Abstract
Objective:
The objective of this study was to develop a low-cost kit for the detection of subclinical mastitis (SCM) and to check its validity, reproducibility, and efficacy at the field level.
Materials and Methods:
A total of 550 quarter milk samples from crossbred dairy cows were collected, of which 400 milk samples were used to validate the newly developed BLRI mastitis test (BMT) kit to justify its efficacy as an individual test kit in detecting SCM based on somatic cell count (SCC) by direct microscopic count (DMC). The efficacy of the newly developed BMT was compared with the California Mastitis Test (CMT) kit. Another 150 milk samples were subjected to SCC determined by DMC and DCC (De Laval cell counter®) categorized by CMT and BMT scores.
Results:
A SCM test kit, namely, BMT kit was successfully developed in this study. The percentage accuracy of CMT and BMT were 76.75% and 75.75%; sensitivity 69.36% and 67.56%; specificity 85.95% and 85.85%; positive predictive value 86.03% and 85.71%; negative predictive value 69.23% and 68%, respectively. A p value of 0.001 was found for both CMT and BMT. However, CMT and BMT had no significant difference in sensitivity (p = 0.778). Average SCCs (cells/ml) determined by DCC and DMC, respectively, were mostly corresponded to the SCC ranges of both CMT and BMT scores.
Conclusion:
The newly developed BMT kit is an independent, cheap, farmer-friendly, first country made, and reliable SCM diagnostic test kit that can be used at field condition.
Keywords: Accuracy, BMT, CMT, efficacy, mastitis, validity
Introduction
Mastitis is considered as one of the most common diseases causing economic losses due to reduced milk production, increased labor costs, increased treatment costs, animal death, and premature culling [1,2]. Subclinical mastitis (SCM) shows no gross clinical signs in the udder of animals. However, this condition acts as a continuous source of infection for other herd mates. However, SCM may affect in decreasing milk quality and quantity causing huge economic loss [3,4]. Annual losses caused mainly by SCM in the USA are estimated at approximately 2 billion dollars, and 526 million dollars in India [5]. In Bangladesh, SCM causes great loss in the dairy industry, which estimates BDT 122.6 (US$ 2.11) million annually [6].
Besides economic losses, SCM also possesses the risk for the transmission of zoonotic diseases like brucellosis, leptospirosis, tuberculosis, and streptococcal sore throat to human [7]. The etiological agents responsible for SCM may vary from place to place and case to case depending on the animal species, breed, parity, production, disease management practices, and climatic condition [8,9]. More than 135 different types of pathogens are reported to be associated with mastitis. Thus, prevention and control of mastitis is a big challenge throughout the world. Several researchers have reported the prevalence, potential risk factors, and comparison of different screening tests for bovine mastitis in Bangladesh [10–17]. However, the screening tests of SCM using commercially available foreign kits need huge money. Therefore, development and validation of a cheap SCM screening kit is time demanding for saving foreign currencies and sustainable development of the dairy industry in Bangladesh. Here, we report a newly developed SCM test kit which is cheap, good in efficiency, and rapid in producing a visible result. The kit was also validated and compared for its applicability as compared to other commercially available SCM test kits in Bangladesh.
Materials and Methods
Ethical statement
The milk samples from the animals were collected by field veterinarians by following the international standard considering animal welfare and ethics.
Development of BLRI mastitis test (BMT) kit
After a series of trials, BMT was developed at the Bangladesh Livestock Research Institute Regional Station, Sirajganj. Composition of the BMT: sodium carbonate (1%), sodium lauryl ethyl sulphate (0.7%), and bromocresol purple (0.01%).
Selection of study area, duration, and study animal
The present study was conducted at Shahjadpur, Sirajganj, Sathia, Pabna, and Mymensingh during July 2017–June 2018. A total of 400 quarters milk samples from 100 apparently healthy crossbred dairy cows were collected. The milk samples were subjected to the screening of SCM by using the newly developed BMT. In addition, 150 milk samples were collected and subjected to somatic cell count (SCC) by direct microscopic count (DMC) and DCC (De Laval cell counter®) to validate the results of BMT and California Mastitis Test (CMT).
