Abstract
Background:
Success in multidrug-resistant tuberculosis (MDR-TB) and HIV treatment requires high medication adherence despite high pill burdens, frequent adverse events and long treatment duration, which may jeopardize adherence. We prospectively compared MDR-TB/HIV co-infected persons to those with MDR-TB alone to determine the impact of concurrent treatment on adherence and outcomes.
Methods:
We assessed medication adherence monthly using 3-day recall, 30-day recall and visual analog scale and examined adherence to monthly study visits (months 0–12). We determined the proportion of participants fully adherent (no reported missed doses) to MDR-TB vs. HIV treatment by each measure. We assessed the association of medication and clinic visit adherence with MDR-TB treatment success (cure or completion, 18–24 months) and HIV virologic suppression.
Results:
Among 200 MDR-TB patients, 63% were female, median age was 33 years, 144 (72%) were HIV-infected, and 81% were receiving ART at baseline. Adherence to medications (81–98% fully adherent across all measures) and clinic visits (80% missed ≤1 visit) was high, irrespective of HIV status. Adherence to ART was significantly higher than to MDR-TB treatment by all self-reported measures (3-day recall: 92% vs. 84%, respectively; p=0.003). In multivariable analysis, the adjusted risk ratio (aRR) of unsuccessful MDR-TB treatment increased with every missed visit: 1.50, 2.25, and 3.37 for unsuccessful treatment, for 1, 2, ≥3 missed visits.
Conclusions:
Adherence to ART was higher than to MDR-TB treatment among persons with MDR-TB/HIV co-infection. Missed clinic visits may be a simple measure for identifying patients at risk of unsuccessful MDR-TB treatment outcome.
Keywords: Multidrug-resistant tuberculosis (MDR-TB), human immunodeficiency virus (HIV), treatment adherence, MDR-TB/HIV co-treatment
INTRODUCTION
Multidrug-resistant tuberculosis (MDR-TB), defined as resistance to at least isoniazid and rifampin, is an increasing global health concern, with an estimated 460,000 persons with MDR-TB in 2017.1 MDR-TB treatment has traditionally involved 4-5 different drugs, including an injected medication for the first six months of treatment, with many toxic side effects and treatment length of approximately two years. The global treatment success rate for MDR-TB was estimated to be between 55%1 and 65%2 from 2009-2016, compared to 82% treatment success for drug-susceptible TB (DS-TB) in 2016, highlighting the need for improved treatment outcomes.1
Worldwide, TB is the leading cause of mortality in patients with HIV.1 Among patients with MDR-TB and HIV, mortality rates are substantially higher, with one study citing a hazard ratio of 5.6 for HIV-infected vs. HIV-uninfected MDR-TB patients.3,4 Treatment for MDR-TB/HIV co-infected patients requires complicated drug regimens with a high pill burden, potential drug-drug interactions, and numerous toxicities from antiretroviral therapy (ART) and MDR-TB medications, such as gastrointestinal symptoms, peripheral neuropathy, depression, and hearing and vision disturbances.5,6 Despite this, concurrent MDR-TB and HIV treatment is feasible and can yield good patient outcomes, even in low-resource settings.7
Medication adherence is critical to successful treatment of both MDR-TB and HIV.8 Non-adherence increases the risk of treatment failure, relapse, and development of further drug resistance.9,10 However, little quantitative evidence exists regarding adherence in MDR-TB/HIV co-treatment, or the effect of non-adherence on treatment outcomes.9 Qualitative evidence suggests longer treatment time, serious adverse events, perception of poor treatment outcomes, stigma and lack of social support, and preferential adherence to ART all contribute to reduced MDR-TB treatment adherence.11,12 Directly-observed therapy (DOT) is recommended as the standard of care for TB to support patient adherence, and a daily injection during MDR-TB treatment’s intensive phase requires DOT. However, with lengthy treatment duration and twice daily dosing of certain drugs implementation in high-burden settings is inconsistent due to logistical and human resource challenges. Despite recent changes in MDR-TB treatment guidelines that now recommend shorter and all-oral regimens,13 the issue of adherence in MDR-TB/HIV co-infected persons remains critical to treatment success.
South Africa has among the highest burdens of TB, MDR-TB and HIV co-infection, harboring approximately 25% of the global TB/HIV burden.14 South Africa had an estimated 14,000 (95% CI: 8,900-20,000) persons with MDR-TB in 2017,1 of whom 70-80% are HIV-infected.7,15 In this prospective observational study, we assessed treatment adherence and measured the association with MDR-TB and HIV outcomes in KwaZulu-Natal, South Africa. We compared persons with MDR-TB/HIV co-infection to those with MDR-TB alone to determine the impact of concurrent treatment on adherence and outcomes.
