Abstract
Purpose:
To compare the technical success and complication rates of push versus pull gastrostomy tubes in cancer patients, and to examine their dependence on operator experience.
Materials and methods:
A retrospective review was performed of 304 cancer patients (170 men, 134 women; mean age 60.3 ±12.6 [SD], range: 19–102 years) referred for primary gastrostomy tube placement, 88 (29%) of whom had a previously unsuccessful attempt at percutaneous endoscopic gastrostomy (PEG) placement. Analyzed variables included method of insertion (push versus pull), indication for gastrostomy, technical success, operator experience, and procedure-related complications within 30 days of placement.
Results:
Gastrostomy tubes were placed for feeding in 189 patients and palliative decompression in 115 patients. Technical success was 91%: 78% after endoscopy had previously been unsuccessful and 97% when excluding failures associated with prior endoscopy. In the first 30 days, there were 29 minor complications (17.2%) associated with push gastrostomies, and only 8 minor complications (7.5%) with pull gastrostomies (P < 0.05). There was no significant difference in major complications (push gastrostomy 5.3%, pull gastrostomy 5.6%). For decompressive gastrostomy tubes, the pull technique resulted in lower rates of both minor and major complications. There was no difference in complications or technical success rates for more versus less experienced operators.
Conclusion:
Pull gastrostomy tube placement had a lower rate of complications than push gastrostomy tube placement, especially when the indication was decompression. The technical success rate was high, even after a failed attempt at endoscopic placement. Both the rates of success and complications were independent of operator experience.
Keywords: Interventional radiology, Gastrostomy, Palliative treatment, Nutritional support, Abdominal decompression
Gastrostomy tubes are primarily used either to manage prolonged nutritional supplementation in patients with inadequate oral intake, or to decompress the stomach in the setting of a small bowel obstruction or gastric outlet obstruction, often in those with end stage metastatic disease [1–5]. The three primary modalities for placement are surgical, endoscopic, and radiological. There have been numerous studies to determine the best method, but the ideal technique remains controversial [6,7].
Overall, percutaneous endoscopic gastrostomy (PEG) remains the most readily available and frequently used method, largely supplanting surgical placement due to a high technical success rate and fewer complications [2,3,8]. However, its use is limited in patients with head, neck, and esophageal cancers, as the tumor might prevent passage of the endoscope, increase the risk of bleeding from passage of the tube across friable tumor, or result in tract seeding around the gastrostomy tube [9–12].
The advent of interventional radiology has given rise to fluoroscopically guided techniques that circumvent these limitations [13]. The push method allows for direct percutaneous access into the stomach and insertion of a gastrostomy tube without going through the mouth [14]. The major drawbacks of this approach are that the tubes are often smaller and an inflatable balloon or pigtail is used for tube retention, resulting in more frequent dislodgement and clogging [2,3,15,16]. The pull technique is a more recently developed hybrid approach that allows for larger tubes and more secure bumpers, but similarly to the PEG, must pass through the oral cavity and incur the risk of tract seeding in patients with head and neck malignancies [3,17–19].
Compared to PEG, radiologically inserted gastrostomies have been shown to have higher technical success rates and similar or decreased risk of complications [2,3,17,20–22]. Indeed, several studies have commented on the appreciable number of patients receiving radiological gastrostomy placements after a previously failed PEG – data that support its use even in patients with difficult anatomy [17,20,22].
While a number of studies have examined PEG vs. pull or PEG vs. push, there is a paucity of literature that evaluates the differences between push and pull radiologic gastrostomies, particularly as it relates to patients requiring palliative decompression [2,20,23–25]. Venting gastrostomy tubes are understudied but are important for palliation of malignant obstruction. They are associated with unique challenges, due to the higher risk of aspiration; the difficulty of draining partially digested food, and the potentially distorted anatomy due to bowel obstruction or post-operative anatomy.
The purpose of this study was to compare the complication rates of push versus pull gastrostomies in a diverse population of cancer patients (including patients requiring either feeding or decompression). We evaluated the technical success of radiological gastrostomies, including patients with a prior failed attempt at PEG placement. In addition, we examined the role that operator experience plays in technical success or complication rates.
Materials and methods
Patients
This is a single-institution retrospective study that was HIPAA compliant and IRB-approved. Informed consent was waived. All patients 18-years or older who underwent attempted primary push or pull gastrostomy tube placement by interventional radiology between July 2000 and October 2015 were included. Gastrostomy tube exchanges and patients with incomplete documentation were excluded.
