Abstract
Background:
Parity is widely recognized as protective for breast cancer, but breast cancer risk may be increased shortly after childbirth. Whether this risk varies with breastfeeding, family history of breast cancer, or specific tumor subtype has rarely been evaluated.
Objective:
To characterize breast cancer risk in relation to recent childbirth.
Design:
Pooled analysis of individual-level data from 15 prospective cohort studies.
Setting:
The international Premenopausal Breast Cancer Collaborative Group.
Participants:
Women younger than 55 years.
Measurements:
During 9.6 million person-years of follow-up, 18 826 incident cases of breast cancer were diagnosed. Hazard ratios (HRs) and 95% CIs for breast cancer were calculated using Cox proportional hazards regression.
Results:
Compared with nulliparous women, parous women had an HR for breast cancer that peaked about 5 years after birth (HR, 1.80 [95% CI, 1.63 to 1.99]) before decreasing to 0.77 (CI, 0.67 to 0.88) after 34 years. The association crossed over from positive to negative about 24 years after birth. The overall pattern was driven by estrogen receptor (ER)–positive breast cancer; no crossover was seen for ER-negative cancer. Breast cancer risk increases after childbirth were pronounced when combined with a family history of breast cancer, and greater for women who were older at first birth or who had more births. Breastfeeding did not modify risk patterns.
Limitations:
Breast cancer diagnoses during pregnancy were not uniformly distinguishable from early postpartum diagnoses. Data on human epidermal growth factor receptor 2 (HER2) oncogene overexpression were limited.
Conclusion:
Compared with nulliparous women, parous women have an increased risk for breast cancer for more than 20 years after childbirth. Health care providers should consider recent childbirth a risk factor for breast cancer in young women.
Parity is widely recognized as protective for breast cancer, but there is evidence that breast cancer risk may increase shortly after childbirth. Whether this risk varies with such factors as breastfeeding, family history of breast cancer, or specific tumor subtype has rarely been evaluated. To characterize breast cancer risk in relation to recent childbirth, this study pooled individual-level data for women younger than 55 years from 15 prospective cohort studies.
Breast cancer is the leading cancer diagnosis among reproductive-aged women worldwide (1). Parity is recognized as a protective factor for breast cancer overall, but this may largely apply to the peak ages of incidence (after age 60 years) and may not be true for younger women. Previous studies have reported that recent childbirth confers a short-term increase in breast cancer risk (2–13), which may last 10 or more years (6, 11, 14–16) and be amplified in women who are older at first birth (6, 11, 15, 16). Evidence for this increased risk often comes from national registry linkage studies in Scandinavian countries (2, 4, 6, 17). Information about such behaviors as breastfeeding is often not available or comes from case–control studies (8–11), where potential risk factors are assessed after diagnosis and parenting responsibilities could differentially deter study participation.
We used combined data from 15 cohort studies to assess breast cancer risk after childbirth. The use of international, prospective data offers a new opportunity to assess the strength and duration of associations between recent childbirth and breast cancer risk while considering the effect of such factors as breastfeeding and family history of breast cancer (5, 18) and to evaluate risk specific to breast cancer subtypes that may be differentially influenced by reproductive history (12, 13, 19). Understanding these patterns may have implications for identifying risk-reducing strategies and vulnerable subgroups.
Methods
We used data from the Premenopausal Breast Cancer Collaborative Group, a pooling project involving 20 prospective cohort studies (20). This work was approved by the relevant institutional review boards.
In brief, participating studies contributed data from women aged younger than 55 years who did not have breast cancer at enrollment; these women were followed prospectively through direct contact or linkage with cancer registries (described previously [20]). Studies contributed (as available) age at enrollment and end of follow-up, demographic characteristics, lifestyle factors, reproductive history, medical conditions, and first-degree family history of breast cancer at enrollment and each follow-up round. Data harmonization and quality control were done by the study coordinating centers in North Carolina and London.
Across 15 cohorts that provided information on women’s ages at childbirth (21–35), 890 269 women (96% of participants) had available information on total number of births and age at most recent birth or were nulliparous. We excluded women who reported a first birth before age 13 years (N = 82), were 50 years or older at study entry and at most recent birth (N = 60), or reached parity greater than 10 births before enrollment (N = 183). These events were considered to have greater potential for data errors. This left 889 944 women for analysis (Supplement Figure 1, available at Annals.org).
Attained age, ages at first and most recent births, and parity at study enrollment were available in all 15 studies (21–35). Twelve studies (21–27, 29, 31, 33–35) assessed pregnancy history in at least 1 follow-up questionnaire after enrollment; the remaining 3 provided pregnancy information at enrollment only. Breastfeeding status was available in 12 studies (21–23, 25–27, 29–34) and family history in 13 (21–27, 29, 31–35). Thirteen studies (21–23, 25–27, 29–35) reported breast cancer stage and estrogen receptor (ER) status.
Statistical Analysis
Parity, time since most recent birth, breastfeeding, and family history of breast cancer were analyzed as time-varying exposures over follow-up. We used Cox proportional hazards regression to calculate hazard ratios (HRs) and 95% CIs for the association between time since most recent birth and breast cancer, with attained age as the underlying time scale (17, 36). Follow-up started at age at study enrollment or the first available follow-up round with information on age at most recent birth and ended at breast cancer diagnosis, death, last follow-up, or age 55 years, whichever occurred first. During follow-up, women were censored at the age they reached parity greater than 10 births (N = 22) or the age they had a birth at age 50 years or older (N = 9) (Supplement Figure 1). Proportional hazards assumptions were assessed by Schoenfeld residuals (37) and were not significantly violated.
We first examined study-specific estimates and calculated a pooled estimate across studies using a random-effects model that weighted estimates by the inverse of the study-specific variance (38–40). Because we detected no significant heterogeneity between studies with the Cochran Q test or I2 statistic (41–43) (Supplement Figure 2, available at Annals.org), we pooled individual-level data and did an aggregated analysis stratified by study cohort. Supplement Table 1 (available at Annals.org) gives characteristics of the individual cohorts.
Time since most recent birth and parity were modeled as time-varying exposures in 1-year and 1-birth increments, respectively. We accounted for additional births during follow-up by resetting time since birth to 0 at the time of each birth. The Supplement (available at Annals.org) gives additional detail on these methods. Quadratic splines (44) were used to examine time since birth as a continuous, nonlinear exposure, defining time with knots at the 5th, 25th, 50th, 75th, and 95th percentiles for the distribution of time since most recent birth for women with a breast cancer diagnosis before age 55 years. In spline models, time since most recent birth was set to 0 for nulliparous women and an indicator term for parity allowed the risk at 0 years since most recent birth to differ between nulliparous and parous women. As an approximation of the 95% CI (in years since most recent birth) for the point where the HR crossed 1.0, we used the points where the lower and upper bounds of the 95% CI for the spline regression crossed 1.0. In categorical models, exposure was defined as nulliparous or 0 to 2.9, 3 to 4.9, 5 to 9.9, 10 to 14.9, 15.0 to 19.9, 20.0 to 24.9, 25.0 to 29.9, or 30 or more years since most recent birth.
