Abstract
Previous studies in other provinces of China (Beijing, Xinjiang, Shanxi, Jiangxi, Shanghai, Guangdong, and Taiwan) suggest that the distributions of lymphoma subtypes differ compared with Western populations. In order to evaluate the characteristics of malignant lymphoma in Sichuan, China, we analyzed case series data from incident lymphoma patients diagnosed in 2008 from three hospitals, including a total of 1629 cases and including only current residents of Sichuan. The median age of diagnosis for cases was 54 years, with a higher proportion of male cases compared with female cases. The most commonly diagnosed subtypes included diffuse large B-cell lymphoma (40.4%), NK/T-cell lymphoma (NKTCL; 11.8%), mixed cellularity Hodgkin lymphoma (7.0%), mantle cell lymphoma (4.8%), and marginal zone B-cell lymphoma (3.9%). Differences in demographic characteristics between Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) cases were apparent for median age at diagnosis (HL: 34 years; NHL: 57 years), and NHLs accounted for nearly all (99.3%) of the 931 cases of extranodal lymphoma. These findings indicate a higher proportion of NKTCL cases and a lower proportion of follicular lymphoma cases (2.3%) in these hospitals in Sichuan, relative to reports from some other provinces within China (e.g., Shanghai and Shanxi) and the USA.
Keywords: lymphoma, China, geographical variation, descriptive epidemiology
Introduction
The highest incidence rates of lymphoma are observed in Western countries, whereas comparatively lower incidence rates have been reported in Eastern Asia [1]. The incidence rates of non-Hodgkin lymphoma (NHL) in men and women from the USA are 15.5/100,000 and 10.8/100,000, respectively, based on data from Cancer Incidence in Five Continents Vol. X, whereas NHL rates in China range from 2.1 to 7.4/100,000 in men and 1.4 to 5.4/100,000 in women across different Chinese registries [2]. Similar differences have been reported for Hodgkin lymphoma (HL), where the incidence rate in China is <1 case/100,000 in both men and women. Nonetheless, lymphoma overall is currently one of the top ten malignancies in China in terms of incidence, with an age-standardized incidence rate of 3.75 cases/100,000 [3].
Case series and registry data have provided evidence for geographic variability in the histological distribution of lymphoma subtypes both between China and Western countries as well as, in some cases, within regions of China [4–7]. One of the most defining differences is a decreased frequency of follicular lymphoma (FL) and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in China compared with the West, while a comparatively higher proportion of NK/T-cell (NKTCL) lymphomas have been reported in Chinese patients [5]. Evaluation of the variation in diagnosed lymphoma subtypes and characteristics of the cases in China compared with the West may provide clues concerning the etiological factors responsible for this disease. The objective of our study was to analyze the histological classification, age, gender, and other characteristics of 1629 newly diagnosed lymphoma cases in 2008 from three main hospitals in Sichuan province, China, with cases restricted to include only current residents of this province.
Methods
Data from 1629 newly diagnosed lymphoma cases were collected from three hospitals in Sichuan, including the West China Hospital of Sichuan University, Sichuan Tumour Hospital, and Sichuan Provincial People’s Hospital from 1 January 2008 to 31 December 2008. Cases included inpatients, outpatients, and referral and consulting cases from outside the hospital. Of the 1629 cases, 1161 were identified from consultation results. All cases were restricted to current residents of Sichuan. A previous evaluation of lymphoma patients diagnosed from 2000 to 2008 at West China Hospital has been conducted but ~50% of the study population was consultant cases from all areas of southwestern China [4].
