Abstract
Tuberous sclerosis (TSC) is an autosomal dominant disorder caused by heterozygous mutations in the TSC1 or TSC2 gene. TSC is often associated with neurological, cognitive, and behavioral deficits. TSC patients also express co-morbidity with anxiety and mood disorders. The mechanism of pathogenesis in TSC is not entirely clear, but TSC-related neurological symptoms are accompanied by excessive glutamatergic activity and altered synaptic spine structures. To address whether extrasynaptic (e)NMDA-type glutamate receptor (NMDAR) antagonists, as opposed to antagonists that block physiological phasic synaptic activity, can ameliorate the synaptic and behavioral features of this disease, we utilized the Tsc2+/− mouse model of TSC to measure biochemical, electrophysiological, histological, and behavioral parameters in the mice. We found that antagonists that preferentially block tonic activity as found at eNMDARs, particularly the newer drug NitroSynapsin, provide biological and statistically significant improvement in Tsc2+/− phenotypes. Accompanying this improvement was correction of activity in the p38 MAPK-TSC-Rheb-mTORC1-S6K1 pathway. Deficits in hippocampal long-term potentiation (LTP), histological loss of synapses, and behavioral fear conditioning in Tsc2+/− mice were all improved after treatment with NitroSynapsin. Taken together, these results suggest that amelioration of excessive excitation, by limiting aberrant eNMDAR activity, may represent a novel treatment approach for TSC.
Keywords: Tuberous Sclerosis, NitroSynapsin, extrasynaptic NMDA receptor, E/I imbalance, hippocampal long-term potentiation
1. Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder manifested by intellectual disability, epilepsy/electrophysiological deficits, and neurobehavioral abnormalities, often producing features of autism spectrum disorder (ASD) (Bolton et al., 2002; Hsieh et al., 2016; Smalley, 1998). Approximately 20-30% of individuals with TSC have very low IQs and require assistance with daily tasks. Roughly 50% of TSC patients have IQs in the normal range, while displaying neuropsychological impairment such as deficits in attention-executive skills, memory and learning (de Vries et al., 2009; Harrison et al., 1999; Prather and de Vries, 2004; Ridler et al., 2007). Psychiatric comorbidities of TSC include mood, anxiety and adjustment disorders (de Vries et al., 2007; Lewis et al., 2004; Muzykewicz et al., 2007; Pulsifer et al., 2007; Raznahan et al., 2006).
Currently, there is no cure or adequate treatment for TSC. The disorder is caused by heterozygous mutations that inactivate one of two genes, Tsc1 or Tsc2. The TSC proteins form the TSC1/2 complex, which inhibits Rheb, a GTPase which activates mammalian target of rapamycin complex 1 (mTORC1). mTORC1 stimulates mRNA translation and cell growth; excessive activation of this pathway due to the inactive TSC1/2 complex may lead to TSC pathological features, such as abnormal signaling at synapses and aberrant subunit composition of AMPA receptors in a variety of neuronal types (Talos et al., 2008). Tsc1 and Tsc2 heterozygous mice have hippocampal-dependent learning and memory deficits (Ehninger et al., 2008; Goorden et al., 2007). In this study, we used the Tsc2+/− mouse model of tuberous sclerosis because of the higher frequency and more severe phenotype of Tsc2 mutations compared to those of Tsc1 in humans although this mouse strain does not manifest epilepsy (Ehninger et al., 2008; Goorden et al., 2007; Nie et al., 2010; Onda et al., 1999). Tsc2+/− mice have been reported to display learning and memory deficits due to hyperactivity of mTOR signaling in addition to abnormalities in long-term potentiation (LTP) in the CA1 region of the hippocampus (Ehninger et al., 2008). Behaviorally, these mice manifest a deficit in hippocampal-dependent context specificity, as tested by fear conditioning (Ehninger et al., 2008).
The mechanism of pathogenesis in TSC is not entirely clear. One important etiological pathway involves overstimulation of mTOR. Hyperactivation of mTOR in TSC results in inhibition of macroautophagy, with consequent loss of developmental synaptic spine pruning and behavioral abnormalities; inhibition of mTOR with rapamycin can rescue these phenotypes (Tang et al., 2014). TSC-related neurological symptoms are accompanied by increased expression of N-methyl-D-aspartate-type glutamate receptors (NMDARs), excessive glutamatergic (excitatory) activity, and altered synaptic spine structures (Lozovaya et al., 2014; Tavazoie et al., 2005). Thus, we hypothesized that NMDAR antagonists might ameliorate neurological symptoms in TSC.
We initially used the drug memantine (Namenda®), which our group had previously developed, characterized, and licensed to pharma, contributing to its FDA-approval for the treatment of moderate-to-severe Alzheimer’s disease (AD) (Lipton, 2006). We had shown that memantine is an Uncompetitive/relatively Fast Off-rate (so-called ‘UFO’) antagonist of the NMDAR (Chen and Lipton, 1997; Chen et al., 1992; Lipton, 2006; Xia et al., 2010). Drugs of this class preferentially inhibit extrasynaptic (e)NMDARs because of their tonic activity, as opposed to the phasic activity of physiologically-activated synaptic NMDARs (Chen and Lipton, 1997; Chen et al., 1992; Emnett et al., 2013; Lipton, 2006; Lipton, 2007; Takahashi et al., 2015; Talantova et al., 2013; Xia et al., 2010). Additionally, we recently developed a more efficacious eNMDAR antagonist, currently designated NitroSynapsin (formerly NitroMemantine YQW-036/NMI-6979), with markedly superior neuroprotective effects than memantine both in vitro and in vivo in multiple animal models (Lipton, 2006; Takahashi et al., 2015; Talantova et al., 2013; Wang et al., 2006)). The new drug takes advantage of additional modulatory sites on the NMDAR that are redox active and undergo S-nitrosylation (transfer of nitric oxide [NO]-related species to critical Cys residues). We and our colleagues had discovered that S-nitrosylation of the NMDAR decreases excessive receptor activity (Choi et al., 2000; Lei et al., 1992; Lipton et al., 1993); in the case of the NitroSynapsin adduct, memantine-like open-channel block by the aminoadamantane moiety serves to target the nitrosylating species specifically to the receptor, avoiding off-target effects of NO (Choi et al., 2000; Lei et al., 1992; Lipton, 2006; Lipton, 2007; Lipton et al., 1993; Talantova et al., 2013; Wang et al., 2006). Thus, this drug avoids systemic side effects of NO, such as cell toxicity, hypotension, effects on platelet aggregation or S-nitrosylation of other proteins, by targeting the NO group to the NMDAR. One of the reasons that neurons treated with memantine can still be damaged is that they depolarize (become positively charged), thereby relieving the channel block of memantine because the drug is also positively charged and similar charges repel (Chen and Lipton, 1997; Chen et al., 1992; Lipton, 2006). Using NitroSynapsin, by virtue of its having a second antagonist function that is not voltage dependent, i.e., S-nitrosylation of redox sites on the receptor that are located outside of the membrane field (Choi et al., 2000; Lei et al., 1992; Lipton, 2006; Lipton et al., 1993), this shortcoming is avoided (Takahashi et al., 2015; Talantova et al., 2013; Wang et al., 2006). Of importance, NitroSynapsin is an aminoadamantane nitrate. Hence, NO-related species are donated as a nitro group from an alkyl nitrate that lacks the toxicity of free radical nitric oxide (NO•) since it has one less electron and consequently manifests a different chemical reactivity (e.g., it does not react with O2• to form toxic ONOO− [peroxynitrite]) (Beckman et al., 1990; Choi et al., 2000; Dawson et al., 1991; Lipton, 2006; Lipton, 2007; Talantova et al., 2013; Wang et al., 2006).