Covariates considered as potential confounders were parity, age at first birth, breastfeeding, infertility, education, oral contraceptive use, and birth cohort. We identified confounding variables using a directed acyclic graph (45, 46) and the prior literature (Supplement Figure 3, available at Annals.org); the minimally sufficient adjustment set was parity and breastfeeding. All models were adjusted for attained age (as the time scale; continuous), study, and parity (1 to 10 births; time-varying). Adjustment for breastfeeding was possible only in analyses limited to the 12 studies with available breastfeeding data.
We evaluated potential effect modification by parity (primiparous [1 birth], biparous [2 births], or multiparous [≥3 births]), age at first birth (<25, 25 to 34, or 35 to 39 years), breastfeeding, and family history of breast cancer. Interactions between these factors and time since most recent birth were assessed using likelihood ratio tests (47). We examined risk for invasive nonmetastatic disease (stage I to III breast cancer) by treating breast cancer of stage 0 (in situ) or IV as a censoring event. Augmentation models were used to assess differences in HRs by ER status by using the Wald test (48).
In repeated analyses, we restricted the cohort to parous women only, censored follow-up at age 45 years or the last age at which pregnancy history was assessed if younger than 45 years (to minimize the potential for additional pregnancies after the most recent questionnaire), excluded each study in turn to identify potentially influential studies, and excluded women with multiple births (for example, twins).
We also calculated the weighted cumulative hazard of breast cancer according to attained age for categories of time since most recent childbirth (nulliparous and 0 to 2.9, 3 to 6.9, 7 to 14.9, 15 to 24.9, and 25 or more years), adjusted for the distribution of parity in the overall pooled sample using an inverse probability of exposure approach, described further in Supplement Figure 4 (available at Annals.org) (49). Because our data were left-truncated, we also provided a standardized weighted cumulative hazard function calculated over a common age interval that had participants in each category of time since most recent birth. The standardized weighted cumulative hazard function starts at 0 at the beginning of the common age interval and cumulates throughout the interval, which allows comparison of the cumulative hazard across categories of time since most recent birth (Supplement Figure 5, available at Annals.org).
Analyses were done with SAS, version 9.3 (SAS Institute); figures were produced in SAS and R (R Foundation for Statistical Computing).
Results
During 9 625 727 person-years of follow-up (mean, 10.8 years [SD, 6.4]), 18 826 incident cases of breast cancer were diagnosed before age 55 years among 889 944 women. At enrollment, 720 555 women were parous; 71 609 women contributed 1 or more births during follow-up. The mean age at study entry was 41.8 years (range, 16.0 to 54.9 years). The last update of pregnancy information occurred at a mean age of 50.0 years (range, 16.0 to 76.7 years). Overall, 12.4% of person-years were contributed by women who reported a family history of breast cancer (Table). For parous women, 72.9% of person-years were contributed by women who reported breastfeeding.
Table.
Distribution of Person-Years Among 889 944 Women in 15 Prospective Cohort Studies, by Exposure Characteristics*
| Variable | Person-Years, n (%)† | Cases of Breast Cancer, n | Stage, n‡ | Type of Breast Cancer, n§ | |||
|---|---|---|---|---|---|---|---|
| 0 | I–III | IV | ER-Positive | ER-Negative | |||
| Total | 9 625 727 (100) | 18 826 | 2319 | 9428 | 247 | 8508 | 2758 |
| Time since most recent birth | |||||||
| Nulliparous | 1 680 909 (17.5) | 3033 | 413 | 1446 | 38 | 1396 | 394 |
| 0–2.9 y | 402 357 (4.2) | 219 | 22 | 125 | 5 | 84 | 57 |
| 3.0–4.9 y | 317 723 (3.3) | 308 | 41 | 173 | 5 | 126 | 58 |
| 5.0–9.9 y | 1 082 408 (11.2) | 1437 | 193 | 753 | 18 | 608 | 216 |
| 10.0–14.9 y | 1 555 448 (16.2) | 2780 | 346 | 1461 | 39 | 1256 | 388 |
| 15.0–19.9 y | 1 839 722 (19.1) | 4185 | 497 | 2152 | 41 | 1917 | 583 |
| 20.0–24.9 y | 1 624 570 (16.9) | 4017 | 495 | 1964 | 48 | 1820 | 597 |
| 25.0–29.9 y | 882 071 (9.2) | 2208 | 231 | 1045 | 42 | 1001 | 350 |
| ≥30.0 y | 240 519 (2.5) | 639 | 81 | 309 | 11 | 300 | 115 |
| Age at first birth | |||||||
| 13–23 y | 3 149 633 (39.1) | 5562 | 598 | 2882 | 81 | 2295 | 921 |
| 24–25 y | 1 493 666 (18.5) | 2851 | 344 | 1428 | 44 | 1260 | 422 |
| 26–29 y | 2 026 933 (25.2) | 4246 | 534 | 2154 | 54 | 2000 | 612 |
| 30–39 y | 1 327 752 (16.5) | 3001 | 407 | 1455 | 30 | 1482 | 395 |
| 40–49 y | 55 027 (0.7) | 130 | 22 | 61 | 0 | 73 | 13 |
| Missing | 1848 (0) | 3 | 1 | 2 | 0 | 2 | 1 |
| Parity | |||||||
| 0 | 1 680 909 (17.5) | 3033 | 413 | 1446 | 38 | 1396 | 394 |
| 1 | 1 569 982 (16.3) | 3190 | 404 | 1451 | 40 | 1412 | 479 |
| 2 | 3 628 391 (37.7) | 7548 | 904 | 3664 | 99 | 3427 | 1111 |
| 3 | 1 890 292 (19.6) | 3639 | 422 | 1994 | 46 | 1669 | 541 |
| 4 | 590 884 (6.1) | 1015 | 130 | 604 | 19 | 429 | 165 |
| 5 | 169 499 (1.8) | 264 | 30 | 174 | 4 | 122 | 45 |
| 6–10 | 95 770 (1.0) | 137 | 16 | 95 | 1 | 53 | 23 |
| Family history of breast cancer | |||||||
| Yes | 1 051 985 (12.4) | 3587 | 666 | 2099 | 37 | 1983 | 545 |
| No | 7 087 732 (83.5) | 11 737 | 1451 | 6736 | 181 | 5168 | 1691 |
| Missing | 346 339 (4.1) | 1536 | 118 | 210 | 11 | 632 | 279 |
| Breastfeeding status (parous women only) | |||||||
| Yes | 5 007 247 (72.9) | 10 096 | 1368 | 5582 | 128 | 5020 | 1491 |
| No | 1 337 496 (19.5) | 2924 | 418 | 1661 | 43 | 1460 | 595 |
| Missing | 519 487 (7.6) | 879 | 109 | 613 | 36 | 390 | 182 |
ER = estrogen receptor.
