Abstract
Congenital heart defects are the most prevalent type of birth defects. The association of air pollution with congenital heart defects is not well understood. We investigated a cohort of 8,969 singleton live births in Lanzhou, China during 2010–2012. Using inverse distance weighting, maternal exposures to particulate matter with diameter ≤10μm (PM10), nitrogen dioxide (NO2), and sulfur dioxide (SO2) were estimated as a combination of monitoring station levels for the time spent at home and the work location. We used logistic regression to estimate the associations, adjusting for maternal age, education, income, BMI, disease, folic acid intake and therapeutic drug use, and smoking; season of conception; fuels for cooking; and temperature. We found significant positive associations of Patent Ductus Arteriosus (PDA) with PM10 during the 1st trimester, 2nd trimester and the entire pregnancy (OR 1st trimester=3.96, 95% Confidence Interval (CI): 1.36, 11.53; OR 2nd trimester=3.59, 95% Confidence Interval (CI): 1.57, 8.22; OR entire pregnancy=2.09, 95% CI: 1.21, 3.62, per interquartile range (IQR) increment for PM10 (IQR=71.2, 61.6, and 27.4 μg/m3 respectively)), and associations with NO2 during 2nd trimester and entire pregnancy (OR 2nd trimester= 1.92, 95% CI: 1.11, 3.34; OR entire pregnancy=2.32, 95% Cl: 1.14, 4.71, per IQR increment for NO2 (IQR=13.4 and 10.9 μg/m3 respectively)). The associations for congenital malformations of the great arteries and pooled cases showed consistent patterns. We also found positive associations for congenital malformations of cardiac septa with PM10 exposures in the 2nd trimester and the entire pregnancy, and SO2 exposures in the entire pregnancy. Results indicate a health burden from maternal exposures to air pollution, with increased risk of congenital heart defects.
Keywords: air pollution, birth defect, China, congenital heart defect, NO2, PM10, SO2
1. Introduction
A growing body of literature links maternal exposure to air pollution to adverse birth outcomes, including low birth weight, preterm births, intrauterine growth retardation and birth defects [1–5]. Birth defects are a leading cause of infant death and disability later in life [6]. The most prevalent group of birth defects, congenital heart defects, has the highest prevalence in Asia (i.e., 9.3 per 1,000 live births) [7]. However, no previous study has been conducted in Asia to investigate the relationship between congenital heart defects and air pollution, despite high pollution levels in this region. Studies on this issue were mainly conducted in western countries and provided inconsistent results [8–20], which might be due to differences in inclusion of subgroups of congenital heart defects, exposure window selection, exposure assessment, and adjustment for confounding factors. In light of the inconclusive association between ambient air pollution and congenital heart defects as well as the lack of studies in areas with both high prevalence of congenital heart defects and high air pollution levels, we conducted a study in Lanzhou, China to fill this gap. To address the challenges of lack of standardized methodology in previous literature, we included multiple pollutants and exposure windows, and conducted extensive sensitivity analyses to test the validity of our results. In addition, detailed information on residential mobility and other risk factors of congenital heart defects was available in our cohort to improve exposure and risk assessment.
Lanzhou, located in Northwest China, is the capital and largest city of Gansu Province, with area of 5058 mile2 and 3.6 million residences. It is also a transportation hub linking the East and the West of China. According to a 2011 World Health Organization report, Lanzhou was the most polluted city in China [21]. Sand storms, emissions from factories and traffic result in high levels of air pollution. Moreover, the city center is surrounded by mountains and hills that rise to 500–600 m. This trough-shaped topography traps air pollutants at the ground level, resulting in well-documented poor air quality [22]. Previous analysis of the characteristics of ambient air pollution in Lanzhou during the study period identified that PM10 concentrations exceeded health-based regulations and guidelines [23–25] and high levels of air pollution in Lanzhou were associated with increased risk of health outcomes such as hospital admissions for respiratory disease [26].
The objective of this study is to investigate whether maternal exposures to PM10, NO2, and SO2 are associated with elevated risks of congenital heart defects in Lanzhou, China. This study is the first to investigate the relationship between birth defects and ambient air pollution in China. We provide analysis of birth defects in relation to levels of ambient air pollution that are higher than many other locations, but relevant globally, especially in areas with rapid urbanization and expanding industry and transportation.
2. Materials and methods
2.1. Study population
We recruited 10,542 women who gave birth at Gansu Provincial Maternity and Child Care Hospital in Lanzhou, China in 2010–2012. The process of enrollment was described elsewhere [27]. Written consent was obtained and then in-person interviews were conducted by trained study interviewers at the hospital. By means of a standardized and structured questionnaire, women provided detailed information on demographics (i.e., residential history, education, family income, and access to prenatal health education), medical and reproductive history (e.g., any conditions of hypertension, coronary heart disease, diabetes, HBV infection; any previous pregnancy history) and lifestyle choices (e.g., active or passive smoking, alcohol drinking, tea consumption, diet, physical exercises and fuels for cooking). Birth outcomes and information on maternal complications (e.g., gestational age, birth weight, and defects; any gestational hypertension and preeclampsia) were abstracted from the medical records. Congenital heart defects (N=73) were ascertained if any of the following subtypes were confirmed: Q20-Q28 (International Classification of Diseases (ICD)-10 code). The cases were diagnosed shortly after birth.
Among the 10,542 women who completed the questionnaires, we excluded women who lived outside Lanzhou (n=1344), had multiple births (n=323), had infants with other birth defects (n=174) or had stillbirths (n=53). Then we excluded women living further than 50km to the closest air pollution monitor (n=127) [14]. Finally, 8,729 singleton live births were included in the analysis. Congenital heart defects were the most common type of birth defect in this cohort (prevalence: 8.4 per 1000 singleton live births). Infants included in the analyses (n=8,729) include 73 pooled cases (any type or multiple congenital heart defects), 54 congenital malformations of the great arteries, 19 congenital malformations of cardiac septa, 1 congenital malformations of cardiac chambers and connections, and 7 other congenital malformations of heart (Supplementary, Table S5).
2.2. Exposure assessment
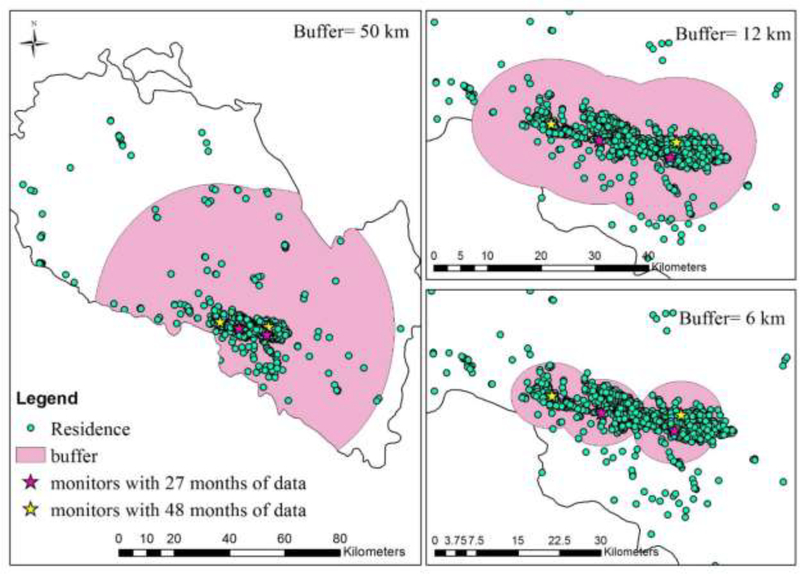
Daily average concentrations of ambient PM10, SO2, and NO2 were obtained from 4 air monitoring stations in Lanzhou, operated by Lanzhou Environmental Monitoring Bureau (Figure 1). These three pollutants were the only ambient air pollutants routinely measured in Lanzhou before 2013. Two monitoring stations, Xigu and Huanghebei, had 48 months of daily observations (April 2009- March 2013) in the study period. The other two stations, Xizhan and Tieluju, provided observations for 27 months (January 2011-March 2013). The monitoring data from the 2 monitors covering the full study period were used in the main analysis.
Figure 1.