One result of the mechanism of action of memantine and particularly of NitroSynapsin is that these antagonists selectively block excessive, tonically-activated eNMDARs rather than the physiological activity of synaptic (s)NMDARs, thus protecting synaptic integrity (Lipton, 2006; Okamoto et al., 2009; Talantova et al., 2013; Xia et al., 2010). Therefore, we hypothesized that this action of memantine and NitroSynapsin might offer a rational treatment for individuals with TSC. We thus investigated if administration of these eNMDARs antagonists could improve synaptic spine structure, electrophysiological properties, and behavioral abnormalities in TSC murine models by affecting the eNMDAR/p38 mitogen-activated protein kinase (MAPK)/MAPK activated protein kinase 2 (APK2)/TSC2 cascade.
2. Materials and methods
2.1. Phosphoproteomics and immunoblotting
For phosphoproteomic analysis, rat cerebrocortical cultures were exposed to NMDA after pharmacological blockade of synaptic NMDARs by simultaneous exposure to bicuculine and MK-801, resulting in stimulation of eNMDARs as detailed previously (Hardingham et al., 2002; Okamoto et al., 2009; Talantova et al., 2013). In this method, synaptic NMDA channels are selectively activated and then blocked by addition of bicuculline (to block inhibitory GABAergic transmission, thus allowing basal activation of excitatory currents) and MK-801 (to block the synaptic NMDA current thus activated). This procedure is then followed by activation of extrasynaptic NMDARs with NMDA. Twenty-four hours later, phosphoproteomic analysis was performed by multidimensional liquid chromatography electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS), as we recently detailed (Singec et al., 2016). Briefly, proteins were re-suspended in 100 mM (NH4)2HCO3/8 M urea containing protease and phosphatase inhibitors, gel-filtered, and protein concentrations measured by Bradford assays. Protein (1 mg) was reduced, alkylated, digested with trypsin, peptides desalted, dried and stored at −80 °C. Peptides were separated by strong cation exchange chromatography (SCX), desalted and dried. Phosphopeptides were enriched, using TiO2 beads, from SCX fractions. LC-ESI-MS/MS was performed on each fraction in duplicate with a C18 column, captive spray source and LTQ Orbitrap Velos mass spectrometer equipped with electron transfer dissociation. MS/MS data was searched against a concatenated forward and reverse ipi.RAT database with Sorcerer-SEQUEST. Static carbamidomethylation of C, differential oxidation of M, differential phosphorylation of S, T, and Y were specified. Protein identifications were filtered at a FDR < 0.01 (ProteinProphet, Institute for Systems Biology, Seattle, WA), including stringent phosphopeptide level verification. NSAF-SpCs, Ln-NSAFs and PSpCs were calculated as described (Singec et al., 2016).
For protein expression and phosphorylation analyses by immunoblot, brain tissue was homogenized in T-PER Tissue Protein Extraction Reagent containing protease inhibitor cocktail (Thermo Scientific, Waltham, MA). Protein concentration was determined using BCA reagent (Thermo Scientific). Equal amounts of samples were separated by gel electrophoresis and transferred to nitrocellulose/PVDF membranes. All antibodies were from Cell Signaling Technologies: phospho-p38 (#9211), p38 (#9212), phospho-MAPKAPK2 (#3007), MAPKAPK2 (#3042), phospho-TSC2 (#3616), TSC2 (#4308), phospho-S6K (#9234), S6K (#2708).
2.2. Animals and drug treatments
Tsc2+/− mice on the C57Bl/6J background were obtained from Dr. Mark F. Bear (Auerbach et al., 2011) and bred in our Institutional Animal Facility with approval of the Animal Care Committee. For electrophysiology studies, hippocampal slices were perfused for 4 hours with vehicle, memantine or NitroSynapsin at 1-2 μM. For neurobehavioral studies, which were followed by histological assessment, animals received via the intraperitoneal route either vehicle or a loading dose of 92 μmol/kg of memantine or NitroSynapsin, followed by 4.6 μmol/kg of drug twice daily for two-and-a-half-days, with the last dose 3 hours prior to the behavioral training session (for a total of five drug injections). This dosing regimen yields an effective concentration of drug at NMDARs in the brain of 1-10 μM (Okamoto et al., 2009; Takahashi et al., 2015; Talantova et al., 2013; Wang et al., 2006). Saline solution (vehicle)-containing the compounds were prepared fresh just prior to administration in each case.
