Abstract
Objectives:
Drug residues in poultry products could lead to the development of antibiotic-resistant bacteria as in any living animal and human alike. Extensive use of antibiotics in animals to promote growth rate, increase feed efficiency, and for prevention of intestinal infections has led to the development of resistant bacteria in the gastrointestinal tract. The present study was conducted to evaluate the effects of biological supplementation of probiotic, phytobiotic, and their combination over antibiotic on growth performance, microbial load, and hematological parameters in Broiler.
Materials and methods:
Sixty-five broiler chicken were divided into five groups (12 birds in each group), namely, group A (basal diet), group B (antibiotic, Renamycin 100®), group C (phytobiotic, Galibiotic®), group D (probiotic, Bio-Top®), and group E (combination, Galibiotic®+Galibiotic®) and five were sacrificed for baseline data on day 0.
Results:
Average final live weight gain was highest in group D (probiotic) than other groups. The feed conversion ratio was highest in group A and lowest in the probiotic group (group C). Blood samples were collected on 14th and 28th day for hematological studies. The mean hematology values regarding the total erythrocyte count, hemoglobin concentration, packed cell volume, and erythrocyte sedimentation rate differed significantly (p < 0.05) among groups. The pH of all the treatment groups was significantly decreased compared to the control group (p < 0.05) where group C was significantly (p < 0.05) lower than all other groups. Highest total viable cell count was observed in control (group A) and total coliform count in phytobiotic (group C) was significantly lower than in other treatment groups (p < 0.05).
Conclusion:
It may conclude that biological supplements have a significant positive impact on growth performance, hematological parameters, and gut microbial load in broiler chicken of which the probiotic showing the best effects. Supplementation of probiotic in feed could be one of the best candidates as an alternative to antibiotics as growth promoter for safe broiler production.
Keywords: Probiotic, phytobiotic, antibiotic, broiler chicken, hematology, bacterial load, intestinal pH
Introduction
Poultry industry plays a vital role for income generation to the rural people of Bangladesh. Tremendous demand for animal protein has caused an expansion of broiler farming. However, to mimic the maximum production, feeding and good management practices are not properly maintained [1]. Modern feeding practice involves the use of phytobiotics, probiotics, antibiotic growth promoters, balanced diet, and so many new concepts [1]. Several animal husbandry practices have been reported to be important risk factors in transmitting antimicrobial zoonotic drug resistance in rural Bangladesh [2]. Antibiotics in poultry production cause antibiotic resistance in birds while the residues may be passed on to human leading to public health hazards. Furthermore, antibiotics also cause imbalance in the intestinal normal flora of birds [3]. As antibiotics are extensively used to promote growth in poultry production or control infectious disease, anti-microbial abuse is considered to be the most vital selecting force to produce resistant bacteria [4]. Ultimately, public health is at high risk and this is why antibiotics are being replaced by alternative growth promoters like phytobiotics, probiotics, etc. [5]. Probiotics have been defined as “Live microorganisms (bacteria or yeasts), which when ingested or locally applied in sufficient numbers confer one or more specified demonstrated health benefits for the host” [3]. In fact, probiotics have been used for quite a long time throughout human history. Probiotics are thought to change the composition of the normal intestinal microflora from a potentially harmful composition towards a microflora that would be beneficial for the host. Previous study reported that probiotics help in the production of lactic acid and hydrogen peroxide which are detrimental to many pathogens, lowering oxidation-reduction potential in the gut which inhibits aerobic pathogens, prevention of toxic amines and ammonia accumulation, production of essential digestive enzymes, production of B-vitamins, and appetite stimulation [6].
In previous studies, several bioactive compounds from mushrooms and plants have been identified as potential candidates that differentially stimulate favorable bacteria such as lactobacilli and bifidobacteria without promoting the growth of pathogenic species [7,8]. Stimulation of these beneficial bacteria could contribute to a balanced gut microflora and may provide an optimal precondition for effective protection against pathogenic microorganisms and an intact immune system [9]. Phytobiotics are compounds from herb spices and their extracts can stimulate appetite, endogenous secretions such as enzymes, and have antimicrobial, coccidiostatic, or anthelmintic activities in monogastric animals [9].