The three hospitals included in our study are the primary hospitals that care for lymphoma cases from all over Sichuan, accounting for about 70% of the total lymphoma cases in the province. Moreover, the Department of Pathology of West China Hospital is one of the leading lymphoma pathology consultation centers in China, as almost all of the newly diagnosed lymphoma specimens are sent here for final histological confirmation. For this reason, the newly diagnosed lymphoma cases in the three hospitals may account for >80% of the total cases in Sichuan, and are therefore believed to be generalizable to the case distribution of the overall province. We analyzed the characteristics for these 1629 cases by age, gender, pathological classification, and urban versus rural residence. Pathological diagnoses were made after consultations with two highly experienced pathologists, and cases were classified according to the 2008 World Health Organization criteria. We further estimated the crude incidence rate of lymphoma in Sichuan using population data from the Sichuan Provincial Bureau of Statistics for 2009 and based on the assumption that the number of cases in our study represents 80% of the total cases diagnosed in the province in 2008 [i.e., (total cases÷ 0.80) ÷population]. The population of Sichuan province in 2008 was 89.078 million, including 46.077million males and 43.001 million females. Among them, 17.35% of the population was under the age of 14 years. The study involved analysis of data abstracted from hospital records without recording of personal identifiers and conformed to the provisions of the Declaration of Helsinki. All statistical analyses were conducted using SPSS version 17.0.
Results
Gender distribution
Of the 1629 incident lymphomas, there were 1033 male and 596 female patients (male : female ratio of 1.73:1). The estimated crude incidence rate of lymphoma for Sichuan in 2008 was approximately 2.29/100,000 for men and women combined. For men and women separately, the estimated crude rates were 2.80/100,000 and 1.73/100,000, respectively. A higher number of male cases relative to female cases were observed for both HL (1.48:1) and NHL (1.76:1). Further, a higher proportion of male cases was observed for the majority of the lymphoma subtypes (Table 1), with the exception of nodular sclerosis HL (NS-CHL; M:F, 1:1.71) and anaplastic large cell lymphoma (ALCL), which had similar proportions of diagnosed males and females (M:F, 1:1.04).
Table 1.
Characteristics of lymphoma subtypes diagnosed in Sichuan, China in 2008
| Subtype | Number of cases |
Gender (male/female) |
Age (years) | Urban/rural | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0– | 12– | 19– | 30– | 40– | 50– | 60– | 70– | 80– | Range (median age) |
||||
| DLBCL | 658 | 1.56 | 5 | 14 | 35 | 83 | 101 | 160 | 159 | 88 | 13 | 9–93 (57) | 1.19 |
| NKTCL | 192 | 2.25 | 1 | 10 | 20 | 49 | 35 | 36 | 26 | 13 | 2 | 12–82 (45) | 2.31 |
| MC-CHL | 114 | 2.00 | 14 | 19 | 18 | 10 | 10 | 20 | 17 | 6 | 0 | 4–78 (39) | 1.37 |
| MCL | 78 | 1.89 | 0 | 0 | 0 | 6 | 9 | 21 | 34 | 8 | 0 | 31–78 (63) | 1.79 |
| MZL | 64 | 1.29 | 0 | 1 | 0 | 6 | 17 | 10 | 14 | 15 | 1 | 17–88 (59) | 3.00 |
| CLL/SLL | 61 | 1.18 | 0 | 0 | 0 | 2 | 8 | 19 | 24 | 7 | 1 | 31–76 (62) | 1.44 |
| PTCL-NOS | 54 | 2.86 | 0 | 3 | 0 | 5 | 8 | 12 | 14 | 11 | 1 | 15–90 (60) | 1.16 |
| ALCL | 51 | 0.96 | 4 | 9 | 3 | 12 | 5 | 6 | 10 | 2 | 0 | 4–75 (37) | 0.82 |
| AITL | 49 | 3.08 | 0 | 0 | 0 | 3 | 1 | 11 | 15 | 16 | 3 | 38–83 (67) | 1.04 |
| NS-CHL | 38 | 0.58 | 1 | 4 | 18 | 7 | 5 | 2 | 1 | 0 | 0 | 6–65 (26) | 1.60 |
| FL | 37 | 1.85 | 0 | 0 | 0 | 6 | 10 | 7 | 10 | 3 | 1 | 31–82 (59) | 1.31 |
| Burkitt | 28 | 1.80 | 13 | 8 | 3 | 1 | 2 | 0 | 0 | 1 | 0 | 2–42 (13) | 1.33 |
| Other/NOS* | 205 | 1.69 | 7 | 10 | 21 | 38 | 25 | 38 | 23 | 40 | 3 | 3–82 (56) | 1.31 |
DLBCL, diffuse large B-cell lymphoma; NKTCL, NK/T-cell lymphoma; MC-CHL, mixed cellularity classical Hodgkin lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; PTCL-NOS, peripheral T–cell lymphoma not otherwise specified; ALCL, anaplastic large cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma, NS-CHL, nodular sclerosis classical Hodgkin lymphoma; FL, follicular lymphoma.
