Abstract
Abstract. Cell cycle time (TC) and the rate of entry of cells into mitosis (rM) in the jejunum and duodenum of young rats were investigated using the stathmokinetic method. The cell cycle times in the jejunum were 24.3 and 28.3 h in light and dark periods, respectively. Cell cycle times in the duodenum were 17.1 and 21.5 h in light and dark periods, respectively. Rates of entry of cells into mitosis in the jejunum were 1.2 and 1.1 cells/cell/h in light and dark periods and rates of entry of cells into mitosis in the duodenum were 1.4 and 1.8 cells/cell/h in light and dark periods, respectively. Although these changes to cell cycle time values are not statistically significant, the variation between the two periods should be considered in relation to its possible biological effects.
INTRODUCTION
The intestinal epithelium undergoes constant cell renewal and in the jejunum and duodenum there are two distinct compartments: intestinal crypts and villi. Proliferation and differentiation of cells occurs in the crypts. On the villi, cells that have migrated from the crypts execute their various functions and subsequently are lost from the tip eliminated by apoptosis.
In the adult rat, the deepest crypts and the tallest villi are found in the duodenum, while the flattest crypts and smallest villi are found in the ileum. This morphological differentiation begins during foetal development from the 14th day before birth (Ono et al. 1989; Yamamoto et al. 1992) and continues until the third week after birth, when the morphofunctional pattern of the upper gut is established (Herbst & Sunshine 1969; Klein & Mckenzie 1983).
Cell kinetic processes in the intestinal epithelium are in flux during the third week after birth (Herbst & Sunshine 1969; Yeh 1977; Trahair 1989) which is the weaning period. During this phase, there is an increase in the number and size of intestinal crypts and villi, in the rate of cell migration from crypt to villus and in a gradual reduction of the cell cycle time (Yeh 1977, 1991). Alterations in cell kinetic processes in the gastrointestinal epithelium during the suckling and weaning period could be caused by changes in circadian rhythm, intake of food, hormonal activity and/or effects of growth factors.
Studies related to the proliferative behaviour of cell populations in the intestinal epithelium of rats during suckling and weaning phases have contributed to the understanding of intestinal development during the young‐to‐adult transition.
Previous investigations carried out by our group have demonstrated that the metaphasic index (MI) in the jejunal epithelium of 18‐day‐old rats is higher in the dark daily period than in the light period (data not published).
Thus, the main aim of this work has been to verify whether there is a relationship between the circadian rhythm, of MI and cell cycle time in the jejunum. In addition, we have obtained the rate of entry of cells into mitosis (rM) in the jejunal epithelium (TC) during dark and light periods of the day. In this work we have also obtained the cell cycle time and rate of entry of cells into mitosis (rM) in the duodenal epithelium of 18‐day‐old rats in light and dark periods.
We have found in the jejunum and duodenum that highest TC values occurred during darkness; for rM values, however, in both jejunum and duodenum there was no statistical difference between values of light and dark periods. These results have demonstrated no unequivocal relationship between the circadian rhythm of MI and cell cycle time in jejunum.
MATERIALS AND METHODS
Five groups, each of three 18‐day‐old Wistar rats, both male and female, were used in each period (light and dark). All animals were maintained with a 12‐h light/dark cycle (06.30–18.30 h) and temperature variation between 23 °C and 25 °C only. Food and water were provided ad libitum.
All animals received an intraperitoneal injection of vincristine sulphate (Oncovin, Lilly do Brasil Itda, São Paulo, Brasil) 0.5 mg/kg body weight at 13.30 h (light) or 01.00 h (dark).
Cohorts were killed at each of the following time points: 15 min, 30 min, 1 h, 1.5 h and 2 h after vincristine injection. To obtain the metaphysic index, 2‐cm tissue samples were collected and processed from each duodenum and jejunum. Cell counting was performed in selected longitudinally sectioned crypts showing the lumen. Approximately 3000 cells per animal were counted and the MIs were obtained as the ratio between arrested metaphases and total number of cells.
For each time point, the MI was adjusted by 0.62 – the Tannock Constant (Tannock 1967; Aldewachi et al. 1975). MI factors were calculated according to the method originally proposed by Puck & Steffen (1962), converting MIs to a logarithmic scale following formula: log value of 1 + MI. MI factors obtained were used to construct regression curves. Where ‘b’ is the slope of the regression curve, calculation of cell cycle time (TC) and rate of entry of cells into mitosis (rM) were performed using the following formulae:
| b = log(1 + MI)/t |
| rM = 1/TC |
| TC = log 2/b(log 2 = 0.3010) |
The rM values were expressed in cells/cell/h.