2.3. Hippocampal slice preparation and multi-electrode array electrophysiology
One-month-old mice underwent cervical dislocation before decapitation following National Institute of Health and Institutional guidelines. After rapid brain dissection, hippocampal slices (300 μm in thickness) were cut as previously described (Akhtar et al., 2016) and placed in ACSF containing the following (in mM): 124 NaCl, 5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 10 glucose (pH 7.4), and bubbled with 95% O2/5% CO2. Slices were equilibrated for ≥1 h prior to recording on the multi-electrode array chamber (MEA60 Multi Channel Systems, Reutlingen, Germany). Slices were held in place with a nylon mesh and perfused with ACSF at 32 °C for the duration of the experiment. Field excitatory postsynaptic potentials were evoked in CA1 pyramidal neurons by monopolar biphasic voltage stimulation delivered at the Schaffer collaterals. Increasing voltage stimulation was used to determine the threshold at which 30% maximal response was obtained in the absence of a population spike. Baseline recordings were obtained with 30 stimuli at 30 s intervals. Potentiation was induced by four bursts at 20 s intervals with each burst consisting of ten stimulatory pulses at 100 Hz. Field excitatory postsynaptic potentials amplitude and initial slope were analyzed at the apical dendrites (stratum radiatum). Percent potentiation was calculated as the ratio of the mean field excitatory postsynaptic potentials initial slope, recorded at various time points after first tetanus stimulation, and divided by the mean field excitatory postsynaptic potentials initial slope of the baseline recording.
2.4. Fear conditioning tests for behavioral assessment
The ‘Freeze Monitor’ from San Diego Instruments (SDI, Inc., San Diego, CA) was used for the fear conditioning experiments, which were performed on 3-month-old Tsc2+/− mice and WT littermates. Three hours after drug injection, mice were placed in the fear conditioning apparatus and allowed to acclimate. Day 1 of testing included a 3-minute baseline reading in which the mice were allowed to explore the chamber. Following the initial 3-minute period, the mice were trained with 2 cue-paired-stimuli (sound, presented for 30 s, immediately followed by a shock), separated by 100 s.
During day 2 of testing, mice were again placed into the fear-conditioning apparatus for 5 min and monitored for freezing episodes without any cue or shock (“test in the training context”). Then, 1 hour later, the mice were placed back into the fear-conditioning chamber, but this time in a novel context (with the addition of different flooring and walls). They were allowed to explore for 5 min, during which their freezing was monitored (“test in the novel context”). After the test, the mice were taken back to their home cage for 1 hour. A final set of tests was performed then on day 2 in which the mice were allowed to explore for 3 min with no cues in the novel environment, after which they were continuously presented with the conditioned cue (sound) for an additional 3 min with their freezing monitored (“test in the novel context + cue”).
For automated assessment of freezing, SDI software was used to measure the first 3 beam breaks in a 5-s interval. A freezing episode was defined as no beam breaks for 1 or more seconds. Data are presented as the number of 5-s intervals during which an instance of freezing was observed divided by the total number of 5-s intervals and multiplied by 100.
2.5. Neurohistological analysis
The same mice that were evaluated in the fear conditioning paradigm were sacrificed after behavioral testing and their brains analyzed by immunohistochemical methods. For immunostaining, three serial sections of corresponding mouse brain regions were analyzed. For assessment of neuronal and synaptic changes, sections were immunolabeled with the following antibodies: microtubule associated protein-2 (MAP2) (Millipore, Billerica, MA) to label neuronal cell bodies and dendrites; NeuN (Millipore) to label neuronal nuclei and cell bodies; synaptophysin (Millipore) to label presynaptic vesicles of neurons; and GFAP to label glial fibrillary acidic protein (Millipore). Primary antibody staining was identified with fluor-tagged secondary antibodies. Immunolabeled, blind-coded sections were imaged serially using a laser-scanning confocal microscopy or deconvolution microscopy (Garden et al., 2002; Kang et al., 2010). Digitized images of optical sections (40 μm in thickness) were analyzed with SlideBook 5.0 software (Intelligent Imaging Innovations, Denver, CO). The hippocampus, including the molecular layer of the dentate gyrus and pyramidal layer of the CA1 region, was analyzed quantitatively for each antibody label in terms of percent area for synaptophysin, MAP2, and GFAP. For NeuN labeling, neuronal cell counts were performed using an optical dissector as an unbiased stereological counting method, as described previously (Li et al., 2008). In serial sections, we counted positive cells under a 63x objective using SlideBook software.
2.6. Statistical analyses
All data were collected by observers masked to the identity of the cells or mice. Data are expressed as mean + s.e.m. in each experiment. Statistical comparisons were made for single comparisons by a two-way Student’s t test, or for multiple comparisons by an analysis of variance (ANOVA) with post hoc Newman-Keuls test. Results were considered significant at a level of P < 0.05.
3. Results
3.1. eNMDAR antagonism can increase TSC2 activation
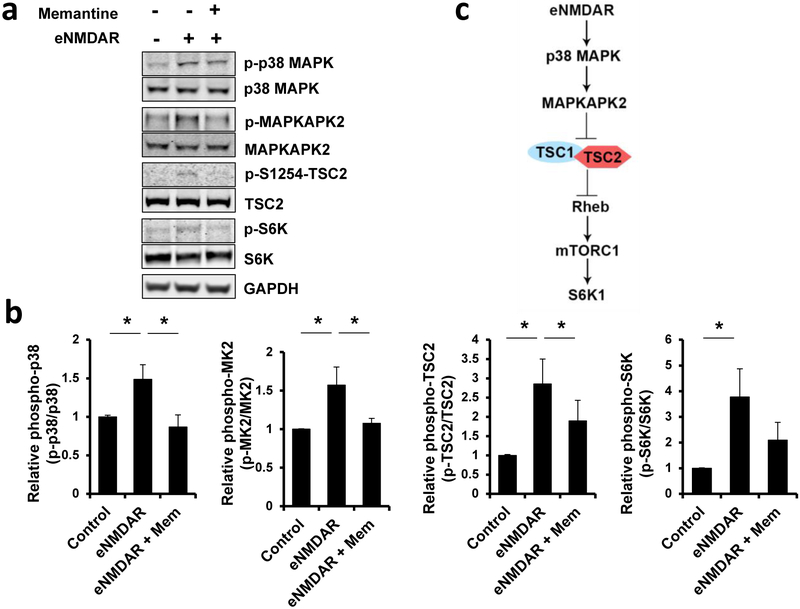
The detailed mechanism of detrimental glutamatergic/eNMDAR signaling that mediates synaptic spine loss remains to be explored. To begin to address this mechanism in an unbiased manner, we initiated a large-scale, quantitative phosphoproteome analysis of eNMDAR signals in cultured rat cerebrocortical neurons (Supplementary Table 1). The reproducible identification of proteins in the TSC-mTOR pathway suggested their potential relevance to synaptic spine pathology in this setting. For example, the phosphoproteomic analysis identified the induction of TSC2 serine-1254 phosphorylation after stimulation of eNMDARs. We confirmed this finding using Western blots from cultures treated with memantine, a well-characterized eNMDAR inhibitor (Fig. 1a,b) (Chen and Lipton, 1997; Chen et al., 1992; Lipton, 2006; Xia et al., 2010). We found that components of the p38 MAPK-MAPKAPK2 cascade, including MAPKAPK2-dependent TSC2 phosphorylation at serine-1254, increased after eNMDAR activation (Fig. 1a,b). Additionally, we found that S6 kinase 1 (S6K1), the canonical target of mTORC1 located downstream of TSC2 in the cascade, is phosphorylated after eNMDAR stimulation.
