Abstract
Rationale & Objective:
The relationship between hypertension, antihypertension medication use, and change in glomerular filtration rate (GFR) over time among individuals with preserved GFR requires investigation.
Study Design:
Observational study.
Setting & Participants:
14,854 participants from the Atherosclerosis Risk in Communities (ARIC) Study.
Predictors:
Baseline hypertension status (1987–1989) was categorized according to the 2017 American College of Cardiology/American Heart Association Clinical Practice Guideline as normal blood pressure, elevated blood pressure, stage 1 hypertension, stage 2 hypertension without medication, or stage 2 hypertension with medication.
Outcomes:
Slope of estimated GFR (eGFR) at 5 study visits over 30 years.
Analytical Approach:
Mixed models with random intercepts and random slopes were fit to evaluate the association between baseline hypertension status and slope of eGFR.
Results:
At baseline, 13.2%, 7.3%, and 19.4% of whites and 15.8%, 14.9%, and 39.9% of African Americans had stage 1 hypertension, stage 2 hypertension without medication, and stage 2 hypertension with medication. Compared with those with normal blood pressure, the annual eGFR decline was greater in people with higher blood pressure (whites: elevated blood pressure, −0.11 mL/min/1.73 m2; stage 1 hypertension, −0.15 mL/min/1.73 m2; stage 2 hypertension without medication, −0.36 mL/min/1.73m2; stage 2 hypertension with medication, −0.17 mL/min/1.73 m2; African Americans: elevated blood pressure, −0.21 mL/min/1.73 m2; stage 1 hypertension, −0.16 mL/min/1.73 m2; stage 2 hypertension without medication, −0.50 mL/min/1.73 m2; stage 2 hypertension with medication, −0.16 mL/min/1.73 m2). The 30-year predicted probabilities of developing chronic kidney disease stage G3a+ with normal blood pressure, elevated blood pressure, stage 1 hypertension, stage 2 hypertension without medication, or stage 2 hypertension with medication among whites were 54.4%, 61.6%, 64.7% 78.1%, and 70.9%, respectively, and 55.4%, 62.8%, 60.9%, 76.1%, and 66.6% among African Americans.
Limitations:
Slope estimated using a maximum of 5 eGFR assessments; differential loss to follow-up.
Conclusions:
Compared to normotension, baseline hypertension status was associated with faster kidney function decline over 30-year follow-up in a general population cohort. This difference was attenuated among people using antihypertensive medications.
Hypertension ranks as the top risk factor for chronic disease worldwide.1 People with hypertension have increased risk for myocardial infarction, stroke, heart failure, and kidney failure.2 According to the 2017 American College of Cardiology/American Heart Association clinical practice guideline, the prevalence of hypertension among US adults was 45.6%.3,4 Hypertension is a risk factor for kidney disease progression in individuals with chronic kidney disease (CKD),5,6 but few studies have addressed the relationship between hypertension and longitudinal change in glomerular filtration rate (GFR) in the general population.7 Furthermore, the extent to which hypertension precedes GFR decline or is simply a consequence of a lower GFR continues to be an area of controversy.8
African Americans have a substantially higher risk for hypertension than whites and, among those with hypertension, poorer hypertension control.9–12 There are also profound racial disparities in kidney disease, with African Americans being approximately 1.5 times more likely to develop advanced CKD and 3 times more likely to develop end-stage kidney disease (ESKD) compared with whites.13–16 Racial disparities may be explained in part by a greater burden of risk factors among African Americans, including higher prevalences of hypertension, diabetes mellitus, and the APOL1 genetic risk variant.17–20 However, it is also possible that the risk relationship between hypertension may be stronger in African Americans than whites, either due to heightened susceptibility to disease or poorer risk factor control.
The purpose of this study was to evaluate the association of hypertension and antihypertensive medication with trajectories of estimated GFR (eGFR) and to assess whether the risk for kidney outcomes associated with hypertension varied by race in a community-based cohort of 14,854 white and African American adults during 30 years of follow-up.
Methods
Study Design and Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort designed to investigate the cause of atherosclerosis and its clinical consequences, as well as examine variability in disease risk according to characteristics of the study population.21 The ARIC Study enrolled 15,792 middle-aged (45–64 years old at baseline) predominantly white and African American men and women from 4 communities in the United States: Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD. The initial examination took place in 1987 to 1989 (baseline; study visit 1). Follow-up examinations occurred at approximately 3-year intervals: 1990 to 1992 (study visit 2), 1993 to 1995 (study visit 3), 1996 to 1998 (study visit 4), more recently in 2011 to 2013 (study visit 5), and 2016 to 2017 (study visit 6). During each study visit, an extensive questionnaire was administered, a clinical examination was conducted, and blood and urine specimens were collected.
In the present study, participants were excluded if they had missing data for hypertension status at baseline, missing measurement of serum creatinine at baseline, eGFR < 60 mL/min/1.73 m2 at baseline, prevalent ESKD, self-reported race other than white or African American, or missing covariates. After these exclusions, the analytic sample size was 14,854 (94% of the original cohort). Study participants provided written documentation of informed consent and study protocols were approved by the institutional review board at each study site.
Assessment of Hypertension Status
Systolic (SBP) and diastolic blood pressure (DBP) were measured twice at visit 4 and 3 times at other visits using a random-zero sphygmomanometer while seated after resting for 5 minutes in a separate quiet room. Participants were requested to avoid vigorous physical activity, cigarette smoking, and consumption of food, caffeinated beverages, and alcohol for 12 hours before the study visit. The appropriate cuff size was selected after measuring arm circumference. The first and second blood pressure values at visit 4 and second and third blood pressure values at other visits were averaged and used in the analysis.