Human health can either be affected directly from antibiotic residues in meat or eggs, which may cause side-effects, or indirectly, through the antibiotic resistance determinants that may spread to a human pathogen [10]. This raises concern and an urgent need to explore better options alternative to antibiotics that will ensure safe and economically viable poultry production. This study could open a new door towards a safe poultry production, efficiency, and cost-benefit to the advantage of the farmer and public health. Considering these above discussions, we aimed to evaluate the beneficial effects of probiotic, phytobiotic, and their combination over antibiotic on growth performance, microbial load, and hematological parameters in broilers.
Materials and Methods
The experiment was carried out in the Departments of Pharmacology, Microbiology and Hygiene, Bangladesh Agricultural University, Mymensingh-2202. All experimental procedures were performed according to the guidelines for the care and use of animals as established by Animal Welfare and Experimentation Ethics Committee, Bangladesh Agricultural University, Mymensingh [Approval number: AWEEC/BAU/2018(11)]. The aim of the present study was to explore the comparative effects of antibiotic, phytobiotic, probiotic, and their combination on growth, production response, intestinal pH modification, and gut bacterial population in broilers were studied. A total of 65 male day-old broilers chicks from Kazi Farms Ltd, Bangladesh of either sex were used for this experiment. Five randomly selected day-old chicks were sacrificed on the first day for baseline bacterial analysis. The rest of the birds were divided into five groups of 12 birds in each group. Group-A control diet, Group-B diet with 0.1% antibiotic [Oxytetracycline hydrochloride, (Renamycin 100®), Renata Ltd. (Animal Health Division), Bangladesh], Group-C diet with 0.1% phytobiotic [Galibiotic®, Square Pharmaceuticals Co. Ltd., Dhaka, Bangladesh], Group-D diet with 0.15% probiotic [Bio-Top®, Pharma and Firm Co. Ltd., Dhaka, Bangladesh], and Group-E diet with phytobiotic + probiotic 0.1% + 0.15%, respectively. Body weights (CAMRY electrical balance, China) were recorded and five birds from each group were sacrificed at 2nd and 4th weeks for pH measurements and bacteriological analysis. The total viable count (TVC), total coliform count (TCC), and total Salmonella count (TSC) of both feces and intestinal contents were recorded [3].
Composition of feed
Hand-mixed manually prepared corn-soya based feed with appropriate formula was used throughout the experimental study. Feed ingredients were collected from local markets of Mymensingh. The broiler chicks were fed with corn-soya based basal diet starting from day 1 (Mashed diet)–day 28 (Regular diet).
Immunization by vaccination
The following vaccination schedule was maintained during the experimental period to prevent the birds from common viral diseases. Vaccines were purchased by FnF Pharmaceuticals Ltd. Bangladesh, and were administered as per manufacturer’s instructions. On 7th day of age, Baby Chick Ranikhate Disease Virus vaccine was administered as eye drop followed by booster doses on 21st and 24th days of age. On 11th day of age, Gumboro vaccine was administered as eye drop.
pH measurement
The pH determination of small and large intestinal contents was measured with Adwa AD 1020 pH meter immediately after slaughter. Intestines were collected in Petri dishes and incised with scissors to measure the pH by inserting a glass electrode probe [3].
Bacterial load count
TVC, TCC, and TSC were determined following the methods of Thomas et al. [11]. Briefly, 900 μl of phosphate buffer solution was taken in eight Eppendorf tubes and 100 μl suspension was used to prepare 10 serial fold dilution of each content. Then 10 μl from each dilution was dropped on plate count agar and incubated in bacteriological incubator at 37oC overnight. Colonies were counted for each dilution and total viable cells were calculated.
Hematological studies
Total erythrocyte count (TEC) was done using a counting chamber following the method described by Lamberg and Rothstein [12] and the result was expressed in million/μl of blood. Estimation of hemoglobin was done based on the acid-hematin method with result read in daylight by observing the height of the liquid in the tube considering the lower meniscus of the liquid column. The result was then expressed in gm%. Packed cell volume (PCV) and erythrocyte sedimentation rate (ESR) were also determined following the methods of Lamberg and Rothstein [12].