Other/NOS cases include those with rare subtypes or those with insufficient pathological files or tissues samples for which a histological diagnosis was not available.
A striking male predominance was observed for angioimmunoblastic T-cell lymphoma (AITL), peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), and NK/T-cell lymphoma (NKTCL) with male to female ratios of 3.08:1, 2.86:1, and 2.25:1, respectively (Table 1).
Age distribution
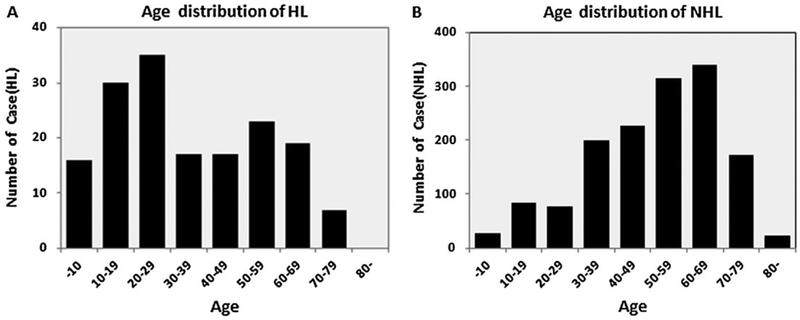
The age distributions and median age of cases by subtype are shown in Table 1. For all lymphomas, the majority of patients were between 60 and 69 years (21.3%), 50 and 59 years (21.0%), and 40 and 49 years (14.5%), while fewer patients were between 12 and 18 years (4.8%) and older than the age of 80years (1.5%). Cases of HL and NHL showed differences in age distributions at diagnosis (Figure 1). For HL, age at diagnosis ranged from 4 to 78 years with a median age of 34 years. A peak at 10–29 years of age and a second small peak at 50–69 years was apparent (Figure 1A). The median age at diagnosis was younger for NS-CHL cases, with a median age of onset of 26 years, compared with mixed cell type [mixed cellularity classical Hodgkin lymphoma (MC-CHL)] cases who had a median age of 39 years at diagnosis; 57.9% of NS-CHL patients had an age of onset between 10 and 29 years, but no second peak was evident in the 50–69 years age group.
Figure 1.
Distributions of Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) by age at diagnosis. Distributions of HL by age at diagnosis (A). Distributions of NHL by age at diagnosis (B)
For NHL patients, the range of ages at diagnosis was between 2 and 93 years with a median of 57 years old. The highest peak in diagnoses was most evident for patients between the ages of 50 and 69 years (Figure 1B). For most pathological types, median ages of onset were between 57 and 63 years, while Burkitt lymphoma (median age 13 years), ALCL (median age 37 years), and NKTCL (median age 45 years) were more likely to be diagnosed in adolescents and young adults. Conversely, AITL (median age 67 years) was most commonly diagnosed in older individuals, as patients >50years old accounted for 91.8% of these cases.
Urban versus rural distribution
According to the patient’s residence address identified from telephone follow-up, 955 patients were from urban areas and 674 were from rural areas (urban: rural ratio of 1.42:1 for lymphoma overall). These ratios for individual lymphoma subtypes are shown in Table 1. For HL, the ratio of urban to rural residence was 1.45:1, and for NHL patients it was 1.32:1. With the exception of ALCL, which had a slightly higher number of patients diagnosed from rural areas (urban : rural =1:1.22), the proportion of other subtypes for both HL and NHL was higher in patients from urban areas. This was particularly the case for NKTCL (urban: rural, 2.31:1) and marginal zone lymphoma (MZL) (urban: rural, 3:1; Table 1).