Sample collection
In this research work, a total of 550 bovine milk samples were collected during morning time. Before the collection of milk, the udder including teat and tips of teat were hygienically washed with water and soaked with 70% alcohol. The milk samples (15 ml from each quarter) were collected in pre-labeled screw-capped vials. CMT and BMT were done at the field prior to milk sample collection. The milk samples were kept in an icebox and transported to the Laboratory of Animal Health, Bangladesh Livestock Research Institute Regional Station, Baghabari, Sirajganj, where maximum tests were performed. As replicas, the samples were kept at 4ºC in a refrigerator for further laboratory investigations at the Department of Microbiology and Hygiene, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, and Department of Medicine, Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Chittagong.
California mastitis test (CMT) and BLRI mastitis test (BMT)
A total of 400 quarter milk samples from 100 crossbred dairy cows were subjected to BMT to justify its efficacy to validate as an individual test kit as compared to CMT in detecting SCM based on SCC through DMC. CMT was performed with commercial CMT kit (Immucell California Mastitis Test Kit, Portland) and the results were scored according to the manufacturer’s instructions. Another study with 150 milk samples, the SCCs were determined by DMC and DCC (De Laval cell counter®) categorized by CMT and BMT scores including average result were performed.
The milk samples were mixed properly for homogenization of cream. A drop (0.01 ml/10 μl) of milk was spread evenly over an area of 12 cm on a microscopic glass slide and was air-dried. Then, the milk fat from the slide was removed. For this, the glass slides were dipped in Xylene for 1–2 min and dried again. The dried slide was immersed in 95% ethanol for 2–5 min. Staining with Broadhurst-Paley stain for at least 5 sec was done if necessary. The leukocytes present in 10 microscopic fields were counted as per the method described by Schalm et al. [18].
The following criteria were used in making the cell count:
Within a field count all nucleated somatic cells including those at the periphery with more than 50% of the cell body in view.
Free nuclei representing more than 50% of the nuclear material are counted.
A cytoplasmic mass without a nucleus and small cell fragments with little nuclear material are not counted.
Animals were considered as positive for mastitis when CMT and BMT score was ≥1+ and SCC value was ≥2 × 105/ ml of milk (threshold value).
The following diagnostic test characteristics were determined using the milk somatic count result as a gold standard control.
Accuracy = TP + TN/TP + FP + TN + FN × 100
Sensitivity = TP/TP + FN × 100
Specificity = TN/TN + FP × 100, PPV = TP/TP + FP × 100, NPV = TN/TN + FN × 100
where: TP = True Positive, FP = False Positive, TN = True Negative, FN = False Negative.
Sensitivity, specificity, and accuracy test
We used CMT as a gold standard. Number of positive and negative quarter’s in CMT and BMT was recorded and sensitivity, specificity, accuracy, and predictive value were calculated, as per the method described by Greiner et al. [19].
Statistical analysis
Data analysis was done using STATA version 12.1 (STATA Corp., College Station, TX). The percentage accuracy of the tests and sensitivity, specificity, and the predictive values of the CMT and BMT results, compared to SCC were calculated using standard two-by-two contingency tables. Data were also analyzed by Chi-square test to observe the significant influence of CMT and BMT.
Results and Discussion
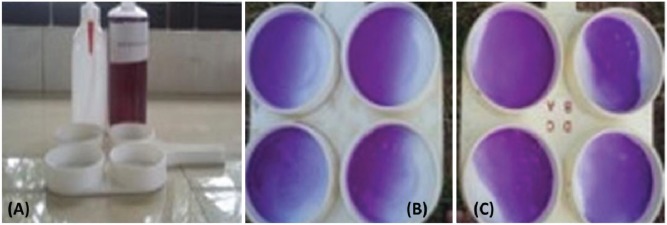
BMT was successfully developed at the Bangladesh Livestock Research Institute Regional Station, Sirajganj. The kit was developed by using locally available reagents (Fig. 1). Thus, costing of the kit was lower as compared to commercially available other mastitis test kit in Bangladesh. Moreover, accuracy, sensitivity, and specificity were almost similar to that of the commercially available mastitis test kit.
Figure 1. (A) BMT kit. (B) Milk testing showing strong positive results by BLRI Mastitis Test kit (B) and (C) California Mastitis Test kit. Both the kits showed similar results with the same milk sample.
The BMT could be considered as a rapid, cow-side, semiquantitative, and inexpensive test. Similar report has been reported by Schalm and Noor-lander [20] and Barnum and Newbould [21] on CMT that has been used for more than 60 years. On the other hand, BMT could be used as an inexpensive test to assess the SCM of animals, as reported by Sargeant et al. [22] on CMT. In another study, we found the CMT as an effective test kit for assessing SCM [13]. However, Sarker et al. [23] and Sumon et al. [17] found only 20.2% and 25% prevalence of SCM in dairy cows, respectively. A medium ranged prevalence of SCM (50.4% and 58%, respectively) was reported by Tripura et al. [24] and Mpatswenumugabo et al. [25]. This variation might be due to differences in management practices of the cows and geographical location.