Statistical analysis
All the collected data were analyzed with the help of Graph Pad Prism 6. The mean differences among the treatment groups were determined by one-way analysis of varience followed by Bonferroni post-hoc test [13].
Results
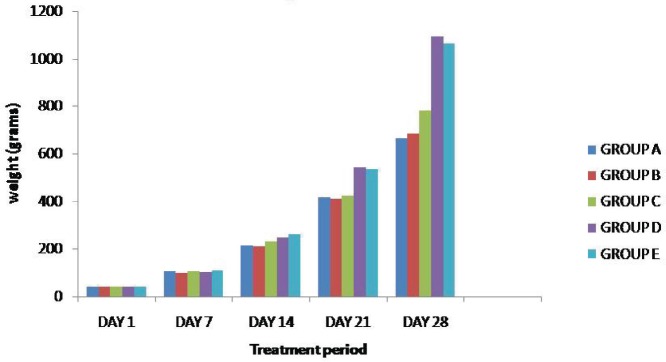
On day 7, the average body weights in different groups of birds were more or less similar. On day 14, the highest body weight was observed in Group E (Phytobiotic + Probiotic) followed by Group D (Probiotic), but on day 28, group D (probiotic) was the highest. Body weight increased significantly (p < 0.05) in all treatment groups (C, D, and E) compared to control (A) group by the end of the experiment (Table 1, Fig. 1). Regarding initial body weight, there was no significant difference among the dietary groups. At the end of 28 days of age, the highest live weight was (1095 ± 6.03 gm/bird) found in broilers of group D (probiotic). This was followed by broilers belonging to group E (1065.0 ± 18.93 gm/bird) and group C (780 ± 27.79 gm/bird), respectively (Table 1, Fig. 1). Another disparity was also seen with group D having the lowest feed conversion ratio at the end of the experiment.
Table 1. Production performance of the broilers supplemented with phytobiotic, probiotic, and their combination.
| Parameters | Treatment groups | ||||
|---|---|---|---|---|---|
| A(Control) | B(Antibiotic) | C (Phytobiotic) | D (Probiotic) | E (Phyto+Probi) | |
| ILW (gm) | 41.25±1.25 | 41.75±1.79 | 41±1.87 | 42±1.58 | 40.50±2.21 |
| FLW (gm) | 665 ± 4.16 | 686 ± 2.08 | 780 ± 27.79 | 1095± 6.03 | 1065.0±18.93 |
| LWG (gm) | 623.75±2.91 | 644.25±1.39 | 739±25.79 | 1053±4.90 | 1024.50±21.25 |
| FI (gm) | 1310 | 1263 | 1352 | 1812 | 1793 |
| FCR | 2.1 | 1.96 | 1.83 | 1.72 | 1.75 |
Data are express as mean ± SE. ILW; Initial live weight. FLW; Final live weight. LWG; Live weight gain. FI; Feed intake. FCR; Feed conversion ratio.
Figure 1. Effects of phytobiotic, probiotics, and their combination on body weight (mean ± SE) in different groups of broilers (n = 12).
Surprisingly, the combination group (E) had the highest weight gain regarding visceral organs (Table 2). This is clearly different from the final live weight gain where group D had the highest with the lowest feed conversion ratio. The probiotic group, however, came second to the combination group regarding visceral organ weight.
Table 2. Relative organ weight of broiler supplemented with phytobiotic, probiotic and their combination (at 28th day).
| Groups | Liver (gm) | Gizzard (gm) | Heart (gm) | Proventriculus (gm) |
|---|---|---|---|---|
| A (Control) | 27 ±1.48 | 21.20±0.75 | 4.40±0.60 | 6.40±0.50 |
| B (Antibiotic) | 25.80±0.86 | 20.27±1.39 | 4.60±0.50 | 5.60±0.50 |
| C (Phytobiotic) | 25 ±1.01 | 20.20±0.87 | 5±0.70 | 6.80±0.37 |
| D (Probiotic) | 40.20 ±0.37 | 23.20±0.86 | 6.20±0.58 | 7.20±0.58 |
| E (Phyto+Probi) | 41±0.71 | 25±0.95 | 7.10±0.54 | 7.80±0.58 |
Data are express as mean ± SE (n = 10).
