Abstract
Vasculitis in Behçet’s disease, termed “vasculo-Behçet’s disease,” is a major cause of mortality and morbidity. We report a case of vasculo-Behçet’s disease complicated by conversion disorder, in which 18F-fluoro-deoxy-glucose positron emission tomography combined with computed tomography (FDG PET/CT) was useful for the diagnosis. A twenty-two-year-old woman recently diagnosed with tonsillitis presented with fever, right foot pain, left equinovarus foot, and numbness in both hands and feet. Laboratory data showed elevated levels of c-reactive protein (CRP). The patient was positive for HLA B51; pathergy testing was also positive. Nerve conduction velocity and electromyography were normal. MRI showed swelling of the left crural muscle group. PET/CT showed intense FDG uptake in the left popliteal artery, demonstrating active vasculitis. The patient was diagnosed with vasculo-Behçet’s disease and treated with corticosteroids, colchicine, and infliximab, which led to obvious improvement of the MRI findings and reduction in CRP. However, left equinovarus foot and numbness in the extremities persisted. She also developed aphonia. They were attributed to psychogenic dystonia and conversion disorder, and psychiatric treatment was effective in relieving those symptoms. We suggest that PET/CT may be useful for the early diagnosis of medium-sized vessel vasculitis in patients with Behçet’s disease.
Keywords: Vasculo-Behçet’s disease, PET/CT, Conversion disorder
Introduction
Behçet’s disease (BD) is a chronic inflammatory disease characterized by ocular lesions, recurrent oral aphthosis, genital ulcers, and other manifestations including skin lesions, gastrointestinal lesions, neurological lesions, and vascular lesions. Vascular manifestations are observed in up to 40% of patients with BD1). Although BD can affect any type and size of vessels, venous lesions are most common. According to the 2012 Revised International Chapel Hill consensus conference nomenclature of vasculitides2), BD is categorized as a “variable vessel vasculitis.”
Conversion disorder is a psychiatric disease that features neurological symptoms that cannot be explained by an organic neurological disorder3,4). Generally, conversion disorder is caused by significant stress or emotional trauma, and the symptoms include paralysis, dystonia, tremors, difficulty walking, blindness, deafness, numbness, and speech problems3). According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)5), it is categorized as a type of somatoform disorder. Although the exact cause is still unknown, significant stress, emotional or physical trauma, and neurological disorders are thought to be risk factors for conversion disorder3).
18F-fluoro-deoxy-glucose (FDG) is an analogue of glucose. FDG accumulates in cancer cells as well as in inflammatory tissues based on their increased glucose uptake and glycolysis6-10). FDG positron emission tomography combined with computed tomography (FDG PET/CT) can image foci of neoplasia or inflammation with increased glucose uptake. FDG PET/CT has been commonly used to detect not only various cancers but also large vessel vasculitis11-14).
We describe a case of vasculo-Behçet’s disease complicated by conversion disorder in which FDG PET/CT was useful for the diagnosis of vasculitis.
Case report
A 22-year-old woman developed a fever of 39°C and severe throat pain 3 weeks prior to presentation. Five days before presentation, the patient experienced temporal pain and tinnitus. Three days before presentation, she experienced pain and numbness in the right foot, and weakness in both feet. Two days before presentation, she developed numbness in the left foot. One day before presentation, numbness occurred in both hands. She had never smoked. Her medical history included atopic dermatitis and recent tonsillitis, for which she took an antibiotic with no improvement.
She was suspected of having Guillain-Barre syndrome, and referred to the department of neurology in our hospital. However, no abnormal findings were shown in nerve conduction velocity or electromyography. The patient was referred to our department for further examination and treatment.
On physical examination, the patient was alert. The temperature was 36.9°C, blood pressure 116/81 mmHg, pulse 78 beats/min, and respiratory rate 16 breaths/min. There was no temporal artery tenderness. The neck was nontender, without adenopathy. The cardiopulmonary examination was normal. The abdomen was soft, flat, nontender, with no masses. The liver and spleen were not palpable. The first metatarsophalangeal joints were swollen.