This kit could be used regularly (every 2 weeks) in individual quarters for the entire herd to detect the presence of SCM. It can also be used to quantify SCC in composite and bulk tank samples. The BMT ingredient reacts with leucocytes (Somatic cells) that are elevated during mastitis. The degree of gel formation was proportional to the increasing number of leucocytes present during mammary gland inflammation. Greater gel formation corresponds to a higher BMT score. BMT results recorded as Negative when mixture remains liquid with no thickening; T (Trace), where slight thickening with padding movement was found, 1 (Weak), where distinct thickening was found, 2 (Distinct), where mixture thickened immediately on moving the center of cup, and 3 (Strong), where distinct gel formation was found, which tends to form a mass.
The prepared working solution of the BMT kit was found unchanged for 2 years in normal environmental temperature and humidity. The cost per test of milk (including reagents and materials) of the kit is 1 (One) BDT. While it needs BDT 25 to 50 by CMT kit. For both CMT and BMT, ≥2 lac cell/ml was considered as the scale of positivity in detecting SCM. The SCM positive of milk samples were detected for both CMT (44.75%; n = 179/400 and BMT (43.75%; n = 173/400) Table 1. All samples were subjected to SCC where 222 samples were positive by DMC. The percentage accuracy of CMT and BMT were 76.75% and 75.75%, respectively Table 1. Similar result was reported by Islam et al. [12] who found 74.49% prevalence of SCM in cattle by CMT. A sensitivity of 69.36% and 67.36% were found for CMT and BMT, respectively Table 2.
Table 1. Percentage accuracy of two indirect tests used for the diagnosis of mastitis.
| Tests | Samples examined | Positive samples (%) | Negative samples (%) | TP (%) | FP (%) | TN (%) | FN (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| CMT | 400 | 179 (44.75) | 221 (55.25) | 154 (86.01) | 25 (13.97) | 153 (69.23) | 52 (23.53) | 76.75 |
| BMT | 400 | 175 (43.75) | 225 (56.25) | 150 (85.71) | 25 (14.28) | 153 (68) | 72 (32) | 75.75 |
TP = True Positive, FP = False Positive, TN = True Negative, FN = False Negative; Accuracy: TP + TN/TP + FP + TN + FN × 100.
Table 2. Agreement and correlation between two tests used for the diagnosis of mastitis with SCC.
| Tests | Sensitivity % | Specificity % | PPV (%) | NPV (%) | p-value | p-value |
|---|---|---|---|---|---|---|
| CMT | 69.37 | 85.95 | 86.03 | 69.23 | 0.001 | 0.776 |
| BMT | 67.56 | 85.85 | 85.71 | 68 | 0.001 |
PPV = Positive predictive value, NPV = Negative predictive value, Sensitivity = TP/TP + FN × 100, Specificity = TN/TN+FP × 100, PPV = TP/TP+FP × 100, NPV = TN/TN + FN × 100.
A p-value of 0.001 was found for both CMT and BMT, and in case of comparison between them, the p-value was 0.776, i.e., insignificant Table 2. CMT and BMT had no difference in sensitivity. Both tests were sensitive to SCC as measured as 2 lac/ml and there was no difference in sensitivity among the test at SCC level above or below 2 lacs/ml Table 3. Somatic cells are normally found in milk, and the number of somatic cells increases when mammary glands are infected. Normal value of somatic cells in healthy udder ranged between 50,000 and 100,000 cells/ml of milk [26]. Sri Balaji et al. [27] reported that the SCC, milk pH, and chloride contents in milk are increased in SCM affected milk samples as compared to that of healthy dairy cows. Average SCC from quarter milk samples measured by CMT score justified the BMT score Table 4.