Pathological subtype composition
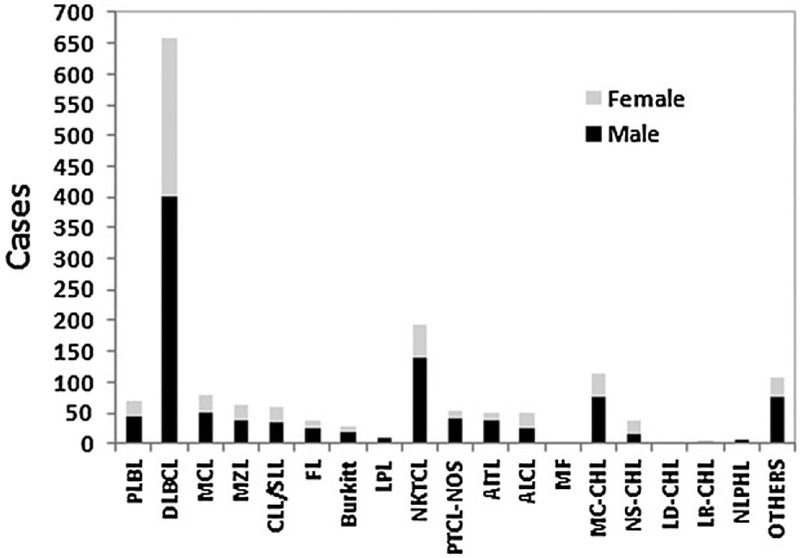
Of the 1629 lymphomas, 164 were pathologically confirmed HL (10.1%) while 1465 were NHL (89.9%). The most common diagnosed subtypes were diffuse large B-cell lymphoma (DLBCL) (n = 658,40.4%), followed by NKTCL (n =192,11.8%), MC-CHL (n =114,7.0%), mantle cell lymphoma (MCL) (n = 78, 4.8%), MZL (n = 64 cases, 3.9%), CLL/SLL (n = 61, 3.7%), PTCL-NOS (n = 54, 3.3%), ALCL (n = 51, 3.1%), AITL (n = 49, 3.0%), NS-CHL (n = 38, 2.3%), and FL (n = 37, 2.3%), as shown in Table 1 and Figure 2.
Figure 2.
Distribution of lymphoid neoplasms by subtype and sex. PLBL, precursor lymphocyte neoplasms; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; FL, follicular lymphoma; LPL, lymphoplasmacytic lymphoma; NKTCL, NK/T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; MF, mycosis fungoides; MC-CHL, mixed cellularity classical Hodgkin lymphoma; NS-CHL, nodular sclerosis classical Hodgkin lymphoma; LD-CHL, lymphocyte depleted classical Hodgkin lymphoma; LR-CHL, lymphocyte rich classical Hodgkin lymphoma; NLPHL, nodular lymphocyte predominant Hodgkin lymphoma
Of the 164 HLs, MC-CHL was the most common diagnosis (69.5%) followed by NS-CHL (23.2%). Conversely, nodular lymphocyte predominant Hodgkin’s lymphoma (NLPHL) and lymphocyte rich classic Hodgkin’s lymphoma (LR-CHL) accounted for a relatively small proportion of HL cases (5.5% and 1.8%, respectively; Figure 2). Of the 1465 NHLs, 67.7% were mature B-cell lymphoma (n = 992), mature T-cell and NK-cell lymphoma accounted for 27.5% (n = 403), and precursor lymphocyte neoplasms (PLBL) accounted for 4.8% (n = 70), including B lymphoblastic leukemia/lymphoma and T lymphoblastic leukemia/lymphoma. Of the B-cell lymphomas, the top five subtypes overall were: DLBCL (66.3%), MCL (7.9%), MZL (6.5%), CLL/SLL (6.2%), and FL (3.7 %), which accounted for a total of 90.5% of the cases. For T-cell and NK-cell lymphoma cases, the most common subtype was NKTCL (47.6%), followed by PTCL-NOS (13.4%), ALCL (12.7%), and AITL (12.2%; Figure 2).
Of the DLBCL cases, 142 cases were further classified as germinal center B-cell lymphoma (GCB) and non-germinal center B-cell lymphoma (non-GCB) according to the immunophenotyping. Whereas GCB (n = 35) was slightly more common in women (male: female, 1:1.05), cases of non-GCB (n =107) were more commonly diagnosed in men (male: female, 1.38:1). The median age of diagnosis was similar for these two subtypes (53 and 56 years, respectively).