Percentages may not sum to 100 due to rounding.
Calculated for studies that provided information on the characteristic of interest and that reflect changes in time-updated exposures, including time since most recent birth, parity, family history, and breastfeeding status.
Available for 11 994 women.
Available for 11 266 women.
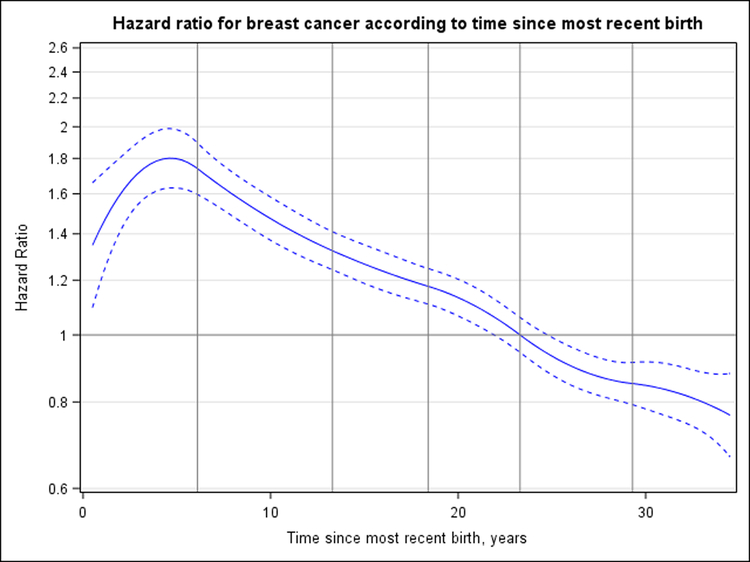
Figure 1 shows the association between time since most recent birth and breast cancer risk, modeled nonlinearly as a continuous exposure. Compared with nulliparous women, parous women had an HR for breast cancer associated with time since most recent birth that peaked 4.6 years after birth (HR, 1.80 [95% CI, 1.63 to 1.99]) before decreasing to its lowest observed point (HR, 0.77 [CI, 0.67 to 0.88]) 34.5 years after birth; the crossover in risk occurred 23.6 years (CI, 21.9 to 25.0 years) after birth. Over a common age interval starting at age 41.5 years, the standardized cumulative hazard of breast cancer per 100 000 person-years among nulliparous women was 1494 at age 45.0 years, 2132 at age 47.5 years, and 2846 at age 50.0 years. For comparison, the standardized cumulative hazard among women who had their most recent child 3 to 6.9 years before was 1496 at age 45.0 years, 2265 at age 47.5 years, and 3060 at age 50.0 years (Supplement Figure 5. This corresponds to 2, 133, and 214 excess cases of breast cancer per 100 000 person-years at each respective age for women whose most recent birth was 3 to 6.9 years before compared with nulliparous women.
Figure 1. HR for breast cancer according to years since most recent birth.
Nulliparous women are the reference group, and HRs are adjusted for attained age, study, and continuous parity. Dashed curves correspond to 95% CIs. Vertical lines represent the quadratic spline knots at 6.1, 13.3, 18.4, 23.3, and 29.3 y after birth. The HR for breast cancer risk peaks at 1.80 (95% CI, 1.63 to 1.99) at 4.6 y, crosses 1 at 23.6 y (CI, 21.9 to 25.0 y), and reaches its lowest observed point (HR, 0.77 [CI, 0.67 to 0.88]) at 34.5 y after birth. HR = hazard ratio.
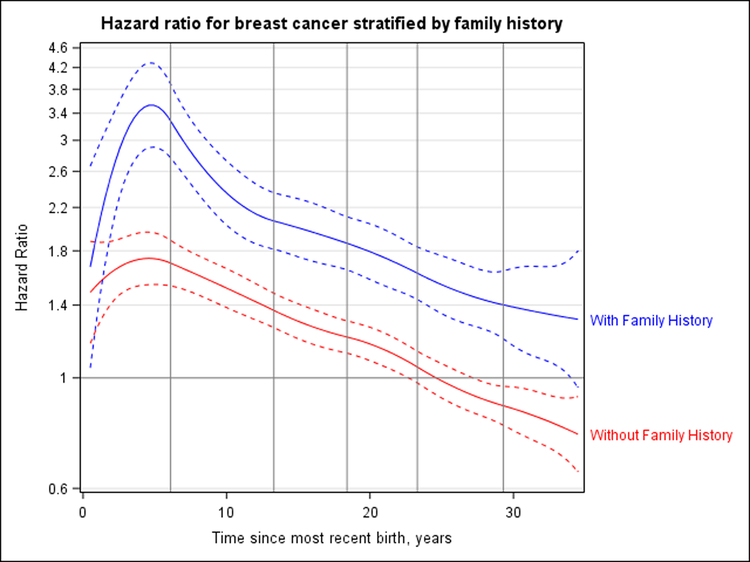
The association between time since most recent birth and breast cancer risk was modified by family history of breast cancer (P = 0.044). Supplement Figure 6 (available at Annals.org) shows analyses done separately for women who did and did not have such a history. The peak HRs associated with time since most recent birth were 1.74 (CI, 1.54 to 1.96) at 4.6 years after birth for women without a family history and 1.82 (CI, 1.48 to 2.24) at 4.9 years in women with a family history. However, compared with women with neither risk factor (that is, nulliparous women without a family history of breast cancer), those with both (parous women with a family history) had a peak HR for breast cancer of 3.53 (CI, 2.91 to 4.29) at 4.9 years after birth (Figure 2).
Figure 2. HR for breast cancer according to years since most recent birth for the joint effect of family history and time since most recent birth.
Nulliparous women without a family history of breast cancer are the reference group, and HRs are adjusted for attained age, study, and continuous parity. Dashed curves correspond to 95% CIs. Vertical lines represent the quadratic spline knots at 6.1, 13.3, 18.4, 23.3, and 29.3 y after birth. Likelihood ratio tests for models with and without interaction terms for time since most recent birth indicated a statistically significant (P = 0.044) interaction with family history of breast cancer. Compared with a common reference group of nulliparous women without such a history, women with a family history of breast cancer had an HR of 3.53 (CI, 2.91, 4.29) 4.9 y after first birth and did not cross over toward a protective effect for ≥30 y. As an approximation of the 95% CI around the crossover point, for women without a family history, the lower bound crosses at 22.9 y and the upper bound at 27.4 y. For women with a family history, the lower bound crosses at 34.2 y and the upper bound does not cross 1 during the 34.5 y of follow-up. HR = hazard ratio.