Table 3. Analytical values of CMT and BMT tests based on somatic cell count used for the comparison between them.
| Test | SCC Count | p-value | p-value | |||
|---|---|---|---|---|---|---|
| <2 lacs | ≥2 lacs | |||||
| Positive | Negative | Positive | Negative | |||
| CMT | 25 | 153 | 154 | 68 | <0.0001 | 0.776 |
| BMT | 25 | 153 | 150 | 72 | <0.0001 | |
| p-value | 1 | 0.683 | ||||
Table 4. Average SCC from quarter milk samples from dairy cows measured by DCC and DMC categorized by CMT score to justify the BMT score.
| CMT score | BMT score | Method | No. | CMT | Method | No. | CMT-BMT |
|---|---|---|---|---|---|---|---|
| SCC (×103) cells/ml | SCC (×103) cells/ml | ||||||
| 0 | 0 | DCC | 10 | 85.50 | DMC | 20 | 55.50 |
| T | T | DCC | 10 | 345.20 | DMC | 20 | 250.50 |
| 1 | 1 | DCC | 10 | 675.50 | DMC | 20 | 550.50 |
| 2 | 2 | DCC | 10 | 1357.50 | DMC | 20 | 1250.0 |
| 3 | 3 | DCC | 10 | 2449.60 | DMC | 20 | 3944.50 |
The newly developed BMT kit could be an independent, cheap, farmer’s friendly, country made, and alternative SCM testing kit having similar accuracy, sensitivity, and specificity as compared to those of CMT. Present findings support the earlier observations [28,29], who reported SCC as the most accurate test for the diagnosis of SCM followed by the modified California mastitis test and the modified white side test. The higher reliability of CMT followed by WST and SFMT was reported by Barua et al. [30].
The SCC and CMT are correlated for diagnosis of SCM, as described by Barbosa et al. [31]. The specificity of CMT and SCC with the standard cultural test was compared by Reddy et al. [21] and observed 100% predictive value with the cultural test of the milk, 84.84% specificity for SCC, and 73.30% for CMT [33]. The comparisons among various diagnostic tests for the detection of SCM performed by Barua et al. [30] indicated that SCM can be identified by different methods like CMT, WST, and SFMT. The BMT kit was also successfully used and validated for the detection of SCM in goats in a conventional and organized farm where similar results were observed (data not shown), confirming that the kit is suitable for the diagnosis of SCM in goats as well. However, we did not check the BMT kit for other animals, except cattle and goats.
BMT kit is cheap, easy, and farmer’s friendly and its reagents are locally available. The kit has five categories of result like CMT (negative, trace, weak, distinct, and strong). The test kit provides the farmer with a simple and rapid method for the detection of increased SCC in the udder. This cheap farm-based test needs no sophisticated equipment and is intended in part for use with good mastitis management practices to control the disease.
Conclusion
BMT is the first country made, reliable, and accurate bovine SCM diagnostic test. It is an independent, cheap, farmer’s friendly, country made, and alternative SCM test kit that shows similar accuracy, sensitivity, and specificity with categorizing scores as generated by CMT. This new kit can be used for the diagnosis of SCM in the field level in Bangladesh.
Acknowledgments
The authors would like to thank the Bangladesh Livestock Research Institute (BLRI) for funding (SRD-04; 2017-2018) this research project and Department of Microbiology and Hygiene, Bangladesh Agricultural University, Mymensingh, and Department of Medicine, Chittagong Veterinary and Animal Sciences University for providing the lab facilities. The authors also would like to thank the farmers and workers for their participation and cooperation.
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contribution
MHK, ME, and MG implemented the study design. HK, MSI, AY, and YA participated in data collection. MHK and MSAS performed the tests. MHK, AY, and YA drafted; ME, RK, and MSI revised the manuscript. KHMNH critically checked the article and corrected the manuscript. All authors read and approved the final version of the manuscript.