METHODS
Study Design and Population
The SHOUT MDR-TB (Survival and HIV OUTcomes in MDR-TB) Study is a prospective observational cohort of MDR-TB patients that compared treatment outcomes among persons co-infected with HIV to those without HIV. Adult MDR-TB participants were recruited from three TB referral hospitals in KwaZulu-Natal Province, South Africa, from 2011 through 2013.
Participants were enrolled based on the following criteria: (1) culture-confirmed MDR-TB (resistance to at least rifampicin and isoniazid); (2) age ≥ 18 years; (3) documented HIV status (HIV-infected or HIV-negative); and (4) initiated on MDR-TB treatment within 14 days of study screening. HIV-infected participants were offered ART according to South African guidelines, which specify that all MDR-TB patients co-infected with HIV should initiate ART within two weeks regardless of CD4 count.16 All HIV-infected participants included this analysis were receiving ART during the study period. Exclusion criteria included: (1) history of previous MDR-TB treatment; (2) resistance to fluoroquinolones or injectable TB medications; (3) current pregnancy; (4) receipt of non-standard TB or HIV treatment; or (5) baseline creatinine >2x upper limit of normal or alanine aminotransferase >5x upper limit of normal.
Medication regimens for HIV and MDR-TB and need for DOT were determined by participants’ clinicians, in accordance with South African national policy;17 research staff were not involved in treatment decisions or DOT administration. The standard South African MDR-TB regimen at the time of the study included kanamycin, ofloxacin or moxifloxacin, ethionamide, terizidone, ethambutol, and pyrazinamide. Kanamycin injections were administered for at least 6 months or 4 months after culture conversion (whichever was longer), and oral medications without kanamycin for 12-18 additional months following culture conversion.17 Most HIV-infected participants received efavirenz-based ART regimens with stavudine and lamivudine at the beginning of the study; stavudine was changed to tenofovir when national guidelines were revised in 2013.18
The participants were followed monthly throughout MDR-TB treatment (typically 24 months). Participant demographics, TB and HIV exposure and treatment history, and other pertinent medical information were obtained at baseline. Treatment response, adverse events, regimen changes, and medication adherence were assessed at each study visit. Sputum was collected for culture and drug-susceptibility testing (DST) at each visit. Viral load and CD4 cell count were measured for HIV-infected participants every three months.
Adherence measurements
Self-Report
Three self-reported measures of medication adherence were collected independently for MDR-TB and HIV treatment regimens at each study visit (i.e, six adherence measures per co-infected participant per study visit). The measures were a: (1) 3-day self-report of the number of pills missed over the last three days; (2) 30-day self-report of the number of pills not taken, lost, given away, or additional pills taken from other sources; and (3) visual analog scale (VAS)19,20, ranging from 1–100%, allowing participants to report their estimated adherence in the past 30 days. We defined “full adherence” as no reported missed medication at any point in the study by each individual measure.21,22 We created two composite adherence measures: (1) full adherence reported by all three individual measures; (2) full adherence as reported by the 3-day recall measure and VAS. Adherence was measured separately for MDR-TB and HIV treatment.
Clinic Visit Attendance
Per South Africa national guidance, MDR-TB and HIV medications were dispensed monthly during the first year of treatment; participants progressing well on MDR-TB treatment had clinic visits every two months during the second year of treatment. Therefore, analysis of clinic visit adherence was limited to the first year of treatment to avoid selection bias.
The number of missed clinic visits within the first six and 12 months of MDR-TB treatment were determined for each participant based on sputum specimen collection and other study visit data to confirm whether a clinic visit occurred each month as scheduled.
Participants with treatment completion were included in the analysis because clinic visit adherence was measured during the first year of treatment only, before the visits at the end of treatment that defined this outcome.22 The date of outcome for participants with treatment interruption was the date of the last clinic visit attended (i.e., prior to the 2 consecutive months of missed visits without return to care).22 Clinic visit adherence was measured up to the date of outcome, so did not include the 2 months of missed visits that define treatment interruption. We conducted a sensitivity analysis by removing each of these two groups of participants from the analysis of clinic visit adherence and treatment outcome.
Outcomes of interest
The primary outcome of interest was the proportion of participants with full adherence to MDR-TB treatment, by HIV status, as indicated by the self-reported measures, composite measures, and clinic visits. Among participants co-infected with HIV, we also measured adherence to HIV treatment using these same measures.
We determined the association between adherence and MDR-TB treatment success (e.g., MDR-TB cure or treatment completion), and TB culture conversion within 60 days of treatment initiation; death, treatment failure or treatment interruption were categorized as unsuccessful treatment.23 Culture conversion was defined as two consecutive negative TB-cultures, 30 days apart.