Locations of Monitors, Distribution of Residences of Births and Buffers of 6, 12, and 50km from Monitors (n=8969).
The full residential history including the start and stop dates for each residence was obtained, so residential mobility was taken into account. If a woman had more than one address during an exposure window, the exposure estimates were weighted based on the number of days at each residence. Further we considered exposures at the location of work.
First, the date of conception was calculated based on the date of birth and gestational age. Daily average concentrations of air pollutants were spatially interpolated using inverse distance weighting (IDW) to assign daily exposures at each residence and work location. IDW is an interpolation method to assign pollution levels to unknown points based on known monitoring data. The following is the IDW formula for pollution levels at point (x, y): , where zi is the pollution levels at the ith monitor and di is the distance from the ith monitor to the point of interest (x, y). Then, exposures were time-weighted averaged as 2/3 of time at home and 1/3 of time at work. Daily exposures were averaged over four exposure windows: week 3–8; 1st, 2nd trimester; and entire pregnancy. Week 3–8 of pregnancy is often considered as a critical window during which the fetus is susceptible to the development of congenital heart defects [28]. The 1st and 2nd trimesters have been analyzed separately in previous studies investigating congenital anomalies and air pollution [15, 16, 29]. In summary, for each pregnancy, 12 variables were estimated for combinations of 3 pollutants (PM10, SO2, and NO2) and 4 exposure windows during pregnancy (week 3–8 of pregnancy, the 1st, 2nd trimester and entire pregnancy).
2.3. Statistical analysis
The associations between maternal exposures to air pollution and risk of congenital heart defects were estimated by logistic regression models. The outcome groups included in the models were pooled cases (ICD 10: Q20–28), congenital malformations of great arteries (ICD 10: Q25), congenital malformations of cardiac septa (ICD 10: Q21), and isolated cases of Patent Ductus Arteriosus (PDA) (ICD 10: Q250). PDA is the most prevalent subtype of congenital malformations of great arteries in our cohort. Exposure variables were included in the models as continuous variables, with separate models for each pollutant. We included the following covariates in the fully adjusted models: maternal age (<25, 25–30, 30–35, ≥35 years), maternal education (less than high school, finished high school, more than high school), monthly family income (<2000, 2000–4000, ≥4000 RMB), mother’s body mass index (BMI) (< 24, ≥24 kg/m3) [30], folic acid intake during the 1st trimester (yes/no), therapeutic drug intake (yes/no), maternal illness (yes/no), smoking status including passive and active smoking (yes/no), fuels for cooking (gas and electricity or not cooking/other), temperature and season of conception [spring (March-May), summer (June-August), fall (September-November), or winter (December-February)].
Periconceptional intake of folic acid containing multiple vitamins has been associated with reduced risk of congenital heart defects [31]. On the other hand, some therapeutic drugs might increase risk of birth defects if ingested during pregnancy [31]. A woman was considered to ingest therapeutic drugs if she took at least one category of the following drugs: thalidomide, antibiotics, antiviral agents, antifungal therapies, anticonvulsants, lithium, benzodiazepines, sympathomimetics, corticosteroids, nonsteroidal anti-inflammatory drugs, female hormones, or angiotension-converting enzyme inhibitors. Maternal illness was used as a binary variable to adjust for the overall health status of the mothers. The following diseases assessed by survey were considered: cardiovascular disease, respiratory diseases, renal diseases, thyroid diseases, anemia, HBV infection, and cirrhosis.
Extensive sensitivity analyses have been conducted to test the validity of our results. 1) To assess the influence of exposure at work, maternal exposures calculated only at home were included in the logistic regression models. 2) Children with other defects were included in the analyses. 3) We explored risks associated with exposure levels higher than Chinese national air quality standard [23]. The fraction of days exceeding the daily Chinese air quality standards were calculated for each woman during each exposure window. 4) We conducted the analyses for women who lived close to monitors (< 6 or 12 km of a monitor) (Figure 1). 5) Maternal exposures were divided into tertiles to explore the hypothesis of a linear exposure-response relationship. 6) We repeated main analyses using data from all 4 monitors, that is, daily exposures were calculated based on 2 monitors before January 1, 2011, and based on all 4 monitors for later days. All statistical analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, NC).
3. Results
3.1. Summary of air pollution exposure and the study subjects
The average concentrations of PM10, NO2, and SO2 were 143.8μg/m3, 41.6μg/m3, and 54.5μg/m3, respectively. Average air pollution concentrations from the 4 monitoring stations were similar, indicating low spatial heterogeneity of air pollution at the city level [26]. The correlations between the three pollutants from the 2 monitors covering the full study period were weak (Supplementary Table S1).
The average and IQR of maternal exposures to PM10, NO2, and SO2 during each exposure window are shown in Table 1. The IQR corresponds to the increments in the following results when air pollution exposures were included as continuous variables. Exposures during week 3–8 of pregnancy were highly correlated with exposures in the 1st trimester (Table 2). Exposures were significantly higher among the infants with than without congenital heart defects (p-value <0.05) (Table 3).
Table 1.
Summary Statistics of Daily Maternal Exposuresa in Lanzhou, 2009–2012 (μg/m3)
| Exposure windows | PM10 Mean (IQR) |
NO2 Mean (IQR) |
SO2 Mean (IQR) |
|---|---|---|---|
| Week 3–8 | 137.5 (79.8) | 44.2 (14.4) | 57.0 (57.6) |
| First trimester | 137.9 (71.2) | 44.1 (13.0) | 57.1 (56.1) |
| Second trimester | 142.3 (61.6) | 43.3 (13.4) | 59.0 (62.3) |
| Entire pregnancy | 140.0 (27.4) | 42.5 (10.9) | 56.4 (19.2) |
Abbreviations: IQR, interquartile range.
The exposures were estimated using the primary approach (i.e., 2 monitors covering the entire period of study). The exposures were estimated for women living within 50km of a monitor (n=8,729).
Table 2.
Correlation Coefficients Between Maternal Exposures During Different Exposure Windowsa.
| 1st trimester | 2nd trimester | Entire pregnancy | ||
|---|---|---|---|---|
| PM10 | Week 3–8 | 0.94 | 0.06 | 0.22 |
| 1st trimester | 0.15 | 0.30 | ||
| 2nd trimester | 0.79 | |||
| NO2 | Week 3–8 | 0.93 | −0.05 | 0.44 |
| 1st trimester | 0.07 | 0.53 | ||
| 2nd trimester | 0.67 | |||
| SO2 | Week 3–8 | 0.97 | −0.11 | −0.05 |
| 1st trimester | 0.02 | 0.05 | ||
| 2nd trimester | 0.79 |
The exposures were estimated for women living within 50km of a monitor (n=8,729) using 2 monitors covering the entire period of study.
Table 3.
Characteristics of Infants with and without congenital heart defects, Lanzhou, 2010–2012a.