Outcome measures
Data related to patient demographics, gastrostomy type, indications and technique, minor and major complications, technical success, and operator characteristics were collected. Clinical notes, imaging, and procedures within one month after gastrostomy tube placement were reviewed for complications. Complications that had a clear connection to the procedure and occurred within 30 days after the initial placement were included and categorized based on SIR guidelines [26]. Minor complications included pericatheter leakage, cellulitis, tube dislodgement, tube occlusion, tube or balloon rupture or fracture, poor functionality, pneumonia or aspiration not requiring tube revision, or pneumoperitoneum not requiring drainage or surgery. Major complications included peritonitis, stomal infection causing sepsis, abscess, aspiration requiring tube revision, hemorrhage, pneumoperitoneum requiring drainage or exchange, gastrointestinal perforation, or any complication leading to permanent catheter removal, prolonged hospitalization, permanent adverse outcomes, or death.
Technique
Both types of gastrostomies were performed under fluoroscopic guidance by a board-certified fellowship-trained interventional radiologist, under moderate sedation or monitored anesthesia care. The choice of push or pull techniques was based on operator preference. Oral barium was not administered for gastrostomy tube placement. Antibiotics for both push and pull gastrostomies consisted of intravenous cefazolin at a dose of 1 g. If the patient was allergic to cephalosporins, or if a severe penicillin allergy was present, then intravenous clindamycin at a dose of 900 mg was administered.
For push gastrostomies, the stomach was insufflated through a nasogastric tube, and two or three T-fasteners were inserted into the stomach. A final needle was used to access the stomach, a guide wire was passed into the stomach, and tract dilation was performed. A 10-to-20 F locking loop tube (Multipurpose drainage catheter, Cook medical, Bloomington, IN) or a 12-to-18 F balloon gastrostomy tube (MIC, Halyard Health, Alpharetta, GA) was then placed over the wire into the stomach.
For pull gastrostomies, a needle was advanced into the insufflated stomach and placement was confirmed with contrast injection. The needle was exchanged over a guide wire for a sheath. A catheter and guide wire were then used through the sheath to cannulate the esophagus. The guide wire was advanced out the patient’s mouth, and the catheter and sheath were removed. A 20–28 F mushroom-type gastrostomy tube (Ponsky, Bard Medical, Covington, GA; or EndoVive, Boston Scientific, Marlborough, MA) was advanced through the mouth, over the guide wire, and into the stomach. Contrast was injected into the tube to verify placement.
Feeding gastrostomy tubes were connected to gravity drainage, and tube feeds started the next day. T-fasteners were typically cut 10–14 days after placement.
Statistical analysis
Demographic and outcomes data were analyzed with Prism 7 (GraphPad Software, Inc., Version 7.0a, April 2, 2016) and Microsoft Excel (2016). Proportions were compared using a two-tailed Fisher’s exact test. Linear regression analysis was employed to determine whether a significant relationship existed between years of experience or number of prior gastrostomy placement procedures and complication rates and technical success. Propensity score matching was used to select pairs of push and pull gastrostomy tube patients with similar clinical characteristics [27]. Patients were matched based on the type of malignancy (head and neck versus other), indication for gastrostomy tube (feeding or decompression), and whether the patient had a failed attempt at endoscopic gastrostomy placement. One-to-one nearest neighbor matching was performed, within 0.2 logit units. A P value of < 0.05 was used as the threshold for statistical significance.
Results
Between July 2000 and October 2015, 304 patients underwent an attempt at primary push or pull gastrostomy placement, 276 (91%) of which were successful. Table 1 highlights the demographics of these patients. There was no significant difference in mean age between push and pull groups (62 vs. 60, P = 0.187), while the gender composition differed (69%, 116/169 men vs. 41%, 44/107 men combined push vs. pull respectively, P < 0.0001). There was a larger percentage of men affected by head and neck malignancies, and more women in the abdominopelvic category as a consequence of gynecological malignancies. Of note, there were a number of patients with gastric cancer who received gastrostomies. These patients had gastric outlet obstruction and were not surgical candidates – decompressive gastrostomy was used for palliation.
Table 1.