The primary HIV outcome of interest was virologic failure, defined as a detectable viral load (>150 copies/mL) 6 months after ART initiation, or loss of viral suppression with two viral loads > 1000 copies/mL during follow up, according to South African guidelines.
Data Analysis
Demographic and clinical characteristics were described using medians and interquartile ranges (IQR). We used Wilcoxon Mann-Whitney test and Fisher’s exact and chi-squared tests to compare MDR-TB participants with and without HIV coinfection. The proportion of participants with full medication adherence was calculated separately for MDR-TB and HIV treatment using the three self-reported measures. The number of missed visits in the first 12 months of treatment was calculated to indicate adherence to clinic visits. Full adherence to MDR-TB medication was compared among HIV-infected and HIV-negative cohorts using chi-squared tests. Among HIV-infected participants, we compared adherence to MDR-TB treatment and ART using McNemar’s test.
Log-binomial regression was used to assess associations of the most valid composite measure with MDR-TB and HIV treatment outcomes, as well as the association of missed clinic visits with TB and HIV treatment outcomes. Variables were selected for inclusion in the final log-binomial models based on previously published literature and assessment of significant bivariate associations (two-sided p-value <0.2).
Ethical Considerations
All participants provided written informed consent. The study was approved by the institutional review boards of Emory University, Albert Einstein College of Medicine, University of KwaZulu-Natal (UKZN), and by KwaZulu-Natal Department of Health. The study was also reviewed in accordance with the Centers for Disease Control and Prevention (CDC) human research protection procedures.
RESULTS
From 2011–2013, we screened 403 patients with confirmed MDR-TB, of whom 206 were enrolled in the parent SHOUT MDR-TB study.24 The most common reasons reported for exclusion were age under 18 years (n=39), lab contaminant (i.e., not started on TB treatment; n=34)), prior treatment MDR-TB treatment (n=28), and too ill to consent (n=26). Six participants were excluded from adherence analysis because they withdrew or died before adherence data could be collected (see Figure A1, Supplemental Digital Content 1). Among the remaining 200 participants in this analysis, the median age was 33 years (IQR 26-41), and 126 (63%) participants were female (Table 1). Two-thirds (65%) reported a prior history of DS-TB treatment, and 144 (72%) were HIV-infected. Among the HIV-infected participants, 116 (81%) were receiving ART at the time of MDR-TB treatment initiation (median duration of 9 months; IQR: 3–30); their median CD4 count was 215 cells/mm3 (IQR: 109–376) and 66% had an undetectable viral load (<150 copies/mL) at baseline. An additional 23 (15%) HIV-infected participants started ART after enrollment. HIV-infected participants were more likely to be female (69% vs. 46%, p=0.0025), and more likely to have a prior history of TB treatment (72% vs. 46%, p<0.001).
Table 1.
Baseline characteristics of study participants with multidrug-resistant tuberculosis (MDR-TB), by HIV status – KwaZulu-Natal, South Africa (N=200).
| Total Cohort (N=200) |
HIV+ (n=144) |
HIV− (n=56) |
p-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Demographics | ||||
| Age (median, IQR) | 33 (26-41) | 34 (29-40) | 27 (21-49) | 0.069* |
| Gender (Female) | 126 (63) | 100 (69) | 26 (46) | 0.002 † |
| Race: Black | 198 (99) | 144 (100) | 54 (96) | 0.077 ‡ |
| Previous History of TB Treatment | 130 (65) | 104 (72) | 26 (46) | <0.001 † |
| Smoking History(current or ever use) | 47 (24) | 35 (24) | 12 (21) | 0.667 † |
| Alcohol History (current or ever use) | 62 (31) | 48 (33) | 14 (25) | 0.253 † |
| Household Member with TB | 35 (18) | 22 (15) | 13 (23) | 0.185 † |
| Healthcare Worker, past 12 months | 13 (7) | 7 (5) | 6 (11) | 0.197 ‡ |
| Mineworker, past 12 months | 6 (3) | 5 (3) | 1 (2) | 1.000 ‡ |
| Prison, past 12 months | 5 (3) | 4 (3) | 1 (2) | 1.000 ‡ |
| HIV Characteristics | ||||
| On antiretroviral therapy (ART) | 116 (81) | |||
| CD4 Count (Median (IQR)), n=139 | 215 (109-376) | |||
| Virologically Suppressed, Baseline, n=97 | 64 (66) |
HIV: Human immunodeficiency virus
Wilcoxon exact test (2-sided p-value)
Chi-square test
Fisher’s exact test (2-sided p-value)
Self-reported Adherence
Overall, self-reported adherence to both MDR-TB and HIV treatment was high (Table 2). Among the full cohort of 200 MDR-TB patients, 94% reported full adherence to MDR-TB treatment on the 30-day recall measure, and 85% reported full adherence according to three-day recall and VAS. Among 139 participants with MDR-TB and HIV on ART, 98% (30-day recall), 92% (three-day recall) and 91% (VAS) reported full adherence to ART.
