Abstract
Objective:
High ambient temperature in poultry is a challenging and fatal stress among environmental factors. It affects the production quality, damages the liver, and increases mortality in broilers. The present study is focused to explore appropriate utilization of Selenium (Se) as a feed additive in broiler chickens against high temperature.
Materials and Methods:
Day-old male broiler chickens (Ross 308) (n = 200) were grouped according to the supplements used in their basal diets such as: corn-soybean basal diet as control (Con), a basal diet containing sodium selenite, basal diet with probiotics, and a basal diet containing selenium-enriched probiotics (SP). At the end of the experimental period of 42 days, the liver was isolated and was used to determine the antioxidant capacity through a spectrophotometer. Inflammatory and anti-inflammatory cytokines production in the liver was measured through a real-time polymerase chain reaction.
Results:
Hepatic analyses revealed the decreased level of malondialdehyde, whereas glutathione, glutathione peroxidase (GSH-Px), and superoxide dismutase levels were increased in the SP group. Furthermore, supplementation of SP significantly up-regulated the mRNA expression of glutathione peroxidase 1 (GPx1), GPx4, IL6, and IL10 and down-regulated the expression of pro-inflammatory cytokines.
Conclusion:
It is thus concluded that SP as a potential nutritive supplement may facilitate hepatic protection by suppressing hepatic oxidation, inflammation, and necrosis during the high ambient temperature of summer.
Keywords: Selenium-enriched probiotics, liver, heat stress, pro-inflammatory cytokines, inflammation, broiler
Introduction
High ambient temperature in poultry has been known as one of the most puzzling and disastrous stress among various environmental conditions, which negatively affects the growth rate, feed intake, survival, and immunity [1]. Various studies have reported that heat stress induces liver inflammation, necrosis, irregular cell membrane, dilated hepatic venules, and dilated sinusoids in rats [2]. It induces the production of intracellular reactive oxygen species (ROS) which cause oxidative damage to the tissues [3]. The ROS triggers cytokines production through activation of the NF-κB pathway which regulates gene expression related to inflammation, stress, and apoptosis [4,5]. The deleterious effects due to heat stress can be reduced by the supplementation of antioxidants [6–8].
The essential trace element, i.e., selenium (Se) has antioxidant, anti-inflammatory, and chemoprotective properties; therefore, its consumption as a feed additive has a significant positive impact on human and animal’s health [9]. The beneficial role of Se depends upon its nature and extent of administration as a feed supplement. Inorganic selenium could be deleterious in higher doses because of its pro-oxidant and pro-inflammatory potential [10]. Selenium selenite (SS) was found toxic at the high dose of 5 mg/kg feed in layers and also at a low dose of 1 mg/kg feed in broilers [11,12]. On the other hand, organic Se from the source of selenium yeast was found safe when even administered in higher doses of 20 mg/kg feed in broilers. Similarly, in vitro studies of human lymphocytes, toxicity were observed with 5 uM/l of inorganic selenium; however, it was not the case with organic selenium used. These studies conclude that organic selenium is safer than inorganic selenium [12]. The US Food and Drug Administration allowed (1974) 0.1 to 0.3 mg of sodium selenite/kg of poultry feed. This range may allow establishing a safe level of Se concentration in poultry meat for human consumption.
Selenium regulates the activation of NF-κB, a transcription factor, which plays a pivotal role in the regulation of inflammatory pathways. Selenium can inhibit NF-κB from binding the inflammation-related genes which eventually reduce the expression of pro-inflammatory cytokines [13]. The anti-inflammatory function of Se might be due to the presence of specific selenoproteins, such as glutathione peroxidase (GPx) which reduces the oxidation induced inflammatory changes in the liver [14,15].
Probiotics such as Saccharomyces cerevisiae (S. cerevisiae) and Lactobacillus acidophilus (L. acidophilus) are practicable therapeutic additives for preventing hepatic diseases caused by alcoholism, viral infection, and metabolic maladies [15,16]. The probiotics (P) and Se were combined to increase the therapeutic potency of Se. Selenium-enriched probiotics (SP) were found to prevent inflammation, fibrosis, and necrosis of rat’s liver by alleviating the oxidative stress [17,18].
Until now, no experimental study is conducted which could explain the hepatoprotective role of SP in broiler chickens kept under high ambient temperature. The present study is designed to explore the hepatoprotective function of SP through its antioxidation and immunomodulation properties in broiler chickens reared in the high ambient temperature of the summer season.