Additionally, 57 pediatric lymphomas were diagnosed. Of these, there were 16 cases of HL (28.1%), of which MC-CHL was the most common subtype (n = 14, 87.5%). Of the pediatric NHLs, the most common subtypes were PLBL (n =13, 31.7%), Burkitt (n = 13, 31.7%), DLBCL (n = 5, 12.2%), and ALCL (n = 4,9.8%).
Primary sites by histological subtype
Of the 1629 lymphoma cases, 698 cases presented in nodal sites, accounting for 42.9% of the cases. Of these, the top three subtypes included HL, AITL, and FL, with proportions of nodal lymphoma of 95.7%, 93.9%, and 83.8%, respectively. NHLs accounted for the majority (99.3%) of the 931 cases of extranodal lymphoma, with the highest percentages observed for NKTCL (97.9%) and MZL (81.2%). Table 2 shows the anatomical sites most commonly involved for the extranodal lymphomas. The gastrointestinal tract, nasopharynx regions, tonsil, and skin represented the most frequently involved extranodal regions, accounting for 24.1%, 18.6%, 5.4%, and 4.2% of cases, respectively. DLBCL was found to be the most common subtype of the 226 lymphomas initially occurring in the gastrointestinal tract (62.8%), followed by MALT (18.6%). NKTCL was the most common subtype of the 174 cases occurring in the nasopharynx (73.0%), and PTCL-NOS was the most common subtype involving the skin.
Table 2.
Primary sites involved in commonly diagnosed histological subtypes of lymphoma in Sichuan, China in 2008
| Type/ location |
Lymph node |
Gastrointestinal tract |
Nasopharynx | Tonsil | Skin | Spleen | Brain | Testis | Marrow | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| HL | 95.7% (157) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.6% (1) | 0.6% (1) | 0.0% (0) | 0.0% (0) | 1.2% (2) | 1.8% (3) |
| DLBCL | 47.6% (313) | 21.6% (142) | 5.9% (39) | 3.6% (24) | 1.5% (10) | 2.6% (17) | 2.4% (17) | 1.8% (12) | 1.8% (12) | 11.1% (73) |
| NKTCL | 2.1% (4) | 6.3% (12) | 66.1% (127) | 11.5% (22) | 9.9% (19) | 1.0% (2) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 3.1% (6) |
| MCL | 51.3% (40) | 16.7% (13) | 2.6% (2) | 3.8% (3) | 0.0% (0) | 1.3% (1) | 0.0% (0) | 0.0% (0) | 9.0% (7) | 15.4% (12) |
| MZL | 18.8% (12) | 65.6% (42) | 0.0% (0) | 0.0% (0) | 1.6% (1) | 1.6% (1) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 12.5% (8) |
| CLL/SLL | 49.2% (30) | 6.6% (4) | 3.3% (2) | 0.0% (0) | 3.3% (2) | 1.6% (1) | 0.0% (0) | 0.0% (0) | 26.2% (16) | 9.8% (6) |
| PTCL | 57.4% (31) | 11.1% (6) | 3.7% (2) | 1.9% (1) | 11.1% (6) | 1.9% (1) | 0.0% (0) | 0.0% (0) | 1.9% (1) | 11.1% (6) |
| AITL | 93.9% (46) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 6.1% (3) | 0.0% (0) |
| ALCL | 52.9% (27) | 9.8% (5) | 3.9% (2) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 9.8% (5) | 23.5% (12) |
| FL | 83.8% (31) | 5.4% (2) | 0.0% (0) | 2.7% (1) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 8.1% (3) |
HL, Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; NKTCL, NK/T-cell lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; PTCL, peripheral T-cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell Lymphoma; FL, follicular lymphoma.
In 142 DLBCL cases with further differentiation of subtypes, 40% of the 35 GCB-DLBCL presented in nodal sites, and the cases originating in extranodal sites mainly involved the gastrointestinal tract (n = 10, 47.6%). In 107 non-GCB-DLBCL cases, 36 cases (33.6%) started in nodal sites at diagnosis. Extranodal sites involved were broad, with the gastrointestinal tract accounting for 16.9%.