Table 2.
Proportion of participants with full adherence* to MDR-TB and HIV medication using three self-reported adherence measures, by HIV status (N=200).
| Full Medication Adherence | |||||
|---|---|---|---|---|---|
| 3-day Recall†, n (%) |
30-day Questionnaire‡, n (%) |
Visual Analog Scale§, n (%) |
Composite Adherence Measure 1‖, n (%) |
Composite Adherence Measure 2□, n (%) |
|
| Combined Cohort | |||||
| MDR-TB Tx (n = 200) | 170 (85) | 188 (94) | 169 (85) | 162 (81) | 164 (82) |
| HIV+ | |||||
| MDR-TB Tx (n = 144) | 121 (84) | 134 (93) | 122 (85) | 116 (81) | 118 (82) |
| ART (n=139) | 128 (92) | 136 (98) | 126 (91) | 123 (88) | 124 (89) |
| P-value¶ | 0.003 | 0.031 | 0.039 | 0.007 | 0.013 |
| HIV− | |||||
| MDR-TB Tx (n = 56) | 49 (88) | 54 (96) | 47 (84) | 46 (82) | 46 (82) |
| P-valueΔ | 0.35 | 0.30 | 0.65 | 0.84 | 0.97 |
MDR-TB: Multidrug-resistant tuberculosis; HIV: Human immunodeficiency virus; Tx: Treatment; ART: Antiretroviral Therapy
Full adherence defined as no reported missed medication at any point in the study
Percent that did not report missing any pills in the last 3 days at any point in the study
Percent that did not report missing any pills in the last 30 days at any point in the study
Based on visual analog scale rating (from 0–100%) of percent of medication taken since last study visit
Composite measure 1 indicates full adherence by all three individual measures (3-day, 30-day, and visual analog scale) throughout the study
Composite measure 2 indicates full adherence by both the 3-day recall measure and the visual analog scale throughout the study
Based on McNemar’s exact test
Based on Fisher's exact test for dichotomous variables comparing MDR-TB treatment adherence among HIV+ and HIV− participants
Bivariate analysis indicated no significant differences in self-reported adherence to MDR-TB treatment between HIV-infected and HIV-negative participants (Table 2). However, among HIV-infected participants, self-reported adherence to ART was significantly higher than that of MDR-TB treatment according to all three individual and both composite measures (Table 2). The three-day recall (84% to MDR-TB treatment vs. 92% to ART, p=0.003) and composite measure of VAS and 3-day recall (82% to MDR-TB treatment vs. 89% to ART, p=0.013) showed the largest differences in adherence.
No significant association was found between self-reported adherence and MDR-TB or HIV treatment outcome (see Table A1, Supplemental Digital Content 2).
Clinic Visit Adherence
During the first 12 months of MDR-TB treatment, 67 (35%) participants missed one or more clinic visits; 25 (37%) of these (13% of the total study population) missed three or more visits (Table 3). Fewer HIV co-infected participants attended every clinic visit during the first 6 months (75% vs. 87% zero missed visits, p=0.15) and 12 months (61% vs. 76% missed zero visits, p=0.30) of MDR-TB treatment than their HIV-negative counterparts, though this was not statistically significant.
Table 3.
Missed clinic visits during the first six and twelve months of MDR-TB treatment, among all participants and by HIV co-infection status (n=191).