Fig. 1. eNMDAR activity triggers a signaling cascade to inactivate TSC2, which is attenuated by memantine.
(A) Western blots showing that eNMDAR stimulation increased phosphorylation/activation of the p38 MAPK/ MAPKAPK2/TSC2/mTORC1/S6K1 cascade. Blockade of eNMDARs with the eNMDAR antagonist memantine (10 μM) reduced this signaling. This experiment was replicated 4 times with similar results and quantified in panel b. (b) Quantification of immunoblots by unpaired t-test; mean ± s.e.m., n = 4, *P < 0.05 (Mem, memantine (c) Model of inhibition of the Tsc pathway after eNMDAR stimulation.
These findings suggest that this pathway is a downstream cascade of eNMDAR activation with the potential to further inhibit TSC2 activity, exacerbating the effects of the mutation. Importantly, inhibition of eNMDARs by memantine significantly decreased phosphorylation of p38 MAPK, MAPKAPK2, TSC2 (serine-1254), and S6K1 (Fig. 1a,b). Taken together, these results prompted us to hypothesize that NMDAR antagonists, such as memantine, may raise TSC GAP activity in a beneficial manner in TSC by inhibiting the p38 MAPK/MAPKAPK2/TSC2 pathway (Fig. 1c).
3.2. eNMDAR inhibition with NitroSynapsin reverses deficits in long-term hippocampal plasticity in Tsc2 heterozygous mice
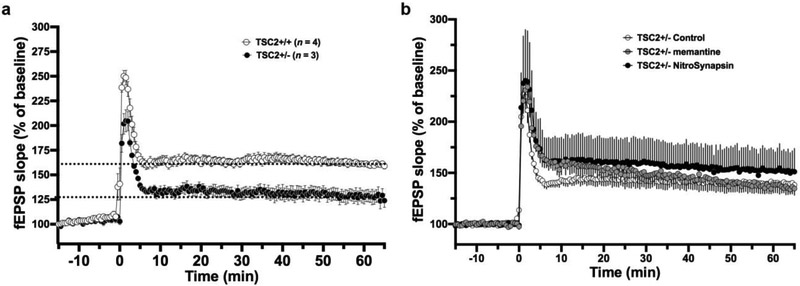
Next, we investigated the proposed molecular signaling cascade in Tsc2+/− mice, which inherently manifest less TSC GAP activity and excessive glutamatergic activity (Auerbach et al., 2011; Gkogkas et al., 2010; Lozovaya et al., 2014; Tavazoie et al., 2005). We examined long-term potentiation (LTP), a form of synaptic plasticity elicited in response to excitatory input and thought to represent an electrical correlate of learning and memory in the hippocampus. Field recordings in the CA1 region of hippocampal slices were used to investigate the effects of Tsc2 mutation on this form of synaptic plasticity. For this purpose, we prepared acute hippocampal slices from one-month-old mice and performed field recordings in a microelectrode array (MEA) chamber perfused with artificial cerebrospinal fluid (ACSF). fEPSPs (field excitatory postsynaptic potentials) were recorded in the CA1 region after evoking LTP via stimulation of the Schaffer collaterals (four repetitions of 100 Hz pulses for one second each). The initial slope of the fEPSP was analyzed to assess LTP. Of note, others have observed that after a weak induction stimulus (100 Hz x 1), late (assessed at 240 min)-LTP is increased in 4-6 mos-old Tsc2+/− mice (Ehninger et al., 2008). However, with a stronger induction protocol as used here, we observed a decrease in LTP, monitored 60 min after induction (Fig. 2a), similar to results that have been reported previously (Gkogkas et al., 2010). Strikingly, we found that a 4-hour treatment with 1-2 μM of the more efficacious eNMDAR antagonist NitroSynapsin, but not memantine, improved LTP in Tsc2+/− mice compared to vehicle control treatment (P < 0.001 at 55-65 min, Fig. 2b).
Fig. 2. Abnormal CA1-LTP in Tsc2+/− mice and improvement with NitroSynapsin.
(a) LTP recorded from hippocampal slices by MEA. fEPSP slope is plotted every 30 s and represents mean ± s.e.m. for Tsc2+/+and Tsc2+/− (n = 7 slices from 7 mice). (b) Effect of treatment with vehicle control vs. 1-2 μM memantine or NitroSynapsin on Tsc+/− mice (n = 13 slices from 13 mice, P < 0.001 for improvement of LTP by NitroSynapsin monitored 55-65 min after induction).