Baseline hypertension status was categorized according to criteria in the 2017 American College of Cardiology/American Heart Association clinical practice guideline as normal blood pressure (SBP < 120 mm Hg and DBP < 80 mm Hg), elevated blood pressure (120 mm Hg ≤ SBP < 130 mm Hg and DBP < 80 mm Hg), stage 1 hypertension (130 mm Hg ≤ SBP < 140 mm Hg or 80 mm Hg ≤ DBP < 90 mm Hg), stage 2 hypertension without medication (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg), and stage 2 hypertension with medication (use of antihypertensive medication in the last 2 weeks).4
Assessment of Kidney Function
Kidney function was assessed by measuring creatinine in serum or plasma specimens collected during each study visit, except for study visit 3. In our study, we used 5 eGFRs (visits 1, 2, 4, 5, and 6) for the estimation of trajectories. The modified kinetic Jaffe method was used for the measurement of creatinine with standardization to the National Institute of Standards and Technology (NIST) standard and calibration across study visits using repeated measurements from a sample of 200 ARIC Study participants.22–24 The CKD Epidemiology Collaboration (CKD-EPI) equation was used to calculate eGFR based on creatinine level.25 For participants who developed incident ESKD (ascertained through linkage to the US Renal Data System [USRDS]), eGFR of 15 mL/min/1.73 m2 was imputed on the date of initiation of renal replacement therapy (transplantation or dialysis).
Assessment of Covariates
Demographic characteristics (date of birth for the calculation of age, sex, race, education, and family income), lifestyle factor (smoking), and medical history (diabetes and coronary heart disease) were ascertained using a questionnaire administered by trained interviewers at the baseline study visit. Study participants brought medications to the study visit and the names of all medications were transcribed, including antihypertensive medications. Body mass index was calculated using weight in kilograms divided by the square of height in meters measured during the study visit. Blood samples that were collected from study participants during the baseline study visit were assayed for the measurement of high-density lipoprotein cholesterol using an enzymatic method after precipitation with dextran sulfate-magnesium and glucose using the modified hexokinase/glucose-6-phosphate dehydrogenase method.26 Diabetes was defined as fasting glucose level ≥ 126mg/dL, nonfasting glucose level ≥ 200 mg/dL, self-report of diagnosed diabetes, or use of diabetes medication in the past 2 weeks.
Statistical Analysis
Baseline characteristics of the study population were compared by hypertension status and racial group using descriptive statistics. Differences were tested using analysis of variance for continuous variables and χ2 tests for categorical variables.
Mixed models were used to evaluate the association between hypertension status at baseline (normal blood pressure/elevated blood pressure/stage 1 hypertension/stage 2 hypertension without medication/stage 2 hypertension with medication) and eGFR trajectories using random intercepts and random slopes to account for individual variations in eGFR at baseline and its change. Because the random slopes had higher variance among African Americans than whites, our models were conducted overall and after stratifying by race. Covariates included in the adjusted models were age (continuous), sex, body mass index (continuous), race-center (Minneapolis, MN, and Washington County, MD, where all participants were white; Jackson, MS, where all participants were African American; and Forsyth County, NC, which recruited both whites and African Americans, and was represented by 2 variables) or center only for race-stratified analysis, smoking (current/former/never), family income (annual income ≥$25,000/<$25,000/not reported), education (high school graduated/not graduated), high-density lipoprotein cholesterol level (continuous), diabetes (yes/no), and history of coronary heart disease (yes/no) at baseline. We tested for interaction by race by adjusting for race and center separately and including a 3-way product term of hypertension category, race, and time.
We examined and plotted the patterns of eGFR change over time (ie, trajectories from best linear unbiased prediction estimates) and estimated the differences in annual eGFR decline according to hypertension status.27 Kernel density plots were used to illustrate the distribution of unadjusted and adjusted annual predicted change in eGFR. The average probability (absolute risk) of developing different stages of CKD (G3a+, eGFR < 60mL/min/ 1.73 m2; G3b+, eGFR < 45 mL/min/1.73 m2; G4+, eGFR < 30 mL/min/1.73 m2) during 30 years of follow-up was estimated for the baseline population based on the baseline covariates. These probabilities were expressed using best linear unbiased predictions from race-stratified models according to hypertension status.28
In sensitivity analysis, we examined the associations between blood pressure category at baseline (SBP < 130 mm Hg and DBP < 80 mm Hg; 130 mm Hg ≤ SBP < 140 mm Hg or 80 mm Hg ≤ DBP < 90 mm Hg; SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg) and eGFR trajectory after adjusting for hypertension medication status at baseline (yes/no). We also examined the associations of interest after imputing eGFR at the time of initiation of renal replacement therapy using eGFR supplied on Centers for Medcare & Medicaid Services Form 2728 to test the robustness of our main results. All analyses were conducted using Stata statistical software, version 13 (StataCorp), and R, version 3.3.3 (R Development Core Team).
Results
Baseline Characteristics
Baseline characteristics of the 14,854 study participants (11,003 whites and 3,851 African Americans) according to hypertension status category and racial group are shown in Table 1. At baseline, 13.2%, 7.3%, and 19.4% of whites and 15.8%, 14.9%, and 39.9% of African Americans were categorized to stage 1 hypertension, stage 2 hypertension without medication, and stage 2 hypertension with medication. In both whites and African Americans, participants with hypertension and particularly those with stage 2 hypertension had higher body mass index (P < 0.001). Individuals with stage 2 hypertension were more likely to have diabetes and annual family income < $25,000 and less likely to be high school graduates (P < 0.001 for all comparisons). Individuals in the stage 2 hypertension with medication category were more likely to have a history of coronary heart disease (P < 0.001). Although there was a statistically significant difference in baseline eGFR by hypertension status (P < 0.001), the absolute difference in eGFRs was relatively small.