Demographics and baseline characteristics of successful gastrostomy placements (n = 276).
| Push gastrostomy | Pull gastrostomy | |
|---|---|---|
| Total | 169 | 107 |
| Demographics | ||
| Men | 116 (69%) | 44 (41%) |
| Women | 53 (31%) | 63 (59%) |
| Mean age (year) | 62 ± 11.3 (SD) [24–102] | 60 ± 13.3 (SD) [22–86] |
| Indication | ||
| Feeding | 145 (86%) | 39 (36%) |
| Decompression | 24 (14%) | 68 (64%) |
| Prior failed percutaneous endoscopic gastrostomy placement | 17 (10%) | 52 (49%) |
| Head and neck malignancies | 134 (79%) | 21 (20%) |
| Abdominopelvic malignancies | 26 (16%) | 69 (64%) |
| Ovarian | 3 | 24 |
| Colon | 5 | 14 |
| Gastric | 2 | 8 |
| Pancreatic | 2 | 6 |
| Sarcoma | 2 | 3 |
| Cholangiocarcinoma | 2 | 2 |
| Gallbladder | 2 | 2 |
| Small bowel | 2 | |
| Uterine | 2 | |
| Other abdominopelvic | 8 | 6 |
| Other malignancies | 9 (5%) | 17 (16%) |
| Esophageal | 2 | 5 |
| Breast | 6 | |
| Lung | 2 | 3 |
| Other | 5 | 3 |
Of the successful placements, there were 169 push and 107 pull gastrostomies. The most frequent clinical indication for push was feeding whereas for pull it was palliative decompression in end stage disease. Although there was a wide spectrum of cancer diagnoses in both groups, the most common category for push was head and neck malignancy compared to abdominopelvic malignancy for pull. Of the successful pull gastrostomies, 49% (52/107) occurred in patients who had previously failed a percutaneous endoscopic gastrostomy (PEG) placement as opposed to 10% (17/169) of the push procedures.
Twenty-eight patients had a failed attempt at radiological gastrostomy placement (Table 2). The most frequent indication was palliative decompression and the most common category of malignancy was abdominopelvic. A prior unsuccessful attempt at PEG placement was made in 68% (19/28) of these patients. Notably, failures of radiological placement were most commonly attributed to the inability to insufflate the stomach (43%, 12/28) or not having a safe window (39%, 11/28), which was often due to interposition of bowel.
Table 2.
Characteristics and baseline demographics of unsuccessful gastrostomy placements (n = 28).
| Demographics | |
| Men | 10 (36%) |
| Women | 18 (64%) |
| Mean age (year) | 56 ± 16.3 (SD) [19–90] |
| Indication | |
| Feeding | 5 (18%) |
| Decompression | 23 (82%) |
| Prior failed percutaneous endoscopic gastrostomy placement | 19 (68%) |
| Head and neck malignancies | 1 (4%) |
| Abdominopelvic malignancies | 25 (89%) |
| Ovarian | 6 |
| Gastric | 6 |
| Colon | 3 |
| Pancreatic | 2 |
| Uterine | 2 |
| Other abdominopelvic | 6 |
| Other malignancies | 2 (7%) |
| Causes of failure | 28 |
| Inability to insufflate stomach | 12 (43%) |
| Interposition of bowel/unsafe window | 11 (39%) |
| Stomach positioned posteriorly or superiorly | 2 (7%) |
| Patient uncooperative or unable to tolerate procedure | 2 (7%) |
| Depth of subcutaneous tissue | 1 (4%) |
The mean size of balloon push gastrostomy tubes (16.4 ± 1.14 [SD] F; range: 12–18 F) was larger than the mean size of the pigtail push gastrostomy tubes (14.0 ± 1.08 [SD] F, range: 10–20 F) (P < 0.0001). The mean size of pull gastrostomy tubes (21 ± 2.56 [SD] F; range: 20–28 F) was larger than the mean size of the push (both balloon and pigtail) gastrostomy tubes (14.5 ± 1.48 [SD] F; range: 10–20 F) (P < 0.0001).
Table 3 illustrates that in the first 30 days, there were 29 minor complications (17.2%, 29/169) in patients who received a push gastrostomy, and 8 minor complications (7.5%, 8/107) in patients who received a pull gastrostomy (P = 0.042). There was no difference in major complications between push and pull gastrostomy tubes. There was also no difference in complications between balloon and pigtail push gastrostomy tubes. For the subset of patients referred for decompression, there were lower rates of both minor and major complications in those who received pull gastrostomies (Table 4).
