev Community Dent. 2018;8:408–417. doi: 10.4103/jispcd.JISPCD_408_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irani S, Dehghan A. Expression of vascular endothelial-cadherin in mucoepidermoid carcinoma: role in cancer development. J Int Soc Prev Community Dent. 2017;7:301–307. doi: 10.4103/jispcd.JISPCD_323_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani S, Jafari B. Expression of vimentin and CD44 in mucoepidermoid carcinoma: A role in tumor growth. Indian J Dent Res. 2018;29:330–340. doi: 10.4103/ijdr.IJDR_184_17. [DOI] [PubMed] [Google Scholar]
- 14.Phi LTH, Sari IN, Yang YG, Lee SH, Jun N, Kim KS, et al. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells int. 2018;2018:5416923. doi: 10.1155/2018/5416923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos A, Ding Z, Loftus JC, Tran NL. Molecular and microenvironmental determinants of glioma stem-like cell survival and invasion. Front Oncol. 2017;7:120. doi: 10.3389/fonc.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell stem cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Fulda S. Regulation of apoptosis pathways in cancer stem cells. Cancer Lett. 2013;338:168–173. doi: 10.1016/j.canlet.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Geng S, Guo Y, Wang Q, Li L, Wang J. Cancer stem-like cells enriched with CD29 and CD44 markers exhibit molecular characteristics with epithelial-mesenchymal transition in squamous cell carcinoma. Arch Dermatol Res. 2013;305:35–47. doi: 10.1007/s00403-012-1260-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim WT, Ryu CJ. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017;50:285–298. doi: 10.5483/BMBRep.2017.50.6.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris KS, Kerr BA. Prostate cancer stem cell markers drive progression, therapeutic resistance, and bone metastasis. Stem Cells int. 2017;2017:8629234. doi: 10.1155/2017/8629234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jablonska-Trypuc A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme inhib Med Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 22.Chetty C, Vanamala SK, Gondi CS, Dinh DH, Gujrati M, Rao JS. MMP-9 induces CD44 cleavage and CD44 mediated cell migration in glioblastoma xenograft cells. Cell Signal. 2012;24:549–559. doi: 10.1016/j.cellsig.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 24.Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. ScientificWorldJournal. 2014;2014:521754. doi: 10.1155/2014/521754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Judd NP, Winkler AE, Murillo-Sauca O, Brotman JJ, Law JH, Lewis JS Jr, et al. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res. 2012;72:365–374. doi: 10.1158/0008-5472.CAN-11-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiozawa Y, Taichman RS. Cancer stem cells and the bone marrow microenvironment. Bonekey Rep. 2012;1:48. doi: 10.1038/bonekey.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazama S, Kishikawa J, Kiyomatsu T, Kawai K, Nozawa H, Ishihara S, et al. Expression of the stem cell marker CD133 is related to tumor development in colorectal carcinogenesis. Asian J Surg. 2018;41:274–278. doi: 10.1016/j.asjsur.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Nomura A, Banerjee S, Chugh R, Dudeja V, Yamamoto M, Vickers SM, et al. CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget. 2015;6:8313–8322. doi: 10.18632/oncotarget.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Song X, Chen Z, Li X, Li M, Liu H, et al. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PloS One. 2013;8:e56380. doi: 10.1371/journal.pone.0056380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura M, Zhang X, Mizumoto Y, Maida Y, Bono Y, Takakura M, et al. Molecular characterization of CD133+ cancer stem-like cells in endometrial cancer. Int J Oncol. 2014;44:669–677. doi: 10.3892/ijo.2013.2230. [DOI] [PubMed] [Google Scholar]
- 31.Baillie R, Tan ST, Itinteang T. Cancer stem cells in oral cavity squamous cell carcinoma: A review. Front Oncol. 2017;7:112. doi: 10.3389/fonc.2017.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao J, Jin Q, Wang XD, Zhu HJ, Ni QC. Aldehyde dehydrogenase 1 expression is correlated with poor prognosis in breast cancer. Medicine. 2017;96:e7171. doi: 10.1097/MD.0000000000007171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Torres M, Allan AL. Aldehyde dehydrogenase as a marker and functional mediator of metastasis in solid tumors. Clin Exp Metastasis. 2016;33:97–113. doi: 10.1007/s10585-015-9755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol. 2012;2012:708036. doi: 10.1155/2012/708036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taniuchi K, Nishimori I, Hollingsworth MA. Intracellular CD24 inhibits cell invasion by posttranscriptional regulation of BART through interaction with G3BP. Cancer Res. 2011;71:895–905. doi: 10.1158/0008-5472.CAN-10-2743. [DOI] [PubMed] [Google Scholar]