Table 1.
Baseline Characteristics of the Study Population According to Baseline Hypertension Status
| White (n = 11,003) |
African American (n = 3,851) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Normal BPa |
Elevated BPa |
Stage 1 HTNa |
Stage 2 HTN w/o MEDa |
Stage 2 HTN w/ MEDa |
Normal BPa |
Elevated BPa |
Stage 1 HTNa |
Stage 2 HTN w/o MEDa |
Stage 2 HTN w/ MEDa |
| No. of participants | 5,341 | 1,281 | 1,448 | 801 | 2,132 | 859 | 273 | 610 | 573 | 1,536 |
| Age, yb | 53.5 ± 5.5 | 56.1 ± 5.6 | 55.0 ± 5.7 | 56.7 ± 5.6 | 56.8 ± 5.4 | 52.2 ± 5.6 | 54.5 ± 6.1 | 52.9 ± 5.6 | 54.6 ± 5.7 | 55.0 ± 5.7 |
| Female sexb | 3,052 (57.1%) | 639 (49.9%) | 630 (43.5%) | 381 (47.6%) | 1,107 (51.9%) | 537 (62.5%) | 1 79 (65.6%) | 328 (53.8%) | 277 (48.3%) | 1,039 (67.6%) |
| Smoking statusb | ||||||||||
| Current | 1,512 (28.3%) | 322 (25.1%) | 290 (20.0%) | 152 (19.0%) | 458 (21.5%) | 268 (31.3%) | 89 (32.7%) | 1 79 (29.3%) | 200 (34.9%) | 416 (27.1%) |
| Former | 1,723 (32.3%) | 456 (35.6%) | 560 (38.7%) | 308 (38.5%) | 826 (38.8%) | 209 (24.4%) | 65 (23.9%) | 161 (26.4%) | 122 (21.3%) | 377 (24.6%) |
| Never | 2,103 (39.4%) | 503 (39.3%) | 598 (41.3%) | 341 (42.6%) | 847 (39.7%) | 379 (44.3%) | 118 (43.4%) | 270 (44.3%) | 251 (43.8%) | 742 (48.3%) |
| High school graduateb | 4,573 (85.7%) | 1,040(81.3%) | 1,214(83.9%) | 637 (79.5%) | 1,660 (77.9%) | 587 (68.4%) | 162 (59.3%) 377 | (62.1%) | 318 (55.7%) | 822 (53.6%) |
| Family income < $25,000/yb | 1,150 (21.5%) | 335 (26.2%) | 345 (23.8%) | 248 (31.0%) | 696 (32.6%) | 456 (53.1%) | 169 (61.9%) | 372 (61.0%) | 389 (67.9%) | 1,054 (68.6%) |
| Centerb | ||||||||||
| Forsyth County | 1,772 (33.2%) | 420 (32.8%) | 386 (26.7%) | 243 (30.3%) | 569 (26.7%) | 135 (15.7%) | 46 (16.8%) | 51 (8.4%) | 49 (8.6%) | 170 (11.1%) |
| Jackson | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 705 (82.1%) | 223 (81.7%) | 553(90.7%) | 518(90.4%) | 1,349 (87.8%) |
| Minneapolis | 1,879 (35.2%) | 433 (33.8%) | 606 (41.9%) | 303 (37.8%) | 656 (30.8%) | 9 (1.0%) | 4 (1.5%) | 1 (0.2%) | 3 (0.5%) | 5 (0.3%) |
| Washington County | 1,690 (31.6%) | 428 (33.4%) | 456 (31.5%) | 255 (31.8%) | 907 (42.5%) | 1 0 (1.2%) | 0 (0.0%) | 5 (0.8%) | 3 (0.5%) | 1 2 (0.8%) |
| SBP, mm Hgb | 106.0 ± 8.5 | 123.9 ± 2.8 | 128.5 ± 7.6 | 148.2 ± 11.8 | 128.0 ± 1 7.5 | 107.9 ± 7.3 | 124.0 ± 2.9 | 126.4 ± 8.1 | 152.2 ± 18.4 | 132.7 ± 21.3 |
| DBP, mm Hgb | 65.9 ± 7.2 | 71.2 ± 6.2 | 79.2 ± 6.7 | 84.2 ± 10.3 | 75.7 ± 10.1 | 69.2 ± 6.1 | 72.4 ± 5.9 | 81.2 ± 5.9 | 91.0 ± 12.8 | 81.8 ± 12.1 |
| BMI, kg/m2b | 25.7 ± 4.1 | 27.3 ± 4.8 | 27.7 ± 5.0 | 28.3 ± 5.4 | 29.1 ± 5.4 | 27.8 ± 5.1 | 29.0 ± 6.0 | 29.0 ± 6.0 | 29.2 ± 6.3 | 31.0 ± 6.4 |
| HDL-C, mg/dLb | 52.4 ± 17.1 | 50.8 ± 1 7.0 | 50.0 ± 16.5 | 50.1 ± 16.8 | 46.2 ± 15.5 | 56.0 ± 1 7.4 | 57.4 ± 1 7.2 | 56.8 ± 19.2 | 56.8 ± 18.9 | 52.8 ± 16.2 |
| DMb | 198 (3.7%) | 88 (6.9%) | 92 (6.4%) | 61 (7.6%) | 326 (15.3%) | 88 (10.2%) | 34 (12.5%) | 65 (10.7%) | 80 (14.0%) | 374 (24.3%) |
| CHDb | 190 (3.6%) | 60 (4.7%) | 51 (3.5%) | 26 (3.2%) | 234 (11.0%) | 24 (2.8%) | 4 (1.5%) | 16 (2.6%) | 12 (2.1%) | 94 (6.1%) |
| eGFR, mL/min/1.73 m2b | 101.5 ± 11.2 | 99.5 ± 10.9 | 99.5 ± 11.3 | 99.0 ± 11.3 | 96.5 ± 12.7 | 114.8 ± 15.5 | 115.7 ± 14.0 | 114.4 ± 15.9 | 113.2 ± 15.7 | 109.6 ± 18.6 |
Note: N = 14,854. Mean ± standard deviation for continuous variables and count (percentage) for categorical variables.