Table 3.
Minor and major complication rates of push and pull gastrostomies.
| Pigtail | Balloon | P valuea | |
|---|---|---|---|
| Minor | 22/13116.8% | 7/3818.4% | > 0.999 |
| Major | 8/131 6.1% | 1/38 2.6% | 0.686 |
| Combined Push | Pull | P valuea | |
| Minor | 29/169 17.2% | 8/1077.5% | 0.042 |
| Major | 9/169 5.3% | 6/1075.6% | > 0.999 |
Calculated using Fisher’s exact test.
Table 4.
Minor and major complication rates by clinical indication.
| Feeding | Combined Push | Pull | P valuea | |
|---|---|---|---|---|
| Minor | 22/145 15.2% | 2/395.1% | 0.172 | |
| Major | 4/145 2.8% | 3/397.7% | 0.166 | |
| Decompression | Combined Push | Pull | P valuea | |
| Minor | 7/24 29.2% | 6/688.8% | 0.035 | |
| Major | 5/24 20.8% | 3/684.4% | 0.026 | |
Calculated using Fisher’s exact test.
After propensity score matching, there were 50 pairs of matched push and pull gastrostomy tubes. There was no significant difference between the push and pull gastrostomy groups, in term of type of malignancy (P = 1), indication for gastrostomy tube placement (P = 1), and whether the patient had a failed attempt at endoscopic gastrostomy placement (P = 1). However, 4 of 50 (8%) pull gastrostomy patients had a minor or major complication, whereas 13 of 50 (26%) push gastrostomy patients had a minor or major complication (P = 0.03).
The most frequent minor complication in push gastrostomies was dislodgement whereas in pull gastrostomies they were tube occlusion, low-grade pericatheter leakage, and dislodgement (Table 5). The most common major complications in push gastrostomies were aspiration with respiratory failure and death, hemorrhage requiring embolization, and pneumoperitoneum requiring drainage; in pull gastrostomies, the most common were significant stomal infections, i.e. abscesses or superficial infections causing sepsis (Table 6). Two push gastrostomy tubes resulted in hemorrhage requiring embolization. In both patients, the site of bleeding was the left gastric artery.
Table 5.
Classifications of minor complications.
| Push | Pull | |
|---|---|---|
| Total | 29 | 8 |
| Dislodgement | 8 | 2 |
| Pneumonia or aspiration not requiring intervention | 7 | 1 |
| Pneumoperitoneum necessitating tube check or repositioning | 3 | 0 |
| Low grade pericatheter leakage | 2 | 2 |
| Tube occlusion | 2 | 2 |
| Inadequate decompression | 2 | 1 |
| Inadequate feeding | 2 | 0 |
| Tube fracture | 1 | 0 |
| Cellulitis | 2 | 0 |
Table 6.
Classifications of major complications.
| Push | Pull | |
|---|---|---|
| Total | 9 | 6 |
| Aspiration with respiratory failure and death | 2 | 0 |
| Aspiration requiring intervention | 0 | 1 |
| Hemorrhage requiring embolization | 2 | 0 |
| Hemoperitoneum | 0 | 1 |
| Pneumoperitoneum requiring drainage | 2 | 1 |
| Pneumoperitoneum with intestinal pneumatosis, subsequent deterioration and death | 1 | 0 |
| Large pneumoperitoneum with ascites, subsequent shock and death | 1 | 0 |
| Septic shock and death | 0 | 1 |
| Significant stomal infection | 1 | 2 |
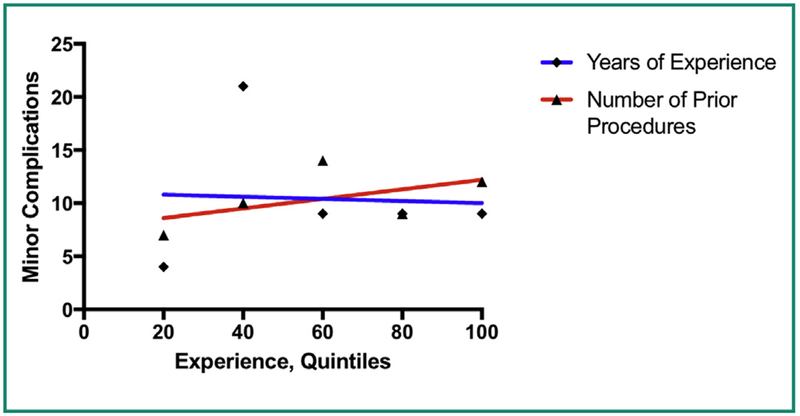
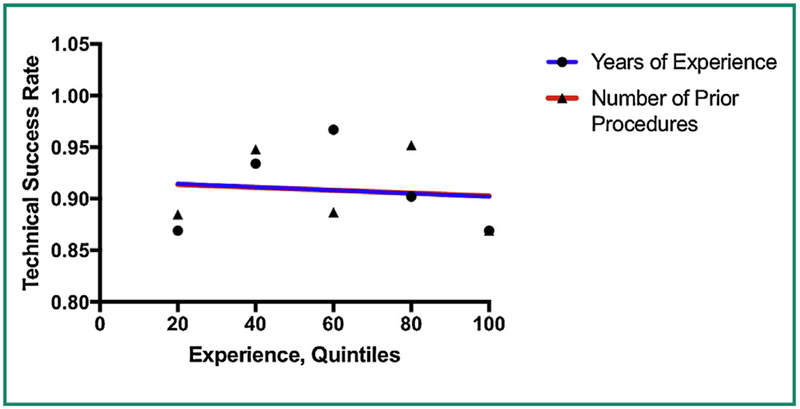
There was no correlation between operator experience and either technical success or complication rates. We examined both number of years of experience with gastrostomy placement, and number of prior gastrostomy placement procedures. The relationship between experience and complications can be seen in the linear regression performed in Fig. 1 – the slope of the line is not significantly different than zero (P = 0.37 and P = 0.90 respectively). For technical success, the linear regression analyses shown in Fig. 2 also failed to show a statistically significant association with experience, with P values of 0.86 and 0.85 respectively.