- 36.Hatano Y, Fukuda S, Hisamatsu K, Hirata A, Hara A, Tomita H. Multifaceted Interpretation of Colon Cancer Stem Cells. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18071446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jolly MK, Tripathi SC, Jia D, Mooney SM, Celiktas M, Hanash SM, et al. Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget. 2016;7:27067–27084. doi: 10.18632/oncotarget.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Da C, Wu K, Yue C, Bai P, Wang R, Wang G, et al. N-cadherin promotes thyroid tumorigenesis through modulating major signaling pathways. Oncotarget. 2017;8:8131–8142. doi: 10.18632/oncotarget.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang R, Zong X. Aberrant cancer metabolism in epithelial-mesenchymal transition and cancer metastasis: Mechanisms in cancer progression. Crit Rev Oncol Hematol. 2017;115:13–22. doi: 10.1016/j.critrevonc.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Pradella D, Naro C, Sette C, Ghigna C. EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression. Mol cancer. 2017;16:8. doi: 10.1186/s12943-016-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sommers CL, Heckford SE, Skerker JM, Worland P, Torri JA, Thompson EW, et al. Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant human breast cancer cell lines. Cancer Res. 1992;52:5190–5197. [PubMed] [Google Scholar]
- 42.Ishiwata T. Cancer stem cells and epithelial-mesenchymal transition: Novel therapeutic targets for cancer. Pathol Int. 2016;66:601–608. doi: 10.1111/pin.12447. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Ye D, Guo W, Yu W, He Y, Hu J, et al. G9a is essential for EMT-mediated metastasis and maintenance of cancer stem cell-like characters in head and neck squamous cell carcinoma. Oncotarget. 2015;6:6887–6901. doi: 10.18632/oncotarget.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. doi: 10.1016/j.ceb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. cancers. 2015;7:2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SK, Hwang JH, Choi KY. Interaction of the Wnt/beta-catenin and RAS-ERK pathways involving co-stabilization of both beta-catenin and RAS plays important roles in the colorectal tumorigenesis. Adv Biol Regul. 2018;68:46–54. doi: 10.1016/j.jbior.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. Journal Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35 Suppl:S244–S275. doi: 10.1016/j.semcancer.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 53.Zhao J, Li J, Schlosser HA, Popp F, Popp MC, Alakus H, Jauch KW, et al. Targeting cancer stem cells and their niche: Current therapeutic implications and challenges in pancreatic cancer. stem cells Int. 2017;2017:6012810. doi: 10.1155/2017/6012810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sangaletti S, Chiodoni C, Tripodo C, Colombo MP. The good and bad of targeting cancer-associated extracellular matrix. Curr Opin Pharmacol. 2017;35:75–82. doi: 10.1016/j.coph.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Webb AH, Gao BT, Goldsmith ZK, Irvine AS, Saleh N, Lee RP, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer. 2017;17:434. doi: 10.1186/s12885-017-3418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markwell SM, Weed SA. Tumor and stromal-based contributions to head and neck squamous cell carcinoma invasion. Cancers. 2015;7:382–406. doi: 10.3390/cancers7010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pietruszewska W, Bojanowska-Pozniak K, Kobos J. Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: an immunohistochemical study. Otolaryngol Pol . 2016;70(3):32–43. doi: 10.5604/00306657.1202546. [DOI] [PubMed] [Google Scholar]