Materials and Methods
Ethical approval
Experimentation upon animals was approved by the Committee for Animal Care and Use of Nanjing Agriculture University (Animal Ethical Number: SYXK (Su) 2011-0036).
Preparation of sodium selenite, probiotics, selenium-en-riched probiotics
Earlier studies have reported that two P strains, including S. cerevisiae and L. acidophilus, have the capability to convert inorganic Se (SS, sodium selenite) from the medium to organic Se (Se-methionine and Se-cysteine) [19]. Probiotics (P) are the simple aerobic fermented forms of both S. cerevisiae and L. acidophilus strains while SP were prepared by aerobic fermentation of both P strains with SS, by utilizing the facilities of Institute of Nutritional and Metabolic Disorders of Domestic Animals and Fowls, Nanjing Agricultural University Jiangsu, China. The colony-forming units (CFU) of L. acidophilus and S. cerevisiae in both products (SP and P) were about 0.25 × 1011/ml and 0.25 × 109/ml, respectively. The total Se contents in the SP, detected with AF-610A atomic fluorescence spectrometer, were 10.0 mg/l and were considered to be a standard diet additive. SS stock solution was prepared from inorganic Se (sodium selenite) containing Se contents of 100 mg/l.
Experimental design
Two hundred a-day-old male broilers (Ross 308) with an average body weight of 45.59 ± 3.9 g were randomly divided into four groups. Each group had five replicates, containing 10 birds per replicate. The broilers were grouped according to the feed given, such as a corn-soybean basal diet (0.11 mg Se/kg feed) (Con), a basal diet containing SS (0.30 mg Se/kg feed), a basal diet containing P, and a basal diet containing (SP, 0.30 mg Se/kg feed). The basal diets were formulated according to the basic requirements of the National Research Council [11,12].
Husbandry practices and ethics of experimental studies
The broilers were reared for the experimental period of 42 days during the hot days of the summer season. The birds were kept in a room provided with a facility of temperature regulation and installation of three floors of identical stainless steel pens of size (1.75 × 1.55 m). The birds were exposed to room temperature of 34°C–36°C for the first 14 days, then they were exposed to high ambient temperature for the rest of the entire experimental period. The average high ambient temperature during the day and night hours ranged from 30°C to 39°C and 26°C to 31°C, respectively. Lighting and cross ventilation were provided for 24 h. The relative humidity inside the room was 60%–80%. All birds were vaccinated against Newcastle disease and infectious bursal disease. Moreover, feed and water were provided ad libitum.
Analysis of antioxidant capacity of liver
Ten birds per group (two/replicate) were slaughtered by cervical dislocation and the liver samples were collected. One-fourth of the liver was stored at −20°C, which was later used for the analyses of antioxidant enzyme activity, Se concentrations, and malondialdehyde (MDA) levels.
Hepatic selenium concentration
Liver Se concentration was measured by the method described earlier [16,20] by using an AFS-930A atomic fluorescence spectrometer (Jitian Analysis Instrument Co., Beijing, China).
Quantitative analysis of GSH-Px, SOD, and MDA in the liver
The glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and MDA were measured by spectrophotometer as described before [21] by following the protocol of commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
Determination of GSH activity of liver homogenate
The glutathione (GSH) activity of liver homogenate was determined from the supernatant by using a protocol of commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China), according to the method used by [22].
Real-time polymerase chain reaction (rtPCR) assay
Following the manufacturer’s guidelines, mRNA was isolated from the frozen liver using RNAiso Plus reagent (TaKaRa). In brief, the pellet from isolated mRNA was resuspended in 30 μl diethyl-pyrocarbonate-treated water. Its concentration was measured by NanoDrop microvolume spectrophotometer (Themofisher Scientific, MA, U.S.A.) and stored at −70°C. cDNA was synthesized with PrimeScript cDNA synthesis kits (Takara Biotechnology, Shiga, Japan) using the manufacturer’s guidelines. A 25 μl reaction mixture for rtPCR was made from 12.5 μl of 2×SYBR Green I PCR Master Mix (TaKaRa BIO INC), 1 μl of each primer (10μM) (Table 1), 10 μl of cDNA, and 0.5 μl of PCR grade water. rtPCR was performed on an ABI Prism 7300 Detection System (Applied Biosystems, CA, U.S.A.). Relative mRNA expression levels of the IL-1β, TNF-α, IFN-ɣ, NF-кB, IL-6, IL-10, GPx1, and GPx4 in the liver were detected by using 2−ΔΔCT method of Livak. All reactions were performed in triplicate [16,20].