Abbreviations: BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; MED, medication; SBP, systolic blood pressure.
Normal BP defined as SBP < 120 mm Hg and DBP < 80 mm Hg. Elevated BP defined as SBP 120 ≤ SBP < 130 mm Hg and DBP < 80 mm Hg. Stage 1 HTN defined as 130 < SBP < 140 mm Hg or 80 ≤ DBP < 90 mm Hg. Stage 2 HTN defined as SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, and this group was stratified by the use of antihypertensive medication in the last 2 weeks.
P value for comparing the HTN groups within each race group < 0.05; P value calculated by analysis of variance for continuous variables and χ2 test for categorical variables.
Average eGFR Trajectories
There was a steady decline in eGFR over time among both whites and African-Americans in each of the 5 hypertension status categories (Fig 1). Compared with individuals without hypertension, slopes of the 30-year trajectory among participants with hypertension were steeper, representing faster eGFR declines. After adjusting for risk factors, the decline in eGFR among individuals in the stage 2 hypertension with medication category was similar to that among those in the stage 1 hypertension category (differences in eGFR decline per year: elevated blood pressure, −0.12 mL/min/1.73 m2; stage 1 hypertension, −0.14 mL/min/1.73 m2; stage 2 hypertension without medication, −0.39 mL/min/1.73 m2; stage 2 hypertension with medication, −0.16 mL/min/1.73 m2).
Figure 1.
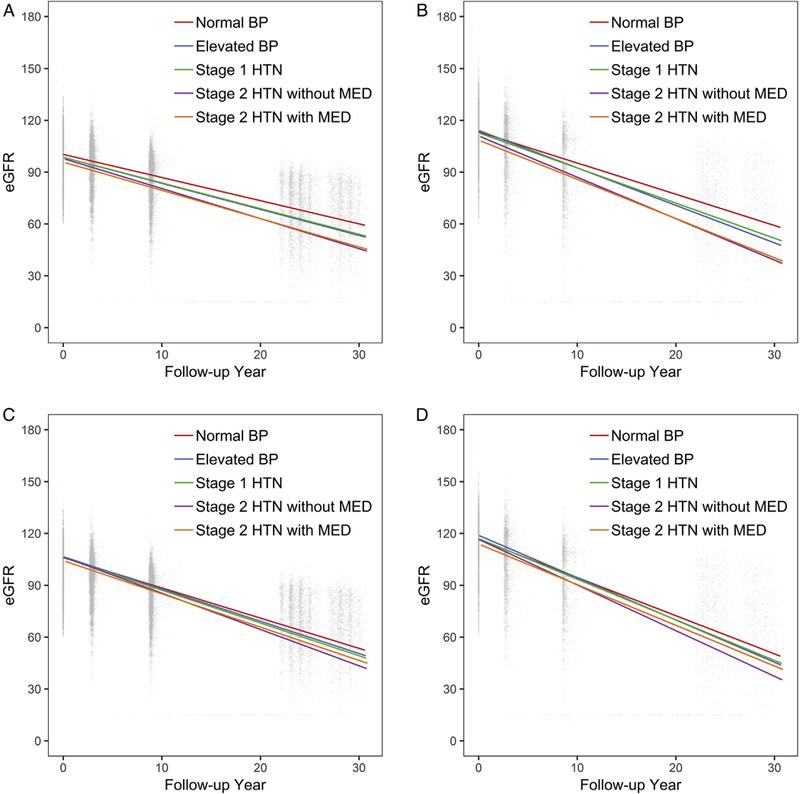
Distribution of estimated glomerular filtration rates (eGFRs; mL/min/1.73 m2) and unadjusted and adjusted eGFR change during 30 years’ follow-up according to baseline hypertension (HTN) status. Unadjusted eGFR change among (A) whites and (B) African Americans and adjusted eGFR change among (C) whites and (D) African Americans. For adjusted eGFR changes, model adjusted for age (centered at 50 years old), sex (reference group: male), center (reference group: Forsyth County, NC), baseline smoking status (reference group: current smoker), baseline education level (reference group: non-high school graduate), baseline annual family income (reference group: <$25,000), baseline body mass index (centered at 25 kg/m2), baseline high-density lipoprotein cholesterol level (centered at 40 mg/dL), baseline history of diabetes (reference group: no diabetes), baseline history of coronary heart disease (reference group: no coronary heart disease), and their interaction with follow-up time. For adjusted predicted average annual changes among African Americans, African Americans in the Minnesota and Washington County cohorts were excluded in the adjusted model because of small numbers. Numbers of participants at each visit are: visit 1: whites, n = 11,003; African Americans, n = 3,851; visit 2: whites, n = 10,297 (93.6% of the original cohort); African Americans, n = 3,224 (83.7% of the original cohort); visit 4: whites, n = 8,616 (78.3%); African Americans, n = 2,373 (61.6%); visit 5: whites, n = 4,758 (43.2%); African Americans, n = 1,375 (35.7%); visit 6: whites, n = 2,995 (27.2%); African Americans, n = 1,013 (26.3%). Total number of eGFR assessments was 49,502, and the median of eGFR assessments was 3 (IQR, 3–4). Abbreviations: BP, blood pressure; MED, medication.