- 58.Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 2014;40:558–566. doi: 10.1016/j.ctrv.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Jenning S, Pham T, Ireland SK, Ruoslahti E, Biliran H. Bit1 in anoikis resistance and tumor metastasis. Cancer Lett. 2013;333:147–151. doi: 10.1016/j.canlet.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun B, Hu C, Yang Z, Zhang X, Zhao L, Xiong J, et al. Midkine promotes hepatocellular carcinoma metastasis by elevating anoikis resistance of circulating tumor cells. Oncotarget. 2017;8:32523–32535. doi: 10.18632/oncotarget.15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piyush T, Rhodes JM, Yu LG. MUC1 O-glycosylation contributes to anoikis resistance in epithelial cancer cells. Cell Death Discov. 2017;3:17044. doi: 10.1038/cddiscovery.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frisch SM, Schaller M, Cieply B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J Cell Sci. 2013;126:21–29. doi: 10.1242/jcs.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao X, Gray S, Pham T, Delgardo M, Nguyen A, Do S, et al. Downregulation of Bit1 expression promotes growth, anoikis resistance, and transformation of immortalized human bronchial epithelial cells via Erk activation-dependent suppression of E-cadherin. Biochem Biophys Res Commun. 2018;495:1240–1248. doi: 10.1016/j.bbrc.2017.11.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8:310. doi: 10.1038/s41467-017-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jablonska J, Lang S, Sionov RV, Granot Z. The regulation of pre-metastatic niche formation by neutrophils. Oncotarget. 2017;8:112132–112144. doi: 10.18632/oncotarget.22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qian JJ, Akcay E. Competition and niche construction in a model of cancer metastasis. PloS One. 2018;13:e0198163. doi: 10.1371/journal.pone.0198163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoye AM, Erler JT. Structural ECM components in the premetastatic and metastatic niche. Am J Physiol Cell Physiol. 2016;310:C955–967. doi: 10.1152/ajpcell.00326.2015. [DOI] [PubMed] [Google Scholar]
- 69.Chen W, Hoffmann AD, Liu H, Liu X. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. NPJ Precis oncol. 2018;2:4. doi: 10.1038/s41698-018-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aguado BA, Bushnell GG, Rao SS, Jeruss JS, Shea LD. Engineering the pre-metastatic niche. Nat Biomed Eng. 2017:1. doi: 10.1038/s41551-017-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang W, Lin P, Han C, Cai W, Zhao X, Sun B. Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J Exp Clin Cancer Res . 2010;29 doi: 10.1186/1756-9966-29-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ping YF, Bian XW. Consice review: Contribution of cancer stem cells to neovascularization. Stem Cells. 2011;29:888–894. doi: 10.1002/stem.650. [DOI] [PubMed] [Google Scholar]
- 74.Chen YS, Chen ZP. Vasculogenic mimicry: a novel target for glioma therapy. Chin J Cancer. 2014;33:74–79. doi: 10.5732/cjc.012.10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Angara K, Borin TF, Arbab AS. Vascular Mimicry: A novel neovascularization mechanism driving anti-angiogenic therapy (AAT) resistance in glioblastoma. Transl Oncol. 2017;10:650–660. doi: 10.1016/j.tranon.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irani S, Salajegheh A, Gopalan V, Smith RA, Lam AK. Expression profile of endothelin 1 and its receptor endothelin receptor A in papillary thyroid carcinoma and their correlations with clinicopathologic characteristics. Ann Diagn Pathol. 2014;18:43–48. doi: 10.1016/j.anndiagpath.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Irani S, Salajegheh A, Smith RA, Lam AK. A review of the profile of endothelin axis in cancer and its management. Crit Rev Oncol Hematol. 2014;89:314–321. doi: 10.1016/j.critrevonc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, Zhang W, et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta. 2014;1845:182–201. doi: 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287–292. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torigata M, Yamakawa D, Takakura N. Elevated expression of Tie1 is accompanied by acquisition of cancer stemness properties in colorectal cancer. Cancer Med. 2017;6:1378–1388. doi: 10.1002/cam4.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao X, Ping Y, Liu Y, Chen K, Yoshimura T, Liu M, et al. Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells. PloS One. 2013;8:e57188. doi: 10.1371/journal.pone.0057188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alshammari A, Eldeib OJ, Eldeib AJ, Saleh W. Adenoid cystic carcinoma of the submandibular gland with rare metastasis to the sternum in a 52-year-old male. Ann Thorac Med. 2016;11:82–84. doi: 10.4103/1817-1737.165307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clinc Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- 84.T LR, Sanchez-Abarca LI, Muntion S, Preciado S, Puig N, Lopez-Ruano G, et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commu Signal . 2016;14:2. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Togarrati PP, Sasaki RT, Abdel-Mohsen M, Dinglasan N, Deng X, Desai S, et al. Identification and characterization of a rich population of CD34(+) mesenchymal stem/stromal cells in human parotid, sublingual and submandibular glands. Sci Rep. 2017;7:3484. doi: 10.1038/s41598-017-03681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marofi F, Vahedi G, Biglari A, Esmaeilzadeh A, Athari SS. Mesenchymal stromal/stem cells: A new era in the cell-based targeted gene therapy of Cancer. Front Immunol. 2017;8:1770. doi: 10.3389/fimmu.2017.01770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fan L, Hu C, Chen J, Cen P, Wang J, Li L. Interaction between mesenchymal stem cells and B-cells. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishihara S, Ponik SM, Haga H. Mesenchymal stem cells in breast cancer: response to chemical and mechanical stimuli. Oncoscience. 2017;4:158–159. doi: 10.18632/oncoscience.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang AI, Schwertschkow AH, Nolta JA, Wu J. Involvement of mesenchymal stem cells in cancer progression and metastases. Curr Vancer Drug Targets. 2015;15:88–98. doi: 10.2174/1568009615666150126154151. [DOI] [PubMed] [Google Scholar]
- 91.Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16 doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kudo-Saito C. Cancer-associated mesenchymal stem cells aggravate tumor progression. Front Cell Dev Biol. 2015;3 doi: 10.3389/fcell.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kubo N, Araki K, Kuwano H, Shirabe K. Cancer-associated fibroblasts in hepatocellular carcinoma. World J Gastroenterol. 2016;22:6841–6850. doi: 10.3748/wjg.v22.i30.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hanley CJ, Noble F, Ward M, Bullock M, Drifka C, Mellone M, et al. A subset of myofibroblastic cancer-associated fibroblasts regulate collagen fiber elongation, which is prognostic in multiple cancers. Oncotarget. 2016;7:6159–6174. doi: 10.18632/oncotarget.6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang TS, Yang XH, Chen X, Wang XD, Hua J, Zhou DL, et al. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. FEBS Lett. 2014;588:2162–2169. doi: 10.1016/j.febslet.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Q, Peng C. Cancer-associated fibroblasts regulate the biological behavior of cancer cells and stroma in gastric cancer. Oncol Lett. 2018;15:691–698. doi: 10.3892/ol.2017.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo J, Hsu H, Tyan S, Li F, Shew J, Lee W, et al. Serglycin in tumor microenvironment promotes non-small cell lung cancer aggressiveness in a CD44-dependent manner. Oncogene . 2017;36:2457–2471. doi: 10.1038/onc.2016.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nair N, Calle AS, Zahra MH, Prieto-Vila M, Oo AKK, Hurley L, et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci Rep. 2017;7 doi: 10.1038/s41598-017-07144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Celia-Terrassa T, Kang Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016;30:892–908. doi: 10.1101/gad.277681.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liao WT, Ye YP, Deng YJ, Bian XW, Ding YQ. Metastatic cancer stem cells: from the concept to therapeutics. Am J Stem Cells. 2014;3:46–62. [PMC free article] [PubMed] [Google Scholar]
- 102.Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer. 2017;16:4. doi: 10.1186/s12943-016-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Onstenk W, Sieuwerts AM, Mostert B, Lalmahomed Z, Bolt-de Vries JB, van Galen A, et al. Molecular characteristics of circulating tumor cells resemble the liver metastasis more closely than the primary tumor in metastatic colorectal cancer. Oncotarget. 2016;7:59058–59069. doi: 10.18632/oncotarget.10175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Umer M, Vaidyanathan R, Nguyen NT, Shiddiky MJA. Circulating tumor microemboli: Progress in molecular understanding and enrichment technologies. Biotechnol Adv. 2018;36:1367–1389. doi: 10.1016/j.biotechadv.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 105.Li N. Platelets in cancer metastasis: To help the “villain” to do evil. Int J Cancer . 2016;138:2078–2087. doi: 10.1002/ijc.29847. [DOI] [PubMed] [Google Scholar]