| Total Cohort |
HIV+ (n=138) |
HIV− (n=53) |
p- value* |
|
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Number of missed visits - first 6 months | ||||
| MDR-TB treatment | 0.15 | |||
| 0 | 149 (78) | 103 (75) | 46 (87) | |
| 1 | 23 (12) | 17 (12) | 6 (11) | |
| 2 | 7 (4) | 7 (5) | 0 (0) | |
| ≥ 3 | 12 (6) | 11 (8.0) | 1 (2) | |
| Number of missed visits - first 12 months | ||||
| MDR-TB treatment | 0.30 | |||
| 0 | 124 (65) | 84 (61) | 40 (76) | |
| 1 | 29 (15) | 24 (17) | 5 (9) | |
| 2 | 13 (7) | 11 (8) | 2 (4) | |
| ≥ 3 | 25 (13) | 19 (14) | 6 (11) |
MDR-TB: Multidrug-resistant tuberculosis; HIV: Human immunodeficiency virus
Fisher's exact test
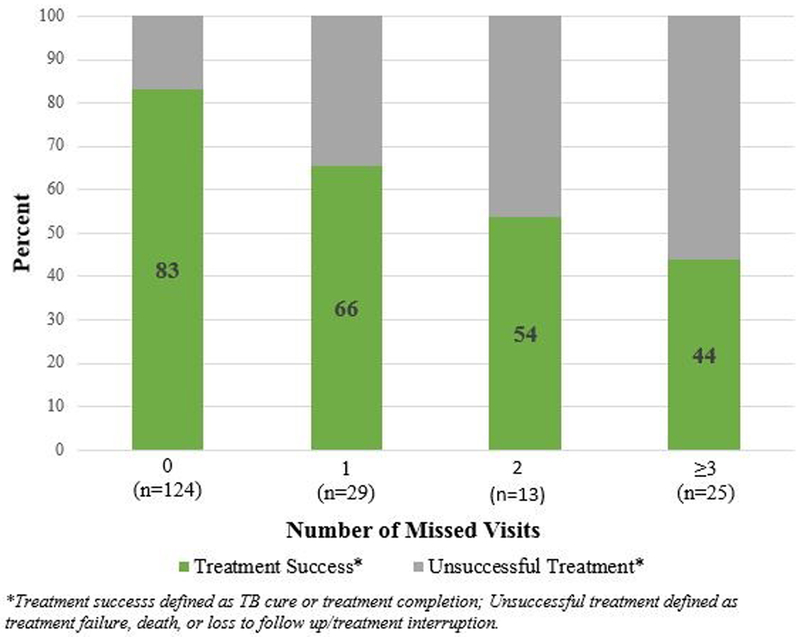
MDR-TB treatment success rates declined from 83% among those with no missed clinic visits to 44% among those who missed 3 or more visits during the first year of treatment (Figure 1, p=0.0001). The risk of unsuccessful treatment was significantly higher with each missed visit during the first year of TB treatment (crude risk ratio: 1.47, 95% CI: [1.26-1.72], 2.17 [1.58 – 3.0], 3.19 [1.99–5.13] among those who missed 1, 2 and 3 or more visits, respectively; Table 4). No association was found between clinic visit attendance and virologic failure among HIV-infected participants (crude risk ratio of virologic failure among those who missed 1 vs. 0 clinic visits, first 12 months of treatment: 0.82, 95% CI: [0.54, 1.24]).
Figure 1. MDR-TB treatment cure rate by number of missed clinic visits during the first twelve months of MDR-TB treatment (n=191).
The rate of MDR-TB treatment success, defined as cure or treatment completion, was graphed by the number of missed clinic visits in the first year of MDR-TB treatment. The MDR-TB treatment success rate decreases with every additional missed clinic visit in the first year of treatment.
Table 4.
Relative risk of unsuccessful MDR-TB treatment outcome* among participants with low clinic visit adherence† compared to those with high clinic visit adherence during the first 12 months of MDR-TB treatment (n=191).
| Crude RR (95% CI) |
p-value‡ | Adjusted RR §
(95% CI) |
p-value‖ | |
|---|---|---|---|---|
| Number of missed visits | <.0001 | <0.0001 | ||
| Zero | REF | REF | ||
| One | 1.47 (1.26, 1.72) | 1.50 (1.27, 1.77) | ||
| Two | 2.17 (1.58, 3.0) | 2.25 (1.60, 3.14) | ||
| Three or more | 3.19 (1.99, 5.13) | 3.37 (2.03, 5.57) |
MDR-TB: Multidrug-resistant tuberculosis
Unsuccessful MDR-TB treatment outcome defined as treatment failure, treatment interruption, transfer for advanced treatment, or death; treatment success defined as cure or treatment completion
Low clinic visit adherence defined as missing 1, 2, or 3 or more visits during the specified treatment time window
Chi squared test
Adjusted for age, gender, and HIV status
Fisher's exact test
In multivariate analysis adjusted for age, gender, and HIV status, the risk of unsuccessful MDR-TB treatment was 50% higher among participants who missed one clinic visit during the first year of treatment than among those with no missed visits (adjusted risk ratio (aRR): 1.50, 95% CI: 1.27–1.77; Table 4). The risk of unsuccessful MDR-TB treatment increased with each additional missed visit, with more than double the risk (aRR; 2.25, 95% CI: 1.60–3.14) among those with two missed visits, and more than three times greater risk (aRR; 3.37, 95% CI: 2.03–5.57) among those with three or more missed visits. When participants with outcomes of treatment interruption or treatment completion were removed from analysis, the direction of the association of missed clinic visits and MDR- TB treatment outcome did not change (sensitivity analysis, results not shown).