Slopes of the regression curves obtained for light and dark time periods were analysed by the Student t‐test, using the statistics program BioEstat 2.0 (Belém, Brasil).
RESULTS
Figure 1 demonstrates an increase in the number of arrested metaphases as a function of time post vincristine in the jejunum (Fig. 1a) and in the duodenum (Fig. 1b) during the light period. Figure 2 indicates an increase in the number of arrested metaphases as a function of time post vincristine in the jejunum (Fig. 2a) and duodenum (Fig. 2b) during the dark period. Table 1 collates values of the cell cycle time (TC) and rates of entry of cells into mitosis (rM) in the jejunum and duodenum at the light and dark periods.
Figure 1.
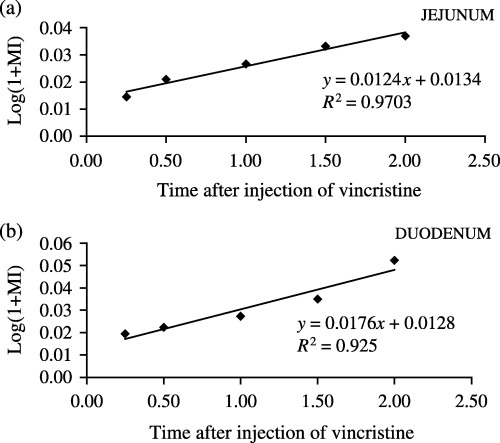
Regression curve obtained from averages of the metaphysic index as function of time post vincristine during the light period (13.45, 14.00, 14.30, 15.00 and 15.30 h). (a) Jejunum, (b) duodenum.
Figure 2.
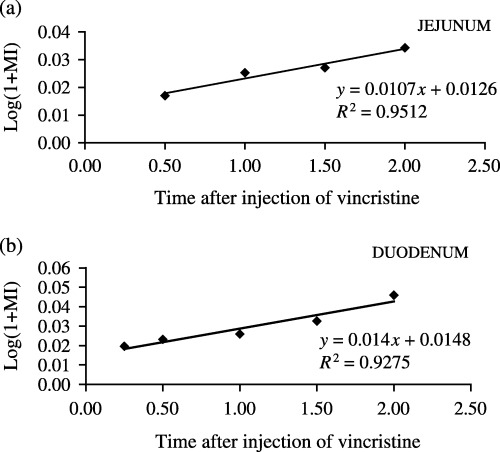
Regression curve obtained from averages of the metaphasic index as function of time post vincristine during the dark period (01.45, 02.00, 02.30, 03.00 and 03.30 h). (a) Jejunum, (b) duodenum.
Table 1.
Cell cycle time (TC) and the rate of cells entry into mitosis (rM) in jejunum and duodenum of 18‐day‐old rats
| Light period | Dark period | |
|---|---|---|
| Jejunum | ||
| TC/h | 24.3 | 28.3 |
| rM (cells/cell/h) | 1.2 | 1.1 |
| Duodenum | ||
| TC/h | 17.1 | 21.5 |
| rM (cells/cell/h) | 1.4 | 1.8 |
Values of TC in the jejunum were 24.3 and 28.3 h at the light and dark periods, respectively, and values of TC in the duodenum were 17.1 and 21.5 h at the light and dark periods, respectively. Values of rM in the jejunum were 1.2 and 1.1 cells/cell/h at the light and dark periods, respectively, and values of rM in the duodenum were 1.4 and 1.8 cells/cell/h at the light and dark periods, respectively.
Statistical analysis comparing slopes of the regression curves obtained from MIs (Fig. 1a and b, Fig. 2a and b) showed no significant difference (P < 0.05) between the TC values at the light and dark periods in the jejunum and duodenum. Also, no statistical differences were observed in TC values between the two regions.
DISCUSSION
In this present work we carried out experiments in weaning rats to determine the cell cycle time (TC) in the jejunum during darkness, to verify whether there was a relationship between peak MI observed at this time of day (previous data not published) and duration of the cell cycle.
The values of TC obtained in the jejunum were 24.3 and 28.3 h at the light and dark periods, respectively, but these differences are not statistically significant. Our analysis concludes that there is no relationship between circadian variation, of MI and TC the jejunum of weaning rats.