3.3. NitroSynapsin treatment of Tsc2 heterozygous mice reduces synaptic loss
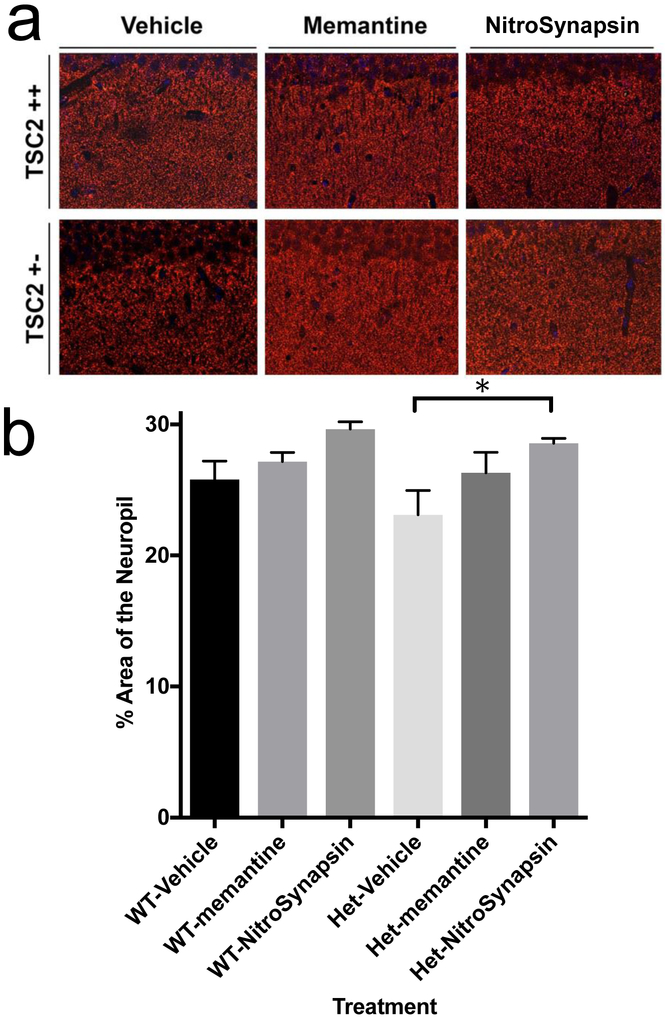
To determine the effects of eNMDAR antagonists on the brain histology of Tsc2+/− mice, we used quantitative confocal immunohistochemistry to analyze coronal brain slices from 3-month-old wild-type (WT) and Tsc2+/− mice that had been treated for two and a half days with vehicle, memantine or NitroSynapsin (as described in the Materials and Methods section). A key feature of TSC is thought to involve loss of synapses. Importantly, Tsc2+/− mice treated with NitroSynapsin manifested significantly more staining for the presynaptic marker synaptophysin (SY38) in the hippocampus than untreated heterozygous littermates, whereas the effect of memantine was not statistically significant (Fig. 3).
Fig. 3. (See also Figures S1-S3). Effects of treatment with memantine or NitroSynapsin on synaptic integrity of Tsc2+/− mice.
Representative histological images and quantification of brain slices from three-month-old TSC2 +/− transgenic mice treated with vehicle, memantine or NitroSynapsin. (a) Staining for SY38 positive synaptic terminals (red) in the hippocampus. (b) Quantification of percent area of synaptophysin immunoreactivity. Low Synaptophysin-positive terminal signal in Tsc2+/− vehicle-treated mice was increased with NitroSynapsin treatment (*P < 0.022). Values are mean + s.e.m. for n = 3 mice evaluated for each treatment by unpaired t-test.
Additional marker analysis included glial fibrillary acidic protein (GFAP, an astrocytic marker under these conditions), NeuN (a neuronal nuclei and cell body marker), and microtubule-associated protein 2 (MAP2, labeling neuronal dendritic spines). Confocal immunohistochemical images of the hippocampus revealed no difference in expression between NeuN (Supplementary Fig. 1), MAP2 (Supplementary fig. 2), or GFAP (Supplementary fig. 3) in WT vs. Tsc2+/− mice. Collectively, these histological results indicate that there was no significant loss of neurons/neuropil or increase in reactive astrocytosis at this stage of the disease in the hippocampus, in contradistinction to the loss of synapses.
3.4. NitroSynapsin improves neurobehavioral phenotypes in Tsc2 heterozygous mice
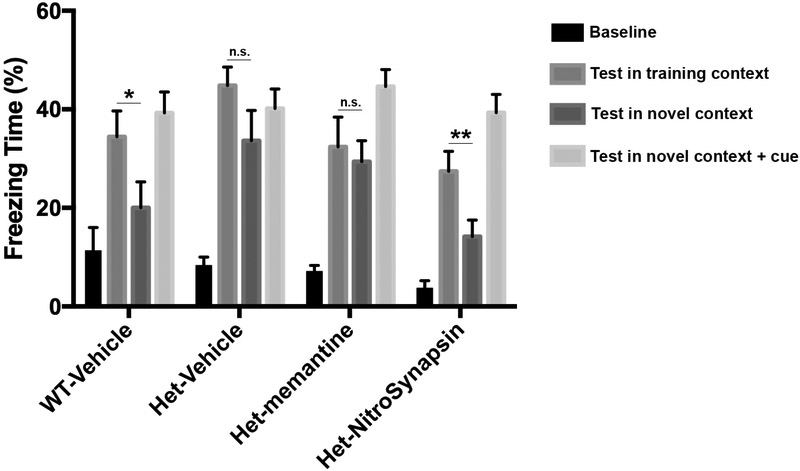
Discrimination between similar contexts is dependent on hippocampal function. We performed fear conditioning tests on three-month-old Tsc2+/+ and Tsc2+/− mice to explore the effects of eNMDAR antagonists on anxiety, and learning and memory deficits in Tsc2+/− mice (Ehninger et al., 2008; Gkogkas et al., 2010). We administered vehicle, memantine or NitroSynapsin for two and a half days, with the last dose of drug occurring 3-hours prior to the first training session (see Materials and Methods for details). On the fear conditioning test (Fig. 4), WT littermate mice exhibited a significantly increased amount of freezing time in the training context over the novel context (P = 0.032), indicating that these animals could discriminate between the two conditions.
Fig. 4. Effect of treatment with memantine or NitroSynapsin on context discrimination in Tsc2+/− mice assessed by the fear conditioning test.