DISCUSSION
Medication adherence has been a pillar of TB and HIV care for decades, with non-adherence leading to drug resistance, clinical deterioration, and ongoing transmission. Consistent adherence to MDR-TB and HIV co-treatment can pose a formidable challenge for patients needing 18–24 months of therapy with 7–8 drugs with toxic side effects. We utilized three self-reported medication adherence measures and clinic visit attendance in a prospective cohort of participants undergoing MDR-TB and HIV co-treatment in KwaZulu-Natal, South Africa.
We found high self-reported adherence to MDR-TB treatment, with no difference between HIV-infected and HIV-uninfected individuals. Among HIV-infected persons, self-reported adherence to ART was higher than to MDR-TB treatment. Regular clinic attendance during the first year of MDR-TB treatment was strongly associated with treatment success, with 2–3 times higher risk for failure among persons who missed 2 or 3 visits, respectively, regardless of HIV status. Together, these findings suggest that MDR-TB and HIV co-treatment is feasible without substantial effect on medication adherence, despite the added pill burden and potential overlapping toxicities. Additionally, patients who miss one or more clinic visits early in the course of MDR-TB treatment may be at higher risk of unsuccessful treatment and may benefit from interventions to improve adherence.
Higher adherence to HIV medication than to TB medication in our study supports previously published findings of preferential adherence to ART over TB treatment.12,25 Qualitative data suggests that negative perceptions of treatment outcomes for drug-resistant TB (DR-TB), social isolation, stigmatization of patients, and inadequate attention to patient education and support compared to HIV care were reasons for non-adherence to DR-TB treatment.12 These barriers were experienced in addition to the challenges of higher pill burden and worse adverse effects of DR-TB medications.12 Patients also expressed feeling personally responsible for adhering to ART, whereas treatment for DR-TB was seen as the responsibility of nurses.12
We found that clinic visit attendance is a strong predictor of TB treatment failure and can be used to identify patients at increased risk of unsuccessful MDR-TB treatment outcome. This objective and less resource-intensive adherence measure is particularly useful in light of the well-established limitations of self-reported adherence data.26,27 Previous work suggests that interventions to improve visit attendance, such as pre-appointment reminders and missed appointment follow-up, are effective ways to improve treatment completion rates in drug-susceptible TB populations.28 Our work expands on the importance of supporting patient attendance at clinic visits for MDR-TB treatment, especially as the global TB community shifts towards more patient-centered TB care.1,29
Self-reported adherence in our study population was high, and none of the self-reported measures were robust predictors of treatment outcome. However, there was a trend towards a protective effect of self-reported adherence on successful MDR-TB treatment outcome, similar to the well-established association of ART adherence with higher virologic suppression rates.30-32 The high adherence levels reported in this study are consistent with previous literature.20,33,34 The closer correlation of the 3-day recall measure with TB treatment outcome than the 30-day recall measure is consistent with evidence suggesting self-report over shorter periods of recall (2-4 days) tends to be more accurate than longer recall periods.35 Shorter recall self-report could serve as a more feasible option in contexts where more objective but costly adherence monitoring, such as electronic devices, cannot be used.35,36
This study had several limitations. First, the sample size was relatively small, and the analysis was limited by the small number of participants defined as non-adherent. This reduced the heterogeneity of adherence categories and limited the power to conduct certain analyses, particularly for the ART adherence measures, which showed the highest levels of adherence. However, the strict cut-offs used to identify “non-adherence” in our study are supported by previous TB adherence literature.25,34 Directly observed therapy was not measured or administered by study staff; however, because its administration was separate from study-related visits and adherence measures, it is unlikely to have impacted findings. Recall and social desirability bias may have limited the accuracy of self-report questionnaires, though the use of the 3-day recall questionnaire minimized recall bias by using a shorter period of recall.35 This analysis was also limited by our inability to include a more objective adherence measure than self-report, such as electronic devices or urine drug levels. However, our analysis of clinic visit attendance provided a more objective measure of treatment adherence and showed a stepwise increased association with treatment outcome. We were not able to determine if missed clinic visits were causative of poor treatment outcome, or if they were a marker for other factors that can affect outcome. However, missing a visit on the day it was originally scheduled did not necessarily mean the participant did not receive any MDR-TB medications for the month in between the missed visit and their next scheduled visit.