Gomes & Alvares (1998) studied cell proliferation in the jejunum of 18‐day‐old rats after 24 h of progressive fasting, by labelling the cell population with [3H]dT at 10.00 h. They calculated TC by the modified method of grain count halving (Bassukas & Schultze 1987). Values of TC in the fed (control group) and fasting groups were 17.2 and 19.5 h, respectively. In a further experiment, a different cell population was labelled with [3H]dT at 23.00 h. Values of TC in the fed and fasting groups were 16.5 and 17.7 h, respectively. Statistical analysis showed that cell cycle times did not differ between both times of the day and treatment studied.
Differences between the values of TC obtained by Gomes & Alvares (1998) and ours in the jejunum could be plausible if there were distinct cell populations cycling through the day. Also, such differences might be explained by methodology used in the study, where kinetic parameters were calculated from separate phases of the cell cycle. However, the value of TC obtained at the light period (17.1 h) in the duodenum here is similar to those obtained by Yeh (1977) with 16‐day‐old rats (TC 17.7 h) and by Al‐Nafussi & Wright (1982) with 18‐day‐old rats (TC 16 h).
A further kinetic parameter measured in this study was the number of cells that entered into mitosis (rM), which is similar between the light (1.2 cells/cell/h) and dark periods (1.1 cells/cell/h) in the jejunum. However, values of rM are higher in the dark period (1.8 cells/cell/h) than in the light period (1.4 cells/cell/h) in the duodenum. Comparison between these regions shows that rM in the jejunum (1.2 cells/cell/h) is similar to that of the duodenum (1.4 cells/cell/h) during the light. Nevertheless, rM in the duodenum (1.8 cells/cell/h) is higher than that in the jejunum (1.1 cells/cell/h) during darkness. The highest rM value at dark in the duodenum during the weaning phase could explain morphological differences between villi and crypts observed in the intestinal regions of adult rats. Gomes & Alvares (1998) concluded that the mechanisms of control of the cell proliferation and migration might be different in young rats and adult mice (Kaur & Potten 1986a,b). In addition, we suggest, as shown for some parts of the gastrointestinal epithelium, that mechanisms that regulate cell proliferation and cell migration involving hormones (Goldfeder & Alvares 1995; Gama & Alvares 1996), prostaglandin (Koelz et al. 1986; Uribe et al. 1987; Uribe & Garberg 1990; 1992, 1995; Kunika et al. 2001) and growth factors (Foltzer et al. 1993; Puccio & Lehy 1988) are already present in the intestinal epithelium leading to the steady‐state of cell proliferation observed in adult rats.
ACKNOWLEDGEMENTS
We are grateful to Ana Maria Kaust of the Foreign Language Department, Universidade Estadual de Ponta Grossa, for her revision of the manuscript. The statistical analysis was performed with the help of Professor Dr Jorim Souza das Virgens Filho of the Informatic Department, Universidade Estadual de Ponta Grossa. Experiments described were accomplished according to current animal welfare laws of Brazil.
REFERENCES
- Aldewachi SH, Wright NA, Appleton RD, Watson JA (1975) The effect of starvation and re‐feeding on cell population kinetics in rat small bowel mucosa. J. Anat. 119, 105. [PMC free article] [PubMed] [Google Scholar]
- Al‐Nafussi AI, Wright NA (1982) Cell kinetics in the mouse small intestine during immediate postnatal life. Virchows Arch. B Cell Pathol. Mol. Pathol. 40, 51. [DOI] [PubMed] [Google Scholar]
- Bassukas ID, Schultze‐Maures B (1987) A modification of the grain‐halving method for detailed analysis of cell kinetic parameters. Cell Tissue Kinet. 20, 527. [DOI] [PubMed] [Google Scholar]
- Foltzer JC, Garaud JC, Nsi‐Emvo E, Raul F (1993) Epidermal growth factor and the maturation of intestinal sucrase in suckling rats. Am. J. Physiol. 265, 459. [DOI] [PubMed] [Google Scholar]
- Gama P, Alvares EP (1996) LHRH and somatostatin effects on the cell proliferation of the gastric epithelium of suckling and weaning rats. Regulatory Peptides 73, 78. [DOI] [PubMed] [Google Scholar]