| Characteristic | Infants without congenial heart defects (n=8656) | Pooled congenital heart defects (n=73) | Congenital malformations of great arteries (n=54)b | Congenital malformations of cardiac septa (n=19) |
|---|---|---|---|---|
| Infant’s gender | ||||
| Male | 4528 (52.3) | 40 (54.8 ) | 29 (53.7) | 13 (68.4) |
| Female | 4099 (47.4) | 32 (43.8) | 25 (46.3) | 6 (31.6) |
| Missing | 29 (0.3) | 1 (1.4) | 0 | 0 |
| Maternal age (years) | ||||
| ≤25 | 1788 (20.7) | 17 (23.3) | 16 (29.6) | 1 (5.3) |
| 25–30 | 4447 (51.4) | 37 (48.0) | 23 (42.6) | 14 (76.7) |
| 30–35 | 1853 (21.4) | 10 (13.7) | 7 (13) | 3 (15.8) |
| >35 | 568 (6.6) | 11 (15.1) | 8 (14.8) | 1 (5.3) |
| Maternal education | ||||
| Less than high school | 1502 (17.4) | 21 (28.8) | 15 (27.8) | 5 (26.3) |
| Finished high school | 1515 (17.5) | 13 (17.8) | 11 (20.4) | 3 (15.8) |
| More than high school | 5486 (63.4) | 38 (52.1) | 27 (50.0) | 10 (52.6) |
| Missing | 153 (1.8) | 1 (1.4) | 1 (1.9) | 1 (5.3) |
| Monthly family income (RMB)c | ||||
| <2000 | 1769 (20.4) | 20 (27.4) | 16 (19.6) | 6 (31.6) |
| 2000–4000 | 4166 (48.1) | 28 (38.4) | 18 (33.3) | 9 (47.4) |
| ≥4000 | 1893 (21.9) | 15 (20.6) | 12 (22.2) | 0 |
| Missing | 835 (9.6) | 10 (13.7) | 8 (14.8) | 4 (21.1) |
| Mother’s BMId (kg/m3) | ||||
| <24 | 7522 (86.9) | 59 (80.8) | 43 (79.6) | 16 (84.2) |
| ≥24 | 897 (10.4) | 10 (13.7) | 8 (14.8) | 2 (10.5) |
| Missing | 237 (2.7) | 4 (5.5) | 3 (5.6) | 0 |
| Maternal folic acid intake in the first trimester | ||||
| Yes | 5840 (67.5) | 41 (56.2) | 25 (46.3) | 10 (52.6) |
| No | 2816 (32.5) | 32 (43.8) | 29 (53.7) | 9 (47.4) |
| Maternal therapeutic drug intake during pregnancy | ||||
| Yes | 447 (5.2) | 7 (9.6) | 5 (9.3) | 3 (15.8) |
| No | 8206 (94.8) | 65 (89.0) | 49 (90.7) | 16 (84.2) |
| Missing | 3 (0.1) | 1 (1.4) | 0 | 0 |
| Maternal illness | ||||
| Yes | 230 (2.7) | 5 (6.9) | 4 (7.4) | 2 (10.5) |
| No | 8426 (97.3) | 68 (93.2) | 50 (92.6) | 17 (89.5) |
| Maternal smoking | ||||
| Yes | 13 (17.8) | 1630 (18.8) | 11 (20.4) | 0 |
| No | 60 (82.2) | 7026 (81.2) | 43 (79.6) | 19 (100) |
| Cooking fuel | ||||
| Gas and electricity or not cooking | 58 (79.5) | 7663 (88.5) | 43 (79.6) | 15 (79.0) |
| Other | 5 (6.9) | 347 (4.0) | 2 (3.7) | 1 (5.3) |
| Missing | 10 (13.7) | 646 (7.5) | 9 (16.7) | 3 (15.8) |
| Season of conception | ||||
| Spring | 13 (17.8) | 1814 (21.0) | 6 (11.1) | 4 (21.1) |
| Summer | 28 (38.4) | 2497 (28.9) | 22 (40.7) | 8 (42.1) |
| Fall | 22 (30.1) | 2367 (27.4) | 17 (31.5) | 5 (26.3) |
| Winter | 10 (13.7) | 1978 (22.9) | 9 (16.7) | 2 (10.5) |
| Pregnancy mobility | ||||
| Move at least once | 266 (3.1) | 2 (2.7) | 1 (1.8) | 1 (5.3) |
| Did not move | 8390 (96.9) | 71 (97.3) | 53 (98.2) | 18 (94.7) |
| Working during pregnancy | ||||
| Work | 3815 (44.1) | 31 (42.5) | 21 (38.9) | 9 (47.4) |
| Not work | 4841 (55.9) | 42 (57.5) | 33 (61.1) | 10 (52.6) |
| Gestational exposure | ||||
| PM10 | 139.9 (19.2) | 151.3 (23.8) | 152.6 (24.3) | 155.0 (24.2) |
| NO2 | 42.4 (6.9) | 44.9 (5.9) | 44.9 (5.6) | 45.5 (4.9) |
| SO2 | 56.4 (12.9) | 60.7 (12.9) | 61.2 (11.5) | 62.6 (11.1) |
Table values are n (column %) for categorical variables and mean (SD) for continuous variables. Percentages may not sum to 100% due to rounding. The primary analysis included women living within 50km of a monitor (n=8,729).
Congenital malformations of the great arteries is a subgroup of congenital heart defects. They include 52 cases with Patent ductus arteriosus, and 2 cases with both Patent ductus arteriosus and Stenosis of pulmonary artery.
1RMB~=0.16USD
BMI cut-off point of overweight is for people living in mainland China.
The characteristics of the study population are shown in Table 3. Distribution of maternal age, folic acid intake, therapeutic drug use, gestational weeks, and maternal illness before pregnancy were significantly different between infants without congenital heart defects and pooled cases (p-value <0.05). Mothers whose infants had any congenital heart defects were more likely to be older than 35 (15.1% vs. 6.6%), or have an illness (6.9% vs. 2.7%) than mothers whose infants did not have congenital heart defects. Folic acid intake during the 1st trimester was more common among mothers with healthy infants (67.5% vs. 56.4%). Therapeutic drug use during pregnancy was more common in women whose infants had any congenital heart defects (9.6% vs. 5.2%). Congenital malformations of the great arteries and cardiac septa had the similar patterns with pooled cases.
3.2. Associations between air pollution and congenital heart defects
The adjusted ORs (95% CI) for congenital heart defects associated with maternal exposure to air pollution are shown in Table 4. In the fully adjusted model, positive associations were observed between isolated PDA and an IQR increase in PM10 exposures during the 1st, 2nd trimester and the entire pregnancy (OR 1st trimester=3.96, 95% CI: 1.36, 11.53; OR 2nd trimester= 3.59, 95% CI: 1.57, 8.22; OR entire pregnancy=2.09, 95% CI: 1.21, 3.62); and an IQR increase in NO2 exposure during the 2nd trimester and entire pregnancy (OR 2nd trimester= 1.92, 95% CI: 1.11, 3.34; OR entire pregnancy=2.32, 95% Cl: 1.14, 4.71). No associations were found during week 3–8 of pregnancy. The results for congenital malformations of great arteries and pooled cases showed similar patterns. Congenital malformations of cardiac septa were positively associated with PM10 exposures during the 2nd trimester and the entire pregnancy (OR 2nd trimester= 5.51, 95% CI: 1.36, 22.35; OR entire pregnancy=2.70, 95% CI: 1.11, 6.57, for IQR increment), and with SO2 exposures during the entire pregnancy (OR entire pregnancy=5.16, 95% CI: 1.01, 26.26, for IQR increment).
Table 4.
Adjusted ORsa (95% CI) for Congenital Heart Defects Associated with IQRb Increases in Air Pollution Exposures During Week 3–8, the 1st, 2nd, 3rd Trimester, and Entire Pregnancyc in Lanzhou, 2010–2012.