Table 1. Primers used for real-time PCR.
| Target gene | Gen Bank accession no. | Primer sequence (5'−3') |
|---|---|---|
| GAPDH | K01458 | Forward: TGAAAGTCGGAGTCAACGGAT Reverse: ACGCTCCTGGAAGATAGTGAT |
| GPx1 | HM590226 | Forward: AACCAATTCGGGCACCAG Reverse: CCGTTCACCTCGCACTTCTC |
| GPx4 | AF498316 | Forward: CATCACCAACGTGGCGTCCAA Reverse: GCAGCCCCTTCTCAGCGTATC |
| NF-κB | M_86930 | Forward: TCA ACG CAG GAC CTA AAG ACA T Reverse: GCA GAT AGC CAA GTT CAG GAT G |
| TNF-α | GU230788.1 | Forward: GCC CTT CCT GTA ACC AGAT G Reverse: ACA CGA CAG CCA AGT CAA CG |
| IL-1β | NM204524 | Forward: TTCATTACCGTCCCGTTG Reverse: GCTTTTATTTCTCCAGTCACA |
| IFN-γ | NM205149 | Forward: ACAGGCAAACAATGGAAGT Reverse: CAGGTCAACAAACATACAACA G |
| IL6 | NM_204628 | Forward: GGTGATAAATCCCGATGAAGT Reverse: CTCCATAAACGAAGTAAAGTCTC |
| IL10 | AJ_621614 | Forward: 5ʹ-CATGCTGCTGGGCCTGAA-3ʹ Reverse: 5ʹ-CGTCTCCTTGATCTGCTTGATG-3 |
Histopathology
The liver tissues were prepared for histopathology examination through H & E staining according to the methods described earlier by [23].
Statistical analysis
The data were analyzed with SPSS Statistics version 19 (SPSS Inc., Chicago, IL, USA) using one-way ANOVA and Duncan multiple range test. A p value <0.05 was considered significant.
Results
Hepatic oxidation analyses
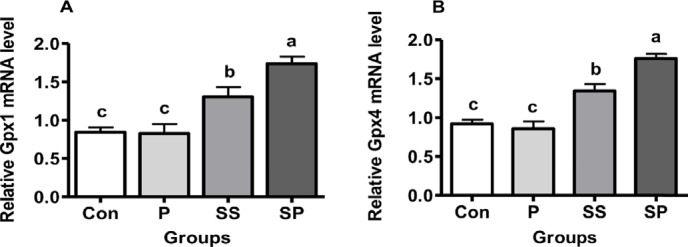
The mRNA expression of GPx1 and GP-x4 was higher in SP and SS groups than its expression in Con and P groups. Their expression was significantly higher in the SP group as well than in the SS group (Fig. 1A and B, p < 0.05).
Figure 1. Effect of P, SS, and SP on the relative mRNA levels of (A) Gpx1 and (B) Gpx4 in the liver of broiler chickens, feeding a basal diet (Con), basal diet plus P, basal diet plus SS, and basal diet plus SP. Each bar represents mean of 10 samples in a group and standard error. The values with unlike superscript letters (a, b, c) in the figure represent different levels of significance at p < 0.05.
Evaluating the liver for Se uptake, we found that the selenium concentration in the liver was significantly increased in SP and SS groups (p < 0.05) compared to Con and P groups. It indicates that the SP diet increases the liver efficiency of selenium uptake (Table 2).