- Goldfeder EM, Alvares EP (1995) Effects of somatostatin and LHRH on cell proliferation of fetal stomach in vitro . Cell Prolif. 28, 194. [Google Scholar]
- Gomes JR, Alvares EP (1998) Cell proliferation and migration in the jejunum of suckling rats submitted to progressive fasting. Bras. J. Med. Biol. Res. 31, 281. [DOI] [PubMed] [Google Scholar]
- Herbst JJ, Sunshine P (1969) Postnatal development of the small intestine of the rat. Changes in mucosal morphology at weaning. Pediatr. Res. 3, 27. [DOI] [PubMed] [Google Scholar]
- Kaur P, Potten CS (1986a) Circadian variation in variation velocity in small intestinal epithelium. Cell Tissue Kinet. 19, 591. [DOI] [PubMed] [Google Scholar]
- Kaur P, Potten CS (1986b) Cell migration velocities in the crypts of the small intestine after cytotoxic insult are not dependent on mitotic activity. Cell Tissue Kinet. 19, 601. [DOI] [PubMed] [Google Scholar]
- Klein RM, Mckenzie JC (1983) The role of cell renewal in the ontogeny of the intestine. II. Regulation of cell proliferation in adult, fetal, and neonatal intestine. J. Pediatr. Gastroenterol. Nutr. 2, 204. [PubMed] [Google Scholar]
- Koelz HR, Fritsche R, Müller OM, Lentze NJ, Halter VF (1986) Structure and function of gastric corpus mucosa in suckling rats after treatment with 16,16‐dimethylprostaglandin E2. Prostaglandin 31, 133. [DOI] [PubMed] [Google Scholar]
- Kunika T, Araki H, Takeeda M, Kato S, Takeuchi K (2001) Prostaglandin E prevents indomethacin‐induced gastric and intestinal damage through different EP receptor subtypes. J. Physiol. (Paris) 95, 157. [DOI] [PubMed] [Google Scholar]
- Ono E, Doy I, Furukawa H, Hirata K, Fujimoto S (1989) The differentiation of entero‐endocrine cells of pre‐ and postnatal rats: light and electron microscopy and immunocytochemistry. Acta. Anat. 149, 81. [DOI] [PubMed] [Google Scholar]
- Puccio F, Lehy T (1988) Oral administration of EGF in suckling rats stimulates cell DNA synthesis in fundic gastric mucosa as well as in intestinal mucosa and pancreas. Regul. Pept. 20, 53. [DOI] [PubMed] [Google Scholar]
- Puck TT, Steffen J (1962) Life cycle analysis of mammalian cells. I. A method for localizing metabolic events within the life cycle, and its application to the action of colcemid and sublethal doses of X‐irradiation. Bioph. J. 3, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock IF (1967) A comparison of the relative efficiencies of various metaphase arrest agents. Expl. Cell Res. 47, 345. [Google Scholar]
- Trahair JF (1989) Remodelling of the rat small intestine mucosa during the suckling period. J. Ped. Gastrointestinal Nutr. 9, 232. [DOI] [PubMed] [Google Scholar]
- Uribe A, Tribukait B, Johanson C (1987) Cell cycle distribution of proliferative and functional cells of the jejunum after treatment with oral E2 prostaglandin. Scand. J. Gastroenterol. 22, 177. [DOI] [PubMed] [Google Scholar]
- Uribe A, Garberg L (1990) Prostaglandin E2‐induced hyperpalasia of the rat anterior epithelium is follow by secondary inhibition of the mitotic activity. Prostaglandin 40, 1. [DOI] [PubMed] [Google Scholar]
- Uribe A, Allam M, Midtvedt T (1992) E2 prostaglandin modulates cell proliferation in the small intestinal epithelium of the rat. Digestion 539, 157. [DOI] [PubMed] [Google Scholar]
- Uribe A, Alam M, Winell‐Kapraali M (1995) Indomethacin inhibits cell proliferation and increases cell loss in rat gastrointestinal epithelium. Diges. Dis. Sci. 40, 2490. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Toyota T, Kataoka K (1992) Electron microscope observations on the formation of primitive villi in the rat small intestine with special reference to intercellular junctions. Arch. Histol. Citol. 55, 551. [DOI] [PubMed] [Google Scholar]
- Yeh KY (1977) Cell kinetics in the small intestine of suckling rats. I. Influence of hypophysectomy. Anat. Rec. 188, 69. [DOI] [PubMed] [Google Scholar]
- Yeh KY, Yeh M, Holt PR (1991) Intestinal lactase expression and epithelial cell transit in hormone‐treated suckling rats. Am. J. Physiol. 260, 389. [DOI] [PubMed] [Google Scholar]