| PM10 | NO2 | SO2 | ||
|---|---|---|---|---|
| Outcome groups | Exposure windows | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Pooled congenital heart defects (N=73) | Week 3–8 | 1.42 (0.70, 2.87) | 1.21 (0.78, 1.89) | 0.78 (0.28, 2.17) |
| 1st trimester | 3.10 (1.28, 7.51) | 1.49 (0.91, 2.44) | 0.54 (0.15, 2.02) | |
| 2nd trimester | 4.23 (2.11, 8.50) | 1.86 (1.18, 2.93) | 0.87 (0.16, 4.62) | |
| Entire pregnancy | 2.28 (1.45, 3.60) | 2.26 (1.26, 4.08) | 1.21 (0.51, 2.86) | |
| Congenital malformation of great arteries (N=54) | Week 3–8 | 1.83 (0.83, 4.03) | 1.18 (0.72, 1.94) | 0.89 (0.28, 2.84) |
| 1st trimester | 4.23 (1.53, 11.65) | 1.45 (0.83, 2.54) | 0.69 (0.26, 1.81) | |
| 2nd trimester | 4.00 (1.80, 8.87) | 1.99 (1.18, 3.36) | 0.87 (0.12, 6.22) | |
| Entire pregnancy | 2.33 (1.37, 3.97 ) | 2.16 (1.12, 4.18) | 1.27 (0.47, 3.48) | |
| Congenital malformation of cardiac septa (N=19) | Week 3–8 | 1.39 (0.35, 5.50) | 0.94 (0.36, 2.42) | 1.46 (0.22, 9.94) |
| 1st trimester | 2.43 (0.43, 13.90) | 0.95 (0.34, 2.63) | 0.84 (0.14, 4.91) | |
| 2nd trimester | 5.51 (1.36, 22.35) | 1.58 (0.65, 3.80) | 2.57 (0.10, 63.78) | |
| Entire pregnancy | 2.70 (1.11, 6.57) | 1.53 (0.52, 4.50) | 5.16 (1.01, 26.26) | |
| Isolated Cases of Patent Ductus Arteriosusd (N=46) | Week 3–8 | 1.72 (0.74, 4.00) | 1.27 (0.76, 2.13) | 0.68 (0.20, 2.39) |
| 1st trimester | 3.96 (1.36, 11.53) | 1.69 (0.94, 3.04) | 0.49 (0.10, 2.38) | |
| 2nd trimester | 3.59 (1.57, 8.22) | 1.92 (1.11, 3.34) | 0.60 (0.08, 4.71) | |
| Entire pregnancy | 2.09 (1.21, 3.62) | 2.32 (1.14, 4.71) | 0.86 (0.29, 2.53) | |
Adjusted for: maternal age, maternal education, monthly family income, mother’s body mass index, folic acid intake during the 1st trimester, therapeutic drug intake, any maternal diseases, smoking status including passive and active smoking, fuel for cooking, season of conception and temperature. For congenital malformations of cardiac septa, covariates of maternal age, income, smoking, and fuels for cooking have categories where the number of cases were ≤1. Therefore, the following changes were made for these covariates in the models: 1) smoking and fuels for cooking were excluded; 2) maternal age was categorized into two groups (≤30 and >30); 3) income was categorized into two groups (<2000 and ≥2000).
IQR: interquartile range of PM10, NO2, and SO2 are specific to each exposure window (Table 1).
The exposures were estimated for women living within 50km of a monitor (n=8,729) using 2 monitors covering the entire period of study.
Patent Ductus Arteriosus (PDA) is the most prevalent subtype of malformations of the great arteries in this cohort. Isolated cases of PDA are the cases with PDA only.
The analyses using exposures calculated only at home, rather than work and home, showed similar results (Table 5). The analyses including children with other defects showed similar results (Table 6). On average during entire pregnancy, Chinese daily air quality standards were exceeded for 32.3%, 6.2%, and 5.3% of days for PM10, NO2, and SO2, respectively. For isolated PDA, positive associations were found for the fraction of days with PM10 levels exceeding daily national standards during the 2nd trimester and the entire pregnancy (Table 7). During the entire pregnancy, for every 40.5% (IQR) increase in the fraction of days with PM10 levels exceeding 150 μg/m3, the odds of having an infant with PDA would be doubled (OR=2.00; 95% CI: 1.16, 3.45). For pooled cases and congenital malformations of great arteries, positive associations were found for the fraction of days with PM10 levels exceeding 150 μg/m3 during the 1st, 2nd, and entire pregnancy (Table 7).
Table 5.
Adjusted ORsa (95% CI) for Congenital Heart Defects Associated with IQRb Increases in Air Pollution Exposures at Homec in Lanzhou, 2010–2012
| PM10 | NO2 | SO2 | ||
|---|---|---|---|---|
| Outcome groups | Exposure windows | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Pooled congenital heart defects (N=73) | Week 3–8 | 1.43 (0.71, 2.88) | 1. 19 (0.80, 1.78) | 0.80 (0.29, 2.23) |
| 1st trimester | 3.02 (1.26, 7.25) | 1.47 (0.90, 2.40) | 0.77 (0.15, 4.02) | |
| 2nd trimester | 4.01 (2.00, 8.01) | 1.81 (1.16, 2.85) | 0.78 (0.15, 4.01) | |
| Entire pregnancy | 2.21 (1.40, 3.50) | 2.18 (1.22, 3.88) | 1.22 (0.53, 2.83) | |
| Congenital malformation of great arteries (N=54) | Week 3–8 | 1.83 (0.84, 4.02) | 1.16 (0.71, 1.91) | 0.89 (0.28, 2.83) |
| 1st trimester | 4.06 (1.49, 11.06) | 1.41 (0.81, 2.47) | 0.69 (0.27, 1.79) | |
| 2nd trimester | 3.81 (1.73, 8.41) | 1.95 (1.16, 3.27) | 0.76 (0.11, 5.28) | |
| Entire pregnancy | 2.26 (1.32, 3.87) | 2.08 (1.09, 3.98) | 1.24 (0.47, 3.31) | |
| Congenital malformation of cardiac septa (N=19) | Week 3–8 | 1.40 (0.36, 5.49) | 0.99 (0.39, 2.52) | 1.61 (0.24, 10.75) |
| 1st trimester | 2.44 (0.44, 13.62) | 0.99 (0.36, 2.71) | 0.89 (0.15, 5.11) | |
| 2nd trimester | 5.11 (1.28, 20.41) | 1.52 (0.64, 3.64) | 2.28 (0.10, 52.89) | |
| Entire pregnancy | 2.58 (1.05, 6.31) | 1.48 (0.51, 4.3) | 5.21 (1.06, 25.47) | |
| Isolated Cases of Patent Ductus Arteriosusd (N=46) | Week 3–8 | 1.73 (0.75, 4.00) | 1.26 (0.75, 2.10) | 0.69 (0.20, 2.39) |
| 1st trimester | 3.80 (1.32, 10.97) | 1.64 (0.92, 2.94) | 0.54 (0.19, 1.52) | |
| 2nd trimester | 3.42 (1.50, 7.80) | 1.88 (1.09, 3.26) | 0.52 (0.07, 4.01) | |
| Entire pregnancy | 2.02 (1.16, 3.53) | 2.22 (1.11, 4.44) | 0.86 (0.30, 2.45) | |
Adjusted for: maternal age, maternal education, monthly family income, mother’s body mass index, folic acid intake during the 1st trimester, therapeutic drug intake, any maternal diseases, smoking status including passive and active smoking, fuel for cooking, season of conception, and temperature. For congenital malformations of cardiac septa, covariates of maternal age, income, smoking, and fuels for cooking have categories where the number of cases were ≤1. Therefore, the following changes were made for these covariates in the models: 1) s moking and fuels for cooking were excluded; 2) maternal age was categorized into two groups (≤30 and >30); 3) income was categorized into two groups (<2000 and ≥2000).
IQR: interquartile range of PM10, NO2, and SO2are specific to each exposure window (Table 1).
The exposures were estimated for women living within 50km of a monitor (n=8,729) using 2 monitors covering the entire period of study.
Patent Ductus Arteriosus (PDA) is the most prevalent subtype of malformations of the great arteries in this cohort. Isolated cases of PDA are the cases with PDA only.
Table 6.
Adjusted ORsa (95% CI) for Congenital Heart Defects Associated with IQRb Increases in Air Pollution Exposures During Week 3–8, the 1st, 2nd, 3rd Trimester, and Entire Pregnancyc Including Children with Other Defects in Lanzhou, 2010–2012.