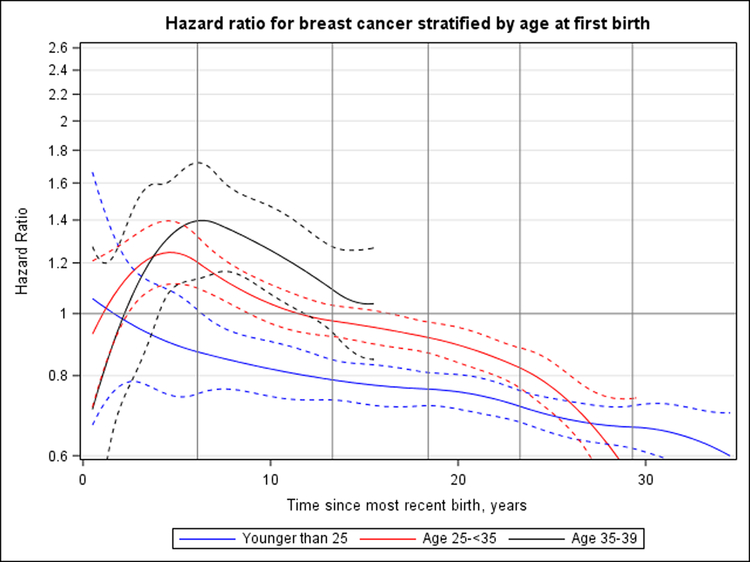
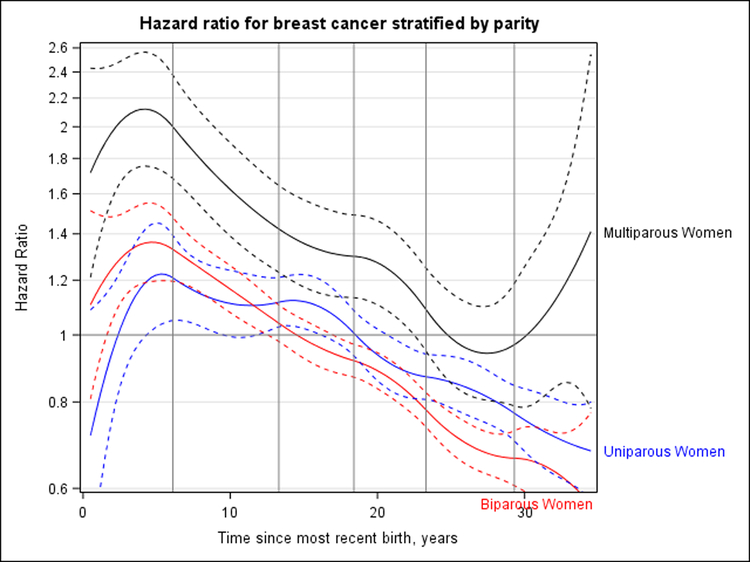
We observed significant heterogeneity in the association between time since most recent birth and breast cancer risk according to age at first birth (P = 0.013) (Figure 3) and parity (P = 0.030) (Figure 4), but not breastfeeding (P = 0.38) (Supplement Figure 7, available at Annals.org). Peak HRs for breast cancer associated with recent childbirth seemed to be higher with increasing age at first birth; women who were youngest at first birth (<25 years) did not have an increased risk for breast cancer compared with nulliparous women (Figure 3). The magnitude of peak HRs was smaller than that seen overall owing to the inability to adjust for parity continuously (1 to 10 births) across age-at-first-birth groups because few women who were older at first birth had 3 or more children. When primiparous, biparous, and multiparous women were evaluated separately, the magnitude of peak HRs was greatest (and the time to crossover toward an inverse association longest) among multiparous women (Figure 4).
Figure 3. HR for breast cancer according to years since most recent birth, stratified by age at first birth (nulliparous or parous with age at first birth <25, 25–34, or 35–39 y).
Nulliparous women are the reference group, and HRs are adjusted for attained age, study, and parity (0, 1, or ≥2 births). Dashed curves correspond to 95% CIs. Vertical lines represent the quadratic spline knots at 6.1, 13.3, 18.4, 23.3, and 29.3 y after birth. Likelihood ratio tests indicated a statistically significant interaction with age at first birth (P = 0.013). All analyses censor at age 55 y; therefore, only the first 15 y of follow-up are analyzed for women in the oldest age group (35–39 y) because they were aged ≥50 y after 15 y. Women who had their first birth before age 25 y had a peak HR for breast cancer of 1.06 (95% CI, 0.67 to 1.66) at <1 y after birth. For women who had their first births at age 25–34 y and 35–39 y, peak HRs were 1.25 (CI, 1.11 to 1.40) at 4.6 y and 1.40 (CI, 1.14 to 1.72) at 6.4 y, respectively, since most recent birth. HR = hazard ratio.
Figure 4. HR for breast cancer according to years since most recent birth, stratified by parity (0, 1, 2, or ≥3 births).
Nulliparous women are the reference group, and HRs are adjusted for attained age and study; estimates for women with ≥3 births are further adjusted for continuous number of births (3–10 births). Dashed curves correspond to 95% CIs. Vertical lines represent the quadratic spline knots at 6.1, 13.3, 18.4, 23.3, and 29.3 y after birth. Likelihood ratio tests indicated a statistically significant interaction with parity (P = 0.030). For uniparous women, the peak HR was 1.22 (95% CI, 1.03 to 1.45) and occurred 5.3 y after last birth; crossover toward an inverse association occurred 18.5 y (CI, 16.5 to 20.9 y) after first birth for uniparous women. For biparous women, the peak HR was 1.36 (CI, 1.19 to 1.55) at 4.6 y after last birth; crossover toward an inverse association occurred 14.8 y (CI, 12.7 to 17.2 y) after last birth. For women with ≥3 births, the peak HR was 2.12 (CI, 1.75 to 2.56) at 4.2 y after last birth, crossover toward an inverse association occurred 25.0 y since last birth, and the lower bound of the CI crossed over at 22.6 y. HR = hazard ratio.
