This letter aims to clarify the definitions of the posterior arcuate fasciculus (pAF) and the vertical occipital fasciculus (VOF). These two tracts are of interest in many different fields, including the neuroscience of language (Catani, Jones, & ffytche, 2005), post-mortem dissection (Vergani, Mahmood, Morris, Mitchell, & Forkel, 2014), reading (Thiebaut de Schotten, Cohen, Amemiya, Braga, & Dehaene, 2014; Yeatman, Rauschecker, & Wandell, 2012), neurosurgery (Bartsch, Geletneky, & Jbabdi, 2013; Martino et al., 2013), and human vision (Takemura et al., 2015; Yeatman et al., 2014). Despite this broad interest, there is not an agreed upon anatomical definition of these two tracts. Here, we use a computational approach to create a reproducible method that delineates these tracts.
Some authors describe the pAF and VOF as separate tracts (Catani et al., 2005; Curran, 1909; Yeatman et al., 2014), but there are reasons why the distinction between the pAF and VOF remains unclear. First, Wernicke’s original semi-schematic image of the VOF (Wernicke, 1881) was in unspecified non-human primate and there is only a partial correspondence between macroanatomical landmarks in human and non-human primates; for example, the fusiform gyrus is hominoid-specific and does not exist in macaque. Second, early descriptions of the VOF (Wernicke, 1881; Obersteiner, 1888; Sachs, 1892) did not clearly define the anterior extent of the tract; in some of these early schematics, fibers extend beyond modern definitions of the occipital lobe and hence, should not be labeled vertical occipital fibers. Third, given the differences in methods, species, and terminology, it is unclear that these early neuroanatomists identified the same boundaries of the VOF. In that era, data were often communicated as schematics, which were produced without reproducible data analysis methods. Of course, modern investigations of white matter tracts should build on the rich insights from more than a century of neuroanatomy, but our studies should not rest on tentative, historical studies that are not directly applicable to our modern understanding of the organization of the human brain.
To identify the pAF and VOF using reproducible methods, we acquired diffusion-weighted magnetic resonance imaging (dMRI) data in 37 healthy participants. We then used constrained spherical deconvolution (CSD) to estimate fiber orientation distribution functions from the diffusion signal in each voxel. We used probabilistic tractography to generate whole-brain connectomes of 500,000 fibers randomly seeded throughout the brain. LiFE (Pestilli, Yeatman, Rokem, Kay, & Wandell, 2014) determined fiber estimates significantly contributing to a predictive model of the diffusion signal, removing fibers with no contribution (false positives). Fiber tract segmentation was performed using Automated Fiber-tract Quantification (AFQ; Yeatman, Dougherty, Myall, Wandell, & Feldman, 2012). Additional routines were written within AFQ to automatically distinguish the VOF from the pAF.
We identify two principal structures: a large arcuate (AF) pathway in the temporal lobe and an adjacent vertical sheet of fibers that extends posterior into the occipital lobe. The pAF is a temporoparietal pathway, intermingled within the AF; it has a vertical orientation, is located posterior to the Sylvian fissure, and parallels the long, arching segment of the AF (Fig. 1). These structures match the AF and pAF definitions in prior work (Catani et al., 2005; Catani & Thiebaut de Schotten, 2008; Thiebaut de Schotten et al., 2014). The vertical (superior-inferior) sheet of white matter extends several centimeters posterior to the pAF and is centered within the occipital lobe. We propose that this planar sheet comprises the VOF.
Fig. 1 –
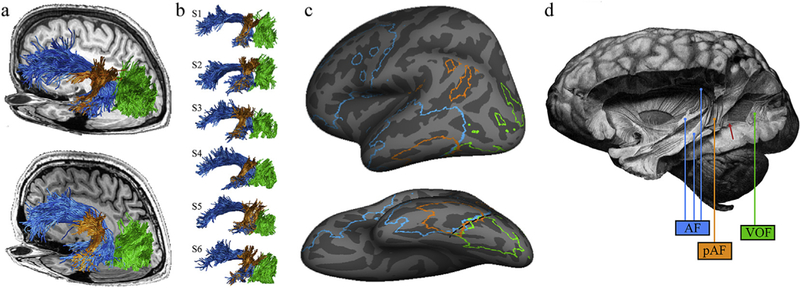
The arcuate fasciculus and the vertical occipital fasciculus. The vertical occipital fasciculus (VOF; green), the arcuate fasciculus (AF; blue) and the posterior segment of the arcuate fasciculus (pAF; orange) are shown in living and post-mortem human brains. (a) The VOF, AF, and pAF are shown for two representative subjects against the background of their T1-weighted anatomy. (b) Renderings of the VOF, AF, and pAF for six additional subjects to illustrate variability. For some subjects, there is a clear separation between the pAF and VOF (S1 and S2), while for others, there is no sharp boundary between the VOF and the pAF (S4 and S5). (c) Cortical endpoints for these three pathways were defined for 37 subjects. Cortical alignment was used to transform each individual’s endpoint map to the FreeSurfer average template (www.freesurfer.net), and regions with consistent, intersubject overlap are shown for each pathway. Outlined cortical extents indicate locations in which the AF, pAF, or VOF terminated in more than 10 subjects. The black dashed line highlights the cortical location where the three pathways converge (posterior occipitotemporal sulcus extending into the lateral fusiform gyrus). (d) Curran’s identification of the VOF, AF, and pAF in the post-mortem human brain (Curran, 1909). Labels have been added for clarity, but Curran distinguished these three tracts. He highlights the fact that the pAF and VOF abut one another, writing: ‘In this dissection, most of this bundle is removed showing a large window through which the fasc. o. f. inf. [IFOF] can be seen. At the anterior of this window a small part of the fasc. trans. occ. [VOF] still remains’. (Bracketed terms have been added to reflect modern terminology). The red arrow highlights the anterior portion of the VOF that Curran describes.