| PM10 | NO2 | SO2 | ||
|---|---|---|---|---|
| Outcome groups | Exposure windows | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Pooled congenital heart defects (N=73) | Week 3–8 | 1.42 (0.70, 2.88) | 1.19 (0.79, 1.78) | 0.77 (0.28, 2.17) |
| 1st trimester | 3.09 (1.27, 7.50) | 1.49 (0.91, 2.46) | 0.56 (0.23, 1.37) | |
| 2nd trimester | 4.21 (2.10, 8.45) | 1.86 (1.18, 2.93) | 0.87 (0.16, 4.67) | |
| Entire pregnancy | 2.32 (1.46, 3.70) | 2.26 (1.26, 4.07) | 1.20 (0.50, 2.84) | |
| Congenital malformation of great arteries (N=54) | Week 3–8 | 1.84 (0.83, 4.05) | 1.17 (0.71, 1.93) | 0.88 (0.27, 2.84) |
| 1st trimester | 4.23 (1.53, 11.68) | 1.45 (0.83, 2.53) | 0.69 (0.26, 1.81) | |
| 2nd trimester | 3.98 (1.80, 8.83) | 1.99 (1.18, 3.36) | 0.87 (0.12, 6.25) | |
| Entire pregnancy | 2.32 (1.36, 3.95) | 2.15 (1.11, 4.16) | 1.26 (0.46, 3.43) | |
| Congenital malformation of cardiac septa (N=19) | Week 3–8 | 1.40 (0.35, 5.53) | 0.94 (0.36, 2.42) | 1.46 (0.21, 9.98) |
| 1st trimester | 2.44 (0.43, 13.92) | 0.95 (0.34, 2.64) | 0.85 (0.14, 4.93) | |
| 2nd trimester | 5.49 (1.36, 22.21) | 1.58 (0.65, 3.81) | 2.61 (0.10, 64.75) | |
| Entire pregnancy | 2.69 (1.11, 6.56) | 1.53 (0.52, 4.50) | 5.12 (1.01, 25.99) | |
| Isolated Cases of Patent Ductus Arteriosusd (N=46) | Week 3–8 | 1.72 (0.74, 4.02) | 1.27 (0.76, 2.12) | 0.68 (0.19, 2.38) |
| 1st trimester | 3.97 (1.36, 11.59) | 1.69 (0.94, 3.03) | 0.48 (0.10, 2.36) | |
| 2nd trimester | 3.58 (1.56, 8.19) | 1.92 (1.11, 3.34) | 0.59 (0.07, 4.73) | |
| Entire pregnancy | 2.08 (1.20, 3.60) | 2.31 (1.14, 4.68) | 0.85 (0.29, 2.49 ) | |
Adjusted for: maternal age, maternal education, monthly family income, mother’s body mass index, folic acid intake during the 1st trimester, therapeutic drug intake, any maternal diseases, smoking status including passive and active smoking, fuel for cooking, season of conception and temperature. For congenital malformations of cardiac septa, covariates of maternal age, income, smoking, and fuels for cooking have categories where the number of cases were ≤1. Therefore, the following changes were made for these covariates in the models: 1) smoking and fuels for cooking were excluded; 2) maternal age was categorized into two groups (≤30 and >30); 3) income was categorized into two groups (<2000 and ≥2000).
IQR: interquartile range of PM10, NO2, and SO2 are specific to each exposure window (Table 1).
The exposures were estimated for women living within 50km of a monitor (n=8,839) using 2 monitors covering the entire period of study.
Patent Ductus Arteriosus (PDA) is the most prevalent subtype of malformations of the great arteries in this cohort. Isolated cases of PDA are the cases with PDA only.
Table 7.
Adjusted ORs for Congenital Heart Defects Associated with IQRa Increase in Fraction of Days Exceeding Daily National Standards During Each Exposure Window for PM10, NO2, and SO2 (150.0 μg/m3 for PM10, 80.0 μg/m3 for NO2, 150.0 μg/m3 for SO2 )b
| PM10 | NO2 | SO2 | ||
|---|---|---|---|---|
| Outcome groups | Exposure windows | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Pooled congenital heart defects (N=73) | Week 3–8 | 1.10 (0.51, 2.40) | 1.12 (0.87, 1.44) | 1.34 (0.97, 1.84) |
| 1st trimester | 2.53 (1.07, 5.99) | 1.38 (0.99, 1.94) | 1.26 (0.63, 2.52) | |
| 2nd trimester | 6.16 (2.54, 14.95) | 1.25 (0.97, 1.61) | 0.85 (0.54, 1.34) | |
| Entire pregnancy | 2.28 (1.44, 3.59) | 1.79 (0.99, 3.25) | 1.20 (0.67, 2.17) | |
| Congenital malformation of great arteries (N=54) | Week 3–8 | 1.48 (0.60, 3.66) | 1.05 (0.80, 1.38) | 1.09 (0/.83, 1.43) |
| 1st trimester | 3.39 (1.23, 9.35) | 1.25 (0.87, 1.80) | 0.89 (0.52, 1.52) | |
| 2nd trimester | 5.41 (1.94, 15.07) | 1.28 (0.95, 1.73) | 0.90 (0.48, 1.70) | |
| Entire pregnancy | 2.88 (1.34, 3.88) | 1.47 (0.74, 2.93) | 0.95 (0.46, 1.93) | |
| Congenital malformation of cardiac septa (N=19) | Week 3–8 | 1.43 (0.31, 6.49) | 1.01 (0.59, 1.73) | 1.39 (0.45, 4.28) |
| 1st trimester | 3.13 (0.59, 16.65) | 1.04 (0.52, 2.09) | 1.59 (0.59, 4.24) | |
| 2nd trimester | 5.50 (0.97, 31.07) | 1.24 (0.75, 2.05) | 0.58 (0.18, 1.86) | |
| Entire pregnancy | 2.95 (1.19, 7.32) | 1.39 (0.45, 4.28) | 3.31 (1.26, 8.69) | |
| Isolated Cases of Patent Ductus Arteriosusc (N=46) | Week 3–8 | 1.27 (0.49, 3.30) | 1.07 (0.81, 1.42) | 1.05 (0.77, 1.42) |
| 1st trimester | 2.53 (0.88, 7.29) | 1.34 (0.91, 1.96) | 0.79 (0.43, 1.45) | |
| 2nd trimester | 4.92 (1.68, 14.44) | 1.29 (0.92, 1.80) | 0.88 (0.45, 1.69) | |
| Entire pregnancy | 2.00 (1.16, 3.45) | 1.58 (0.76, 3.24) | 0.81 (0.37, 1.76) | |
The IQR of fraction of days exceeding daily national standards during week 3–8, 1st, 2nd trimester, and entire pregnancy for PM10 are 45.2%, 40.5%, 34.5%, 13.1%. The IQR for NO2 during the above exposure windows are 9.5%, 9.5%, 8.3%, 9.4%. The IQR for SO2 during the above exposure windows are 7.1%, 9.5%, 10.7%, 3.4%.
The exposures were estimated for women living within 50km of a monitor (n=8,729) using 2 monitors covering the entire period of study.
Patent Ductus Arteriosus (PDA) is the most prevalent subtype of malformations of the great arteries in this cohort. Isolated cases of PDA are the cases with PDA only.
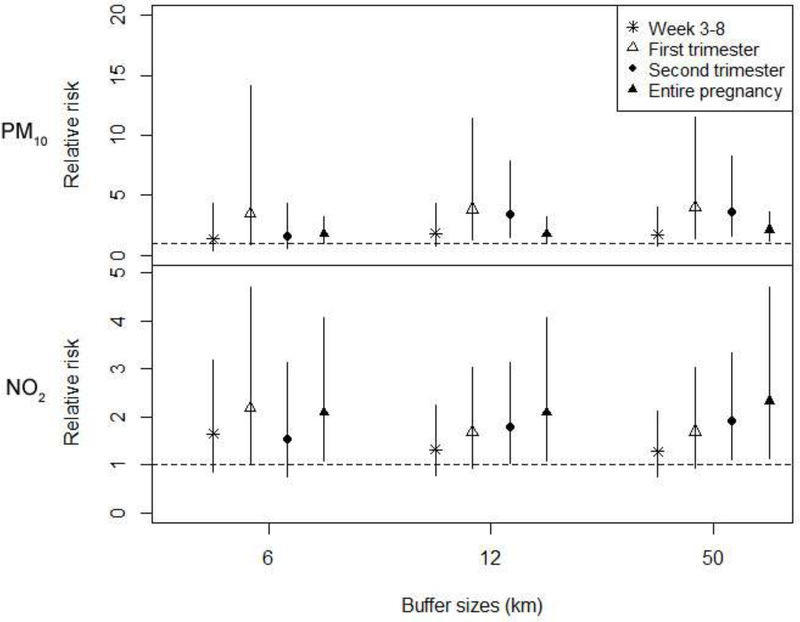
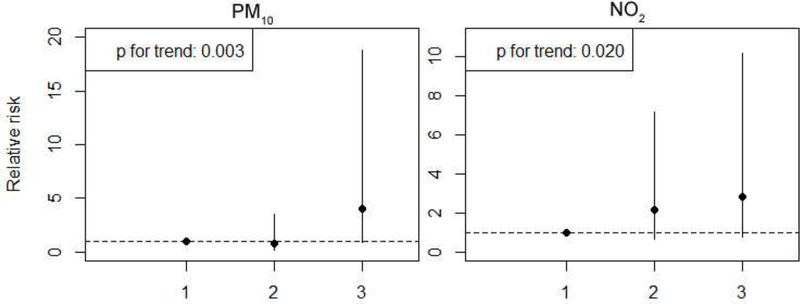
Most of the women lived within 12km of a monitor. The number of cases and total births living within 6, 12, and 50 km of a monitor, and the distribution of distance between residence and the closest monitor are shown in Supplementary Table S3. The sensitivity analyses for women living within 6 or 12km of a monitor showed consistent results (results for isolated PDA shown in Figure 2). Linear exposure-response patterns were confirmed between isolated PDA and PM10 and NO2 exposures during the 2nd trimester (p for trend <0.05) (Figure 3). The sensitivity analyses using monitoring data from all 4 monitors showed consistent results (Supplementary Table S2).