Discussion
Based on our data, the estimated crude incidence rate of lymphoma in Sichuan was approximately 2.29/100,000, which is somewhat lower than the crude incidence rate of 6.68/100,000 reported for lymphoma in China (age-standardized rate 3.75/100,000) [3]. Our data indicated a relatively high proportion of NKTCL and a lower proportion of FL compared with the West, and suggested distinct demographic characteristics of the examined subtypes by factors such as age, gender, and residence.
Our data showed a male to female ratio of 1.73:1 for all lymphoma, which is relatively consistent with previous studies in China, but the male predominance in Sichuan surmounts what has been reported in Japan (1.2:1), Korea (1.6:1), and North America (~1.25:1) [8–14]. Among the lymphoma cases derived from T-cells or NK cells, a high proportion of males was evident. The distribution of ALCL in our study was similar between men and women, which is inconsistent with other Asian populations where a higher proportion of cases was observed in men [12], and is also quite different from the previous reports in Sichuan that included patients only from West China Hospital, where the male to female ratio was reported to be 1.5:1 and 1.78:1 [4,15].
A high proportion of lymphoma cases in our population was concentrated in people between 30 and 79 years, especially between the ages of 50 and 69 years, while patients <30 years and >80 years old at diagnosis were rare. Although malignant lymphoma represents a large proportion of pediatric and adolescent tumors, its incidence is lower in these groups than in adults [16]. Given the average life expectancy of 75 years in Sichuan, it is not unexpected that the proportion of cases over 80 years old was low. The median age of HL was 34 years old, similar to in the USA. The age distribution of HL had a peak at 10–29 years of age and a second peak at 50–69 years, which is inconsistent with previous observations from Sichuan based on one hospital where the proportion of HL cases gradually declined with age [4]. Conversely, the age distribution of NHL cases was concentrated in those 50–70 years and showed a single-peak distribution. Among specific NHL subtypes, NKTCL, Burkitt, and ALCL patients were diagnosed at a younger age, while AITL patients were older, which is consistent with previous reports elsewhere in China [6–9].
A higher incidence of lymphoma in China has previously been reported for people in urban compared with rural regions [17], which is consistent with our data. The proportions of nearly all subtypes were higher in urban areas, and MZL and NKTCL specifically were 2–3 times higher in urban residents than in rural ones. There are several potential reasons for these observations. Rural patients may seek treatment less consistently than patients in urban areas, leading to a decrease in early detection rates in these patients. The differences may also be the result of environmental factors that differentially affect those living in urban compared with rural areas, such as increased exposure to solvents, which have been associated with NHL risk in some studies [18]. ALCL was the only subtype in which there were a higher proportion of cases in rural areas, but the reason for this remains unclear given that the etiology of ALCL specifically is not well understood.
We observed that, compared with NS-CHL, MC-CHL was the most commonly diagnosed HL subtype. Parkin also reported MC-CHL predominance in developing countries, while NS-CHL accounts for the marked peak in developed countries [19]. For NHL, B-cell lymphoma was observed to account for the majority of diagnoses, similar to previous reports in China [20,21]. DLBCL was the most common subtype reported, which is consistent with reports in other Chinese populations [7,11,20,22], including in a previous case series of patients in the Sichuan region (53.3%) [4]. Further, the proportion of GCB cases observed in our study, about 25%, was similar to a previous case series in Shanghai (22%) [23], but less than in Western countries (50%) [24].
The most obvious differences in the subtype distributions observed in our study, as compared with those in Western populations, was for FL and NKTCL. However, some other notable differences were observed including a smaller proportion of CLL/SLL (3.7% in Sichuan compared with >10% generally observed in analyses in the USA) and a slightly higher proportion of several T-cell subtypes including PTCL-NOS, ALCL, and AITL compared with the US SEER population [25]. While the etiology of these specific T-cell subtypes is not well-understood, evidence suggests that the lower incidence of CLL/SLL in Chinese may be largely driven by differences in the risk allele frequencies of associated genetic variants [26]. On the other hand, it is apparent from our data that some subtypes, such as Burkitt lymphoma, are rare in both the Chinese and US populations, and therefore the extent of the geographic variation is not as apparent. The fact that geographic differences are notable for some subtypes but not others suggests a complex etiological profile for lymphoma that likely varies by disease subtype [27]. Subtype-specific associations with putative genetic and environmental risk factors will require further study in large-scale epidemiological studies of Chinese individuals to elucidate the reasons for these observed descriptive patterns.