Freezing time of three-month-old WT and Tsc+/− transgenic mice treated with vehicle, memantine or NitroSynapsin in fear-conditioning trials. All four groups of mice tested (WT-vehicle, Het-vehicle, Het-memantine, and Het-NitroSynapsin) showed freezing in the “training context” and the “novel context + cue,” indicating that their conditioned fear responses were unaffected by genotype or drugs. Additionally, WT mice displayed context discrimination between the training and novel contexts (*P = 0.032). In contrast, vehicle- or memantine-treated Tsc2+/− mice showed deficits in this behavior, resulting in the lack of discrimination between the training and novel contexts (n.s. = no significant difference). NitroSynapsin treatment improved this phenotype, normalizing context discrimination in Tsc2+/− mice (**P = 0.023). Values are mean + s.e.m. (n = 7-12 mice per group). Abbreviations: Training context = training environment in which the mice were previously trained with a shock preceded by a sound cue. Novel context = new flooring and walls in the test chamber. Novel context + cue = novel context with sound cue.
In contrast, Tsc2+/− mice were deficient in this form of context discrimination, confirming a previously reported phenotype (Ehninger et al., 2008). Remarkably, while memantine failed to mitigate this deficit, NitroSynapsin improved the ability to discriminate context in Tsc2+/− mice to normal WT levels (P = 0.023). As a control, all four groups of mice tested (WT-vehicle, Het-vehicle, Het-memantine, and Het-NitroSynapsin) displayed freezing to the conditioned cue in the novel context, indicating that hippocampal-independent fear responses were unaffected by genotype or drugs.
4. Discussion
In the present study, our phosphoproteomic analysis of glutamatergic/eNMDAR-mediated signaling identified upregulation of the p38 MAPK-MAPKAPK2 cascade, which is involved in the transcriptional activation of plasticity-associated genes (Eales et al., 2014; Waltereit et al., 2006). Glutamatergic signaling has been implicated in multiple forms of autism, including that associated with TSC (Kelleher et al., 2012; Lozovaya et al., 2014; Rojas, 2014; Tavazoie et al., 2005). Additional potentially excitotoxic factors in TSC are a significant loss of excitatory amino acid transporter (EAAT) activity in astrocytes with subsequent reduction in synaptic and extrasynaptic glutamate uptake (Wong et al., 2003; Zheng et al., 2010). Further, potential effects of metabotropic glutamate receptors have been reported given that these receptors are known to be aberrantly expressed in TSC tubers (Boer et al., 2008). Since the p38 MAPK pathway is known to inactivate TSC2, as found in TSC, inhibition of eNMDARs should increase TSC2 activity. We therefore hypothesized that a therapeutic approach with a repurposed drug that targets the eNMDAR might affect the mTOR pathway. Thus, we evaluated the potential application of the eNMDAR antagonists memantine and NitroSynapsin for the treatment of tuberous sclerosis using the well-known Tsc2+/− mouse model. Initially, through an unbiased proteomics approach, we found that inhibition of eNMDARs could modulate the levels of all members in the p38 MAPK/MAPKAPK2/TSC/Rheb /mTORC1/SK61 pathway, resulting in increased TSC1/TSC2 signaling. Since this effect was reversed by memantine in a cell-based model system, we reasoned that such an approach could possibly compensate for partial TSC deficiency, as found in the Tsc2+/− mouse model and in human TSC brains. In fact, we observed that short-term (4 hour) application of the new, improved drug NitroSynapsin, but not memantine, was able to reverse deficits in synaptic plasticity, as monitored approximately1 hour after induction of LTP in CA1 hippocampus. Importantly, a two-and-a-half-day treatment with NitroSynapsin reversed the synaptic loss observed in the hippocampus of Tsc2+/− mice as monitored by synaptophysin staining. Previous studies have emphasized the prevalence of anxiety-related disorders and impaired hippocampal-dependent memory in TSC (Chevere-Torres et al., 2012; de Vries et al., 2007; Ehninger et al., 2008; Ehninger and Silva, 2011; Lewis et al., 2004; Muzykewicz et al., 2007; Pulsifer et al., 2007; Raznahan et al., 2006). In this regard, we found that Tsc2+/− mice displayed a lack of discrimination between the training context and the novel context in the fear conditioning test, consistent with previous reports (Ehninger et al., 2008). However, a two-and-a-half-day treatment with NitroSynapsin, but not with memantine, normalized context discrimination in the Tsc2+/− mice. There is precedent for modification in fear conditioning behavior after short-term drug treatment with NMDAR antagonists (Costa et al., 2008). In our case, the normalization in context discrimination may represent improved hippocampal memory in Tsc2+/− mice after treatment with NitroSynapsin. Fear conditioning is a quite robust neurobehavioral test in mice, and the fact that the eNMDAR antagonist NitroSynapsin was of benefit in this paradigm is quite encouraging in terms of potential therapeutic intervention in patients with TSC. Note that we monitored electrophysiological parameters and LTP in 1 month-old animals, and behavior at 3 months. We followed this paradigm because we and others have found that the electrophysiological effects are more sensitive to the pathological process and therefore allow measurement earlier in disease progression than the behavioral changes. Along these lines, synaptic impairment begins prior to behavioral abnormality; hence, we used younger animals for LTP measurement compared to the behavioral tests.
Previously, in human studies NMDAR antagonists were known to exhibit a number of clinically-intolerable side effects, including hallucinations, dissociative/out of body feelings, and even coma, particularly with the use of high affinity agents that blocked synaptic NMDAR function. In contrast, we have shown that drugs with relatively rapid off-rates from the receptor, such as the aminoadamantane compounds memantine and NitroSynapsin, although of low affinity, retain high selectivity for the receptor at micromolar concentrations and are well tolerated. These attributes allow aminoadamantanes to avoid the usual clinical side effects of high-affinity NMDAR antagonists, in part by preferring eNMDARs because of their tonic activity (Okamoto et al., 2009; Takahashi et al., 2015). Moreover, activation of eNMDARs is known to be associated with synaptic pathology, whereas their inhibition by drugs like the aminoadamantanes can protect from synapse loss (Hardingham and Bading, 2010; Okamoto et al., 2009; Parsons and Raymond, 2014; Takahashi et al., 2015; Talantova et al., 2013).