Despite these limitations, we demonstrated that clinic visit attendance in the first year of MDR-TB treatment is a simple, strong predictor of TB treatment outcome in patients with and without HIV co-infection. Our data suggest that missed clinic visits early in treatment can be used to identify and better support patients at risk of unsuccessful treatment in the absence of highly sensitive medication adherence tools, especially in resource-constrained settings. We also found preferential adherence to ART over MDR-TB treatment, which may reflect underlying misperceptions about HIV treatment as compared to MDR-TB treatment by patients and/or healthcare providers. Further understanding of this differential adherence is essential to ensure successful treatment of both diseases. Timely adherence support for MDR-TB patients with any clinic non-attendance and application of lessons learned from HIV treatment support are critical next steps to improve outcomes for MDR-TB patients with and without HIV coinfection.
Supplementary Material
Supplemental Digital Content 1: “Supplemental Figure A1_JAIDS MS No. QAIV19965_Revision.jpg”
Supplemental Digital Content 2: “Supplemental Table A1_JAIDS MS No. QAIV19965_Revision.docx”
Acknowledgments:
We thank the participants and their families who consented to participate in this study. We are also grateful to the study team at the University of KwaZulu-Natal and South African Medical Research Council for their tireless efforts in data collection, record abstraction, participant recruitment and interviews.
Conflicts of Interest and Sources of Funding: This study was funded by the US National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH; R01AI087465 and R01AI089349, both to N. R. G.). It was also supported in part by grants from NIH/NIAID to J. C. M. B. (K23AI083088, R01AI114304), N. R. G. (K24AI114444), the Emory University TB Research Unit (U19AI111211) and CFAR (P30AI050409), Albert Einstein College of Medicine (CFAR P30AI124414), Albert Einstein College of Medicine and Montefiore Medical Center ICTR (UL1TR001073), and the Atlanta CTSI (UL1TR000454). All authors: no reported conflicts of interest.
Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention, U.S. National Institutes of Health, or the U.S. Department of Health and Human Services.
Footnotes
Presented in part at the 48th World Conference on Lung Health, Guadalajara, Mexico, October 11-14, 2017 (Abstract: OA-134-12).
References
- 1.World Health Organization. Global Tuberculosis Report 2018. Geneva: World Health Organization; 2018. Available at: https://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Ahmad N, Ahuja SD, Akkerman OW, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet (London, England). 2018;392(10150):821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manda SO, Masenyetse LJ, Lancaster JL, et al. Risk of Death among HIV Co-Infected Multidrug Resistant Tuberculosis Patients, Compared To Mortality in the General Population of South Africa. Journal of AIDS & clinical research. 2013;Suppl 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cegielski JP, Kurbatova E, van der Walt M, et al. Multidrug-Resistant Tuberculosis Treatment Outcomes in Relation to Treatment and Initial Versus Acquired Second-Line Drug Resistance. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(4):418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. The Journal of infectious diseases. 2007;196 Suppl 1:S86–107. [DOI] [PubMed] [Google Scholar]
- 6.Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2004;8(11):1382–1384. [PubMed] [Google Scholar]
- 7.Brust JC, Lygizos M, Chaiyachati K, et al. Culture conversion among HIV co-infected multidrug-resistant tuberculosis patients in Tugela Ferry, South Africa. PloS one. 2011;6(1):e15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kliiman K, Altraja A. Predictors of poor treatment outcome in multi- and extensively drug-resistant pulmonary TB. The European respiratory journal. 2009;33(5):1085–1094. [DOI] [PubMed] [Google Scholar]
- 9.van den Boogaard J, Boeree MJ, Kibiki GS, et al. The complexity of the adherence-response relationship in tuberculosis treatment: why are we still in the dark and how can we get out? Tropical medicine & international health : TM & IH. 2011;16(6):693–698. [DOI] [PubMed] [Google Scholar]
- 10.Brust JC, Shah NS, Scott M, et al. Integrated, home-based treatment for MDR-TB and HIV in rural South Africa: an alternate model of care. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2012;16(8):998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shringarpure KS, Isaakidis P, Sagili KD, et al. “When Treatment Is More Challenging than the Disease”: A Qualitative Study of MDR-TB Patient Retention. PloS one. 2016;11(3):e0150849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daftary A, Padayatchi N, O’Donnell M. Preferential adherence to antiretroviral therapy over tuberculosis treatment: a qualitative study of drug-resistant TB/HIV co-infected patients in South Africa. Glob Public Health. 2014;9(9):1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO treatment guidelines for multidrug- and rifampicin-resistant tuberculosis: 2018 update. [Pre-final text]. Geneva: World Health Organization; 2018. Available at: https://www.who.int/tb/publications/2018/WHO.2018.MDR-TB.Rx.Guidelines.prefinal.text.pdf?ua=1. [Google Scholar]
- 14.Kanabus A Information about Tuberculosis: TB Statistics-Global, regional, and high burden. 2016; Available at: http://tbfacts.org/tb-statistics/.