- 106.Leblanc R, Peyruchaud O. The role of platelets and megakaryocytes in bone metastasis. J Bone Oncol. 2016;5:109–111. doi: 10.1016/j.jbo.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elaskalani O, Berndt MC, Falasca M, Metharom P. Targeting platelets for the treatment of cancer. Cancers. 2017:9. doi: 10.3390/cancers9070094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qin L, Zhao D, Xu J, Ren X, Terwilliger EF, Parangi S, et al. The vascular permeabilizing factors histamine and serotonin induce angiogenesis through TR3/Nur77 and subsequently truncate it through thrombospondin-1. Blood. 2013;121:2154–2164. doi: 10.1182/blood-2012-07-443903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kizer NT, Hatem H, Nugent EK, Zhou G, Moore K, Heller P, et al. Chemotherapy response rates among patients with endometrial cancer who have elevated serum platelets. Int J Gynecol Cancer. 2015;25:1015–1022. doi: 10.1097/IGC.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elaskalani O, Falasca M. The role of platelet-derived ADP and ATP in promoting pancreatic cancer cell survival and gemcitabine resistance. Cancers. 2017:9. doi: 10.3390/cancers9100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol. 2017;11:40–61. doi: 10.1002/1878-0261.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lou XL, Sun J, Gong SQ, Yu XF, Gong R, Deng H. Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res. 2015;27:450–460. doi: 10.3978/j.issn.1000-9604.2015.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coussens LM, Zitvoge L, Palucka AK. Neutralizing tumor-promoting Chronic Inflammation: A Magic Bullet? Sci. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dionne LK, Driver ER, Wang XJ. Head and neck cancer stem cells: From identification to tumor immune network. J Dent Res. 2015;94:1524–1531. doi: 10.1177/0022034515599766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 116.Liu JY, Peng CW, Yang GF, Hu WQ, Yang XJ, Huang CQ, et al. Distribution pattern of tumor associated macrophages predicts the prognosis of gastric cancer. Oncotarget. 2017;8:92757–92769. doi: 10.18632/oncotarget.21575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Y, Zhou N, Yu X, Zhang X, Li S, Lei Z, et al. Tumacrophage: macrophages transformed into tumor stem-like cells by virulent genetic material from tumor cells. Oncotarget. 2017;8:82326–82343. doi: 10.18632/oncotarget.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, Baral R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2017;316:1–10. doi: 10.1016/j.cellimm.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 119.Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res. 2010;16:800–813. doi: 10.1158/1078-0432.CCR-09-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, et al. Are Mast Cells MASTers in Cancer? Front Immunol. 2017;8:424. doi: 10.3389/fimmu.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Siiskonen H, Poukka M, Bykachev A, Tyynela-Korhonen K, Sironen R, Pasonen-Seppanen S, et al. Low numbers of tryptase+ and chymase+ mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Res. 2015;25:479–485. doi: 10.1097/CMR.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 122.Pittoni P, Tripodo C, Piconese S, Mauri G, Parenza M, Rigoni A, et al. Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res. 2011;71:5987–5997. doi: 10.1158/0008-5472.CAN-11-1637. [DOI] [PubMed] [Google Scholar]
- 123.Zaidi M, Mallick A. A study on assessment of mast cells in oral squamous cell carcinoma. Ann Med Health Sci Res. 2014;4:457–460. doi: 10.4103/2141-9248.133479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gudiseva S, Santosh ABR, Chitturi R, Anumula V, Poosarla C, Baddam VRR. The role of mast cells in oral squamous cell carcinoma. Contemp Oncol. 2017;21:21–29. doi: 10.5114/wo.2017.65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Laishram D, Rao K, Devi HSU, Priya NS, Smitha T, Sheethal HS. Mast cells and angiogenesis in malignant and premalignant oral lesions: An immunohistochemical study. J Oral Maxillofac Pathol. 2017;21:229–238. doi: 10.4103/jomfp.JOMFP_111_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma P, Pan Y, Li W, Sun C, Liu J, Xu T, et al. Extracellular vesicles-mediated noncoding RNAs transfer in cancer. J Hematol Oncol. 2017;10:57. doi: 10.1186/s13045-017-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Plebanek MP, Angeloni NL, Vinokour E, Li J, Henkin A, Martinez-Marin D, et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun. 2017;8:1319. doi: 10.1038/s41467-017-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun F, Wang JZ, Luo JJ, Wang YQ, Pan Q. Exosomes in the Oncobiology, Diagnosis, and Therapy of Hepatic Carcinoma: A New Player of an Old Game. BioMed Res Int. 2018;2018:2747461. doi: 10.1155/2018/2747461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santos JC, Lima NDS, Sarian LO, Matheu A, Ribeiro ML. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci Rep. 2018;8:829. doi: 10.1038/s41598-018-19339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hon KW, Abu N, Ab Mutalib NS, Jamal R. Exosomes As Potential Biomarkers and Targeted Therapy in Colorectal Cancer: A Mini-Review. Front Pharmacol. 