Figure 2.
Adjusted ORs (95% CI) for Isolated Cases of Patent Ductus Arteriosus Associated with IQR increases in PM10 and NO2 exposure during each exposure window for women living within 6 km, 12 km, and 50km of a monitor.
Note: The exposures were estimated using 2 monitors covering the entire period of study.
Figure 3.
Adjusted ORs (95% CI) for Isolated Cases of Arteries Defects Associated with Tertiles of Air Pollution Exposures During the 2nd trimester.
Note: The point reflects central estimates; vertical lines represent 95% confidence intervals. Estimates represent the risk of congenital heart defects for pollutant exposures in a given tertile compared to the first (lowest) tertile. The exposures were estimated for women living within 50km of a monitor (n=8729) using 2 monitors covering the entire period of study. The 2nd trimester was chosen because we found the associations in this trimester were stronger for PM10 and NO2 (Table 4).
4. Discussion
In this birth cohort study, we found positive associations of PM10 and NO2 exposures during the 2nd and entire pregnancy with isolated PDA, congenital malformations of great arteries, and pooled cases in Lanzhou, China in 2009–2012. The association for PM10 exposures in the 1st trimester was also significant. We did not find significant associations for exposures during week 3–8 of pregnancy. Results from sensitivity analyses were consistent.
Mechanisms of teratogenicity of air pollutants remain unclear, but several hypotheses have been proposed for effects of air pollutants on fetal development, including oxidative stress, pulmonary and placental inflammations, increased blood coagulation and viscosity, and oxidation of lipids and proteins by NO2, as reviewed by Polichetti et al. [2]. Congenital anomalies may share the similar etiological pathway with early fetal growth [1].
Our results were consistent with a meta-analysis on air pollution and congenital anomalies, which found positive PM10 associations with coarctation of the aorta (CoA) (a type of congenital malformations of the great arteries), Ventricular Septal Defects (VSD) and Atrial Septal Defects (ASD) (types of congenital malformations of cardiac septa); and associations of NO2 with CoA [1]. We also compared our results with 13 previous original epidemiologic studies investigating the relationship between air pollution exposures and congenital heart defects (Table 8). These studies were identified by searching three scientific literature databases: Web of Science, Scopus, and PubMed [32–34]. Last search was conducted on March 27, 2015. Searches were performed for the following terms: 1) at least one of the following: “air pollution”, “air pollutant”, “air pollutants”, “particulate matter”, PM10, SO2, or NO2; and 2) “heart defect*” or “cardiac defect*”, where * represents any combination of characters. Studies only investigating combined congenital anomalies as a composite outcome variable were not included in this table.
Table 8.
Comparisons Between This Study and Previous Studies Investigating the Effects of Air Pollution Exposure on Congenital Heart Defects.
| Study location (time period) | Study design (No. of births) | Pollutants studied | Exposure window | Air pollutant exposure estimation methods | Regression models (No. of confounders) | Main results related to congenital heart defects | Study |
|---|---|---|---|---|---|---|---|
| Lanzhou, China (2009–2012) | Cohort (8969) | PM10, NO2, SO2 | week 3–8; 1st trimester; entire pregnancy | Inverse distance weighting to estimate levels at work and residence. Accounted for residential mobility. | Logistic regression (12) | Significant associations for: PM10 exposures during 1st trimester and entire pregnancy and pooled cases; NO2 exposures during entire pregnancy with pooled cases. | This study |
| Southern California, US (1987–1993) | Case-control (14,198) | CO, NO2, O3, PM10 | 1st, 2nd, and 3rd month; 2nd and 3rd trimester; 3-month period prior to conception | Assigned most relevant monitor to each zip code of maternal residence | Logistic regression (10) | Does-response patterns for: 2nd-month CO and ventricular septal defects; 2nd month O3 exposure and aortic artery and valve defects, pulmonary artery and valve anomalies, and conotruncal defects. | Ritz et al. (2002) |
| Texas, US (1997–2000) | Population-based case-control (7381) | CO, NO2, O3, PM10 | week 3–8 | Closest monitor | Logistic regression (14) | Significant associations for: CO and tetralogy of Fallot; PM10 and isolated atrial septal defects; SO2 and isolated ventricular septal defects. | Gilboa et al. (2005) |
| Georgia, US (1986–2003) | Cohort (715,500) | CO, NO2, O3, PM10, SO2 | week 3–7 | Central monitoring station | Poisson generalized linear models (2) | Significant association for PM10 and patent ductus arteriosus. | Strickland et al. (2009) |
| Brisbane, Australia (1998–2004) | Case-control (150,308) | CO, NO2, O3, PM10, SO2 | week 3–8 | Closest monitor | Conditional logistic regression (7) | No association between air pollution and cardiac defects. | Hansen et al. (2009) |
| Northern Health Region, UK (1985–1990) | Case-control (245,825) | Black smoke, SO2 | 1st trimester | Average from monitors within 10 km of residence | Logistic regression (3) | Significant negative association for SO2 and congenital heart disease. | Rankin et al. (2009) |
| Northeast England, UK (1985–1996) | Case-control (12,688) | Black smoke, SO2 | week 3–8 | Estimation of weekly exposure using 2-stage spatiotemporal model | Logistic regression (5) | Significant association for BS and congenital malformations of cardiac chambers and connections. | Dadvand et al. (2010) |
| Northeast England, UK (1993–2003) | Case-control (19,036) | CO, NO2, O3, PM10, SO2, NO | week 3–8 | Closest monitor to residential postcode | Logistic regression (5) | Significant associations for: CO and NO and ventricular septal defect and cardiac septa malformations; CO and congenital pulmonary valve stenosis; NO and pooled cases. | Dadvand et al. (2011) |
| UK (1991–1999) | Case-control (759,993) | NO2, PM10, SO2 | 20 weeks | Annual mean at census ward level | Poisson regression (3) | Significant association for SO2 and tetralogy of Fallot. | Dolk et al. (2009) |
| Tel-Aviv region, Israel (2000–2006) | Case-control (135,527) | CO, NO2, O3, PM10, PM2.5, SO2 | week 3–8 | Weekly estimates based on inverse distance weighting | Logistic regression (8) | Significant association for PM10 and pooled cases. Significant inverse association for PM2.5 and isolated patent ductus arteriosus. | Agay-Shay et al. (2013) |
| San Joaquin Valley, CA, US (1997–2006) | Population-based case-control (1,671) | CO, NO, NO2, PM10, PM2.5, O3 | first 2 months | Inverse distance-squared weighting; traffic density indicators representing traffic counts within 300m of early pregnancy residence. Accounted for residential mobility. | Logistic regression (3) | Significant associations for: PM10 with pulmonary valve stenosis and perimembranous ventricular septal defects; PM2.5 and transposition of the great arteries; traffic density with muscular ventricular septal defects and perimembranous ventricular septal defects. Inverse associations for: PM2.5 and perimembranous ventricular septal defects; CO and secundum atrial septal defects. | Padula et al. (2013) |
| Barcelona, Spain (1994–2006) | Case control (5,238) | NOx, NO2, PM10, PM2.5, PM10–2.5, PM2.5 absorbance | week 3–8 | Land use regression models. | Logistic regression (4) | Significant association for NO2 and coarctation of the aorta. | Schembari et al. (2014) |
| Nine states, US (1997–2006) | Case control (7,960) | CO, NO2, O3, SO2, fine and coarse PM | week 2–8 | Closet monitor within 50 km to residence | Hierarchical regression (3) | Positive associations for: NO2 and coarctation of the aorta and pulmonary valve stenosis; fine PM and hypoplastic left heart syndrome. Negative association for fine PM and atrial septal defects. | Stingone et al. (2014) |
| Texas (2002–2006) | Population-based case-control (1,423,483) | PM2.5, O3 | 1st trimester | Hierachical Bayesian model combining data from air monitors with modeled air pollution estimates from CMAQ. Accounted for residential mobility for NBDPS database. | Logistic regression (5) | Significant inverse association for O3 and septal heart defects. | Vinikoor-Imler et al. (2015) |
The ORs for isolated PDA (the most prevalent subtype of arteries malformations in our cohort) were reported in three studies [8, 15, 19]. One study found a positive association for PM10, consistent with our results [19]. Coarctation of aorta, another subtype of arteries malformations, was found to be positively associated with NO2 and SO2 [13, 17, 18]; this subtype was not found in our cohort. Pooled arteries defects were investigated in five studies, with none finding significant associations, which is not consistent with our result [9–13]. Pooled septal defects were investigated in ten previous studies [8–14, 16, 18, 19], which found positive associations for PM10 with ASD, and perimembranous VSD [12, 14, 18]. This is consistent with our result during the entire pregnancy. However, septal defects had a small sample size (14 with complete covariate information). The ORs for pooled cases were reported in six studies [8, 9, 11, 15–17]. Our results are consistent with a study in Israel [8], which also found positive associations for PM10; however, no studies showed positive associations for NO2 or SO2. In fact, SO2 was found to have protective effects in two studies [9, 15].