Similar results were found when examining whites and African Americans separately (whites: elevated blood pressure, −0.11 mL/min/1.73 m2; stage 1 hypertension, −0.15 mL/min/1.73 m2; stage 2 hypertension without medication, −0.36 mL/min/1.73 m2; stage 2 hypertension with medication, −0.17 mL/min/1.73 m2; African Americans: elevated blood pressure, −0.21 mL/min/ 1.73m2; stage 1 hypertension, −0.16 mL/min/1.73 m2; stage 2 hypertension without medication, −0.50 mL/min/ 1.73m2; stage 2 hypertension with medication, −0.16 mL/min/1.73 m2; Table 2). There was no interaction in the association between hypertension and eGFR decline with race except for the stage 2 hypertension with medication category (P for interaction = 0.01).
Table 2.
Difference in eGFR Decline Per Year According to Hypertension Categories in the Total Population and by Race
| Normal BPa | Elevated BPa | Stage 1 HTNa | Stage 2 HTN w/o MEDa |
Stage 2 HTN w/ MEDa |
|
|---|---|---|---|---|---|
| All | n = 6,200 | n = 1,554 | n = 2,058 | n = 1,374 | n = 3,668 |
| Unadjusted | Ref | −0.22 (−0.29 to −0.16) | −0.24 (−0.30 to −0.19) | −0.58 (−0.65 to −0.50) | −0.49 (−0.53 to −0.44) |
| Adjustedb,c | Ref | −0.12 (−0.18 to −0.06) | −0.14 (−0.19 to −0.08) | −0.39 (−0.46 to −0.31) | −0.16 (−0.21 to −0.11) |
| White | n = 5,341 | n = 1,281 | n = 1,448 | n = 801 | n = 2,132 |
| Unadjusted | Ref | −0.17 (−0.22 to −0.11) | −0.14 (−0.20 to −0.09) | −0.40 (−0.47 to −0.32) | −0.30 (−0.35 to −0.24) |
| Adjustedd | Ref | −0.11 (−0.1 7 to −0.05) | −0.15 (−0.20 to −0.09) | −0.36 (−0.44 to −0.28) | −0.1 7 (−0.22 to −0.11) |
| African American | n = 859 | n = 273 | n = 610 | n = 573 | n = 1,536 |
| Unadjusted | Ref | −0.36 (−0.59 to −0.13) | −0.23 (−0.40 to −0.05) | −0.59 (−0.78 to −0.40) | −0.46 (−0.61 to −0.32) |
| Adjustedd,e | Ref | −0.21 (−0.43 to 0.01) | −0.16 (−0.33 to 0.01) | −0.50 (−0.69 to −0.31) | −0.16 (−0.31 to −0.01) |
Note: Differences (95% confidence intervals) in eGFR decline (mL/min/1.73 m2) per year, compared to normal BP as reference.
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HTN, hypertension; MED, medication; Ref, reference; SBP, systolic blood pressure.
Normal BP defined as SBP < 120 mm Hg and DBP < 80 mm Hg. Elevated BP defined as 120 < SBP < 130 mm Hg and DBP < 80 mm Hg. Stage 1 HTN defined as 130 ≤ SBP < 140 mm Hg or 80 ≤ DBP < 90 mm Hg. Stage 2 HTN defined as SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, and this group was stratified by the use of antihypertensive MED in the last 2 weeks.
Model adjusted for age, sex, race-center, baseline body mass index, baseline smoking status, baseline education level, baseline annual family income, baseline high-density lipoprotein cholesterol level, baseline history of diabetes, baseline history of coronary heart disease, and their interaction with follow-up time.
P value for interaction race × elevated BP × time = 0.16, race × stage 1 HTN × time = 0.95, race × stage 2 HTN without MED × time = 0.08, race × stage 2 HTN with MED × time = 0.01.
Model adjusted for age, sex, center, baseline body mass index, baseline smoking status, baseline education level, baseline annual family income, baseline high-density lipoprotein cholesterol level, baseline history of diabetes, baseline history of coronary heart disease, and their interaction with follow-up time.
African Americans in the Minnesota and Washington County cohorts were excluded in the adjusted model because of small numbers.
Variation in Annual Change in eGFR
Figure 2 shows the distribution of eGFR declines by blood pressure category. The unadjusted median annual eGFR changes of white and African American participants, respectively, were −1.32 (interquartile range [IQR], −1.51 to −1.11) and −1.79 (IQR, −2.07 to −1.45) mL/min/1.73 m2 per year among those with normal blood pressure, −1.48 (IQR, −1.67 to −1.31) and −2.10 (IQR, −2.34 to −1.77) mL/min/1.73 m2 per year among those with elevated blood pressure, −1.47 (IQR, −1.66 to −1.26) and −2.00 (IQR, −2.28 to −1.62) mL/min/1.73 m2 per year among those with stage 1 hypertension, −1.71 (IQR, −1.93 to −1.51) and −2.39 (IQR, −2.64 to −1.94) mL/min/1.73 m2 per year among those with stage 2 hypertension without medication, and-1.61 (IQR, −1.81 to −1.40) and −2.25 (IQR, −2.55 to −1.79) mL/min/1.73 m2 per year among those covariates. Compared with whites, African Americans had with stage 2 hypertension with medication. Overlap was similar differences by hypertension status but larger mean greater when eGFR trajectories were adjusted for baseline and variance of the annual eGFR decline.