2017;8:583. doi: 10.3389/fphar.2017.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blackwell RH, Foreman KE, Gupta GN. The Role of Cancer-Derived Exosomes in Tumorigenicity & Epithelial-to-Mesenchymal Transition. Cancers. 2017:9. doi: 10.3390/cancers9080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weidle UH, Birzele F, Kollmorgen G, Ruger R. The Multiple Roles of Exosomes in Metastasis. Cancer Genomics Proteomics. 2017;14:1–15. doi: 10.21873/cgp.20015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Armstrong D, Wildman DE. Extracellular Vesicles and the Promise of Continuous Liquid Biopsies. J Pathol Transl Med. 2018;52:1–8. doi: 10.4132/jptm.2017.05.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Irani S. miRNAs signature in head and neck squamous cell carcinoma metastasis: A literature review. J Dent. 2016;17:71–83. [PMC free article] [PubMed] [Google Scholar]
- 135.Maroof H, Irani S, Ariana A, Vider J, Gopalan V, Lam AK. Interactions of vascular endothelial growth factor and p53 with miR-195 in thyroid carcinoma: possible therapeutic targets in aggressive thyroid cancers. Curr Cancer Drug Targets. 2018 doi: 10.2174/1568009618666180628154727. [DOI] [PubMed] [Google Scholar]
- 136.Qu W, Chen X, Wang J, Lv J, Yan D. MicroRNA-1 inhibits ovarian cancer cell proliferation and migration through c-Met pathway. Clin Chim Acta. 2017;473:237–244. doi: 10.1016/j.cca.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 137.Lei L, Chen C, Zhao J, Wang H, Guo M, Zhou Y, et al. Targeted Expression of miR-7 Operated by TTF-1 Promoter Inhibited the Growth of Human Lung Cancer through the NDUFA4 Pathway. Mol Ther Nucleic Acids. 2017;6:183–197. doi: 10.1016/j.omtn.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu W, Liu S, Liang Y, Zhou Z, Liu X. MiR-7 inhibits progression of hepatocarcinoma by targeting KLF-4 and promises a novel diagnostic biomarker. Cancer Cell Int. 2017;17:31. doi: 10.1186/s12935-017-0386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ding Y, Pan Y, Liu S, Jiang F, Jiao J. Elevation of MiR-9-3p suppresses the epithelial-mesenchymal transition of nasopharyngeal carcinoma cells via down-regulating FN1, ITGB1 and ITGAV. Cancer Biol Ther. 2017;18:414–424. doi: 10.1080/15384047.2017.1323585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li H, Zhou H, Luo J, Huang J. MicroRNA-17-5p inhibits proliferation and triggers apoptosis in non-small cell lung cancer by targeting transforming growth factor beta receptor 2. Exp Ther Med. 2017;13:2715–2722. doi: 10.3892/etm.2017.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shi C, Ren L, Sun C, Yu L, Bian X, Zhou X, et al. miR-29a/b/c function as invasion suppressors for gliomas by targeting CDC42 and predict the prognosis of patients. Br J Cancer. 2017;117:1036–1047. doi: 10.1038/bjc.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fan MJ, Zhong YH, Shen W, Yuan KF, Zhao GH, Zhang Y, et al. MiR-30 suppresses lung cancer cell 95D epithelial mesenchymal transition and invasion through targeted regulating Snail. Eur Rev Med Pharmacol Sci. 2017;21:2642–2649. [PubMed] [Google Scholar]
- 143.Ge F, Wang C, Wang W, Liu W, Wu B. MicroRNA-31 inhibits tumor invasion and metastasis by targeting RhoA in human gastric cancer. Oncol Rep. 2017;38:1133–1139. doi: 10.3892/or.2017.5758. [DOI] [PubMed] [Google Scholar]
- 144.Zhou J, Xu D, Xie H, Tang J, Liu R, Li J, et al. miR-33a functions as a tumor suppressor in melanoma by targeting HIF-1α. Cancer Biol Ther. 2015;16:846–855. doi: 10.1080/15384047.2015.1030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun H, Tian J, Xian W, Xie T, Yang X. miR-34a inhibits proliferation and invasion of bladder cancer cells by targeting orphan nuclear receptor HNF4G. Dis Markers. 2015;2015:879254. doi: 10.1155/2015/879254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xin J, Yue Z, Zhang S, Jiang Z, Wang P, Li Y, et al. miR-99 inhibits cervical carcinoma cell proliferation by targeting TRIB2. Oncol Lett. 2013;6:1025–1030. doi: 10.3892/ol.2013.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou SM, Zhang F, Chen XB, Jun CM, Jing X, Wei DX, et al. miR-100 suppresses the proliferation and tumor growth of esophageal squamous cancer cells via targeting CXCR7. Oncol Rep. 2016;35:3453–3459. doi: 10.3892/or.2016.4701. [DOI] [PubMed] [Google Scholar]