Figure 1.
Linear regression shows no significant correlation between operator experience (based on number of prior gastrostomy tubes placed or years of experience) and rates of minor complications.
Figure 2.
Linear regression shows no significant correlation between operator experience (based on number of prior gastrostomy tubes placed or years of experience) and technical success rate.
Discussion
This study compared the technical success and complication rates of push versus pull gastrostomies in a patient population with cancer diagnoses, and also evaluated whether more experienced operators had better outcomes.
A randomized trial of push versus pull gastrostomy tubes showed a comparable rate of complications between the two techniques, while two retrospective comparisons of push versus pull gastrostomy tubes showed a lower rate of complications with the pull technique [22–24]. The randomized trial used 14 F pigtail gastrostomy tubes and did not examine balloon-type push gastrostomy tubes, and none of the prior studies included a significant number of patients who required gastric decompression. Furthermore, one of these retrospective studies included gastrostomy tubes placed through pre-existing tracts, which substantially reduces the inherent risk of the procedure [22].
In our study, we found that pull gastrostomies had a lower rate of minor complications compared to push gastrostomies, and the same rate of major complications. For palliative decompression, both minor and major complication rates were lower for pull gastrostomy tubes. There was no difference in complications between pigtail and balloon-type push gastrostomy tubes.
In our patient population, 64% (68/107) of the patients who received a pull gastrostomy did so for palliative decompression, compared to 14% (24/169) for push gastrostomy. In addition, more of the pull gastrostomy tubes were placed after a failed attempt at endoscopic placement. Thus, the pull gastrostomy patients on average had more advanced disease (requiring palliative decompression) and more challenging anatomy (failed PEG placement). Despite these challenges, the pull gastrostomy tubes still had a lower minor complication rate.
We corrected for differences between the push and pull gastrostomy groups by using propensity score matching. After selecting matched pairs of patients using the propensity score, the push and pull gastrostomy groups were indistinguishable in terms of type of malignancy, indication for gastrostomy placement, and whether the patient had a failed attempt at endoscopic placement. However, the pull gastrostomy group had a lower rate of complications, compared to the push gastrostomy group.
As expected, the most frequent minor complication of the push gastrostomies was dislodgement – the locking loop tubes and inflatable balloons are not as secure as the mushroom retention mechanism. The pull gastrostomy cohort had a lower minor complication rate, and in particular, they had a lower rate of dislodgement and aspiration. The lower rate of aspiration might be due to the larger mean size of the pull gastrostomy tubes (resulting in better decompression), but the lower rate of dislodgement is likely due to the more secure retention mechanism.