Figure 2.
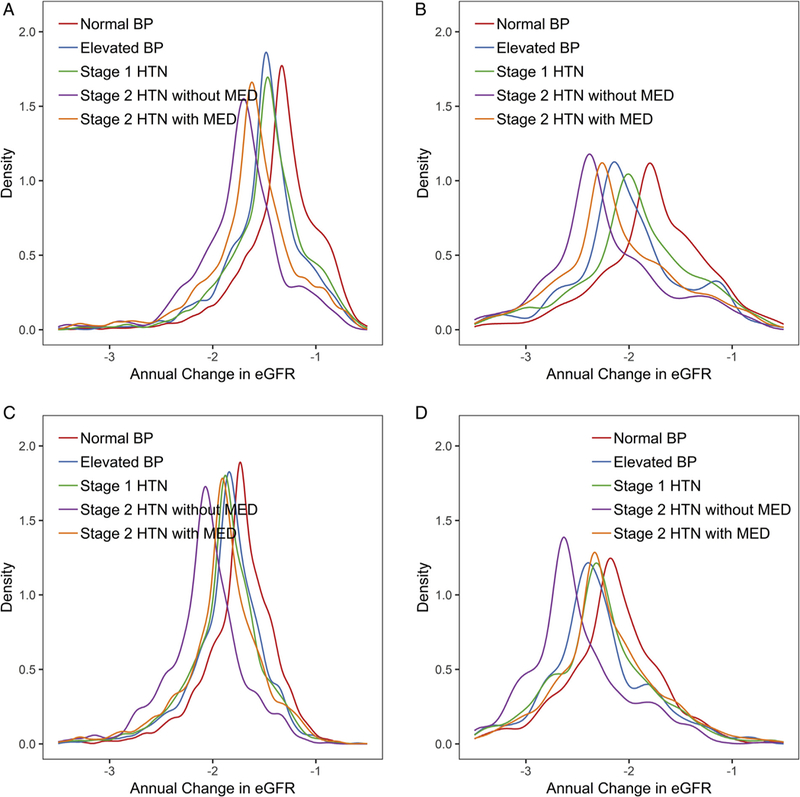
Distribution of predicted average annual change in estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) within the Atherosclerosis Risk in Communities (ARIC) Study population using baseline covariates according to baseline hypertension (HTN) status. Unadjusted predicted average annual change among (A) whites and (B) African Americans and adjusted predicted average annual change among (C) whites and (D) African Americans. For adjusted predicted average annual changes, slopes were estimated from a mixed model adjusted for age (centered at 50 years old), sex (reference group: male), center (reference group: Forsyth County, NC), baseline smoking status (reference group: current smoker), baseline education level (reference group: non–high school graduate), baseline annual family income (reference group: <$25,000), baseline body mass index (centered at 25 kg/m2), baseline high-density lipoprotein cholesterol level (centered at 40 mg/dL), baseline history of diabetes (reference group: no diabetes), baseline history of coronary heart disease (reference group: no coronary heart disease), and their interaction with follow-up time. For adjusted predicted average annual changes among African Americans, African Americans in the Minnesota and Washington County cohorts were excluded in the adjusted model because of small numbers. Abbreviations: BP, blood pressure; MED, medication.
Predicted Probability of CKD
The predicted probability of CKD of 30 years was generally higher among people with hypertension (Table 3). African Americans had a similar predicted risk for developing stage G3a+ but a greater predicted risk for developing stage G3b+ and G4+ CKD compared with whites. The average 30-year predicted probabilities of developing CKD stage G3a+ (eGFR < 30 mL/min/1.73 m2) with normal blood pressure, elevated blood pressure, stage 1 hypertension, stage 2 hypertension without medication, or stage 2 hypertension with medication were 54.4%, 61.6%, 64.7%, 78.1%, and 70.9%, respectively, among whites and 55.4%, 62.8%, 60.9%, 76.1%, and 66.6%, respectively, among African Americans; those of stage G4+ were 7.0%, 9.0%, 10.1%, 15.8%, and 12.4% among whites and 22.3%, 26.0%, 25.4%, 32.5%, and 27.5% among African Americans.
Table 3.
Average Predicted Probability of Developing CKD Over 30 Years Follow-up According to Hypertension Status Among Participants Based on Baseline Covariates
| White |
African Americana |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CKD Stage |
Normal BP |
Elevated BP |
Stage 1 HTN |
Stage 2 HTN w/o MED |
Stage 2 HTN w/ MED |
Normal BP |
Elevated BP |
Stage 1 HTN |
Stage 2 HTN w/o MED |
Stage 2 HTN w/ MED |
| G3a+b | 54.4 | 61.6 | 64.7 | 78.1 | 70.9 | 55.4 | 62.8 | 60.9 | 76.1 | 66.6 |
| G3b+c | 22.5 | 27.8 | 30.0 | 41.8 | 35.0 | 35.2 | 41.6 | 40.1 | 52.4 | 44.5 |
| G4+d | 7.0 | 9.0 | 10.1 | 15.8 | 12.4 | 22.3 | 26.0 | 25.4 | 32.5 | 27.5 |
Abbreviations: BP, blood pressure; CKD, chronic kidney disease; HTN, hypertension; MED, medication.