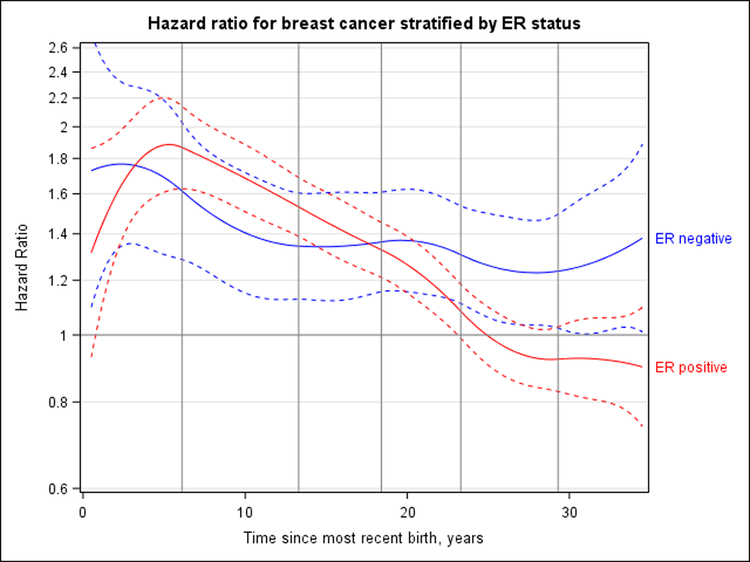
The association between time since most recent birth and breast cancer risk differed by ER status (P < 0.001) (Figure 5). Risk for ER-negative breast cancer was highest 2.2 years after birth (HR, 1.77 [CI, 1.34 to 2.33]) and decreased to an HR of 1.38 (CI, 1.01 to 1.88) at 34.5 years after birth but did not cross over to a protective association. The pattern for ER-positive breast cancer, which accounted for 76% of all breast cancer cases, was similar to the overall results. Additional adjustment for breastfeeding history changed results only slightly for ER-negative breast cancer risk and did not detectably change ER-positive risk (Supplement Figure 8, available at Annals.org). In risk models for ER-negative breast cancer, the test for interaction between time since most recent birth and breastfeeding was statistically significant (P = 0.020). Risk for ER-negative cancer was generally higher for parous women compared to nulliparous women, regardless of breastfeeding status, although the pattern of risk with increasing time since most recent childbirth was less consistent among women who never breastfed, potentially because of smaller sample sizes (Supplement Table 2, available at Annals.org).
Figure 5. HR for ER-positive and ER-negative breast cancer according to years since most recent birth, adjusted for attained age, study, and parity (continuous).
Nulliparous women are the reference group. Dashed curves correspond to 95% CIs. Vertical lines represent the quadratic spline knots at 6.1, 13.3, 18.4, 23.3, and 29.3 y after birth. Tests for interaction with ER status were statistically significant (P < 0.001). ER-negative breast cancer risk peaked 2.2 y after birth (HR, 1.77 [95% CI, 1.34 to 2.33]), and the HR decreased to 1.38 (CI, 1.01 to 1.88) at 34.5 y after birth but did not cross over to a protective association. ER-positive breast cancer had a peak HR of 1.88 (CI, 1.62 to 2.20) at 5.3 y, crossing the null value at 25.0 y and reaching an HR of 0.90 (CI, 0.74 to 1.09) at 34.5 y. ER = estrogen receptor; HR = hazard ratio.
Supplement Table 3 (available at Annals.org) shows analyses according to ER status and restricted to parous women. In models that defined the reference group as women 10 to 14.9 years from most recent birth, we continued to see a long-term crossover toward a protective association for ER-positive (but not ER-negative) breast cancer. Hazard ratios for breast cancer were similar in models that included all breast cancer diagnoses (stages 0 to IV) or that censored in situ (stage 0) or distant (stage IV) diagnoses (Supplement Table 4, available at Annals.org).
Results were essentially unchanged in sensitivity analyses that censored women at the last age where pregnancy was assessed if it was less than 45 years (to minimize the potential for additional pregnancies after the most recent questionnaire), excluded 1 study at a time, or excluded multiple births (data not shown).
Discussion
Our analysis combined individual-level data from about 890 000 women from 15 prospective cohort studies across 3 continents to investigate breast cancer risk in reproductive-aged women. Compared with women who had not given birth, parous women had an elevated breast cancer risk that peaked around 5 years after childbirth and lasted about 20 years. Our results provide evidence that, overall, this association is not modified by breastfeeding and that it varies according to ER expression, age at first birth, and family history of breast cancer.
To our knowledge, the effect of breastfeeding on breast cancer risk after childbirth has not been directly addressed before. Breastfeeding has been associated with an estimated 12% to 25% lower risk for premenopausal breast cancer overall (50, 51) and is thought to be particularly beneficial in reducing risk for ER-negative breast cancer, which is relatively more common at young ages than older ages. Although higher parity is associated with an overall increase in risk for ER-negative breast cancer (13, 52, 53), parous women who breastfeed have comparable risk to nulliparous women (13), suggesting that breastfeeding may mitigate parity-related increases in risk for ER-negative cancer.
In the current analysis of 12 international studies, risks for both ER-positive and ER-negative breast cancer were elevated for 20 years after most recent birth in parous compared with nulliparous women, regardless of breastfeeding. With longer follow-up, the expected inverse association between childbirth and breast cancer became apparent for ER-positive breast cancer, but risk remained elevated for ER-negative disease. These findings are consistent with a sustained increase in risk for ER-negative breast cancer for at least 25 years after birth in parous compared with nulliparous women, as reported in a pooled analysis of 4 U.S. studies that enrolled African American women (12). However, our findings disagree with that study’s report of no increase in ER-positive breast cancer in the first 15 years after last birth.
Familial breast cancer tends to occur at a younger age than breast cancer in women without a genetic predisposition. Family history might therefore modify associations between recent childbirth and breast cancer risk. A study in Denmark (5) found stronger associations between recent childbirth (<5 years prior) and breast cancer risk among women with a mother or sister diagnosed with breast cancer than among those without. In Norway (18), short-term elevations in risk after childbirth were more apparent in women with a family history of breast cancer than in a common reference group of nulliparous women without such a history, although differences were not statistically significant. In our analysis, women with both a recent birth and a family history of breast cancer had a 3.5-fold increase in breast cancer risk compared with women with neither characteristic.
The large number of cases in our pooled analysis allowed us to evaluate potential variation in the association between recent childbirth and breast cancer according to modifiable behaviors, familial susceptibility, and clinical subtypes. These considerations can rarely be addressed in individual studies because of the lower incidence and correspondingly small numbers of breast cancer diagnoses at young ages. However, calendar month was not uniformly available for ages at childbirth and breast cancer diagnosis, so we could not distinguish breast cancer cases diagnosed during pregnancy from those diagnosed in the months immediately postpartum. The small number of breast cancer cases and births that occurred at same integer age (N = 39) resulted in wide CIs for the HR estimate for the first year after childbirth. Our analyses do not address breast cancer risk after age 55 years because of limits of the data provided to the Premenopausal Breast Cancer Collaborative Group (20). We did not address associations according to intervals between births; 1 prior study has suggested that longer intervals could magnify childbirth-related increases in breast cancer risk (17). Available breastfeeding information was not specific to each birth; therefore, if women breastfed some children but not others, breastfeeding status may be misclassified for the most recent birth. Finally, we had limited data on HER2 oncogene overexpression and could not evaluate whether associations differed by the HER2 status of the tumor. In a case-only study of Hispanic women, those within 10 years of their last full-term pregnancy (vs. >10 years) had higher risk for HER2-positive disease (OR, 1.78 [CI, 1.08 to 2.93]) than for cancer that is ER- or progesterone receptor–positive and HER2-negative (19).