Neurologic examination showed normal cranial nerve function and cerebellar function. Deep tendon reflexes were 2+ at the biceps, triceps, brachioradialis, patellar, and Achilles tendons. A Babinski sign was not present. A left equinovarus foot was noted (Figure 1). The left foot offered strong resistance to passive movement. Generalized weakness of the lower extremities was noted when testing the quadriceps, iliopsoas, adductors, hamstrings, and the gastrocnemius-soleus muscle groups. On sensory examination, there was reduced sensation of temperature, pinprick, and light touch in the lower extremities. Reduced sensation of vibratory and position were shown in both 5th fingers and toes.
Figure 1.
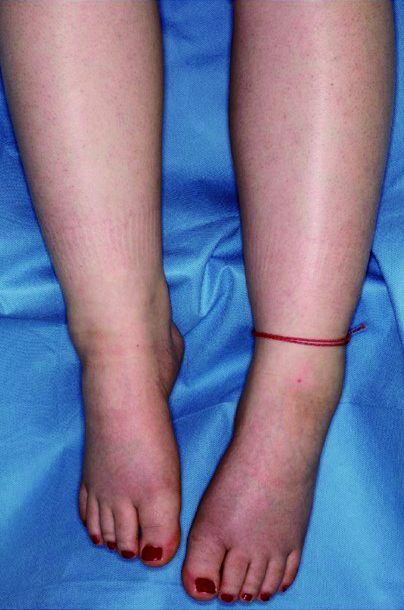
Equinovarus on the left side.
Blood tests showed an increased leukocyte count (10,200/μL) and platelet levels (38.9 × 104/μL). C-reactive protein (9.11 mg/dL) and erythrocyte sedimentation rate (49 mm/h) were also increased. Liver and renal functions were normal. Antinuclear antibodies (ANAs, titer:< 160) were negative. Levels of autoantibodies, including anti-dsDNA antibody, anti-Sm antibody, anti-ssA antibody, anti-ssB antibody, proteinase-3 antineutrophil cytoplasmic antibodies (PR3-ANCA), myeloperoxidase antineutrophil cytoplasmic antibodies (MPO-ANCA), and antiphospholipid antibodies (anti cardiolipin IgG antibody, anti β2-glycoprotein I antibody, lupus anticoagulant) were normal. Clioglobulin was negative. HLA serotyping showed B51. Urinalysis was normal. Blood and urinary microbiological cultures were negative. A lumbar puncture showed a normal cell count (2/μL), glucose (58 mg/dL), total protein levels (17 mg/dL). MBP and IL-6 levels were normal (MBP < 31.3 pg/mL, IL-6 1.4 pg/mL). There were no abnormalities in microbiological cultures or cytology.
For the pathergy test, the skin was subjected to two needle pricks using sterile 21-gauge needles after cleaning with 70% ethanol. After 48 hours, pustules had formed at the site of the needle pricks, which were interpreted as positive.
Audiometry showed mild sensorineural hearing loss on the left.
Gallium-67 scintigraphy showed gallium accumulation in the first metatarsophalangeal joints.
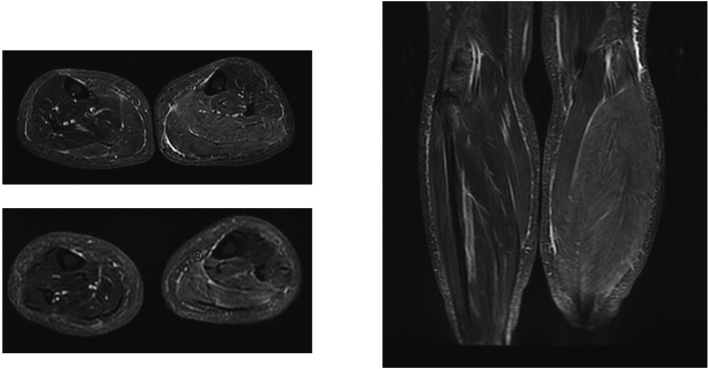
Brain and spinal cord magnetic resonance imaging (MRI) were normal. MRI of the lower legs showed swelling and high intensity in the crural musculature of the left lower leg in the slow-tau inversion recovery (STIR) sequence, which suggests myositis, myofasciitis, or edema (Figure 2).
Figure 2.
MRI of the lower legs shows swelling and high intensity of crural musculature in the left lower leg on slow tau inversion recovery (STIR).