The sites of onset were notably different for various subtypes, and DLBCL was found to be the most common subtype for both extranodal and nodal lymphomas. The proportion of nodal lymphomas for GCB was slightly higher than that of non-GCB, contrary to Lu’s report [28], which could be the result of the small samples or the potential for selection bias. NKTCL and MZL cases were mainly of extranodal onset with the remaining subtypes primarily involving the lymph nodes. Almost all of the HL cases were nodal onset, similar to the report of Groves et al. [29]. The proportion of extranodal lymphoma in our study, nearly 58%, was higher compared with the US proportion of 27% [29]. On the other hand, 58% of cases were reported to be extranodal in a study conducted in Beijing, while a similar percentage of NHLs, ~54%, were extranodal in a previous report from Sichuan [4,30]. Differences in the geographical distribution of the extranodal lymphomas may be related to environmental factors, although other factors such as disease misclassification or missed diagnoses could also partially explain the findings.
Overall, our study suggests a high proportion of NKTCL cases that could explain the higher proportions of extranodal cases in Sichuan, and a low proportion of FL cases as compared with Western countries. In particular, the frequency of FL was only 2.3% of diagnosed lymphomas, which is lower than proportions reported in some other Chinese provinces such as Shanghai [7] and Shanxi [20], and also lower than in Western countries [25]. While these data provide an indication of geographic variation in lymphoma subtype patterns, a limitation is that we were unable to calculate age-adjusted incidence rates for specific subtypes in Sichuan because we did not have data on all cases diagnosed in the province. However, we believe that our case series is generalizable to that of overall Sichuan province given that we included the three primary hospitals that diagnose the majority of cases and also restricted the study population to cases who were current residents of Sichuan. Our findings suggest the need for additional etiological studies of lymphoma in the Chinese population to evaluate potential explanations for the geographic differences in the descriptive epidemiology.
Footnotes
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- 1.Muller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol 2005; 84(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 2.Forman D, Bray F, Brewster DH, et al. (eds). Cancer Incidence in Five Continents Vol. X (electronic version). IARC: Lyon, 2013. http://ci5.iarc.fr. last accessed Jan. 2, 2015. [Google Scholar]
- 3.Chen W, Zheng R, Zhang S, et al. Report of incidence and mortality in China cancer registries, 2009. ChinJ Cancer Res 2013; 25(1): 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang QP, Zhang WY, Yu JB, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol 2011; 6: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. American J Clinical Pathol 2012; 138(3): 429–434. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Song B, Fan T, et al. Pathological and clinical characteristics of 1,248 non-Hodgkin’s lymphomas from a regional cancer hospital in Shandong, China. Asian Pacific J Cancer Prevention 2011; 12(11): 3055–3061. [PubMed] [Google Scholar]
- 7.Gross SA, Zhu X, Bao L, et al. A prospective study of 728 cases of non-Hodgkin lymphoma from a single laboratory in Shanghai, China. Int J Hematol 2008; 88(2): 165–173. [DOI] [PubMed] [Google Scholar]
- 8.Xiao HB, Wang S. Analysis on pathological subtypes of 200 cases of malignant lymphoma in Hunan western Hunan area. Hainan Med 2008; 19(6): 137–139. [Google Scholar]
- 9.Wang JF, Wang YZ, Chen ZW, Taylor RC. Prevalence of lymphoma subtypes in Shanxi according to latest WHO classification. Chin J Pathol 2006; 35(4): 218–223. [PubMed] [Google Scholar]
- 10.Liang GZ, Zhuang H, Ruizhen G, et al. Distribution of various malignant lymphomas in Guizhou. J Clin Exp Pathol 1996; 12(4): 320–322. [Google Scholar]