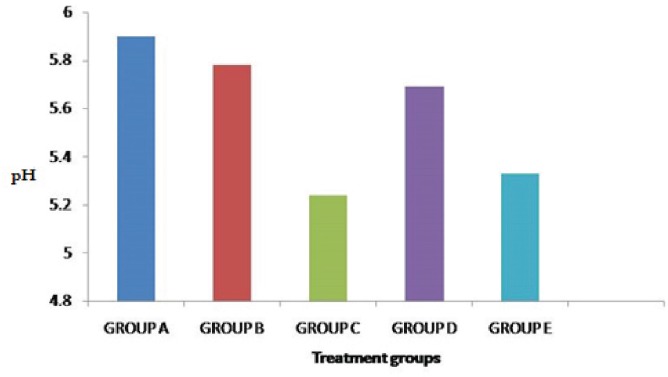
The pH of all the treatment groups was significantly decreased compared to the control group (p < 0.05). However, on day 28, we observed that intestinal pH in Group C was significantly (p < 0.05) lower than all other groups (data not shown). Group E (combination) was second highest followed by group D (probiotic).
TVC count increased in group E by day 28. However, second-highest TVC was observed in control (group A). No growth of Salmonella sp. was observed on day 28 in all the treatment groups. TCC in phytobiotic (Group C) was significantly (p < 0.05) lower than all other groups (A, B, D, and E) by day 28 (Table 3).
Table 3. Average CFU from intestinal content in different groups (n = 12) of broilers at 15th and 28th day, respectively.
| Groups | 15th day | 28th day | ||||
|---|---|---|---|---|---|---|
| TVC (CFU/gm sample) | TSC (CFU/gm sample) | TCC (CFU/gm sample) | TVC (CFU/gm sample) | TSC (CFU/gm sample) | TCC (CFU/gm sample) | |
| Group A | 1.10 × 109 | 2.50 × 109 | 1.06 × 109 | 1.70 × 109 | 0 | 68.00 × 103 |
| Group B | 0.11 × 109 | 1.60 × 109 | 0.12 ×1 09 | 0.6 × 109 | 0 | 7.00 × 103 |
| Group C | 0.12 × 109 | 8.00 × 109 | 1.32 × 109 | 1.26 × 109 | 0 | 0.024 × 103 |
| Group D | 2.00 × 109 | 6.60 × 109 | 1.28 × 109 | 0.54 × 109 | 0 | 48.00 × 103 |
| Group E | 0.06 × 109 | 12.60 × 109 | 1.38 × 109 | 1.80 × 109 | 0 | 0.38 × 103 |
TVC, TCC, and TSC and the results of hematological parameters; total erythrocyte count, packed cell volume, hemoglobin concentration, and erythrocyte sedimentation rate (TEC, PCV, Hb, and ESR), respectively, are presented in Table 4. Significant difference (p < 0.05) among the mean values of TEC, Hb, PCV, and ESR were observed among the different treatment groups at different age. TEC Significantly (p < 0.05) increased in the probiotics treated group D compared to the control group by day 28. The highest packed cell volume (22.00% ± 1.03%) was recorded in the combination group (E) and the lowest (18.00% ± 1.02%) in group C by day 28. No Significant (p < 0.05) differences were observed among the probiotics treated group compared to control groups. Highest erythrocyte sedimentation rate (7.50 ± 1.7 mm) recorded in group (B) and the lowest (5.55 ± 1.35 mm) in group A by the end of the experiment and no significant (p < 0.05) differences were observed among the probiotics treated group and control groups.