Air pollution levels in Lanzhou were substantially higher than levels in Atlanta (US), Brisbane (Australia), Barcelona (Spain), four regions in England, and the Tel-Aviv region in Israel, except for NO2 in Barcelona [8, 11, 13, 17, 19]. PDA is the most prevalent subtype in our cohort (52.7 per 10,000), which was higher than that reported in previous studies (Texas, US: 2.5 per 10,000; four regions, UK, 4.6 per 10,000; the Tel-Aviv region, Israel: 12.6 per 10,000; Guangdong Province, China: 20.1 per 10,000) [8, 15, 19, 35]. These differences might contribute to the variation in results.
We found positive associations for PM10 with isolated PDA, pooled arteries defects, and pooled cases during 1st and 2nd trimester, supported by extensive sensitivity analyses. This is consistent with a Korean study finding the same pattern for pooled congenital anomalies [29]. However, we did not find positive associations during week 3–8. One possible explanation was that cardiac lesions or defects observed in our cohort were those more likely to be developed in later stage of fetal development. Although severe and complex cardiac malformations develop during first 6 to 7 weeks, growing evidence suggests that other defects may occur or progress in utero with advancing gestational age [36–38]. A recent study analyzing scans of the hearts of healthy fetuses found that the heart has distinct left and right chambers by eighth week of pregnancy, but does not have fully organized muscle tissue until the 20th week [38]. The most prevalent cases in this cohort, PDA, might be related to exposure to air pollution later in pregnancy. Under normal condition, ductus arteriosus constricts immediately after birth so that infant lungs could supply oxygen after umbilical cord is cut [39]. However, ductus arteriosus could fail to achieve permanent closure due to abnormal levels of agents (e.g., glucocorticoids) regulating the initial contraction of ductal muscle, especially among preterm births [39, 40]. In our cohort, preterm birth was found to be positively associated with higher PM10 during entire and late course of pregnancy [41]. Other prevalent subtypes in our cohort, including VSD and Stenosis of pulmonary artery, might also develop or progress later in pregnancy [36, 37]. Additional research is needed to further investigate the physiological pathways through which air pollution affects birth defects for different types of malformations and timing of exposure during pregnancy. The exposure timeframe most relevant may vary by birth defect, and is challenging to study given correlations among exposures. For example, in this study exposure in week 3–8 and the first trimester were highly correlated (Table 2), although results were somewhat different (Table 4).
To our knowledge, this is the first study investigating the associations of air pollution with congenital heart defects in Asia. One advantage of this study is the detailed residential history, work address, and lifestyle choices reported by the women. Most studies of air pollution and pregnancy outcomes estimate exposure based on residence at time of birth, which introduces exposure misclassification as women move during pregnancy [1, 42, 43]. Residential mobility was only considered in one previous study on congenital heart defects and air pollution, which did not report the improvement due to incorporating residential mobility [14]. The percentage of women who moved during pregnancy in our study is 3.1%, which is lower than the ~30% reported in the Western World [2]. Considering residential mobility might not have significant influence on the exposure estimation in this study. This is the first study considering maternal exposures at work locations on this topic. In general, central effect sizes for PM10 and NO2 estimated only using exposures at home were slightly smaller than using exposures at both home and work. However, in general the 95% CI are reduced slightly when considering the home address only. Although we incorporated exposure at work locations in the exposure assessment, in an attempt to improve the exposure estimate, the actual exposure is based on time at work, home, shopping, etc. Berkson error in the assessment of exposure (i.e. error that is statistically independent from the observed variable) can occur if our exposure estimates include part of the true exposure, whereas classical measurement error, that includes the true exposure as well as noise [44]. Berkson error typically results in an unbiased but more variable health effect estimates, whereas classical error can result in biased estimates with incorrect estimates of standard error [45]. Additional research is needed to better understand potential sources of exposure misclassification and their impacts on results. For example, we did not account for exposure during commuting and our study lacks information on activity patterns. The linear distance between home and work averaged 7.5km (IQR: 6.6 km). There was no significant difference in the distance from home to work between infants with and without congenital heart defects (p-value: 0.82). Therefore, misclassification due to time spent commuting might have minimal impact on results. Future studies could investigate other factors influencing maternal exposures during commuting, such as means of transportation.
This is also the first study to control for therapeutic drug intake during pregnancy, a known risk factor for congenital heart defects [31]. Other confounders, including maternal folic acid intake, illness, smoking and household cooking fuel, were also seldom considered in previous studies. The percentage of women smoking during pregnancy in Lanzhou (20%) did not differ significantly between infants with and without congenital heart defects. This is consistent with previous studies in the US, which reported comparable or smaller percentage of women smoking (<19%) [12, 14, 18, 20]. However, one study in Barcelona reported higher percentage of women smoking during pregnancy (42%) and found positive associations for smoking with pooled cases [17].
On the other hand, the accuracy of exposure estimation might be limited by the lack of monitors in rural areas in Lanzhou city. However, more than 95% of the women lived within 10km of a monitor. Sensitivity analysis using the data from all 4 monitors and using women living within 6 or 12km of a monitor all showed consistent results. Compared to some previous studies, the sample size in our study was smaller, which might lead to less statistical power, although the detail provided by the cohort study allows better exposure estimation and adjustment of covariates. Another limitation is the lack of information on congenital anomalies for termination of pregnancies.
Studies with data from more monitoring stations and more types of air pollutants are needed to fully understand the impacts of the air pollution mixture on congenital heart defects in China, and such studies may be possible as the air pollution monitoring system has become more extensive in China since 2013. Future studies in Asia and elsewhere should analyze the subtypes of congenital heart defects because they are anatomically, clinically and epidemiologically heterogeneous [46].
In summary, we found significant associations between gestational exposures to PM10 and NO2 and congenital heart defects risk after controlling for demographic variables, maternal health status and maternal lifestyle choices. This present study is the first on this issue conducted in Asia, where air pollution levels and prevalence of congenital heart defects are both high. These findings contribute to the modest body of scientific literature regarding the impacts of air pollution on birth defects.
Supplementary Material
Acknowledgements
This work was supported by internal funding from the Gansu Provincial Maternity and Child Care Hospital; Gansu Provincial Science and Technology Department Grant [1204WCGA021]; and the National Institutes of Health [K02HD70324, R01ES019587].