Several biological explanations for a transient increase in breast cancer risk after childbirth have been proposed. Proliferation of breast cells during pregnancy could promote accelerated development of latent initiated tumor cells (46, 54). In this way, a greater magnitude of risk conferred by older age at first birth could be due to a higher proportion of initiated cells at older ages. The postpartum breast microenvironment, characterized by lactational involution, may also facilitate cancer cell migration and metastasis; the observation that breast tumors diagnosed postpartum have more advanced stages at diagnosis than those diagnosed during pregnancy supports this mechanism (55–58). Although the higher proportion of advanced-stage tumors could also be due to less timely detection of breast cancer in lactating women, our similar results in analyses limited to stage I to III cancer and stratified by breastfeeding suggest that differential detection after childbirth is not the sole cause.
Breast cancer is the most common cancer type in reproductive-aged women. We report an increased risk for breast cancer after childbirth that can last more than 20 years. This risk may be enhanced when a woman is older at first birth or has a family history of breast cancer, and it is not mitigated by breastfeeding. Women and health care professionals should take these factors into account when considering individual risk profiles for breast cancer.
Supplementary Material
Acknowledgment:
The authors thank all study participants, staff, and participating cancer registries, as well as Chelsea Anderson, Niclas Håkansson, Melissa House, and Allison Iwan. The Nurses’ Health Study and Nurses’ Health Study II thank study participants, staff, and the following state cancer registries: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. The Black Women’s Health Study obtained pathology data on breast cancer from state cancer registries in Arizona; California; Colorado; Connecticut; Delaware; Washington, DC; Florida; Georgia; Illinois; Indiana; Kentucky; Louisiana; Maryland; Massachusetts; Michigan; New Jersey; New York; North Carolina; Oklahoma; Pennsylvania; South Carolina; Tennessee; Texas; and Virginia. The authors assume full responsibility for analyses and interpretation of these data. They thank the National Cancer Institute Cohort Consortium for facilitating this collaboration.
Financial Support:
In part by the Avon Foundation (02-2014-080); funding from Breast Cancer Now and the U.K. National Health Service to the Royal Marsden–Institute of Cancer Research National Institute for Health Research Biomedical Research Centre; the Institute of Cancer Research, London; the National Institute of Environmental Health Sciences (Z01 ES044005) and National Cancer Institute (UM1 CA176726, UM1 CA186107, UM1 CA164974, R01 CA058420, R01 CA092447, and P50 CA168504) of the National Institutes of Health; the National Center for Advancing Translational Sciences (KL2-TR001109); the National Program of Cancer Registries of the Centers for Disease Control and Prevention and the Department of Energy; the Swedish Research Council and Swedish Cancer Society; the Japanese Ministry of Health, Labour and Welfare; the Hellenic Health Foundation; a Karolinska Institutet Distinguished Professor Award (2368/10–221); Cancer Council Victoria and the Australia National Health and Medical Research Council (209057, 396414, and 504711); the state of Maryland; and the Maryland Cigarette Restitution Fund. The coordination of EPIC (the European Prospective Investigation into Cancer and Nutrition) is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by the Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, and Institut National de la Santé et de la Recherche Médicale (France); German Cancer Aid, German Cancer Research Center, and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sport, Netherlands Cancer Registry, LK Research Funds, Dutch Prevention Funds, Zorg Onderzoek Nederland, World Cancer Research Fund, and Statistics Netherlands (the Netherlands); ERC-2009-AdG 232997, Health Research Fund (FIS), PI13/00061 to Granada, PI13/01162 to EPIC-Murcia, regional governments of Andalucía, Asturias, Basque Country, Murcia, and Navarra, and ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Research Council, and county councils of Skåne and Västerbotten (Sweden); and Cancer Research UK (14136 to EPIC-Norfolk and C570/A16491 and C8221/A19170 to EPIC-Oxford) and the Medical Research Council (1000143 to EPIC-Norfolk and MR/M012190/1 to EPIC-Oxford) (United Kingdom).
Disclosures: Dr. Schoemaker reports grants from the United Kingdom–based charity Breast Cancer Now during the conduct of the study. Dr. Cai reports grants from the Avon Foundation during the conduct of the study. Ms. Wright reports grants from Breast Cancer Now during the conduct of the study. Dr. Jones reports grants from Breast Cancer Now during the conduct of the study. Dr. Giles reports grants from the National Health and Medical Research Council during the conduct of the study. Dr. Key reports grants from Cancer Research UK during the conduct of the study. Dr. Milne reports grants from the Australian National Health and Medical Research Council during the conduct of the study. Dr. Swerdlow reports grants from Breast Cancer Now during the conduct of the study. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18-1323.
Role of the Funding Source
The funding sources for this analysis had no role in the design, conduct, or interpretation of the study.
Primary Funding Source: Avon Foundation (02-2014-080)
Footnotes
Supplement. Supplementary Material
Contributor Information
Hazel B. Nichols, University of North Carolina Gillings School of Global Public Health, Chapel Hill, North Carolina.
Minouk J. Schoemaker, The Institute of Cancer Research, London, United Kingdom.
Jianwen Cai, University of North Carolina Gillings School of Global Public Health, Chapel Hill, North Carolina.
Jiawei Xu, University of North Carolina Gillings School of Global Public Health, Chapel Hill, North Carolina.
Lauren B. Wright, The Institute of Cancer Research, London, United Kingdom.
Mark N. Brook, The Institute of Cancer Research, London, United Kingdom.
Michael E. Jones, The Institute of Cancer Research, London, United Kingdom.
Hans-Olov Adami, Karolinska Institutet, Stockholm, Sweden.
Laura Baglietto, University of Pisa, Pisa, Italy.
Kimberly A. Bertrand, Slone Epidemiology Center at Boston University, Boston, Massachusetts.
William J. Blot, Vanderbilt-Ingram Cancer Center and Vanderbilt University School of Medicine, Nashville, Tennessee.
Marie-Christine Boutron-Ruault, Institut National de la Santé et de la Recherche Médicale (INSERM) U1018, Institut Gustave Roussy Centre for Research in Epidemiology and Population Health, University Paris-Saclay, and University Paris-Sud, Villejuif, France.