Table 4. Hematological values of broilers at 2 weeks and 4 weeks, respectively.
| Groups | TEC | HB | PCV | ESR | ||||
|---|---|---|---|---|---|---|---|---|
| 14th day | 28thday | 14th day | 28thday | 14th day | 28thday | 14th day | 28thday | |
| Negative control (A) | 2.7 ± 0.02ab | 2.5 ± 0.02 | 7.3 ± 0.10 | 7.3 ± 0.13 | 20.0 ± 2.0 | 20.5 ± 1.50 | 5.5 ± 1.50 | 5.6 ± 1.35 |
| Positive control (B) | 2.1 ± 0.02b | 2.2 ± 0.02ab | 5.0 ± 0.20abc | 6.1 ± 0.10a | 20.1 ± 1.01 | 21.5 ± 1.55 | 7.0 ± 1.05 | 7.5 ± 1.7 |
| Phytobiotic (C) | 2.43 ± 0.02a | 2.8 ± 0.02b | 6.5 ± 0.05c | 5.7 ± 0.12ac | 20.0 ± 1.0 | 18.0 ± 1.02 | 5.5 ± 0.55 | 6.5 ±1.57 |
| Probiotic (D) | 2.4 ± 0.01b | 2.9 ± 0.01a | 6.5 ± 0.15b | 7.2 ± 0.05c | 21.5 ± 0.50 | 21.5 ± 0.57 | 6.0 ± 1.03 | 6.0 ±1.08 |
| Combined (E) | 2.2 ± 0.01 | 2.5 ± 0.03 | 6.7 ± 0.10a | 7.5 ± 0.07a | 18.5 ± 1.50 | 22.0 ± 1.03 | 6.5 ± 1.53 | 6.2 ±1.6 |
Data are expressed as mean ± SE (n = 10).
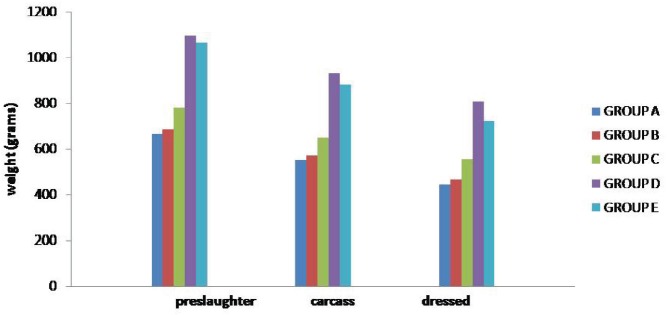
Figure 2. Pre-slaughter weight, carcass weight, and dressing weight (mean ± SE) gain in different groups (n = 12) at day 28.
Figure 3. Effects of phytobiotic, probiotic, and their combination on intestinal pH at day 28 (n = 12).
Discussion
Based on this experiment, we found probiotic supplement alone could give the best weight gain with a lower feed conversion ratio than all other forms of supplements by the end of the experiment. Like our findings, higher body weight gains for probiotic fed broilers were also reported by Ripon et al. [3]; Kamruzzaman et al. [14]; Islam et al. [16]. This also resembles that of Islam et al. [15] who stated that body weight gain was higher in probiotics fed birds. There was a surprising disparity in our experiment where visceral organ weights were higher in the combination group (E) against our expectation of group (D). However, there are conflicting results in the literature concerning the efficacy of probiotics, prebiotics, and plant extracts for growth performance in broilers. In this regard, the previous study has shown that no observable change in body weight gain and feed conversion of broilers by supplementing diets with probiotics, prebiotics, plant extracts, and essential oils [17]. In contrast, the present study data with respect to body weight gain are not so high compared to previous studies probably due to feed formulation variations, environmental conditions, age, and sex of birds. The increased body weight may be due to the stimulatory effects of probiotics on digestibility, feed utilization, and influence of gut microflora. The increased rate of body weight gains in any of the two probiotic-containing groups might be due to increased feed intake, feed consumption, utilization, digestion, absorption, and metabolism of supplied feed nutrients essential for their health and body weight gain. Improved body weight gain in combination group may be due to the synergistic action of probiotic and phytobiotic [5]. Our data suggest that probiotic or combination of probiotic and phytobiotic have a positive effect on weight gain in broilers.