Table 2. Effect of Se-enriched probiotics on selenium (Se) tissue deposition, activities of GSH-Px and SOD and levels of GSH and MDA in the liver under heat stress conditions.
| Parameters | Treatments | |||
|---|---|---|---|---|
| Con | P | SS | SP | |
| Se liver deposition (ng/g) | 351.1 ± 23.6c | 371.4 ± 19.6c | 574.3 ± 7.1b | 700.5 ± 6.3a |
| GSH (mg/g) | 4.55 ± 0.68c | 5.00 ± 0.29c | 10.81 ± 1.09b | 16.57 ± 1.14a |
| GSH-Px (u/mg) | 6.43 ± 0.40c | 6.15 ± 0.32c | 11.53 ± 0.68b | 19.29 ± 1.00a |
| SOD (u/mg) | 61.71 ± 3.90c | 87.85 ± 3.43b | 92.42 ± 3.28b | 132.60 ± 1.69a |
| MDA (nmol/mg) | 1.53 ± 0.09a | 0.388 ± 0.04b | 0.398 ± 0.04b | 0.094 ± 0.02c |
Effect of P, SS, and SP on selenium tissue deposition (ng/g), GSH level (mg/g protein), GSH-Px activity (u/mg protein), SOD activity (u/mg protein), and MDA levels (nmol/mg protein) of the liver in heat stressed broiler chickens, feeding of basal diet without Se (Con group), the basal diet with the addition of P group, the basal diet with the addition of SS group, and the basal diet with the addition of SP group. Mean values with their standard errors. The values with unlike superscript letters (a, b, c) in the graph were different (p < 0.05).
We also found that the level of MDA was significantly decreased (p < 0.05) in the SP group as compared with Con, SS, and P groups. No significant decrease was observed between SS and P groups; however, it was higher than the SP group and lower than the Con group (p < 0.05, Table 2).
Regarding the expression of GSH, GSH-Px, and SOD in the liver, SP showed their highest level compared with Con, P, and SS groups (Table 2, p < 0.05). The SOD and GSH levels were higher in SS and P groups compared with Con group although their levels were lower than the SP group but were indifferent in each other (Table 2, p < 0.05). The GSH-Px level was also higher in the SS group than in the Con group (Table 2, p < 0.05).
Hepatoprotective and anti-inflammatory effects of SP
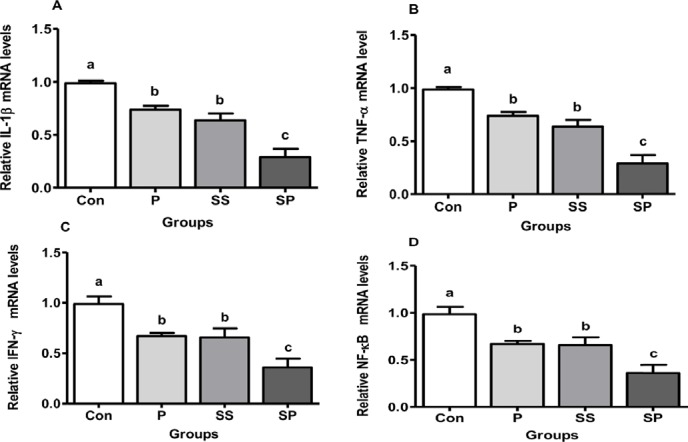
The mRNA expression of pro-inflammatory cytokines (i.e., IL-1β, TNF-α, IFN-ɣ) and NF-кB was down-regulated in P, SS, and SP groups compared with Con group. No difference was observed between P and SS groups; however, it was higher than the SP group and lower than the Con group (Fig. 2, p < 0.05).
Figure 2. Effect of P, SS, and SP on the relative mRNA levels of liver IL-1β (A), TNF-α (B), IFN-ɣ (C), and NF-кB (D) in broiler chickens, Control (Con), P, SS, and SP. Each bar represents mean of 10 samples in a group and standard error. The values with unlike superscript letters (a, b, c) in the figure represent different levels of significance at p < 0.05.
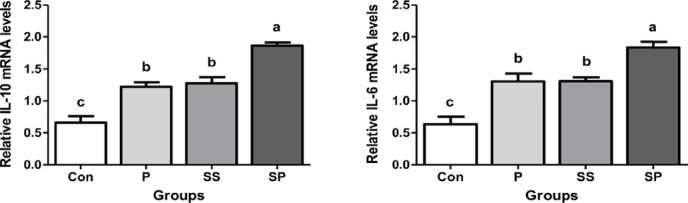
We further assessed the expression of hepatoprotective (IL-6) and anti-inflammatory (IL-10) genes in the liver. Surprisingly, our results showed that the mRNA expression of IL-6 and IL-10 was significantly up-regulated in the SP group than in other groups. No significance was observed between P and SS groups (Fig. 3, p < 0.05).