References
- [1].Chishty MA, Arshad M, Avais M, Ijaz M. Cross-sectional epidemiological studies on mastitis in cattle and buffaloes of tehsil. Gojra Pakistan. 2007;26:50–5. [Google Scholar]
- [2].Hashemi M, Kafi M, Safdarian M. The prevalence of clinical and subclinical mastitis in dairy cows in the central region of Fars province, south of Iran. Iran J Vet Res. 2011;12:3. [Google Scholar]
- [3].Swinkels JM, Hogeveen H, Zadoks RN. A Partial budget model to estimate economic benefits of lactational treatment of subclinical Staphylococcus aureus mastitis. J Dairy Sci. 2005;88:4273–87. doi: 10.3168/jds.S0022-0302(05)73113-1. https://doi.org/10.3168/jds.s0022-0302(05)73113-1. [DOI] [PubMed] [Google Scholar]
- [4].Halasa T, Huijps K, Osteras O, Hogeveen H. Economic effects of bovine mastitis and mastitis management. A review. Vet Quart. 2007;29:18–31. doi: 10.1080/01652176.2007.9695224. https://doi.org/10.1080/01652176.2007.96952 24. [DOI] [PubMed] [Google Scholar]
- [5].Varshney JP, Naresh R. Evaluation of a homeopathic complex in the clinical management of udder diseases of riverine buffaloes. Homeopathy. 2004;93(01):17–20. doi: 10.1016/j.homp.2003.11.007. https://doi.org/10.1016/j.homp.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [6].Kader MA, Samad MA, Saha S. Influence of host level factors on prevalence and economics of subclinical mastitis in dairy cows in Bangladesh. Indian J Dairy Sci. 2003;56:235–40. [Google Scholar]
- [7].Radostits OM, Blood DC, Gay CC, Hinchiff KW, Handerson JA. Veterinary Medicine. 9th. WB Saunders Company; London, UK: 2000. Mastitis; pp. 603–700. [Google Scholar]
- [8].Islam MA, Islam MZ, Islam MA, Rahman MS, Islam MT. Prevalence of subclinical mastitis in dairy cows in selected areas of Bangladesh. Bangladesh J Vet Med. 2011;9:73–8. https://doi.org/10.3329/bjvm.v9i1.11216. [Google Scholar]
- [9].Rabbani AFMG, Samad MA. Host determinants based comparative prevalence of subclinical mastitis in lactating Holstein-Friesian cross cows and Red Chittagong cows in Bangladesh. Bangladesh J Vet Med. 2011;8:17–21. https://doi.org/10.3329/bjvm.v8i1.7397. [Google Scholar]
- [10].Badiuzzaman M, Samad MA, Siddiki SHMF, Islam MT, Saha S. Subclinical mastitis in lactating cows: comparison of four screening tests and effect of animal factors on its occurrence. Bangladesh J Vet Med. 2015;2:41–50. https://doi.org/10.3329/bjvm.v13i2.26627. [Google Scholar]
- [11].Islam MA, Rahman AKMA, Rony SA, Islam MS. Prevalence and risk factors of mastitis in lactating dairy cows at Baghabari milk shed area of Sirajganj. Bangladesh J Vet Med. 2010;2:157–62. https://doi.org/10.3329/bjvm.v8i2.11200. [Google Scholar]
- [12].Islam NN, Farzana Z, Chowdhury AMMA, Mannan A, Kamaruddin KM, Siddiki AMAMZ, et al. Characterization of bovine subclinical mastitis caused by Staphylococcus aureus in southern Bangladesh by bacteriological and molecular approaches. Asian J Biol Sci. 2014;7:1–12. http://doi.org/10.3923/ajbs.2014.1.12. [Google Scholar]
- [13].Kabir MH, Ershaduzzaman M, Giasuddin M, Islam MR, Nazir KHMNH, Islam MS, et al. Prevalence and identification of subclinical mastitis in cows at BLRI Regional Station, Sirajganj, Bangladesh. J Adv Vet Anim Res. 2017;4(3):295–300. http://doi.org/10.5455/javar.2017.d227. [Google Scholar]
- [14].Rahman MA, Bhuiyan MMU, Kamal MM, Shamsuddin M. Prevalence and risk factors of mastitis in dairy cows. Bangladesh Vet J. 2009;26:54–60. https://doi.org/10.3329/bvet.v26i2.4951. [Google Scholar]
- [15].Rahman MM, Islam MR, Uddin MB, Aktaruzzaman M. Prevalence of subclinical mastitis in dairy cows reared in Sylhet district of Bangladesh. Int J Biol Res. 2010;1:23–8. [Google Scholar]
- [16].Rahman MM, Munsi N, Kabir MH, Ekram F, Rahman MT, Saha S. Prevalence of subclinical mastitis in cows at Anwara, a coastal upazila of Chittagong district in Bangladesh. J Vet Adv. 2014;4:594–8. [Google Scholar]
- [17].Sumon SMMR, Ehsan MA, Islam MT. Subclinical mastitis in dairy cows: somatic cell counts and associated bacteria in Mymensingh, Bangladesh. J Bangladesh Agric Univ. 2017;15:266–71. https://doi.org/10.3329/jbau.v15i2.35073. [Google Scholar]