Although gastrostomy tubes are mostly placed endoscopically in the United States [2], we have shown that radiologically inserted gastrostomies have a high technical success rate and low rate of complications. Of patients referred for radiological placement after a failed attempt at PEG, 78% (69/88) successfully received a gastrostomy – these were anatomically challenging patients, yet radiological techniques were still successful most of the time. The overall technical success rate for radiological gastrostomies was 91% (276/304); 97% (295/304) when excluding patients who had a previous attempt at PEG placement.
We also found that less experienced operators had the same rates of technical success and complications as more experienced operators. To our knowledge, there is currently no data in the literature that looks specifically at these metrics. Our data show that both push and pull radiological gastrostomies can be safely performed by fellowship-trained interventional radiologists at all levels of experience, with a high rate of technical success, even after a prior failed attempt at endoscopy. This suggests that radiological techniques should become a primary modality for gastrostomy placement.
There were a number of limitations with this study. First, this was a retrospective analysis at a single cancer center. Second, we only examined complications within the first 30 days, and thus did not evaluate longer-term complications such as tract seeding or tissue breakdown around chronic gastrostomy tubes.
In conclusion, pull gastrostomy tube placement may be preferable to push gastrostomy tube placement, due to a lower rate of complications, especially when the indication is decompression. However, push gastrostomy tube placement may be preferable in patients with airway issues or head and neck cancer. The technical success rate was high, even after a failed attempt at endoscopic placement. Both the rates of success and complications were independent of operator experience.
Acknowledgments
Funding
This work was funded in part through an NIH/NCI Cancer Center Support Grant (P30 CA008748).
Footnotes
Disclosure of interest
The authors declare that they have no competing interest.
References
- [1].Couch M, Lai V, Cannon T, Guttridge D, Zanation A, George J, et al. Cancer cachexia syndrome in head and neck cancer patients: part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck 2007;29:401–11. [DOI] [PubMed] [Google Scholar]
- [2].Laasch HU, Wilbraham L, Bullen K, Marriott A, Lawrance JAL, Johnson RJ, et al. Gastrostomy insertion: comparing the options-PEG, RIG or PIG? Clin Radiol 2003;58:398–405. [DOI] [PubMed] [Google Scholar]
- [3].Stavroulakis T, Walsh T, Shaw PJ, McDermott CJ. Gastrostomy use in motor neurone disease (MND): a review, meta-analysis and survey of current practice. Amyotroph Lateral Scler and Frontotemporal Degener 2013;14:96–104. [DOI] [PubMed] [Google Scholar]
- [4].Herman LL, Hoskins WJ, Shike M. Percutaneous endoscopic gastrostomy for decompression of the stomach and small bowel.Gastrointest Endosc 1992;38:314–8. [DOI] [PubMed] [Google Scholar]
- [5].Pothuri B, Montemarano M, Gerardi M, Shike M, Ben-Porat L,Sabbatini P, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005;96:330–4. [DOI] [PubMed] [Google Scholar]
- [6].Wollman B, D’Agostino HB, Walus-Wigle JR, Easter DW, Beale A. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology 1995;197:699–704. [DOI] [PubMed] [Google Scholar]
- [7].Rustom IK, Jebreel A, Tayyab M, England RJA, Stafford ND. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: a comparison study in head and neck cancer patients. J Laryngol Otol 2006;120:463–6. [DOI] [PubMed] [Google Scholar]
- [8].Gauderer MW, Ponsky JL, Izant RJJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg 1980;15:872–5. [DOI] [PubMed] [Google Scholar]
- [9].Peghini PL, Guaouguaou N, Salcedo JA, Al-Kawas FH. Implantation metastasis after PEG: case report and review. Gastrointest Endosc 2000;51:480–2. [DOI] [PubMed] [Google Scholar]
- [10].Sinclair JJ, Scolapio JS, Stark ME, Hinder RA. Metastasis of head and neck carcinoma to the site of percutaneous endoscopic gastrostomy: case report and literature review. JPEN J Parenter Enteral Nutr 2001;25:282–5. [DOI] [PubMed] [Google Scholar]