Figure 3. Effect of P, SS, and SP on the relative mRNA levels of liver IL-10 (A) and IL-6 (B) of broiler chickens, Control (Con), P, SS, and SP. Each bar represents mean of 10 samples in a group and standard error. The values with unlike superscript letters (a, b, c) in the figure represent different levels of significance at p < 0.05.
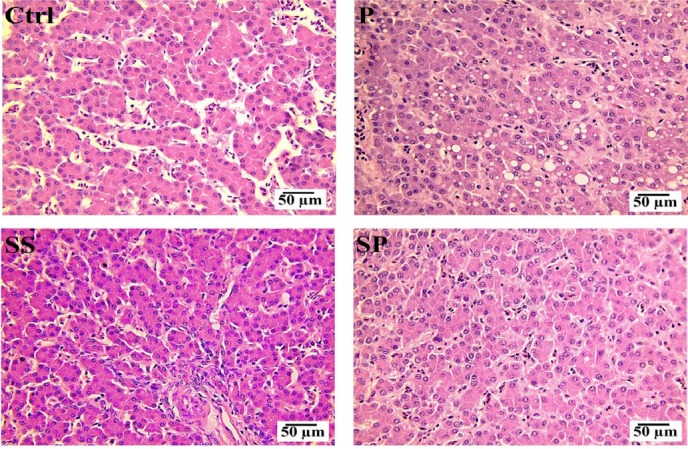
Upon further histopathological studies of the sections of the liver showed that the inflammation and necrosis were significantly reduced in SP group, then in P and SS groups, as compared with Con group (Fig. 4).
Figure 4. H & E staining of liver sections from P, SS, SP, and Con groups for the assessment of inflammation and necrosis. SP supplementation significantly reduced hepatic inflammation and necrosis induced by oxidative stress under a heat stress condition when compared to Con, P, and SS groups.
Discussion
As an essential trace element, Se is required for the synthesis of antioxidant system GPx1 which prevents the oxidative cell injury. In human beings, Se deficiency could be the cause of neoplasia, infertility, bone diseases, nervous diseases, and cardiac failure [24,25]. The present study delineates the hepatoprotective effects of SP used as a feed additive in birds reared in high ambient temperature environment. No untoward effect was observed upon probiotic bacteria due to Se while preparing the conjugated SP.
Expression of liver enzymes helps to understand liver functionality. The low level of these enzymes indicates healthy liver function. Selenium increases the production of GPx1 and GPx4, which in turn repair the DNA, improves the immune and anti-inflammatory responses, as well as enhances resistance against diseases [14]. Previously, the Se feed supplementation has significantly increased the liver GPx1 and GPx4 of male turkey [26]. In the present study, we found that GPx1 and GPx4 levels were increased in SP group which is consistent with the earlier studies conducted upon heat stressed piglets [16,27]. Thus, it can be assumed from the present data that SP feed supplementation promotes anti-oxidation which eventually prevents hepatocytes from lipid peroxidation and reduces MDA levels.
The protective role of Se might be mediated through specific selenoproteins such as GPx. These selenoproteins reduce oxidation induced colon tumor of mice [28]. Therefore, the present findings conclude that hepatoprotective effects against heat stress may be associated with Se modulated up-regulation of GPx1 and GPx4 in male broiler chickens.
High ambient temperature induces ROS production which eventually causes lipid peroxidation [7,23]. As our results clearly demonstrated that SP feed supplementation increases Se uptake by the liver. MDA level was significantly reduced which indicates that oxidative stress and lipid peroxidation were significantly reduced. We also found a higher level of GSH, GSH-Px, and SOD in the SP group which again confirms the protective role of Se (SP) against heat stress.