- [18].Schalm OW, Carrol EJ, Jain NC. 1st. Lea and Febiger; Philadelphia: 1971. Bovine mastitis. [Google Scholar]
- [19].Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristics analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. https://doi.org/10.1016/S0167-5877(00)00115-X. [DOI] [PubMed] [Google Scholar]
- [20].Schalm OW, Noorlander DO. Experiments and obser-vations leading to development of the California Mastitis Test. J Am Vet Med Assoc. 1957;130:199–204. [PubMed] [Google Scholar]
- [21].Barnum DA, Newbould FHS. The use of the California Mastitis Test for the detection of bovine mastitis. Can Vet J. 1965;2:83–90. [PMC free article] [PubMed] [Google Scholar]
- [22].Sargeant JM, Leslie KE, Shirley JE, Pulkrabek BJ, Lim GH. Sensitivity and specificity of somatic cell count and California mastitis test for identifying intramammary infection in early lactation. J Dairy Sci. 2001;84:2018–24. doi: 10.3168/jds.S0022-0302(01)74645-0. https://doi.org/10.3168/jds.S0022-0302(01)74645-0. [DOI] [PubMed] [Google Scholar]
- [23].Sarker SC, Parvin MS, Rahman AK, Islam MT. Prevalence and risk factors of subclinical mastitis in lactating dairy cows in north and south regions of Bangladesh. Trop Anim Health Prod. 2013;45:1171–6. doi: 10.1007/s11250-012-0342-7. https://doi.org/10.1007/s11250-012-0342-7. [DOI] [PubMed] [Google Scholar]
- [24].Tripura TK, Sarker SC, Roy SK, Parvin MS, Sarker RR, Rahman AKMA, et al. Prevalence of subclinical mastitis in lactating cows and efficacy of intramammary infusion therapy. Bangladesh J Vet Med. 2014;12(1):55–61. https://doi.org/10.3329/bjvm.v12i1.20464. [Google Scholar]
- [25].Mpatswenumugabo JP, Bebora LC, Gitao GC, Mobegi VA, Iraguha B, Kamana O, et al. Prevalence of subclinical mastitis and distribution of pathogens in dairy farms of Rubavu and Nyabihu districts, Rwanda. J Vet Med. 2017 doi: 10.1155/2017/8456713. Article ID 8456713; https://doi.org/10.1155/2017/8456713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Skrzypek R, Wtowski J, Fahr RD. Factors affecting somatic cell count in cow bulk tank milk—a case study from Poland. J Vet Med A Physiol Pathol Clin Med. 2004;51(3):127–31. doi: 10.1111/j.1439-0442.2004.00611.x. https://doi.org/10.1111/j.1439-0442.2004.00611.x. [DOI] [PubMed] [Google Scholar]
- [27].Sri Balaji N, Saravanan R, Senthilkumar A, Srinivasan G. Effect of subclinical mastitis on somatic cell count and milk profile changes in dairy cows. Int J Sci Environ Technol. 2016;5(6):4427–31. [Google Scholar]
- [28].Sharma N, Maiti SK, Pandey V. Sensitivity of indirect tests in the detection of subclinical mastitis in buffaloes. Vet Pract. 2008;9:29–31. [Google Scholar]
- [29].Swain DK, Kushwah MS, Dang AK. Neutrophil surface adhesion molecule and toll like receptor dynamics in crossbred cows suffering from Staphylococcus aureus subclinical and clinical mastitis. J Adv Vet Anim Res. 2016;3(2):99–105. http://doi.org/10.5455/javar.2016.c136. [Google Scholar]
- [30].Barua M, Prodhan MAM, Islam K, Chowdhury S, Hasanuzzaman M, Imtiaz MA, et al. Sub-clinical mastitis prevalent in dairy cows in Chittagong district of Bangladesh: detection by different screening tests. Vet World. 2014;7(7):483–8. https://doi.org/10.14202/vetworld.2014.483-488. [Google Scholar]
- [31].Barbosa CB, Benadetti E, Ribeiro SC, Guimaraes EC, Ribeiro SC. The relationship between somatic cell count (SCC) and result of the California Mastitis Test (CMT) to diagnose bovine mastitis. Biosci J. 2002;18:93–102. [Google Scholar]
- [32].Reddy LV, Choudhuri PC, Hamza PA. Sensitivity, specificity and predictive values of various indirect tests in the diagnosis of subclinical mastitis. Indian Vet J. 1998;75:1004–5. [Google Scholar]
- [33].Pyorala S. Indicators of inflammation in the diagnosis of mastitis. Vet Res. 2003;5:565–78. doi: 10.1051/vetres:2003026. https://doi.org/10.1051/vetres:20030260. [DOI] [PubMed] [Google Scholar]