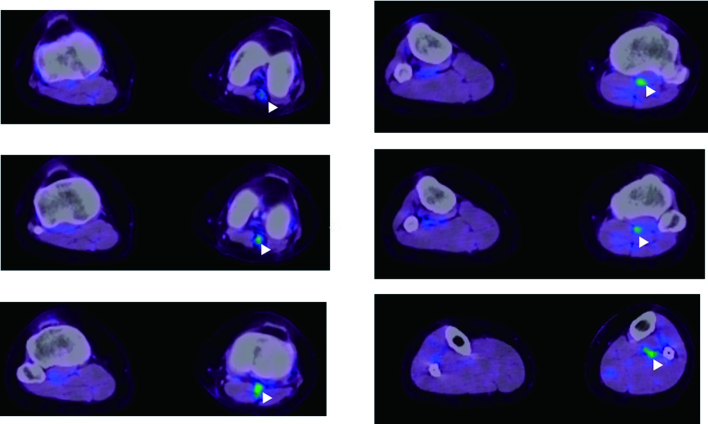
PET/CT was performed because of suspected large vessel vasculitis such as Cogan’s syndrome or Takayasu disease. FDG PET/CT demonstrated intense FDG uptake in the left popliteal artery, demonstrating active vasculitis (Figure 3).
Figure 3.
Fused PET/CT images of the patient. The PET images are presented using a rainbow scale and are superimposed on the CT images, which are shown in gray scale. The white arrowheads indicate the locations of the active inflammation. The images demonstrate intense FDG accumulation in the left popliteal artery.
The patient was diagnosed with BD (incomplete type) because of the arthritis and vasculitis, according to the Behçet’s Disease Research Committee Criteria in Japan15). Positive HLA B51 and positive pathergy testing also supported the diagnosis.
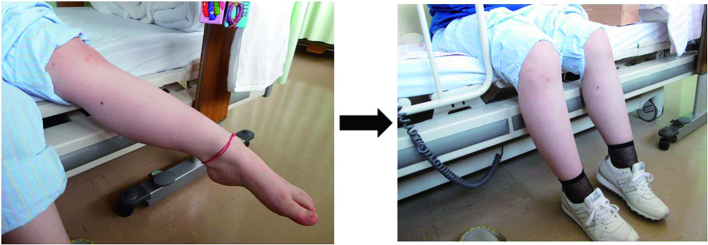
The patient was treated with intravenous methylprednisolone (1,000 mg/day for 3 days) followed by 60 mg of prednisolone p.o., 1 mg of colchicine p.o., and intravenous infliximab. These therapies led to obvious improvement of MRI findings in the left lower leg (Figure 4) and reduction of CRP. However, left equinovarus foot as well as numbness in the extremities did not improve. The patient then developed aphonia. We referred the patient to the Department of Psychiatry, where she was diagnosed with psychogenic dystonia and conversion disorder. Left equinovarus foot, numbness, and aphonia improved with psychiatric treatment (Figure 5).
Figure 4.
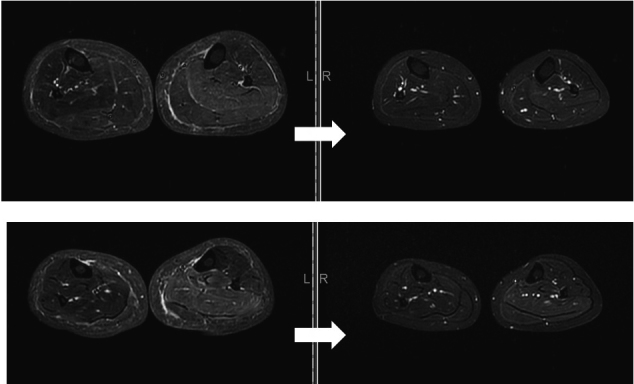
Improvement in MRI findings in the left lower leg.
Figure 5.
Equinovarus improved after treatment by a psychiatrist.
Discussion
In this case, the differential diagnosis for vasculitis of the left popliteal artery included Takayasu arteritis, Cogan’s syndrome, ANCA-associated vasculitis, polyarteritis nodosa (PN), and vasculo-Behçet disease (VBD). Takayasu arteritis and Cogan’s syndrome were excluded because of the lack of large vessel vasculitis. ANCA-associated vasculitis was excluded because of the negative ANCA and lack of organ involvement. PN was also excluded from the differential diagnosis because of lack of organ involvement. Although our patient lacked common symptoms such as ocular lesions or oral and/or genital aphthosis, the diagnosis of BD (incomplete type) was made according to the Behçet’s Disease Research Committee Criteria in Japan.