African Americans in the Minnesota and Washington County cohorts were excluded in the adjusted model because of small numbers.
eGFR < 60 mL/min/1.73 m2.
eGFR < 45 mL/min/1.73 m2.
eGFR < 30 mL/min/1.73 m2.
Sensitivity Analysis
Replacing hypertension stages with 2 separate variables, blood pressure categories and antihypertensive medication, showed similar results. In the total population, individuals with higher blood pressures had significantly greater declines in eGFR during the 30 years of follow-up (120 ≤ SBP < 130 mm Hg and DBP < 80 mm Hg, −0.15 mL/min/1.73 m2 per year; 130 ≤ SBP < 140 mm Hg or 80 ≤ DBP < 90 mm Hg, −0.14 mL/min/1.73 m2 per year; SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, −0.42 mL/min/1.73 m2 per year) compared with those with normal blood pressure (SBP < 120 mm Hg and DBP < 80 mm Hg), after adjusting for risk factors and hypertension medication status (Table S1). Results were similar when examining whites and African Americans separately. After accounting for blood pressure categories, hypertension medication was not associated with eGFR decline in the total population or in analyses stratified by race (P = 0.9 for overall, 0.4 among whites, and 0.2 among African Americans). When eGFR at the initiation of renal replacement therapy was imputed from the 2728 form rather than using a value of 15 mL/min/1.73 m2, results were nearly identical to those in the main analysis (Table S2).
Discussion
In this community-based population of 14,854 middle-aged adults, participants with hypertension at baseline experienced faster eGFR declines than those without hypertension during 30 years of follow-up. The risk for developing CKD was greater with hypertension, especially stage 2 hypertension, in both whites and African Americans. Although there was no difference by race in the association between hypertension and eGFR decline, African Americans had higher mean and dispersion in the rate of decline and risk for developing CKD stage G3b or worse, which translated into a greater absolute risk difference between those with and without hypertension.
The current study adds to the existing literature by demonstrating that hypertension is a risk factor for eGFR decline during 30 years of follow-up in a population with relatively preserved eGFR. We defined hypertension at baseline to definitively establish the temporal relationship between onset of hypertension and eGFR decline. Other studies suggest that this is a conservative approach.29
Previous work in the Chronic Renal Insufficiency Cohort (CRIC) Study evaluated baseline blood pressure as a predictor of eGFR decline,29 but this study included only participants with CKD.6 The Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated that higher SBP and variable pulse pressure were significantly associated with cystatin C—based eGFR (eGFRcys) decline in participants who attended a 5-and 10-year visit.30 High blood pressure was also an independent predictor of age-adjusted annual eGFR decline over 10 years among Italian individuals with preserved eGFR and type 2 diabetes.31
Our study demonstrates the variability in slope by hypertension and race among middle-aged men and women with preserved eGFR, finding that eGFR decline is more variable among African Americans than whites. The higher variability in eGFR decline among African Americans resulted in a disproportionately greater probability of the development of advanced CKD despite relatively small differences in annual eGFR decline. Greater variability in quality of medical care or control of risk factors among African Americans may play a role in the greater hetero-geneity of disease progression.32,33
Our finding that African Americans had a similar probability of early-stage CKD compared with whites but greater probability of late-stage CKD is consistent with previous studies that found significantly faster kidney disease progression in African Americans compared with whites.34 Previous prediction models developed among populations of healthy individuals also showed higher risk for developing ESKD for African Americans.35 The difference may be attributable to both biological differences and treatment barriers.20,36,37 In particular, genetic variants of the apolipoprotein L1 gene (APOL1) have been associated with worse kidney outcomes, and carriers of these variants are overwhelmingly of African descent.20,38
The mechanism underlying the association between hypertension and eGFR decline is not fully understood, but may be due in part to higher intraglomerular pressure and progressive arteriosclerosis.39,40 However, low GFR may also increase blood pressure due to impairments in salt and water excretion. For this reason, we evaluated blood pressure only at visit 1 to maintain strict temporality. Others have shown that time-updated blood pressure is more strongly associated with kidney disease risk, which may be due in part to this bidirectional association.6
There are several strengths to this study. The ARIC Study is a large prospective cohort with 30 years of follow-up and representation from 4 US communities with both whites and African Americans. The long duration of the study allows for the characterization of eGFR decline in a population that was generally healthy at the outset. Multiple established risk factors were collected in a research protocolized manner.
There are also several study limitations to acknowledge. There were only up to 5 eGFRs for the estimation of long-term trajectories. That said, there are few longitudinal population-based cohorts that have had more frequent eGFR assessments over 30 years. Participants may be healthier than the general population, and antihypertensive medication use was self-reported, which may have introduced some misclassification. Hypertension treatment practice has changed over time, and we used baseline rather than time-varying hypertension status. This approach was chosen to reflect a clear temporal relationship between hypertension and subsequent eGFR decline and reduce the possibility of introducing time-varying confounding. Participants who develop ESKD are less likely to survive to attend subsequent study visits; we included an estimate of their trajectory by imputing eGFR at the time of ESKD onset as identified through linkage to the USRDS registry. There is the potential for differential loss to follow-up by baseline hypertension status. Our analysis did not attempt to capture acute kidney injury occurring during follow-up, precluding inferences about the impact of acute kidney injury on eGFR decline. For blood pressure measurement, we characterized hypertension based on 2 measurements at the same occasion rather than 2 or more occasions as stipulated by the 2017 American College of Cardiology/American Heart Association clinical practice guideline. Also, blood pressure was measured using a random-zero sphygmomanometer. Last, because ARIC only included whites and African Americans, our results are not applicable to other ethnic groups.