Miren Dorronsoro, Public Health Direction and Biodonostia Research Institute and Centro de Investigación Biomédica en Red de Epidemiología y Salud Pública, Basque Regional Health Department, San Sebastian, Spain.
Laure Dossus, International Agency for Research on Cancer, Lyon, France.
A. Heather Eliassen, Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health, Boston, Massachusetts.
Graham G. Giles, Cancer Council Victoria and University of Melbourne School of Population and Global Health, Melbourne, Victoria, Australia.
Inger T. Gram, University of Tromsø, The Arctic University of Norway, Tromsø, Norway.
Susan E. Hankinson, University of Massachusetts Amherst School of Public Health and Health Sciences, Amherst, Massachusetts.
Judy Hoffman-Bolton, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Rudolf Kaaks, German Cancer Research Center, Heidelberg, Germany.
Timothy J. Key, University of Oxford, Oxford, United Kingdom.
Cari M. Kitahara, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Susanna C. Larsson, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden.
Martha Linet, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Melissa A. Merritt, School of Public Health, Imperial College London, London, United Kingdom.
Roger L. Milne, Cancer Council Victoria and University of Melbourne School of Population and Global Health, Melbourne, Victoria, Australia.
Valeria Pala, Cancer Registry of Norway, Institute of Population-Based Cancer Research, and University of Oslo, Oslo, Norway, and University of Southern California, Los Angeles, California.
Julie R. Palmer, Slone Epidemiology Center at Boston University, Boston, Massachusetts.
Petra H. Peeters, University Medical Center Utrecht, Utrecht, the Netherlands.
Elio Riboli, School of Public Health, Imperial College London, London, United Kingdom.
Malin Sund, Umeå University, Umeå, Sweden.
Rulla M. Tamimi, Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health, Boston, Massachusetts.
Anne Tjønneland, Danish Cancer Society Research Center, Copenhagen, Denmark.
Antonia Trichopoulou, Hellenic Health Foundation, Athens, Greece.
Giske Ursin, Norwegian University of Science and Technology, Trondheim, Norway.
Lars Vatten, Norwegian University of Science and Technology, Trondheim, Norway.
Kala Visvanathan, Johns Hopkins Bloomberg School of Public Health and Johns Hopkins School of Medicine, Baltimore, Maryland.
Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; University of Tromsø, The Arctic University of Norway, Tromsø, Norway; and Folkhälsan Research Center, Helsinki, Finland.
Alicja Wolk, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden.
Wei Zheng, Vanderbilt-Ingram Cancer Center and Vanderbilt University School of Medicine, Nashville, Tennessee.
Clarice R. Weinberg, National Institute of Environmental Health Sciences, National Institutes of Health, Durham, North Carolina.
Anthony J. Swerdlow, The Institute of Cancer Research, London, United Kingdom.
Dale P. Sandler, National Institute of Environmental Health Sciences, National Institutes of Health, Durham, North Carolina.
References
- 1.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev. 2017;26:444–57. doi: 10.1158/1055-9965.EPI-16-0858 [DOI] [PubMed] [Google Scholar]
- 2.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331:5–9. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsen G, Heuch I, Kvåle G. The short-term and long-term effect of a pregnancy on breast cancer risk: a prospective study of 802,457 parous Norwegian women. Br J Cancer. 1995;72:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leon DA, Carpenter LM, Broeders MJ, Gunnarskog J, Murphy MF. Breast cancer in Swedish women before age 50: evidence of a dual effect of completed pregnancy. Cancer Causes Control. 1995;6:283–91. [DOI] [PubMed] [Google Scholar]
- 5.Wohlfahrt J, Olsen JH, Melby M. Breast cancer risk after childbirth in young women with family history (Denmark). Cancer Causes Control. 2002;13:169–74. [DOI] [PubMed] [Google Scholar]
- 6.Kauppila A, Kyyrönen P, Lehtinen M, Pukkala E. Dual effect of short interval between first and second birth on ductal breast cancer risk in Finland. Cancer Causes Control. 2012;23:187–93. doi: 10.1007/s10552-011-9868-7 [DOI] [PubMed] [Google Scholar]
- 7.Williams EM, Jones L, Vessey MP, McPherson K. Short term increase in risk of breast cancer associated with full term pregnancy. BMJ. 1990;300:578–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruzzi P, Negri E, La Vecchia C, Decarli A, Palli D, Parazzini F, et al. Short term increase in risk of breast cancer after full term pregnancy. BMJ. 1988;297:1096–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh C, Pavia M, Lambe M, Lan SJ, Colditz GA, Ekbom A, et al. Dual effect of parity on breast cancer risk. Eur J Cancer. 1994;30A:969–73. [DOI] [PubMed] [Google Scholar]
- 10.Chie WC, Hsieh C, Newcomb PA, Longnecker MP, Mittendorf R, Greenberg ER, et al. Age at any full-term pregnancy and breast cancer risk. Am J Epidemiol. 2000;151:715–22. [DOI] [PubMed] [Google Scholar]
- 11.Cummings P, Weiss NS, McKnight B, Stanford JL. Estimating the risk of breast cancer in relation to the interval since last term pregnancy. Epidemiology. 1997;8:488–94. [DOI] [PubMed] [Google Scholar]
- 12.Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst. 2014;106. doi: 10.1093/jnci/dju237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer JR, Boggs DA, Wise LA, Ambrosone CB, Adams-Campbell LL, Rosenberg L. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev. 2011;20:1883–91. doi: 10.1158/1055-9965.EPI-11-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borges VF, Schedin PJ. Pregnancy-associated breast cancer: an entity needing refinement of the definition [Editorial]. Cancer. 2012;118:3226–8. doi: 10.1002/cncr.26643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schedin P Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–91. [DOI] [PubMed] [Google Scholar]
- 16.Innes KE, Byers TE. First pregnancy characteristics and subsequent breast cancer risk among young women. Int J Cancer. 2004;112:306–11. [DOI] [PubMed] [Google Scholar]
- 17.Albrektsen G, Heuch I, Hansen S, Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albrektsen G, Heuch I, Thoresen S, Kvåle G. Family history of breast cancer and short-term effects of childbirths on breast cancer risk. Int J Cancer. 2006;119:1468–74. [DOI] [PubMed] [Google Scholar]