The Behçet’s Disease Research Committee Criteria in Japan, which are the most frequently used criteria in Japan, describe four major clinical features (recurrent oral aphthoid ulcers, skin lesions, ocular symptoms, genital ulcers) and five minor ones (arthritis, epididymitis, gastrointestinal lesions, vascular lesions, and central nervous system lesions)15). Vascular lesions generally occur in the first 5 years after diagnosis of BD (33%)1,16). In 7%-30% of VBD cases, however, vascular lesions occur as the presenting manifestation16,17).
When a vascular lesion is the presenting symptom of BD, as the criteria give more weight to ocular lesions, the diagnosis is occasionally difficult. Although the presence of HLA-B51 and a positive pathergy test is clinically useful, they are not included in the criteria. Therefore, the patient presenting with a vascular lesion needs to be examined for any of the other four minor symptoms (arthritis, epididymitis, gastrointestinal lesions, and central nervous system lesions). Although our patient did not complain of any joint pain, physical examination showed swollen metatarsophalangeal joints. In addition, Ga scintigraphy showed increased isotope uptake in the metatarsophalangeal joints. Therefore, we thought our patient had arthritis. As a result, the diagnosis of BD (incomplete type) was made. Positive HLA B51 and the positive pathergy test also supported the diagnosis.
In addition to the symptoms of VBD, the patient had left equinovarus foot, numbness in the extremities, and aphonia, which were attributed to psychogenic dystonia and conversion disorder. Psychogenic dystonia is a condition with symptoms resembling dystonia but which are caused by psychological factors. It has been reported that psychogenic dystonia can be a form of conversion disorder. Conversion disorder is a psychiatric illness in which neurological signs affect voluntary motor or sensory function without organic lesions. Generally, it is caused by significant stress or emotional trauma, and the risk factors for conversion disorder include being young, female, with family history of conversion disorder, having a neurological disorder, previous physical disability, exposure to other disabled subjects, and significant anxiety3,18). Our patient had several risk factors for conversion disorder, including female gender, youth, and exposure to other disabled subjects (nurse). Significant anxiety over the symptoms caused by vasculitis could be a trigger for conversion disorder. For most patients with conversion disorder, it is difficult to realize their inner conflict. Therefore, psychotherapy, which clarifies the emotional basis of symptoms, is thought to be essential. In addition to psychotherapy, the usefulness of physical therapy and medication for underlying psychiatric issues (such as depression or anxiety) are also reported.
VBD is classified as a specific type of BD. Aneurysm formation, arterial occlusion, and venous thrombosis are well-observed clinical manifestations of VBD. The frequency of vascular involvement in patients with BD ranges from 13% to 25%, and occurs more commonly in males. The mean age at onset of vascular lesions in BD is 29.5 y19,20). According to a report from Turkey, superficial venous thrombosis is the most common venous complication (53%) and deep vein thrombosis is the second most common (30%)16). Although the frequency of arterial involvement in VBD is less than that of venous involvement (1 to 7%), the mortality rate in patients with arterial lesions is higher21,22). Aneurysm or occlusion in the aorta or pulmonary artery can be a cause of sudden death.
In our case, PET/CT detected inflammation of the popliteal artery, while MRI failed to detect it. In the imaging studies for the diagnosis of vasculitis, CT is useful for showing the changes of vessel wall thickness, calcification, and thrombi11,23). MRI is also valid for evaluation of structural changes in vessels, such as aneurysms and stenosis; however, it is not sensitive for inflammation in grossly normal blood vessels11,24). Therefore, MRI may be not useful for the initial diagnosis of patients with vasculitis without obvious structural abnormalities.
Recently, FDG PET/CT has been increasingly used for the diagnosis of large vessel vasculitis (LVV), and provides high sensitivities and specificities (giant cell arteritis: sensitivities ranging 77%-92%, specificities ranging 89%-100%11,13), Takayasu arteritis: sensitivity 92%, specificity 100%11,25). In particular, it is noteworthy that PET/CT is useful for the diagnosis of vasculitis in patients presenting with nonspecific symptoms. However, it should be noted that the Japanese national health insurance system allows the use of PET/CT not for initial diagnosis of LVV, but for the evaluation and localization of disease activity in patients already diagnosed with LVV.