- 11.Xiao C, Su ZL, Wu QL, et al. Clinical and pathological reassessment of 493 cases of non-Hodgkin’s lymphomas according to current WHO classification of lymphoid neoplasm. Chin J Pathol 2005; 34(1): 22–27. [PubMed] [Google Scholar]
- 12.Ko YH, Kim CW, Park CS, et al. REAL classification of malignant lymphomas in the republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Hematolymphoreticular Study Group of the Korean Society of Pathologists. Revised European-American lymphoma. Cancer 2008; 83(4): 806–812. [PubMed] [Google Scholar]
- 13.Aoki R, Karube K, Sugita Y, et al. Distribution of malignant lymphoma in Japan: analysis of 2260 cases, 2001–2006. Pathol Int 2008; 58(3): 174–182. [DOI] [PubMed] [Google Scholar]
- 14.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006; 107(1): 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Gong YP, Yang X, et al. Clinical characteristics of 1126 cases of malignant lymphoma. J Leukemia Lymphoma (Chinese) 2010; 19(5): 290–292. [Google Scholar]
- 16.Jaglowski SM, Liden E, Termuhlen AM, Flynn JM. Lymphoma in adolescents and young adults. Semin Oncol 2009; 36(5): 381–418. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Li GC, Zhang YL, Zhang SW, Yang NN. An analysis of the incidence and mortality with malignant lymphoma in China during 2003–2007. Chin Cancer 2012; 21(3): 190–196. [Google Scholar]
- 18.Bassig BA, Lan Q, Rothman N, Zhang Y, Zheng T. Current understanding of lifestyle and environmental factors and risk of non-Hodgkin lymphoma: an epidemiological update. J Cancer Epidemiol 2012; 2012: 978930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118(12): 3030–3044. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Young L, Win W, Taylor CR. Distribution and ZAP-70 expression of WHO lymphoma categories in Shanxi, China: a review of 447 cases using a tissue microarray technique. Appl Immunohistochem Mol Morphol 2005; 13(4): 323–332. [DOI] [PubMed] [Google Scholar]
- 21.Yang SE, Li X, Zhao B, Jia C, Zhang G. The clinical and pathological analysis of 1,012 cases of non-Hodgkins lymphoma. Chin JClin Oncol 2009; 36(24): 1412–1415. [Google Scholar]
- 22.Chen WL, Tsai WC, Chao TY, et al. The clinicopathological analysis of 303 cases with malignant lymphoma classified according to the World Health Organization classification system in a single institute of Taiwan. Ann Hematol 2010; 89(6): 553–562. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Chen H, Fu K, Zhu XZ, Irons R. Prevalence of germinal center B-cell-like and non-germinal center B-cell-like types of diffuse large B-cell lymphoma in Shanghai, China. Chin JPathol 2010; 39(5): 313–318. [PubMed] [Google Scholar]
- 24.Shiozawa E, Yamochi-Onizuka T, Takimoto M, Ota H. The GCB subtype of diffuse large B-cell lymphoma is less frequent in Asian countries. Leukemia Res 2007; 31(11): 1579–1583. [DOI] [PubMed] [Google Scholar]
- 25.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER 9 Regs Research Data, Nov 2013 Sub (1973-2011) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total U.S., 19692012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission. (www.seer.can-cer.gov).
- 26.Lan Q, Au WY, Chanock S, et al. Genetic susceptibility for chronic lymphocytic leukemia among Chinese in Hong Kong. Eur J Haematol 2010; 85(6): 492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton LM, Slager SL, Cerhan JR, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI 2014; 2014(48): 130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu JB, Li XQ, Zhang PH, et al. Nodal versus extranodal diffuse large B-cell lymphoma: comparison of clinicopathologic features, immunophenotype and prognosis. Chin J Pathol 2007; 36(7): 470–473. [PubMed] [Google Scholar]
- 29.Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: non-Hodgkin’s lymphoma incidence by histologic subtype in the United States from 1978 through 1995. JNCI 2000; 92(15): 1240–1251. [DOI] [PubMed] [Google Scholar]
- 30.Ji XL, Shen M. Extranodal lymphoma in China: an analysis of 943 cases. Chinese J Cancer 1999; 05: 570–572. [Google Scholar]