- 148.Wang P, Liu X, Shao Y, Wang H, Liang C, Han B, et al. MicroRNA-107-5p suppresses non-small cell lung cancer by directly targeting oncogene epidermal growth factor receptor. Oncotarget. 2017;8:57012–57023. doi: 10.18632/oncotarget.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kong R, Ma Y, Feng J, Li S, Zhang W, Jiang J, et al. The crucial role of miR-126 on suppressing progression of esophageal cancer by targeting VEGF-A. Cell Mol Biol Lett. 2016;21:3. doi: 10.1186/s11658-016-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang S, Wa Q, Pan J, Peng X, Ren D, Huang Y, et al. Downregulation of miR-141-3p promotes bone metastasis via activating NF-κB signaling in prostate cancer. J Exp Clin Cancer Res. 2017:36. doi: 10.1186/s13046-017-0645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou LL, Dong JL, Huang G, Sun ZL, Wu J. MicroRNA-143 inhibits cell growth by targeting ERK5 and MAP3K7 in breast cancer. Braz J Med Biol Res. 2017;50:e5891. doi: 10.1590/1414-431X20175891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen GM, Zheng AJ, Cai J, Han P, Ji HB, Wang LL. microRNA-145-3p inhibits non-small cell lung cancer cell migration and invasion by targeting PDK1 via the mTOR signaling pathway. J Cell Biochem. 2018;119:885–895. doi: 10.1002/jcb.26252. [DOI] [PubMed] [Google Scholar]
- 153.Cao H, Liu Z, Wang R, Zhang X, Yi W, Nie G, et al. miR-148a suppresses human renal cell carcinoma malignancy by targeting AKT2. Oncol Rep. 2017;37:147–154. doi: 10.3892/or.2016.5257. [DOI] [PubMed] [Google Scholar]
- 154.Shuang Y, Li C, Zhou X, Huang Y, Zhang L. MicroRNA-195 inhibits growth and invasion of laryngeal carcinoma cells by directly targeting DCUN1D1. Oncol Rep. 2017;38:2155–2165. doi: 10.3892/or.2017.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fang JF, Zhao HP, Wang ZF, Zheng SS. Upregulation of RASAL2 promotes proliferation and metastasis, and is targeted by miR-203 in hepatocellular carcinoma. Mol Med Rep. 2017;15:2720–2726. doi: 10.3892/mmr.2017.6320. [DOI] [PubMed] [Google Scholar]
- 156.Wang L, Shen YF, Shi ZM, Shang XJ, Jin DL, Xi F. Overexpression miR-211-5p hinders the proliferation, migration, and invasion of thyroid tumor cells by downregulating SOX11. J Clin Lab Anal. 2017:32. doi: 10.1002/jcla.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guo C, Zhao D, Zhang Q, Liu S, Sun MZ. miR-429 suppresses tumor migration and invasion by targeting CRKL in hepatocellular carcinoma via inhibiting Raf/MEK/ERK pathway and epithelial-mesenchymal transition. Sci Rep. 2018:8. doi: 10.1038/s41598-018-20258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Song G, Zhang H, Chen C, Gong L, Chen B, Zhao S, et al. miR-551b regulates epithelial-mesenchymal transition and metastasis of gastric cancer by inhibiting ERBB4 expression. Oncotarget. 2017;8:45725–45735. doi: 10.18632/oncotarget.17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang L, Sun J, Wang B, Ren JC, Su W, Zhang T. MicroRNA-10b Triggers the Epithelial-Mesenchymal Transition (EMT) of Laryngeal Carcinoma Hep-2 Cells by Directly Targeting the E-cadherin. Appl Biochem Biotechnol. 2015;176:33–44. doi: 10.1007/s12010-015-1505-6. [DOI] [PubMed] [Google Scholar]
- 160.Wang Z, Yao W, Li K, Zheng N, Zheng C, Zhao X, et al. Reduction of miR-21 induces SK-N-SH cell apoptosis and inhibits proliferation via PTEN/PDCD4. Oncol Lett. 2017;13:4727–4733. doi: 10.3892/ol.2017.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cui S, Liao X, Ye C, Yin X, Liu M, Hong Y, et al. ING5 suppresses breast cancer progression and is regulated by miR-24. Mol Cancer. 2017;16:89. doi: 10.1186/s12943-017-0658-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ji C, Liu H, Yin Q, Li H, Gao H. miR-93 enhances hepatocellular carcinoma invasion and metastasis by EMT via targeting PDCD4. Biotechnol Lett. 2017;39:1621–169. doi: 10.1007/s10529-017-2403-5. [DOI] [PubMed] [Google Scholar]
- 163.Han X, Saiyin H, Zhao J, Fang Y, Rong Y, Shi C, et al. Overexpression of miR-135b-5p promotes unfavorable clinical characteristics and poor prognosis via the repression of SFRP4 in pancreatic cancer. Oncotarget. 2017;8:62195–62207. doi: 10.18632/oncotarget.19150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ji H, Tian D, Zhang B, Zhang Y, Yan D, Wu S. Overexpression of miR-155 in clear-cell renal cell carcinoma and its oncogenic effect through targeting FOXO3a. Exp Ther Med. 2017;13:2286–2292. doi: 10.3892/etm.2017.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu J, Xing Y, Rong L. miR-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the PTEN/PI3K/AKT pathway. Oncol Rep. 2018;39:1631–1639. doi: 10.3892/or.2018.