Until recently, PET/CT could only detect inflammatory lesions of the aorta and large vessels due to its limited spatial resolution13,14). Improvements in spatial resolution allow PET/CT to detect FDG uptake in smaller arteries. Our report suggests that PET/CT is useful not only for the early diagnosis of large vessel vasculitis, but also for that of medium-sized vessel vasculitis.
References
- 1.Kural-Seyahi E, Fresko I, Seyahi N, et al. The long-term mortality and morbidity of Behcet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore), 82: 60-76, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Bavon PA, et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum, 65: 1-11, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Ali S, Jabeen S, Pate RJ, et al. Conversion Disorder-Mind versus Body: A Review. Innov Clin Neurosci, 12: 27-33, 2015. [PMC free article] [PubMed] [Google Scholar]
- 4.Blitzstein SM. Recognizing and treating conversion disorder. Virtual Mentor, 10: 158-160, 2008. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric A, American Psychiatric A, Force DSMT. Diagnostic and statistical manual of mental disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2017. [Google Scholar]
- 6.Otsuka H, Morita N, Yamashita K, Nishitani H. FDG-PET/CT for diagnosis and follow-up of vasculitis. J Med Invest, 54: 345-349, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Familiari D, Glaudemans AW, Vitale V, et al. Can sequential 18F-FDG PET/CT replace WBC imaging in the diabetic foot ? J Nucl Med, 52: 1012-1019, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Mostard RL, Voo S, van Kroonenburgh MJ, et al. Inflammatory activity assessment by F18 FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med, 105: 1917-1924, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med, 51: 1937-1949, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher JW, Kymes SM, Gould M, et al. A comparison of the diagnostic accuracy of 18F-FDG PET and CT in the characterization of solitary pulmonary nodules. J Nucl Med, 49: 179-185, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A. The use of 18F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol, 2013: 623036. Doi: 10.1155/2013/623036. Epub 2013 Aug 21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs M, Briel M, Daikeler T, et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging, 39: 344-353, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Zerizer I, Tan K, Khan S, Barwick T, et al. Role of FDG-PET and PET/CT in the diagnosis and management of vasculitis. Eur J Radiol, 73: 504-509, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Treglia G, Mattoli MV, Leccisotti L, Ferraccioli G, Giordano A. Usefulness of whole-body fluorine-18-fluorodeoxyglucose positron emission tomography in patients with large-vessel vasculitis: a systematic review. Clin Rheumatol, 30: 1265-1275, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Kurokawa M, Suzuki N. Behcet’s disease. Clin Exp Med, 4: 10-20, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Sarica-Kucukoglu R, Akdag-Kose A, Kayabal IM, et al. Vascular involvement in Behcet’s disease: a retrospective analysis of 2319 cases. Int J Dermatol, 45: 919-921, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Melikoglu M, Kural-Seyahi E, Tascilar K, Yazici H. The unique features of vasculitis in Behcet’s syndrome. Clin Rev Allergy Immunol, 35: 40-46, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Hafeiz HB. Hysterical conversion: a prognostic study. Br J Psychiatry, 136: 548-551, 1980. [DOI] [PubMed] [Google Scholar]
- 19.Fei Y, Li X, Lin S, et al. Major vascular involvement in Behcet’s disease: a retrospective study of 796 patients. Clin Rheumatol, 32: 845-852, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi H. What Is Vascular Behcet’s Disease ? Ann Vasc Dis, 11: 52-56, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saadoun D, Asli B, Wechsler B, et al. Long-term outcome of arterial lesions in Behcet disease: a series of 101 patients. Medicine (Baltimore), 91: 18-24, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Rokutanda R, Kishimoto M, Okada M. Update on the diagnosis and management of Behcet’s disease. Open access rheumatology: research and reviews, 7: 1-8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bau JL, Ly JQ, Borstad GC, Lusk JD, Seay TM, Beall DP. Giant cell arteritis. AJR Am J Roentgenol, 181: 742, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Bertagna F, Bosio G, Caobelli F, Motta F, Biasiotto G, Giubbini R. Role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography for therapy evaluation of patients with large-vessel vasculitis. Jpn J Radiol, 28: 199-204, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Webb M, Chambers A, AL-Nahhas, A, et al. The role of 18F-FDG PET in characterising disease activity in Takayasu arteritis. Eur J Nucl Med Mol Imaging, 31: 627-634, 2004. [DOI] [PubMed] [Google Scholar]