The increase in hemoglobin concentration and PCV on days 28 and 14, respectively, was in agreement with the findings of Islam et al. [15] and Kamruzzaman et al. [14] who stated that the mean values of TEC, Hb, and PCV increased significantly (p < 0.05) in probiotics treated groups. However, our results differ from Shareef and Al-Dabbagh [18] and Mohan et al. [19] who stated that there was no significant change in hematological parameters as a result of probiotics supplementation.
The decreased intestinal pH in group C recorded in present experiment resembles the result of Jamroz et al. [8] who reported that dietary supplementation of plant extracts significantly reduced intestinal pH when used as broiler feed additives. The decreased pH in phytobiotic treatment group C indicates that medium-chain fatty acids found in phytobiotics reduce the number of pathogenic bacteria in the gut while increasing the beneficial Lactobacilli to produce more lactic acid which makes the gut more acidic, thus reducing the growth of pathogenic bacteria. The second highest TVC in group A by day 28 may be due to overgrowth of pathogenic bacteria in the untreated group (A). The increase TVC count in group E indicates that probiotic stimulated the growth of beneficial cecal bacteria such as lactobacilli in broiler chickens and by combined effects with phytobiotic containing MCFAs, the number of potentially harmful bacteria such as Bacteroides spp. was reduced. The increase in TVC count in group (E) has similarity with the work of Guo et al. [7] who stated that supplementation with plant, mushroom extracts, and other probiotics stimulated the growth of beneficial cecal bacteria such as lactobacilli in broiler chickens and reduced the number of potentially harmful bacteria such as Bacteroides spp. and E. coli. The findings of the present study of TCC in group C support the study of Jamroz et al. [8] who stated that dietary supplementation of plant extracts containing capsaicin, carvacol, and cinnamic aldehyde reduced C. perfringens and E. coli counts in colonic contents to the same degree as birds treated with avilamycin and oxytetracycline. Guo et al. [7,20] stated that mushroom extracts in broiler chickens increased Bifidobacteria and lactobacilli and decreased E. coli in cecal content.
The findings of the present study also support the finding of Zhang et al. [21], Jamroz et al. [22], Thomas et al. [11] and Islam et al. [16], who found lower total viable count with the introduction of probiotics in the feed. Decreased total coliform count in treated groups was in agreement with findings demonstrated by Vicente et al. [23]. These results were in harmony with the result of Jamroz et al. [22] who reported that adding 0.5%–1.5% FA to broiler diet reduced significantly crop and cecal pH. However, Hernandez et al. [24] and Al-Natour and Alshawabkeh [25] found insignificant reduction in the intestinal pH for broiler when used 0.5%–1.5% FA. This could be due to the difference in doses and sources of MCFA in the diet. This result indicates that both probiotic and phytobiotic have beneficial effects to improve the gut health of chicken by increasing the beneficial bacterial load.
In addition, in the present study, we observed that phytobiotic and its combination with probiotic supplementation showed a significant influence on growth performance, carcass yield, and dressing percentage after 28 days of treatment. However, phytobiotic (0.1%) alone checked the growth of pathogenic Salmonella Sp. and significantly lower intestinal pH and the total coliform count. This result indicates the improvement of gut health of broiler. Therefore, it might be concluded that phytobiotic may be an effective growth promoter in exchange of antibiotics as growth promoter in the broiler industry to maintain food safety.
Further dose-dependent studies of probiotic and phytobiotic with a larger number of birds in farm level and their molecular studies are strongly recommended.
Conclusion
Supplementation of probiotic in feed could be one of the best candidates as an alternative to antibiotics as growth promoter. In addition, phytobiotic or combination of probiotic and phytobiotic in broiler feed could also be the alternative potential candidates to antibiotics as growth promoter for safe broiler production.