- [11].Coletti D, Genuit T, Ord R, Engroff S. Metastasis to the percutaneous endoscopic gastrostomy site in the patient with headand neck cancer: a case report and review of the literature. J Oral Maxillofac Surg 2006;64:1149–57. [DOI] [PubMed] [Google Scholar]
- [12].Thorburn D, Karim SN, Soutar DS, Mills PR. Tumour seeding following percutaneous endoscopic gastrostomy placement in head and neck cancer. Postgrad Med J 1997;73:430–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ho SG, Marchinkow LO, Legiehn GM, Munk PL, Lee MJ. Radiological percutaneous gastrostomy. Clin Radiol 2001;56:902–10. [DOI] [PubMed] [Google Scholar]
- [14].Lowe AS, Laasch HU, Stephenson S, Butterfield C, Goodwin M, Kay CL, et al. Multicentre survey of radiologically inserted gastrostomy feeding tube (RIG) in the UK. Clin Radiol 2012;67:843–54. [DOI] [PubMed] [Google Scholar]
- [15].Laasch HU, Martin DF. Radiologic gastrostomy. Endoscopy 2007;39:247–55. [DOI] [PubMed] [Google Scholar]
- [16].Shin JH, Park A-W. Updates on percutaneous radiologic gastrostomy/gastrojejunostomy and jejunostomy. Gut Liver 2010;4:S25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chavada G, El-Nayal A, Lee F, Webber SJ, Mcalindon M, Walsh T,et al. Evaluation of two different methods for per-oral gastrostomy tube placement in patients with motor neuron disease(MND): PIG versus PEG procedures. Amyotroph Lateral Scler 2010;11:531–6. [DOI] [PubMed] [Google Scholar]
- [18].Szymski GX, Albazzaz AN, Funaki B, Rosenblum JD, Hackworth CA, Zernich BW, et al. Radiologically guided placement of pull-type gastrostomy tubes. Radiology 1997;205:669–73. [DOI] [PubMed] [Google Scholar]
- [19].Kahriman G, Ozcan N, Donmez H. Fluoroscopy-guided placement of pull-type mushroom-retained gastrostomy tubes in 102 patients. Diagn Interv Imaging 2017;98:715–20. [DOI] [PubMed] [Google Scholar]
- [20].Allen JA, Chen R, Ajroud-Driss S, Sufit RL, Heller S, Siddique T, et al. Gastrostomy tube placement by endoscopy versus radiologic methods in patients with ALS: a retrospective study of complications and outcome. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:308–14. [DOI] [PubMed] [Google Scholar]
- [21].Grant DG, Bradley PT, Pothier DD, Bailey D, Caldera S, Baldwin DL, et al. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol 2009;34:103–12. [DOI] [PubMed] [Google Scholar]
- [22].de Baere T, Chapot R, Kuoch V, Chevallier P, Delille JP, Domenge C, et al. Percutaneous gastrostomy with fluoroscopic guidance: single-center experience in 500 consecutive cancer patients.Radiology 1999;210:651–4. [DOI] [PubMed] [Google Scholar]
- [23].Yip D, Vanasco M, Funaki B. Complication rates and patency of radiologically guided mushroom gastrostomy, balloon gastrostomy, and gastrojejunostomy: a review of 250 procedures. Cardiovasc Intervent Radiol 2004;27:3–8. [DOI] [PubMed] [Google Scholar]
- [24].Yang Y, Schneider J, Düber C, Pitton MB. Comparison of fluoroscopy-guided Pull-type percutaneous radiological gastrostomy (Pull-type-PRG) with conventional percutaneous radiological gastrostomy (Push-type-PRG): clinical results in253 patients. Eur Radiol 2011;21:2354–61. [DOI] [PubMed] [Google Scholar]
- [25].Bernstein OA, Campbell J, Rajan DK, Kachura JR, Simons ME, Beecroft JR, et al. Randomized trial comparing radiologic pigtail gastrostomy and peroral image-guided gastrostomy: intra- and postprocedural pain, radiation exposure, complications, and quality of life. J Vasc Interv Radiol 2015;26:1680–6. [DOI] [PubMed] [Google Scholar]
- [26].Itkin M, DeLegge MH, Fang JC, McClave SA, Kundu S, Janne d’Othee B, et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of interventional radiology and American gastroenterological association (AGA) Institute, with endorsement by Canadian interventional radiological association (CIRA) and Cardiovascular and interventional radiological society of Europe (CIRSE). J Vasc Interv Radiol 2011;22:1089–106. [DOI] [PubMed] [Google Scholar]
- [27].Ensor JE. Addressing confounders in retrospective studies. J Vasc Interv Radiol 2017;28:558–60. [DOI] [PubMed] [Google Scholar]