In summary, hypertension status is an important risk factor for future eGFR decline and the development of kidney disease in community-dwelling white and African American adults. Our study highlights the potential importance of preventing and treating hypertension as a strategy to preserve eGFR. Population-level efforts to lower blood pressure may help reduce the onset of kidney disease.
Supplementary Material
Differences in eGFR decline per year according to BP categories in the total population and by race.
Differences in eGFR decline per year according to hypertension categories in the total population and by race with imputing the eGFR from the CMS 2728 form.
Acknowledgements:
The authors thank the staff and participants of the ARIC Study for important contributions.
Support: Dr Rebholz is supported by a Mentored Research Scientist Development Award from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH; K01 DK107782) and a grant from the National Heart, Lung, and Blood Institute (NHLBI; R21 HL143089). The ARIC Study has been funded in whole or in part with federal funds from the NHLBI, NIH, Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201 700003I, H HSN268201700004I, and HHSN268201700005I. None of the funders had any role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit this report for publication.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Disclaimer: Some of the data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Peer Review: Received August 27, 2018. Evaluated by 2 external peer reviewers and a statistician, with direct editorial input from an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form February 12, 2019. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859): 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black HR. The burden of cardiovascular disease: following the link from hypertension to myocardial infarction and heart failure. Am J Hypertens. 2003;16(9, pt 2):4s–6s. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Carey RM, Gidding S, et al. Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137(2):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. [DOI] [PubMed] [Google Scholar]
- 5.Stephens-Shields AJ, Spieker AJ, Anderson A, et al. Blood pressure and the risk of chronic kidney disease progression using multistate marginal structural models in the CRIC Study. StatMed. 2017;36(26):4167–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson AH, Yang W, Townsend RR, et al. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med. 2015;162(4): 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inker LA, Shafi T, Okparavero A, et al. Effects of race and sex on measured GFR: the Multi-Ethnic Study of Atherosclerosis. Am J Kidney Dis. 2016;68(5):743–751. [DOI] [PubMed] [Google Scholar]
- 8.Tedla FM, Brar A, Browne R, Brown C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens. 2011;2011:132405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole MB, Wright B, Wilson IB, Galarraga O, Trivedi AN. Longitudinal analysis of racial/ethnic trends in quality outcomes in community health centers, 2009–2014. J Gen Intern Med. 2018;33(6):906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke. 2006;37(5):1171–1178. [DOI] [PubMed] [Google Scholar]
- 11.Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348(2):135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon SS, Burt V, Louis T, Carroll MD. Hypertension Among Adults in the United States, 2009–2010 NCHS data brief no. 107. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2012. [Google Scholar]
- 13.Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3)(suppl 1):S1–S390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muntner P, Newsome B, Kramer H, et al. Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(1):101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CA, Katz R, DeBoer I, et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol. 2011;22(7):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis. 2013;62(2):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-Year MRFIT findings. JAMA. 1997;277(16): 1293–1298. [PubMed] [Google Scholar]
- 18.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26(8):2392–2399. [DOI] [PubMed] [Google Scholar]
- 19.Grams ME, Rebholz CM, Chen Y, et al. Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol. 2016;27(9):2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 22.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 23.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39(5):920–929. [DOI] [PubMed] [Google Scholar]
- 24.Parrinello CM, Grams ME, Couper D, et al. Recalibration of blood analytes over 25 years in The Atherosclerosis Risk in Communities Study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61(7): 938–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28(6):1379–1388. [PubMed] [Google Scholar]
- 27.Robinson GK. That BLUP is a good thing: the estimation of random effects. Stat Sci. 1991;6(1):15–32. [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. [Google Scholar]
- 29.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8): 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Judson GL, Rubinsky AD, Shlipak MG, et al. Longitudinal blood pressure changes and kidney function decline in persons without chronic kidney disease: findings from the MESA Study. Am J Hypertens. 2018;31(5):600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zoppini G, Targher G, Chonchol M, et al. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol. 2012;7(3): 401–408. [DOI] [PubMed] [Google Scholar]
- 32.Gulley SP, Rasch EK, Chan L. Difference, disparity, and disability: a comparison of health, insurance coverage, and health service use on the basis of race/ethnicity among US adults with disabilities, 2006–2008. Med Care. 2014;52(10)(suppl 3):S9–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redmond N, Baer HJ, Hicks LS. Health behaviors and racial disparity in blood pressure control in the National Health and Nutrition Examination Survey. Hypertension. 2011;57(3): 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–290. [DOI] [PubMed] [Google Scholar]
- 35.Grams ME, Sang Y, Levey AS, et al. Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med. 2016;374(5):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crews DC, Liu Y, Boulware LE. Disparities in the burden, outcomes, and care of chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23(3):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall YN. Racial and ethnic disparities in end stage renal disease: access failure. Clin J Am Soc Nephrol. 2012;7(2): 196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40(10):1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keane WF, Eknoyan G. Proteinuria, Albuminuria, Risk, Assessment, Detection, Elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33(5):1004–1010. [DOI] [PubMed] [Google Scholar]
- 40.Yoshioka T, Rennke HG, Salant DJ, Deen WM, Ichikawa I. Role of abnormally high transmural pressure in the permselectivity defect of glomerular capillary wall: a study in early passive Heymann nephritis. Circ Res. 1987;61(4):531–538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences in eGFR decline per year according to BP categories in the total population and by race.
Differences in eGFR decline per year according to hypertension categories in the total population and by race with imputing the eGFR from the CMS 2728 form.