Dietary supplementation of antioxidant products (e.g., Selenium) may inhibit NF-κB binding with inflammation-related genes and accordingly diminishes cytokine production and reduces inflammation. Previously, NF-κB inhibition and reduced inflammation due to selenium supplementation have been reported in rodents [29,30]. The production of pro-inflammatory cytokines, i.e., IL-1β, TNF-α, and IFN-ɣ are associated with the activation of NF-κB [31,32]. It supports our present study where TNF-α and IFN-ɣ, as well as NF-κB, were down-regulated with the SP diet. Certainly, our findings are consistent with a previous study where the SP diet significantly protected the liver fibrosis by attenuating liver oxidation and inflammation in rats [15]. Additionally, our studies showed that SP supplementation facilitated the upregulation of IL-10, which has been reported as an inhibitor of oxidative stress and pro-inflammatory cytokines (i.e. TNF-α, IL-1β, and IFN-γ) [33]. Furthermore, it has been reported that organic Se supplements can improve avian immunity by up-regulating the ability of immunocompetent cells (through IL-10) to respond to inflammation [34]. Alternatively, the assessment of the mRNA expression of IL-6 also indicated that the SP diet has a significant hepatoprotective effect which is in agreement with earlier studies [35,36]. Moreover, our histopathological examination of the liver also depicted that a SP diet may help to cope with high ambient temperature induced hepatic damage. At the end of our study, these outcomes were found to be strongly associated with mortality and total weight gain in the male broilers (Ross 308). Aggregately, our findings closely correlate with previous findings in other animals, which concluded that SP supplementation protects liver through immunomodulation and anti-oxidation in pigs and rats [23,37].
Conclusion
The present study describes SP as a potential nutritive supplement in broilers kept under high ambient temperature. We found that SP is a potential nutritive supplement, capable of protecting the liver by enhancing the antioxidant and immune system to suppress hepatic oxidation, inflammatory response, and necrosis through the regulation of heat stress-induced activation of ROS and associated NF-κB pathway. In addition, it provides a novel strategy to formulate poultry feed in tropical and subtropical regions to overcome the pathogenesis associated with summer stress in broiler production.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31272627, 31472253), the Research Fund for Doctoral Program of Higher Education in China (20110097110014, 20120097130002), and Higher Education Commission of Pakistan.
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ contribution
AZK, MH, SB, and SA helped in the execution of experiment, management and care of birds, and collection of data; IUK, S, MT, IH, NU, and MAK helped in data assembly, analysis and evaluation, and manuscript writing; KH and RL provided technical guidance and funds for the execution of the experiment.
References
- [1].Niu ZY, Liu FZ, Yan QL, Li WC. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci. 2009;88:2101–07. doi: 10.3382/ps.2009-00220. https://doi.org/10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- [2].Attia YA, Abdalah AA, Zeweil HS, Bovera F, Tag El-Din AA, Araft MA. Effect of inorganic or organic selenium supplementation on productive performance, egg quality and some physiological traits of dual-purpose breeding hens. Czech J Anim Sci. 2010;55:505–19. https://doi.org/10.17221/1702-CJAS. [Google Scholar]
- [3].Chauhan SS, Celi P, Fahri TT, Leury BJ, Dunshea FR. Dietary antioxidants at supranutritional doses modulate skeletal muscle heat shock protein and inflammatory gene expression in sheep exposed to heat stress. J Anim Sci. 2014;92:4897–908. doi: 10.2527/jas.2014-8047. https://doi.org/10.2527/jas.2014-8047. [DOI] [PubMed] [Google Scholar]
- [4].Montilla R, Isabel S. Iowa State University; 2013. The effects of heat stress in redox balance and inflammatory signaling in porcine skeletal muscle’, Sandra Isabel Rosado Montilla. [Google Scholar]