- 19.Cruz GI, Martínez ME, Natarajan L, Wertheim BC, Gago-Dominguez M, Bondy M, et al. Hypothesized role of pregnancy hormones on HER2+ breast tumor development. Breast Cancer Res Treat. 2013;137:237–46. doi: 10.1007/s10549-012-2313-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols HB, Schoemaker MJ, Wright LB, McGowan C, Brook MN, McClain KM, et al. The Premenopausal Breast Cancer Collaboration: a pooling project of studies participating in the National Cancer Institute Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2017;26:1360–9. doi: 10.1158/1055-9965.EPI-17-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boggs DA, Rosenberg L, Adams-Campbell LL, Palmer JR. Prospective approach to breast cancer risk prediction in African American women: the Black Women’s Health Study model. J Clin Oncol. 2015;33:1038–44. doi: 10.1200/JCO.2014.57.2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clavel-Chapelon F; E3N Study Group. Cohort profile: the French E3N Cohort Study. Int J Epidemiol. 2015;44:801–9. doi: 10.1093/ije/dyu184 [DOI] [PubMed] [Google Scholar]
- 23.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. [DOI] [PubMed] [Google Scholar]
- 24.Doody MM, Freedman DM, Alexander BH, Hauptmann M, Miller JS, Rao RS, et al. Breast cancer incidence in U.S. radiologic technologists. Cancer. 2006;106:2707–15. [DOI] [PubMed] [Google Scholar]
- 25.Gallicchio L, Visvanathan K, Burke A, Hoffman SC, Helzlsouer KJ. Nonsteroidal anti-inflammatory drugs and the risk of developing breast cancer in a population-based prospective cohort study in Washington County, MD. Int J Cancer. 2007;121:211–5. [DOI] [PubMed] [Google Scholar]
- 26.Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- 27.Hennekens CH, Speizer FE, Rosner B, Bain CJ, Belanger C, Peto R. Use of permanent hair dyes and cancer among registered nurses. Lancet. 1979;1:1390–3. [DOI] [PubMed] [Google Scholar]
- 28.Krokstad S, Langhammer A, Hveem K, Holmen TL, Midthjell K, Stene TR, et al. Cohort profile: the HUNT Study, Norway. Int J Epidemiol. 2013;42:968–77. doi: 10.1093/ije/dys095 [DOI] [PubMed] [Google Scholar]
- 29.Lund E, Dumeaux V, Braaten T, Hjartåker A, Engeset D, Skeie G, et al. Cohort profile: the Norwegian Women and Cancer Study—NOWAC—Kvinner og Kreft. Int J Epidemiol. 2008;37:36–41. [DOI] [PubMed] [Google Scholar]
- 30.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. [DOI] [PubMed] [Google Scholar]
- 31.Roswall N, Sandin S, Adami HO, Weiderpass E. Cohort profile: the Swedish Women’s Lifestyle and Health Cohort. Int J Epidemiol. 2017;46:e8. doi: 10.1093/ije/dyv089 [DOI] [PubMed] [Google Scholar]
- 32.Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, Cai Q, Schlundt DG, et al. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97:972–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Swerdlow AJ, Jones ME, Schoemaker MJ, Hemming J, Thomas D, Williamson J, et al. The Breakthrough Generations Study: design of a long-term UK cohort study to investigate breast cancer aetiology. Br J Cancer. 2011;105:911–7. doi: 10.1038/bjc.2011.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, et al. ; Sister Study Research Team. The Sister Study cohort: baseline methods and participant characteristics. Environ Health Perspect. 2017;125:127003. doi: 10.1289/EHP1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolk A, Bergström R, Hunter D, Willett W, Ljung H, Holmberg L, et al. A prospective study of association of monounsaturated fat and other types of fat with risk of breast cancer. Arch Intern Med. 1998;158:41–5. [DOI] [PubMed] [Google Scholar]
- 36.Breslow NE, Day NE. Statistical methods in cancer research. Volume II—the design and analysis of cohort studies. IARC Sci Publ. 1987:1–406. [PubMed] [Google Scholar]
- 37.Schoenfeld D Chi-squared goodness-of-fit tests for the proportional hazards model. Biometrika. 1980. 67:145–53. [Google Scholar]
- 38.Harville DA. Maximum likelihood approaches to variance component estimation and to related problems. J Am Stat Assoc. 1977;72:320–38. [Google Scholar]
- 39.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 40.Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163:1053–64. [DOI] [PubMed] [Google Scholar]
- 41.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 42.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 43.Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7:51–61. [DOI] [PubMed] [Google Scholar]
- 44.Witte JS, Greenland S. A nested approach to evaluating dose-response and trend. Ann Epidemiol. 1997;7:188–93. [DOI] [PubMed] [Google Scholar]
- 45.Glymour MM, Greenland S. Causal diagrams In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 46.Adami HO, Persson I, Ekbom A, Wolk A, Pontén J, Trichopoulos D. The aetiology and pathogenesis of human breast cancer. Mutat Res. 1995;333:29–35. [DOI] [PubMed] [Google Scholar]
- 47.Buse A The likelihood ratio, Wald, and Lagrange multiplier tests: an expository note. Am Stat. 1982;36:153–7. [Google Scholar]
- 48.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–32. [PubMed] [Google Scholar]
- 49.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–9. [DOI] [PubMed] [Google Scholar]
- 50.Martin RM, Middleton N, Gunnell D, Owen CG, Smith GD. Breast-feeding and cancer: the Boyd Orr cohort and a systematic review with meta-analysis. J Natl Cancer Inst. 2005;97:1446–57. [DOI] [PubMed] [Google Scholar]
- 51.Stuebe AM, Willett WC, Xue F, Michels KB. Lactation and incidence of premenopausal breast cancer: a longitudinal study. Arch Intern Med. 2009;169:1364–71. doi: 10.1001/archinternmed.2009.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Work ME, John EM, Andrulis IL, Knight JA, Liao Y, Mulligan AM, et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer. 2014;110:1367–77. doi: 10.1038/bjc.2013.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adami HO, Signorello LB, Trichopoulos D. Towards an understanding of breast cancer etiology. Semin Cancer Biol. 1998;8:255–62. [DOI] [PubMed] [Google Scholar]
- 55.Johansson AL, Andersson TM, Hsieh CC, Cnattingius S, Lambe M. Increased mortality in women with breast cancer detected during pregnancy and different periods postpartum. Cancer Epidemiol Biomarkers Prev. 2011;20:1865–72. doi: 10.1158/1055-9965.EPI-11-0515 [DOI] [PubMed] [Google Scholar]
- 56.Stensheim H, Møller B, van Dijk T, Fosså SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45–51. doi: 10.1200/JCO.2008.17.4110 [DOI] [PubMed] [Google Scholar]
- 57.Johansson AL, Andersson TM, Hsieh CC, Jirström K, Dickman P, Cnattingius S, et al. Stage at diagnosis and mortality in women with pregnancy-associated breast cancer (PABC). Breast Cancer Res Treat. 2013;139:183–92. doi: 10.1007/s10549-013-2522-1 [DOI] [PubMed] [Google Scholar]
- 58.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–15. doi: 10.1038/nm.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.