We thank all the study personnel from the Gansu Provincial Maternity and Child Care Hospital for their exceptional efforts on study subject recruitment, and Dr. Joshua Warren of Yale University.
References
- 1.Vrijheid M, et al. , Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ Health Perspect, 2011. 119(5): p. 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polichetti G, et al. , Effects of Ambient Air Pollution on Birth Outcomes: An Overview. Critical Reviews in Environmental Science and Technology, 2013. 43(12): p. 1223–1245. [Google Scholar]
- 3.Sram RJ, et al. , Ambient air pollution and pregnancy outcomes: A review of the literature. Environmental Health Perspectives, 2005. 113(4): p. 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glinianaia SV, et al. , Particulate air pollution and fetal health a systematic review of the epidemiologic evidence. Epidemiology, 2004. 15(1): p. 36–45. [DOI] [PubMed] [Google Scholar]
- 5.Maisonet M, et al. , A review of the literature on the effects of ambient air pollution on fetal growth. Environmental Research, 2004. 95(1): p. 106–115. [DOI] [PubMed] [Google Scholar]
- 6.Mathews TJ and MacDorman MF, Infant mortality statistics from the 2007 period linked birth/infant death data set. Natl Vital Stat Rep, 2011. 59(6): p. 1–30. [PubMed] [Google Scholar]
- 7.van der Linde D, et al. , Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol, 2011. 58(21): p. 2241–7. [DOI] [PubMed] [Google Scholar]
- 8.Agay-Shay K, et al. , Air pollution and congenital heart defects. Environmental Research, 2013. 124: p. 28–34. [DOI] [PubMed] [Google Scholar]
- 9.Dadvand P, et al. , Ambient air pollution and congenital heart disease: A register-based study. Environmental Research, 2011. 111(3): p. 435–441. [DOI] [PubMed] [Google Scholar]
- 10.Dadvand P, et al. , Association Between Maternal Exposure to Ambient Air Pollution and Congenital Heart Disease: A Register-based Spatiotemporal Analysis. American Journal of Epidemiology, 2011. 173(2): p. 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolk H, et al. , Ambient air pollution and risk of congenital anomalies in England, 1991–1999. Occupational and Environmental Medicine, 2010. 67(4): p. 223–227. [DOI] [PubMed] [Google Scholar]
- 12.Gilboa SM, et al. , Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. American Journal of Epidemiology, 2005. 162(3): p. 238–252. [DOI] [PubMed] [Google Scholar]
- 13.Hansen CA, et al. , Ambient Air Pollution and Birth Defects in Brisbane, Australia. Plos One, 2009. 4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padula AM, et al. , Ambient Air Pollution and Traffic Exposures and Congenital Heart Defects in the San Joaquin Valley of California. Paediatric and Perinatal Epidemiology, 2013. 27(4): p. 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rankin J, et al. , Maternal exposure to ambient air pollutants and risk of congenital anomalies. Environmental Research, 2009. 109(2): p. 181–187. [DOI] [PubMed] [Google Scholar]
- 16.Ritz B, et al. , Ambient air pollution and risk of birth defects in southern California. American Journal of Epidemiology, 2002. 155(1): p. 17–25. [DOI] [PubMed] [Google Scholar]
- 17.Schembari A, et al. , Traffic-Related Air Pollution and Congenital Anomalies in Barcelona. Environmental Health Perspectives, 2014. 122(3): p. 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stingone JA, et al. , Maternal exposure to criteria air pollutants and congenital heart defects in offspring: results from the national birth defects prevention study. Environ Health Perspect, 2014. 122(8): p. 863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strickland MJ, et al. , Ambient Air Pollution and Cardiovascular Malformations in Atlanta, Georgia, 1986–2003. American Journal of Epidemiology, 2009. 169(8): p. 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinikoor-Imler LC, et al. , An exploratory analysis of the relationship between ambient ozone and particulate matter concentrations during early pregnancy and selected birth defects in Texas. Environ Pollut, 2015. 202: p. 1–6. [DOI] [PubMed] [Google Scholar]
- 21.WHO, Database: outdoor air pollution in cities. 2011.
- 22.Ta W, et al. , Gaseous and particulate air pollution in the Lanzhou Valley, China. Sci Total Environ, 2004. 320(2–3): p. 163–76. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Environmental Protection of the People’s Republic of China.(2012)National ambient air quality standards in China.
- 24.U.S. Environmental Protection Agency. (2012) National Ambient Air Quality Standards. http://www.epa.gov/air/criteria.html Accessed 30 March 2015.
- 25.WHO. (2006). Air quality guidelines for particulate matter, ozone, nitrogen dioxide, and sulfur dioxide. Geneva: WHO. [PubMed] [Google Scholar]
- 26.Zhang Y, M.L., Bravo MA, Jin L, Nori-Sarma A, Xu Y, Guan D, Wang C, Chen M, Wang X, Tao W, Qiu W, Zhang Y, Bell ML., Air quality in Lanzhou, a major industrial city in China: Characteristics of air pollution and review of existing evidence on air pollution and health. Water, Air, & Soil Pollution, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J, et al. , Passive smoking and preterm birth in urban China. Am J Epidemiol, 2014. 180(1): p. 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opitz JM and Clark EB, Heart development: an introduction. Am J Med Genet, 2000. 97(4): p. 238–47. [PubMed] [Google Scholar]
- 29.Kim OJ, et al. , PM10 and pregnancy outcomes: a hospital-based cohort study of pregnant women in Seoul. J Occup Environ Med, 2007. 49(12): p. 1394–402. [DOI] [PubMed] [Google Scholar]
- 30.Barba C, et al. , Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet, 2004. 363(9403): p. 157–163. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins KJ, et al. , Noninherited risk factors and congenital cardiovascular defects: Current knowledge a scientific statement from the American Heart Association Council on cardiovascular disease in the young. Circulation, 2007. 115(23): p. 2995–3014. [DOI] [PubMed] [Google Scholar]
- 32.Scientific T, Web of knowledge. 2014.
- 33.Medicine, U.S.N.L.o., PubMed. 2014.
- 34.SciVerse, Scopus. 2012.
- 35.Liu XQ, et al. , Current prevalence rate of congenital heart disease in 12 month-old and younger infants among four regions of Guangdong province. Zhonghua Xin Xue Guan Bing Za Zhi, 2013. 41(4): p. 337–40. [PubMed] [Google Scholar]
- 36.Trines J and Hornberger LK, Evolution of heart disease in utero. Pediatr Cardiol, 2004. 25(3): p. 287–98. [DOI] [PubMed] [Google Scholar]
- 37.Yagel S, et al. , Congenital heart defects: natural course and in utero development. Circulation, 1997. 96(2): p. 550–5. [DOI] [PubMed] [Google Scholar]
- 38.Pervolaraki E, et al. , Antenatal architecture and activity of the human heart. Interface Focus, 2013. 3(2): p. 20120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coceani F and Baragatti B, Mechanisms for ductus arteriosus closure. Semin Perinatol, 2012. 36(2): p. 92–7. [DOI] [PubMed] [Google Scholar]
- 40.Clyman RI, et al. , Permanent anatomic closure of the ductus arteriosus in newborn baboons: the roles of postnatal constriction, hypoxia, and gestation. Pediatr Res, 1999. 45(1): p. 19–29. [DOI] [PubMed] [Google Scholar]
- 41.Zhao N, et al. , Ambient air pollutant PM10 and risk of preterm birth in Lanzhou, China. Environ Int, 2015. 76: p. 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell ML and Belanger K, Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol, 2012. 22(5): p. 429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slama R, et al. , Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect, 2008. 116(6): p. 791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheppard L, et al. , Exposure and measurement contributions to estimates of acute air pollution effects. Journal of Exposure Analysis and Environmental Epidemiology, 2005. 15(4): p. 366–376. [DOI] [PubMed] [Google Scholar]
- 45.Sheppard L, et al. , Confounding and exposure measurement error in air pollution epidemiology. Air Qual Atmos Health, 2012. 5(2): p. 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Botto LD, et al. , Seeking causes: Classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol, 2007. 79(10): p. 714–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.