- [5].Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatol. 2015;61:1066. doi: 10.1002/hep.27332. https://doi.org/10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Arrigo T, Leonardi S, Cuppari C, Manti S, Lanzafame A, D‘Angelo G, et al. Role of the diet as a link between oxidative stress and liver diseases. World J Gastroenterol. 2015;21:384–95. doi: 10.3748/wjg.v21.i2.384. https://doi.org/10.3748/wjg.v21.i2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jang IS, Young HK, Moon YS, Sohn SH. Effects of vitamin C or E on the pro-inflammatory cytokines, heat shock protein 70 and antioxidant status in broiler chicks under summer conditions. Asian Australas J Anim Sci. 2014;27:749–56. doi: 10.5713/ajas.2013.13852. https://doi.org/10.5713/ajas.2013.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kamboh AA, Hang SQ, Bakhetgul M, Zhu WY. Effects of genistein and hesperidin on biomarkers of heat stress in broilers under persistent summer stress. Poult Sci. 2013;92:2411–8. doi: 10.3382/ps.2012-02960. https://doi.org/10.3382/ps.2012-02960. [DOI] [PubMed] [Google Scholar]
- [9].Pappas AC, Zoidis E, Surai PF, Zervas G. Selenoproteins and maternal nutrition. Comp Biochem Physiol Part B Biochem Mol Biol. 2008;151:361–72. doi: 10.1016/j.cbpb.2008.08.009. https://doi.org/10.1016/j.cbpb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [10].Spallholz JE, Hoffman DJ. Selenium toxicity: cause and effects in aquatic birds. Aquatic Toxicol. 2002;57:27–37. doi: 10.1016/s0166-445x(01)00268-5. https://doi.org/10.1016/S0166-445X(01)00268-5. [DOI] [PubMed] [Google Scholar]
- [11].Dale N. National research council nutrient requirements of poultry—ninth revised edition. J Appl Poult Res. 1994;3:101. https://doi.org/10.1093/japr/3.1.101. [Google Scholar]
- [12].Edens FW, Sefton AE. Organic selenium in animal nutrition–utilisation, metabolism, storage and comparison with other selenium sources. J Appl Anim Res. 2016;4 https://doi.org/10.1017/jan.2016.5. [Google Scholar]
- [13].Duntas LH. The evolving role of selenium in the treatment of Graves’ disease and ophthalmopathy. J Thyroid Res. 2012b;2012:736161. doi: 10.1155/2012/736161. https://doi.org/10.1155/2012/736161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang Z, Aaron HR, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16:705–43. doi: 10.1089/ars.2011.4145. https://doi.org/10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu Y, Liu Q, Ye G, Khan A, Liu J, Gan F, et al. Protective effects of Selenium-enriched probiotics on carbon tetrachloride-induced liver fibrosis in rats. J Agric Food Chem. 2015;63:242. doi: 10.1021/jf5039184. https://doi.org/10.1021/jf5039184. [DOI] [PubMed] [Google Scholar]
- [16].Gan F, Ren F, Chen X, Lv C, Pan C, Ye G, et al. Effects of selenium-enriched probiotics on heat shock protein mRNA levels in piglet under heat stress conditions. J Agric Food Chem. 2013;61:2385. doi: 10.1021/jf300249j. https://doi.org/10.1021/jf300249j. [DOI] [PubMed] [Google Scholar]
- [17].Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622. doi: 10.1152/ajplung.00477.2005. https://doi.org/10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- [18].Liu Y, Liu Q, Ye G, Khan A, Liu J, Gan F, et al. Protective effects of selenium-enriched probiotics on carbon tetrachloride-induced liver fibrosis in rats. J Agric Food Chem. 2014;63:242–49. doi: 10.1021/jf5039184. https://doi.org/10.1021/jf5039184. [DOI] [PubMed] [Google Scholar]
- [19].Pophaly SD, Singh P, Kumar H, Tomar SK, Singh R. Selenium enrichment of lactic acid bacteria and bifidobacteria: a functional food perspective. Trends Food Sci Technol. 2014;39:135–45. https://doi.org/10.1055/s-0034-1369079. [Google Scholar]
- [20].Khan AZ, Shahnawaz K, Hamid M, Afzal S, Parveen F, Yunhuan L, et al. Effects of selenium-enriched probiotics on heart lesions by influencing the mRNA expressions of selenoproteins and heat shock proteins in heat stressed broiler chickens. Pak Vet J. 2016;36:460–64. [Google Scholar]
- [21].Ahmad HJ, Tian J, Wang J, Khan MA, Wang Y, Zhang L, et al. Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J Agric Food Chem. 2012;60:7111–20. doi: 10.1021/jf3017207. https://doi.org/10.1021/jf3017207. [DOI] [PubMed] [Google Scholar]
- [22].Hamid M, Liu D, Abdulrahim Y, Liu Y, Qian G, Khan A, et al. Amelioration of CCl4-induced liver injury in rats by selenizing Astragalus polysaccharides: role of proinflammatory cytokines, oxidative stress and hepatic stellate cells. Res Vet Sci. 2017;114:202–11. doi: 10.1016/j.rvsc.2017.05.002. https://doi.org/10.1016/j.rvsc.2017.05.002. [DOI] [PubMed] [Google Scholar]