Acknowledgments
This work was supported by Special Allocation Project for the Year 2015–2016 under the Ministry of Science & Technology to Kazi Rafiqul Islam (Number and date of sanction order: 39.00.0000.009.002.057.2015-2016/BS-17/946, Date: 08-12-2015). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Md. Faisal Ferdous and Md. Shafiul Arefin were NST fellow.
Conflict of interest
Md. Faisal Ferdous and Md. Shafiul Arefin have equal contribution to this work. All other authors declare that they have no conflict of interest.
Authors’ contribution
MFF and MSA performed the experiments and wrote the manuscript; KR, MTH, and MUA designed and supervised the research work, and KR prepared and finalized the draft of the manuscript; MMRR, MRS, MMR, and MHR did the statistical analysis and revised the draft. All the authors read and approved the final manuscript.
References
- [1].Alkhalf A, Alhaj M, Al-homidan I. Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi J Biol Sci. 2010;17(3):219–25. doi: 10.1016/j.sjbs.2010.04.005. https://doi.org/10.1016/j.sjbs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roess AA, Winch PJ, Nabeel AA, Afsana A, Dilara A, Shams EA, et al. Animal husbandry practices in rural Bangladesh: potential risk factors for antimicrobial drug resistance and emerging diseases. Am J Trop Med Hyg. 2013;89(5):965–70. doi: 10.4269/ajtmh.12-0713. https://doi.org/10.4269/ajtmh.12-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ripon MMR, Rashid MH, Rahman MM, Ferdous MF, Arefin MS, Sani AA, et al. Dose-dependent response to phytobiotic supplementation in feed on growth, hematology, intestinal pH, and gut bacterial load in broiler chicken. J Adv Vet Anim Res. 2019;6(2):253–9. doi: 10.5455/javar.2019.f341. https://doi.org/10.5455/javar.2019.f341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Okeke IN, Lamikanra A, Edelman R. Socio-economic and behavioral factors leading to acquired bacterial resistance to antimicrobrial agents in developing countries. Emerg Infect Dis. 1999;5:18–27. doi: 10.3201/eid0501.990103. https://doi.org/10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alimohamadi K, Taherpour K, Ghasemi HA, Fatahnia F. Comparative effects of using black seed (Nigella sativa), cumin seed (Cuminum cyminum), probiotic or prebiotic on growth performance, blood haematology and serum biochemistry of broiler chicks. J Anim Physiol Anim Nutr. 2014;98(3):538–46. doi: 10.1111/jpn.12115. https://doi.org/10.1111/jpn.12115. [DOI] [PubMed] [Google Scholar]
- [6].Singh BP, Chauhan RS. Role of probiotics in control of poultry diseases. Pashudhan. 2004;17(8):3. [Google Scholar]
- [7].Guo FC, Williams BA, Kwakkel RP, Li HS, Li XP, Luo JY, et al. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult Sci. 2004;83:175–82. doi: 10.1093/ps/83.2.175. https://doi.org/10.1093/ps/83.2.175. [DOI] [PubMed] [Google Scholar]
- [8].Jamroz D, Orda J, Kamel C, Wiliczkiewicz A, Wertelecki T, Skorupinska J. The influence of phytogenic extracts on performance, nutrient digestibility, carcass characteristics, and gut microbial status in broiler chickens. J Anim Feed Sci. 2003a;12:583–96. https://doi.org/10.22358/jafs/67752/2003. [Google Scholar]
- [9].Wenk C. Herbs and botanicals as feed additive in monogastric animals. Asian-Aust J Anim Sci. 2003;16:282–9. https://doi.org/10.5713/ajas.2003.282. [Google Scholar]