The vertical sheet of fibers we identify as the VOF matches the labeling of the VOF from over 100 years ago (Curran (1909)) based on human dissection (Fig. 1). As Curran noted, the pAF and VOF often abut one another (Fig. 1D). With dMRI measurements, the separation of the pAF and VOF is the main source of inter-subject variability. This separation is also affected by analysis method: with deterministic tractography and a tensor model of the diffusion signal, the VOF and pAF are separated by about 1 cm; a model characterizing multiple populations of crossing fibers in the voxel (CSD) results in VOF and pAF tracts that are much closer to one another. As the latter aligns more closely with post-mortem dissection, we believe this methodology produces a more accurate reflection of the relationship between the pAF and VOF.
We share our algorithm used to separate the VOF from the pAF (https://github.com/YeatmanLab/AFQ). Future studies implementing high-resolution dMRI may determine a definitive boundary between the two pathways in the cases in which the VOF and pAF are hard to distinguish from one another (the parameter controlling the boundary between the pAF and AF can be set by the user and we have set the default based on our current measurements). Even in these cases where the boundary between the two pathways may not always be clear, we believe that there is value in distinguishing them. For example, in terms of function, the VOF connects dorsal and ventral visual regions and is believed to carry signals that are important for a variety of perceptual functions (Takemura et al., 2015; Yeatman et al., 2014), while the pAF is intermingled with the arcuate and principally terminates in cortical regions that are important for language (Catani et al., 2005; Catani & Thiebaut de Schotten, 2008).
Our computational approach to neuroanatomy discriminates the pAF from the VOF, and our shared algorithm is written to follow reproducible research practices; other researchers can apply these methods to published or shared data (https://scarlet.stanford.edu/nims; http://www.humanconnectomeproject.org/data/, http://arxiv.org/abs/1502.06900) and with our published code. We hope that consensus based on shared computational methods and data will help our field standardize definitions of white matter fascicles in the living human brain. The pioneers of neuroanatomy were frequently in conflict over the definition of fiber tracts, such as the VOF, because the technology that was available at the time made it difficult to communicate and share findings among labs (Yeatman et al., 2014). Advances in computational and data sharing technologies now allow us to ensure that future work on the pAF and VOF are, in fact, describing the same fiber tracts.
Acknowledgments
This work was supported by 1R01EY02391501A1 (KW and KGS) and NSF-BCS1228397 (BAW). We thank Franco Pestilli, Ariel Rokem, and Aviv Mezer for useful comments on prior versions of the manuscript.
REFERENCES
- Bartsch AJ, Geletneky K, & Jbabdi S (2013). The temporoparietal fiber intersection area and wernicke perpendicular fasciculus. Neurosurgery, 73, E381–E382. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, & ffytche DH (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57, 8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, & Thiebaut de Schotten M (2008). A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex, 44, 1105–1132. [DOI] [PubMed] [Google Scholar]
- Curran EJ (1909). A new association fiber tract in the cerebrum. With remarks on the fiber tract dissection method of studying the brain. The Journal of Comparative Neurology and Psychology, 19, 645–656. [Google Scholar]
- Martino J, da Silva-Freitas R, Caballero H, Marco de Lucas E, Garcia-Porrero JA, & Vazquez-Barquero A (2013). Fiber dissection and diffusion tensor imaging tractography study of the temporoparietal fiber intersection area. Neurosurgery, 72, 87–97. discussion 97–8. [DOI] [PubMed] [Google Scholar]
- Obersteiner H (1888). Anleitung beim Studium des baues der nervösen centralorgane im gesunden und kranken zustande. Leipzig, Germany: Toeplitz & Deuticke. [Google Scholar]
- Pestilli F, Yeatman JD, Rokem A, Kay KN, & Wandell BA (2014). Evaluation and statistical inference for human connectomes. Nature Methods, 11, 1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs H (1892). Das hemispharenmark des menschlichen grosshirns. I. Der hinterhauptlappen. Breslau. Universität. Psychiatrische und nervenklinik. Hinterhauptlappen. Leipzig, Germany: G. Thieme. [Google Scholar]
- Takemura H, Rokem A, Winawer J, Yeatman JD, Wandell BA, & Pestilli F (2016). A major human white matter pathway between dorsal and ventral visual cortex. Cerebral Cortex, 26(5), 2205–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, & Dehaene S (2014). Learning to read improves the structure of the arcuate fasciculus. Cerebal Cortex, 24, 989–995. [DOI] [PubMed] [Google Scholar]
- Vergani F, Mahmood S, Morris CM, Mitchell P, & Forkel SJ (2014). Intralobar fibres of the occipital lobe: a post mortem dissection study. Cortex, 56, 145–156. [DOI] [PubMed] [Google Scholar]
- Wernicke C (1881). Lehrbuch der gehirnkrankheiten für ärzte und studierende. Kassel: Verlag von Theodor Fischer. [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, & Feldman HM (2012). Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One, 7, e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Rauschecker AM, & Wandell BA (2012). Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain and Language, 12(2), 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Weiner KS, Pestilli F, Rokem A, Mezer A, & Wandell BA (2014). The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proceedings of the National Academy of Sciences of the United States of America, 111(48), E5214–E5223. [DOI] [PMC free article] [PubMed] [Google Scholar]