- [23].Nido SA, Shituleni SA, Mengistu BM, Liu Y, Khan AZ, Gan F, et al. Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol Trace Elem Res. 2016;171:399–409. doi: 10.1007/s12011-015-0552-8. https://doi.org/10.1007/s12011-015-0552-8. [DOI] [PubMed] [Google Scholar]
- [24].Pieczyńska J, Grajeta H. The role of selenium in human conception and pregnancy. J Trace Elem Med Biol. 2015;1(29):31–8. doi: 10.1016/j.jtemb.2014.07.003. https://doi.org/10.1016/j.jtemb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- [25].Wang X, Hassan W, Jabeen Q, Khan GJ, Iqbal F. Interdependent and independent multidimensional role of tumor microenvironment on hepatocellular carcinoma. Cytokine. 2018;103:150–9. doi: 10.1016/j.cyto.2017.09.026. https://doi.org/10.1016/j.cyto.2017.09.026. [DOI] [PubMed] [Google Scholar]
- [26].Sunde RA, Hadley KB. Phospholipid hydroperoxide glutathione peroxidase (Gpx4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp Biol Med. 2010;235:23. doi: 10.1258/ebm.2009.009262. https://doi.org/10.1258/ebm.2009.009262. [DOI] [PubMed] [Google Scholar]
- [27].Gan F, Chen X, Liao SF, Lv C, Ren F, Ye G, et al. Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J Agric Food Chem. 2014;62:4502. doi: 10.1021/jf501065d. https://doi.org/10.1021/jf501065d. [DOI] [PubMed] [Google Scholar]
- [28].Krehl S, Loewinger M, Florian S, Kipp AP, Banning A, Wessjohann LA, et al. Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis. 2012;33:620–8. doi: 10.1093/carcin/bgr288. https://doi.org/10.1093/carcin/bgr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Duntas LH. The evolving role of selenium in the treatment of graves’ disease and ophthalmopathy. J Thyroid Res. 2012a;212:736161. doi: 10.1155/2012/736161. https://doi.org/10.1155/2012/736161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang F, Yu W, Hargrove JL, Greenspan P, Dean RG, Taylor EW, et al. Inhibition of TNF-α induced ICAM-1, VCAM-1 and E-selectin expression by selenium. Atherosclerosis. 2002;161:381–86. doi: 10.1016/s0021-9150(01)00672-4. https://doi.org/10.1016/S0021-9150(01)00672-4. [DOI] [PubMed] [Google Scholar]
- [31].Abdollahi E, Momtazi AA, Johnston TP, Sahebkar A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades? J Cell Physiol. 2018;233(2):830–48. doi: 10.1002/jcp.25778. https://doi.org/10.1002/jcp.25778. [DOI] [PubMed] [Google Scholar]
- [32].Rahman I, Gilmour PS, Jimenez LA, Biswas SK, Antonicelli F, Aruoma OI. Ergothioneine inhibits oxidative stress- and TNF-alpha-induced NF-kappa B activation and interleukin-8 release in alveolar epithelial cells. Biochem Biophys Res Commun. 2003;302:860. doi: 10.1016/s0006-291x(03)00224-9. https://doi.org/10.1016/S0006-291X(03)00224-9. [DOI] [PubMed] [Google Scholar]
- [33].Mollazadeh H, Cicero AF, Blesso CN, Pirro M, Majeed M, Sahebkar A. Immune modulation by curcumin: the role of interleukin-10. Crit Rev Food Sci Nutr. 2019;2;59(1):89–101. doi: 10.1080/10408398.2017.1358139. https://doi.org/10.1080 /10408398.2017.1358139. [DOI] [PubMed] [Google Scholar]
- [34].Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27:89–93. doi: 10.1111/j.1440-1746.2011.07003.x. https://doi.org/10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ansar S. Effect of selenium on the levels of cytokines and trace elements in toxin-mediated oxidative stress in male rats. Biol Trace Elem Res. 2016;169:129–33. doi: 10.1007/s12011-015-0403-7. https://doi.org/10.1007/s12011-015-0403-7. [DOI] [PubMed] [Google Scholar]
- [36].Tsuji PA, Carlson BA, Anderson CB, Seifried HE, Hatfield DL, Howard MT. Dietary selenium levels affect selenoprotein expression and support the interferon-γ and IL-6 immune response pathways in mice. Nutrients. 2015;7:6529–49. doi: 10.3390/nu7085297. https://doi.org/10.3390/nu7085297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Y, Wu Y, Wang B, Cao X, Fu A, Li Y, et al. Effects of probiotic Bacillus as a substitute for antibiotics on antioxidant capacity and intestinal autophagy of piglets. AMB Express. 2017;7:52. doi: 10.1186/s13568-017-0353-x. https://doi.org/10.1186/s13568-017-0353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