Additionally, in empirical studies, aminoadamantanes like memantine have been reported to improve excitatory/inhibitory (E/I) imbalance in synaptic transmission (Martina et al., 2013). Importantly, E/I imbalance is thought to contribute to the neurobehavioral deficits of various forms of ASD via several molecular pathways (Chao et al., 2010; Han et al., 2013; Han et al., 2014; Penagarikano et al., 2011), but recent studies have suggested that change in the E/I ratio reflects a homeostatic response rather than the pathological phenomenon itself (Antoine et al., 2019). In any event, excessive excitation or impoverished inhibition from the underlying genetic defect could causally contribute to the pathology, and this is the process treated here. For example, in Tsc-deficient mice, impaired inhibitory synaptic function leads to relative hyperexcitability in the hippocampus (Bateup et al., 2013). However, despite initial enthusiasm, memantine has not been found to be effective for ASD in advanced human clinical trials, leading to termination of one trial for lack of efficacy (Chez et al., 2007; Fung and Hardan, 2015). Similarly, in the current study, although memantine is capable of inhibiting tonic eNMDAR overexcitability to some degree, it was not effective in the TSC2 mouse model. To improve upon memantine, we recently synthesized a novel series of drugs based on dual memantine-like action and redox-based inhibition of extrasynaptic NMDARs. The lead in this series of compounds, now designated NitroSynapsin, has been found to prevent synaptic loss, improve synaptic function, and prevent excessive excitation (Takahashi et al., 2015; Talantova et al., 2013). Moreover, the increased efficacy of NitroSynapsin over previous aminoadamantanes at inhibiting hyperfunctioning extrasynaptic NMDARs is thought to represent its mechanism for improving the function of compromised synapses with resultant correction of excessive excitation.
5. Conclusion
In summary, we sought to determine if administration of the FDA-approved drug memantine or the more efficacious eNMDAR antagonist, NitroSynapsin, would mitigate neurological manifestations in a mouse model of TSC. We discovered that NitroSynapsin offered significant benefit over memantine in an electrophysiological readout of synaptic plasticity, in neurohistological analysis of synapses, and in neurobehavioral assessments of anxiety and memory. These results suggest that NitroSynapsin should be tested as a potential therapeutic for the neurological aspects of TSC in an effort to improve quality of life.
Supplementary Material
Supplementary Fig. 1. Effect of treatment with memantine or NitroSynapsin on neuronal counts in the hippocampus of Tsc2+/− mice.
Supplementary Fig. 2. Effect of treatment with memantine or NitroSynapsin on MAP2-labeled neuropil of Tsc2+/− mice.
Supplementary Fig. 3. Effect of treatment with memantine or NitroSynapsin on GFAP-labeled astrocytes Tsc2+/− mice.
Supplementary Table 1. Phosphoproteomic analysis after eNMDAR stimulation of rat cortical neurons compared to control cultures.
Highlights:
Agents that preferentially block tonic extrasynaptic NMDA receptor activity ameliorate tuberous sclerosis phenotypes in mice.
Aberrant activity in the p38 MAPK-TSC-Rheb-mTORC1-S6K1 pathway of Tsc2+/− mice is rescued by NitroSynapsin treatment.
NitroSynapsin treatment corrects deficits in hippocampal long-term potentiation (LTP), histological loss of synapses, and behavioral fear conditioning in Tsc2+/− mice.
Limiting excessive extrasynaptic NMDA receptor activity may be a treatment for tuberous sclerosis.
Acknowledgements
We thank Weiping Tan and Maria Talantova for technical assistance. This work was supported in part by a Department of Defense (Army) grant, W81XWH-13-0053, and by NIH grant P01 HD29787 to S.A.L.).
Abbreviations:
- ACSF
artificial cerebrospinal fluid
- ASD
autism spectrum disorder
- E/I
excitatory/inhibitory
- eNMDAR
extrasynaptic NMDA-type glutamate receptor
- fEPSPs
field excitatory postsynaptic potentials
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- MAPKAPK2
mitogen-activated protein kinase-activated protein kinase 2
- MEA
microelectrode array
- TSC
Tuberous sclerosis complex
- WT
wild-type
Footnotes
Competing interests
S.A.L. is the named inventor on patents for memantine (Namenda®) and NitroSynapsin for the treatment of neurodegenerative diseases. Following Harvard University guidelines, he participates in a royalty sharing agreement with his former institution Harvard Medical School/Boston Children’s Hospital for the licensing of memantine patents to Forest Laboratories/Actavis/Allergan. S.A.L. was also a Scientific Co-Founder of Adamas Pharmaceuticals, Inc., which has agreements with Forest Laboratories/Actavis/Allergan for codeveloping long-lasting formulations of memantine (NamendaXR®) and the combination of memantine and donepezil (Namzaric®). The authors declare that they have no other competing interests. The new eNMDAR antagonist, NitroSynapsin, can be obtained through an MTA by writing to S.A.L. and Panorama Research, Inc., Sunnyvale, CA 94089.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akhtar MW, et al. , 2016. Elevated glucose and oligomeric beta-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat Commun. 7, 10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine MW, Langberg T, Schnepel P, Feldman DE 2019. Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models. Neuron 101, 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD, et al. , 2011. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 480, 63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, et al. , 2013. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron. 78, 510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, et al. , 1990. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 87, 1620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer K, et al. , 2008. Cellular localization of metabotropic glutamate receptors in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Neuroscience. 156, 203–15. [DOI] [PubMed] [Google Scholar]
- Bolton PF, et al. , 2002. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 125, 1247–55. [DOI] [PubMed] [Google Scholar]
- Chao HT, et al. , 2010. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 468, 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HS, Lipton SA, 1997. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol. 499 (Pt 1), 27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HS, et al. , 1992. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 12, 4427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevere-Torres I, et al. , 2012. Impaired social interactions and motor learning skills in tuberous sclerosis complex model mice expressing a dominant/negative form of tuberin. Neurobiol Dis. 45, 156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chez MG, et al. , 2007. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. J Child Neurol. 22, 574–9. [DOI] [PubMed] [Google Scholar]
- Choi YB, et al. , 2000. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci. 3, 15–21. [DOI] [PubMed] [Google Scholar]
- Dawson VL, et al. , 1991. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 88, 6368–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries PJ, et al. , 2009. Neuropsychological attention deficits in tuberous sclerosis complex (TSC). Am J Med Genet A. 149a, 387–95. [DOI] [PubMed] [Google Scholar]
- de Vries PJ, et al. , 2007. The psychopathologies of children and adolescents with tuberous sclerosis complex (TSC): a postal survey of UK families. Eur Child Adolesc Psychiatry. 16, 16–24. [DOI] [PubMed] [Google Scholar]