- 15.Buthelezi S Situational analysis of TB drug resistance in KwaZulu-Natal province: Republic of South Africa. Paper presented at: 2nd Meeting of the Global XDR TB Task Force 2008. [Google Scholar]
- 16.Department of Health: Republic of South Africa. The South African Antiretroviral Treatment Guidelines. 2010. Available at: https://apps.who.int/medicinedocs/documents/s19153en/s19153en.pdf.
- 17.Department of Health: Republic of South Africa. Management of Drug-Resistant Tuberculosis: Policy Guidelines. 2011.
- 18.Department of Health: Republic of South Africa. Management of Drug-Resistant Tuberculosis: Policy Guidelines (Updated- January 2013). Available at: https://www.health-e.org.za/wp-content/uploads/2014/06/MDR-TB-Clinical-Guidelines-Updated-Jan-2013.pdf.
- 19.Finitsis DJ, Pellowski JA, Huedo-Medina TB, et al. Visual analogue scale (VAS) measurement of antiretroviral adherence in people living with HIV (PLWH): a meta-analysis. Journal of behavioral medicine. 2016;39(6):1043–1055. [DOI] [PubMed] [Google Scholar]
- 20.Nackers F, Huerga H, Espie E, et al. Adherence to self-administered tuberculosis treatment in a high HIV-prevalence setting: a cross-sectional survey in Homa Bay, Kenya. PloS one. 2012;7(3):e32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterberg L, Blaschke T. Adherence to medication. The New England journal of medicine. 2005;353(5):487–497. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson BJ, Rowe BH, Haynes RB, et al. The rational clinical examination. Is this patient taking the treatment as prescribed? Jama. 1993;269(21):2779–2781. [PubMed] [Google Scholar]
- 23.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2005;9(6):640–645. [PubMed] [Google Scholar]
- 24.Brust JCM, Shah NS, Mlisana K, et al. Improved Survival and Cure Rates With Concurrent Treatment for Multidrug-Resistant Tuberculosis-Human Immunodeficiency Virus Coinfection in South Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(8):1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell MR, Wolf A, Werner L, et al. Adherence in the treatment of patients with extensively drug-resistant tuberculosis and HIV in South Africa: a prospective cohort study. Journal of acquired immune deficiency syndromes (1999). 2014;67(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical therapeutics. 1999;21(6):1074–1090; discussion 1073. [DOI] [PubMed] [Google Scholar]
- 27.Williams AB, Amico KR, Bova C, et al. A proposal for quality standards for measuring medication adherence in research. AIDS and behavior. 2013;17(1):284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Abba K, Alejandria MM, et al. Reminder systems to improve patient adherence to tuberculosis clinic appointments for diagnosis and treatment. Cochrane Database Syst Rev. 2014(11):CD006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell MR, Daftary A, Frick M, et al. Re-inventing adherence: toward a patient-centered model of care for drug-resistant tuberculosis and HIV. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2016;20(4):430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of internal medicine. 2000;133(1):21–30. [DOI] [PubMed] [Google Scholar]
- 31.Genberg BL, Wilson IB, Bangsberg DR, et al. Patterns of antiretroviral therapy adherence and impact on HIV RNA among patients in North America. AIDS (London, England). 2012;26(11):1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannheimer S, Friedland G, Matts J, et al. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;34(8):1115–1121. [DOI] [PubMed] [Google Scholar]
- 33.Oyugi JH, Byakika-Tusiime J, Charlebois ED, et al. Multiple validated measures of adherence indicate high levels of adherence to generic HIV antiretroviral therapy in a resource-limited setting. Journal of acquired immune deficiency syndromes (1999). 2004;36(5):1100–1102. [DOI] [PubMed] [Google Scholar]
- 34.Mkopi A, Range N, Lwilla F, et al. Validation of indirect tuberculosis treatment adherence measures in a resource-constrained setting. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2014;18(7):804–809. [DOI] [PubMed] [Google Scholar]
- 35.Wilson IB, Lee Y, Michaud J, et al. Validation of a New Three-Item Self-Report Measure for Medication Adherence. AIDS and behavior. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruslami R, van Crevel R, van de Berge E, et al. A step-wise approach to find a valid and feasible method to detect non-adherence to tuberculosis drugs. Southeast Asian J Trop Med Public Health. 2008;39(6):1083–1087. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1: “Supplemental Figure A1_JAIDS MS No. QAIV19965_Revision.jpg”
Supplemental Digital Content 2: “Supplemental Table A1_JAIDS MS No. QAIV19965_Revision.docx”