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Polioudakis D, Abell NS, Iyer VR. MiR-191 Regulates Primary Human Fibroblast Proliferation and Directly Targets Multiple Oncogenes. PloS One. 2015;10:e0126535. doi: 10.1371/journal.pone.0126535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liang H, Yan X, Pan Y, Wang Y, Wang N, Li L, et al. MicroRNA-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol Cancer. 2015;14:58. doi: 10.1186/s12943-015-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yao S. MicroRNA biogenesis and their functions in regulating stem cell potency and differentiation. Biol Proced Online. 2016;18:8. doi: 10.1186/s12575-016-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fu S, Chen HH, Cheng P, Zhang CB, Wu Y. MiR-155 regulates oral squamous cell carcinoma Tca8113 cell proliferation, cycle, and apoptosis via regulating p27Kip1. Eur Rev Med pharmacol Sci. 2017;21:937–944. [PubMed] [Google Scholar]
- 170.Henson BJ, Bhattacharjee S, O’Dee DM, Feingold E, Gollin SM. Decreased expression of miR-125b and miR-100 in oral cancer cells contributes to malignancy. Genes Chromosomes Cancer. 2009;48:569–582. doi: 10.1002/gcc.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wei Z, Liu Y, Wang Y, Zhang Y, Luo Q, Man X, et al. Downregulation of Foxo3 and TRIM31 by miR-551b in side population promotes cell proliferation, invasion, and drug resistance of ovarian cancer. Med Oncol. 2016;33:126. doi: 10.1007/s12032-016-0842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hung PS, Tu HF, Kao SY, Yang CC, Liu CJ, Huang TY, et al. miR-31 is upregulated in oral premalignant epithelium and contributes to the immortalization of normal oral keratinocytes. Carcinogenesis. 2014;35:1162–1171. doi: 10.1093/carcin/bgu024. [DOI] [PubMed] [Google Scholar]
- 173.Chen H, Li L, Wang S, Lei Y, Ge Q, Lv N, et al. Reduced miR-126 expression facilitates angiogenesis of gastric cancer through its regulation on VEGF-A. Oncotarget. 2014;5:11873–1185. doi: 10.18632/oncotarget.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sasahira T, Kurihara M, Bhawal UK, Ueda N, Shimomoto T, Yamamoto K, et al. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br J Cancer. 2012;107:700–706. doi: 10.1038/bjc.2012.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu Y, Wang J, Meng X, Xie H, Tan J, Guo X, et al. A positive feedback loop promotes HIF-1alpha stability through miR-210-mediated suppression of RUNX3 in paraquat-induced EMT. J Cell Mol Med. 2017;21:3529–3539. doi: 10.1111/jcmm.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8:45200–45212. doi: 10.18632/oncotarget.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110:1199–1210. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang Z, Tan Y, Yu W, Zheng S, Zhang S, Sun L, et al. Small role with big impact: miRNAs as communicators in the cross-talk between cancer-associated fibroblasts and cancer cells. Int J Biol Sci. 2017;13:339–348. doi: 10.7150/ijbs.17680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang Z, Xie Q, He D, Ling Y, Li Y, Li J, et al. Circular RNA: new star, new hope in cancer. BMC Cancer. 2018;18:834. doi: 10.1186/s12885-018-4689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang YK, Yu JC. Circulating microRNAs and long non-coding RNAs in gastric cancer diagnosis: An update and review. World J Gastroenterol. 2015;21:9863–9886. doi: 10.3748/wjg.v21.i34.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Agliano A, Calvo A, Box C. The challenge of targeting cancer stem cells to halt metastasis. Semin Cancer Biol. 2017;44:25–42. doi: 10.1016/j.semcancer.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 182.Schneck H, Gierke B, Uppenkamp F, Behrens B, Niederacher D, Stoecklein NH, et al. EpCAM-Independent Enrichment of Circulating Tumor Cells in Metastatic Breast Cancer. PloS One. 2015;10:e0144535. doi: 10.1371/journal.pone.0144535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Iimmunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Montazerian M, Yasari F, Aghaalikhani N. Ovarian extracellular MicroRNAs as the potential non-invasive biomarkers: An update. Biomed Pharmacother. 2018;106:1633–1640. doi: 10.1016/j.biopha.2018.07.073. [DOI] [PubMed] [Google Scholar]
- 185.Irani S. New insights into Oral Cancer Prevention: A review article. Int J Prev Med. 2019 doi: 10.4103/ijpvm.IJPVM_403_18. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Irani S, Meschi M, Goodarzi A. Influence of education on oro-dental knowledge among school hygiene instructors. J Dent Res Dent Clin Dent Prospects. 2009;3:56–59. doi: 10.5681/joddd.2009.013. [DOI] [PMC free article] [PubMed] [Google Scholar]