- Eales KL, et al. , 2014. The MK2/3 cascade regulates AMPAR trafficking and cognitive flexibility. Nat Commun. 5, 4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, et al. , 2008. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 14, 843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ, 2011. Increased levels of anxiety-related behaviors in a Tsc2 dominant negative transgenic mouse model of tuberous sclerosis. Behav Genet. 41, 357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emnett CM, et al. , 2013. Indistinguishable synaptic pharmacodynamics of the N-methyl-D-aspartate receptor channel blockers memantine and ketamine. Mol Pharmacol. 84, 935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung LK, Hardan AY, 2015. Developing Medications Targeting Glutamatergic Dysfunction in Autism: Progress to Date. CNS Drugs. 29, 453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, et al. , 2002. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 22, 4015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkogkas C, et al. , 2010. Translational control mechanisms in long-lasting synaptic plasticity and memory. J Biol Chem. 285, 31913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorden SM, et al. , 2007. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 62, 648–55. [DOI] [PubMed] [Google Scholar]
- Han K, et al. , 2013. SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature. 503, 72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, et al. , 2014. Enhancement of inhibitory neurotransmission by GABAA receptors having alpha2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron. 81, 1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H, 2010. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 11, 682–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, et al. , 2002. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 5, 405–14. [DOI] [PubMed] [Google Scholar]
- Harrison JE, et al. , 1999. Cognitive deficits in normally intelligent patients with tuberous sclerosis. Am J Med Genet. 88, 642–6. [PubMed] [Google Scholar]
- Hsieh DT, et al. , 2016. Tuberous sclerosis complex: Five new things. Neurology: Clinical Practice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, et al. , 2010. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Ann Neurol. 68, 342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ 3rd, et al. , 2012. High-throughput sequencing of mGluR signaling pathway genes reveals enrichment of rare variants in autism. PLoS One. 7, e35003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei SZ, et al. , 1992. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 8, 1087–99. [DOI] [PubMed] [Google Scholar]
- Lewis JC, et al. , 2004. Genotype and psychological phenotype in tuberous sclerosis. J Med Genet. 41, 203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. , 2008. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc. Natl. Acad. Sci. U. S. A 105, 9397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, 2006. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 5, 160–70. [DOI] [PubMed] [Google Scholar]
- Lipton SA, 2007. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 8, 803–8. [DOI] [PubMed] [Google Scholar]
- Lipton SA, et al. , 1993. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 364, 626–32. [DOI] [PubMed] [Google Scholar]
- Lozovaya N, et al. , 2014. Selective suppression of excessive GluN2C expression rescues early epilepsy in a tuberous sclerosis murine model. Nat Commun. 5, 4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, et al. , 2013. Selective Pharmacological Modulation of Pyramidal Neurons and Interneurons in the CA1 Region of the Rat Hippocampus. Front Pharmacol. 4, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykewicz DA, et al. , 2007. Psychiatric comorbid conditions in a clinic population of 241 patients with tuberous sclerosis complex. Epilepsy Behav. 11, 506–13. [DOI] [PubMed] [Google Scholar]
- Nie D, et al. , 2010. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci. 13, 163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, et al. , 2009. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 15, 1407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda H, et al. , 1999. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 104, 687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Raymond LA, 2014. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 82, 279–93. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, et al. , 2011. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 147, 235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather P, de Vries PJ, 2004. Behavioral and cognitive aspects of tuberous sclerosis complex. J Child Neurol. 19, 666–74. [DOI] [PubMed] [Google Scholar]
- Pulsifer MB, et al. , 2007. Psychological profile of adults with tuberous sclerosis complex. Epilepsy Behav. 10, 402–6. [DOI] [PubMed] [Google Scholar]
- Raznahan A, et al. , 2006. Psychopathology in tuberous sclerosis: an overview and findings in a population-based sample of adults with tuberous sclerosis. J Intellect Disabil Res. 50, 561–9. [DOI] [PubMed] [Google Scholar]
- Ridler K, et al. , 2007. Neuroanatomical correlates of memory deficits in tuberous sclerosis complex. Cereb Cortex. 17, 261–71. [DOI] [PubMed] [Google Scholar]
- Rojas DC, 2014. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J Neural Transm (Vienna). 121, 891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, et al. , 2016. Quantitative Analysis of Human Pluripotency and Neural Specification by In-Depth (Phospho)Proteomic Profiling. Stem Cell Reports. 7, 527–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley SL, 1998. Autism and tuberous sclerosis. J Autism Dev Disord. 28, 407–14. [DOI] [PubMed] [Google Scholar]
- Takahashi H, et al. , 2015. Pharmacologically targeted NMDA receptor antagonism by NitroMemantine for cerebrovascular disease. Sci Rep. 5, 14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talantova M, et al. , 2013. Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A. 110, E2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, et al. , 2014. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 83, 1131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF, et al. , 2005. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 8, 1727–34. [DOI] [PubMed] [Google Scholar]
- Waltereit R, et al. , 2006. Enhanced episodic-like memory and kindling epilepsy in a rat model of tuberous sclerosis. J Neurochem. 96, 407–13. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. , 2006. The pharmacology of aminoadamantane nitrates. Curr Alzheimer Res. 3, 201–4. [DOI] [PubMed] [Google Scholar]
- Wong M, et al. , 2003. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 54, 251–6. [DOI] [PubMed] [Google Scholar]
- Xia P, et al. , 2010. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 30, 11246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, et al. , 2010. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med. 2, 52ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Effect of treatment with memantine or NitroSynapsin on neuronal counts in the hippocampus of Tsc2+/− mice.
Supplementary Fig. 2. Effect of treatment with memantine or NitroSynapsin on MAP2-labeled neuropil of Tsc2+/− mice.
Supplementary Fig. 3. Effect of treatment with memantine or NitroSynapsin on GFAP-labeled astrocytes Tsc2+/− mice.
Supplementary Table 1. Phosphoproteomic analysis after eNMDAR stimulation of rat cortical neurons compared to control cultures.