- [10].Bengtsson-Palme J, Kristiansson E, Joakim Larsson DG. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol Rev. 2018;42(1):68–80. doi: 10.1093/femsre/fux053. https://doi.org/10.1093/femsre/fux053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thomas P, Sekhar AC, Upreti R, Mujawar MM, Pasha SS. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast CFU enumeration and single colony isolation from diverse samples. Biotechnol Rep. 2015;8:45–55. doi: 10.1016/j.btre.2015.08.003. https://doi.org/10.1016/j. btre.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lamberg SL, Rothstein R. 1st. AG Publishing Company Inc; Westport, CT: 1977. Laboratory manual of hematology and urine analysis. [Google Scholar]
- [13].Rafiq K, Fan YY, Sherajee SJ, Takahashi Y, Matsuura J, Hase N, et al. Chymase activities and survival in endotoxin-induced human chymase transgenic mice. Int J Med Sci. 2014;11(3):222–5. doi: 10.7150/ijms.7382. https://doi.org/10.7150/ijms.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kamruzzaman SM, Kabir SML, Rahman MM, Islam MW, Reza MA. Effect of probiotics and antibiotic supplementation on body weight and haemato biochemical parameters in broiler. Bangladesh J Vet Med. 2005;3:100–4. https://doi.org/10.3329/bjvm.v3i2.11303. [Google Scholar]
- [15].Islam MN. Effects of probiotics supplementation on growth performance and certain haemato-biochemical parameters in broiler chickens. Bangladesh J Vet Med. 2004;2(1):39–43. https://doi.org/10.3329/bjvm.v2i1.1933. [Google Scholar]
- [16].Islam MA, Kabir SML, Rahman BM, Das SK, Hossain KMM, Mustafa MMH, et al. The viability of dietary (Bactosac) influencing growth parameters, cellular alteration in intestinal wall and immune response of broilers. Curr Res J Biol Sci. 2014;6:128–33. https://doi.org/10.19026/crjbs.6.5510. [Google Scholar]
- [17].Botsoglou NA, Florou-Paneri P, Christaki E, Fletouris DJ, Spais AB. Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues. Br Poult Sci. 2002;43:223–30. doi: 10.1080/00071660120121436. https://doi.org/10.1080/00071660120121436. [DOI] [PubMed] [Google Scholar]
- [18].Shareef M, Al-Dabbagh ASA. Effect of probiotic (Saccharomyces cerevisiae) on performance of broiler chicks. Iraqi J Vet Sci. 2009;23:23–9. [Google Scholar]
- [19].Mohan B, Kadirevel R, Natarajan A, Bhaskaran M. Effect of probiotic supplementation on growth, nitrogen utilization, and serum cholesterol in broilers. Br Poult Sci. 1996;37:395–401. doi: 10.1080/00071669608417870. https://doi.org/10.1080/00071669608417870. [DOI] [PubMed] [Google Scholar]
- [20].Guo FC, Savelkoul HFJ, Kwakkel RP, Williams BA, Verstegen MWA. Immunoactive, medicinal properties of mushroom and herb polysaccharides and their potential use in chicken diets. World Poult Sci J. 2003;59:427–40. https://doi.org/10.1079/WPS20030026. [Google Scholar]
- [21].Zhang AW, Lee DB, Lee SK, Lee KW, Song KB, Lee CH. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult Sci. 2005;84(7):1015–21. doi: 10.1093/ps/84.7.1015. [DOI] [PubMed] [Google Scholar]
- [22].Jamroz D, Wertelecki TJ, Orda A, Wiliczkiewicz A, Skorupinska J. Influence of phytogenic extracts on gut microbial status in chickens. 14th European Symposium on Poultry Nutrition; 2003b; pp. 176–8. [Google Scholar]
- [23].Vicente JL, Avina L, Torres-Rodriguez A, Hargis B, Tellez G. Effect of a lactobacillus spp based probiotics culture product on broiler chicks performance under commercial conditions. Int J Poult Sci. 2007;6:154–6. https://doi.org/10.3923/ijps.2007.154.156. [Google Scholar]
- [24].Hernandez F, Madrid J, Garcia V, Orengo J, Megias MD. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult Sci. 2004;83:169–74. doi: 10.1093/ps/83.2.169. https://doi.org/10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- [25].Al-Natour M, AL-Shawabkeh KM. Using varying levels of formic acid to limit growth of Salmonella gallinarum in contaminated broiler food. Asian-Aust J Anim Sci. 2005;18:390–5. https://doi.org/10.5713/ajas.2005.390. [Google Scholar]