Abstract
Polyphenols may play a chemopreventive role in colorectal cancer (CRC); however, epidemiological evidence supporting a role for intake of individual polyphenol classes, other than flavonoids is insufficient. We evaluated the association between dietary intakes of total and individual classes and subclasses of polyphenols and CRC risk and its main subsites, colon and rectum, within the European Prospective Investigation into Cancer and Nutrition (EPIC) study. The cohort included 476,160 men and women from 10 European countries. During a mean follow-up of 14 years, there were 5,991 incident CRC cases, of which 3,897 were in the colon and 2,094 were in the rectum. Polyphenol intake was estimated using validated centre/country specific dietary questionnaires and the Phenol-Explorer database. In multivariable-adjusted Cox regression models, a doubling in total dietary polyphenol intake was not associated with CRC risk in women (HRlog2 = 1.06, 95 % CI 0.99-1.14) or in men (HRlog2 = 0.97, 95 % CI 0.90-1.05), respectively. Phenolic acid intake, highly correlated with coffee consumption, was inversely associated with colon cancer in men (HRlog2 = 0.91, 95 % CI 0.85-0.97) and positively associated with rectal cancer in women (HRlog2 = 1.10, 95 % CI 1.02-1.19); although associations did not exceed the Bonferroni threshold for significance. Intake of other polyphenol classes was not related to colorectal, colon or rectal cancer risks. Our study suggests a possible inverse association between phenolic acid intake and colon cancer risk in men and positive with rectal cancer risk in women.
Keywords: Polyphenols, intake, diet, colorectal cancer, prospective cohort, EPIC
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of death from cancer worldwide, with 1.4 million new cases and 694,000 deaths in 2012 (1). Lifestyle (physical inactivity, body fatness, tobacco smoking and alcohol consumption) and dietary factors, such as a high intake of red and processed meat and low intake of fruit and vegetables, are known to increase CRC risk (2).
Polyphenols are bioactive compounds naturally contained in plant-based foods, such as tea, coffee, wine, fruit, vegetables, whole-grain cereals, and cocoa (3). Experimental studies have shown anti-carcinogenic properties of polyphenols against CRC through several plausible biological mechanisms including modulation of nuclear factor (NF)-κB genes involved in inflammation and carcinogenesis, reduction of oxidative damage to lipids and DNA, induction of phase I and II enzymes, inhibition of angiogenesis, stimulation of DNA repair and apoptosis (4–7). Based on their chemical backbone, polyphenols are divided into 4 main classes: flavonoids, phenolic acids, lignans, and stilbenes (3). Polyphenols can be absorbed in the small intestine, although the vast majority, from 50 to 99% depending on the polyphenol, transit down to the colon where they can be metabolized by the gut microbiota and partially absorbed in the con as small phenolic acids (8). Furthermore, polyphenols can modulate gut microbiota, both in quantity and type of species (9). Imbalanced gut microbiota, called dysbiosis, can alter both metabolism and absorption of polyphenols, and may also induce aberrant molecular signalling, triggering the CRC pathogenesis (10).
To date, several case-control studies suggest an inverse association between flavonoid and lignan intake and CRC risk (3). However, no association in cohort studies has been observed so far (3;11;12) including our previous results in the European Prospective Investigation into Cancer and Nutrition (EPIC) study with a shorter follow-up (13); except for the Iowa Women’s Health study, in which an inverse association between flavanol intake and rectal cancer risk was shown (14). To our knowledge, there is only one case-control study investigating the relationships with other polyphenol classes, such as phenolic acids, stilbenes and other minor subclasses in Japan (15). In this previous study, intakes of coffee polyphenols and consequently coffee consumption were inversely associated with CRC risk in men and women, especially with colon cancer (15).
The Phenol-Explorer (www.phenol-explorer.eu) (16), a food composition database on all known dietary polyphenols, greatly facilitates the assessment of relationships between polyphenol intake and chronic disease risk. The aim of the present study was to investigate the associations between the intake of total polyphenols and individual polyphenol subclasses and CRC risk and by subsite (colon and rectum) in the EPIC study, a large cohort with a high variability in polyphenol intake and a long follow-up (17).
Materials and Methods
Subjects and study design
EPIC is an on-going cohort consisting of 521,324 adult participants, mostly recruited from the general population, enrolled between 1992 and 2000 from 23 centres in 10 European countries: Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden and the United Kingdom (18). All participants gave written informed consent, and the study was approved by the local ethics committees in the participating countries and the ethical review board of the International Agency for Research on Cancer (IARC). We excluded participants with prevalent cancer other than non-melanoma skin cancer at baseline or with missing information on date of diagnosis or incomplete follow-up data (n=29,332), missing data on dietary or lifestyle factors (n=6,259), extreme energy intake and/or expenditure (participant in the top or the bottom 1% of the distribution of the ratio of total energy intake to energy requirement; n=9,573). In the current analysis, 476,160 men and women were included.
Identification and follow-up of colorectal cancer cases
Cancer cases were identified through population cancer registries in Denmark, Italy, the Netherlands, Norway, Spain, Sweden and the United Kingdom. In France, Germany, Greece and Naples-Italy, a combination of methods was used including health insurance records, cancer and pathology registries, and by active follow-up of study participants and their next of kin. Vital status was collected from regional or national mortality registries.
Cancer incidence data were coded according to the 10th revision of the International Statistical Classification of Diseases, Injuries and Causes of Death (ICD-10) and the second revision of the International Classification of Diseases for Oncology (ICDO-2). Proximal colon cancers included those within the cecum, appendix, ascending colon, hepatic flexure, transverse colon, and splenic flexure (C18.0–18.5). Distal colon cancers included those within the descending (C18.6) and sigmoid (C18.7) colon. Overlapping (C18.8) and unspecified (C18.9) lesions of the colon were grouped among all colon cancers only (C18.0-C18.9). Cancer of the rectum included tumours occurring at the recto sigmoid junction (C19) and rectum (C20). Five hundred and fourteen cases were censored because they were carcinoma in situ (n=193), non-adenocarcinoma, mixed types or not well defined (n=312), unknown histology of the cancer (n=5), or a CRC originating from other organs (n=4).
Dietary assessment and data collection
At recruitment, validated country/centre-specific dietary questionnaires were used for recording habitual diet over the previous 12 months (18;19). Most centres utilized a self-administered food frequency questionnaire. In the remaining centres (Greece, Spain, and Ragusa and Naples-Italy), a face-to-face diet history questionnaire was employed to collect dietary information. In Malmö-Sweden, a method combining a food frequency questionnaire with a 7-day dietary diary and 1h interview was used. Total energy, alcohol, and nutrient intakes were estimated by using the standardized EPIC Nutrient Database (20).
Lifestyle questionnaires were collected to obtain information on lifetime and smoking status, physical activity classified according to the Cambridge Physical Activity Index (21), education, menstrual and reproductive history. Height and weight were measured at baseline in all centres except for Norway, France, and the majority of participants in EPIC-Oxford where anthropometric measures were self-reported (18).
Polyphenol intake
Dietary polyphenol intake was estimated using the Phenol-Explorer database (16) accounting for cooking and processing of foods via retention factors (22), as previously described (17;23). Total polyphenols was calculated as the sum of all classes of polyphenols: flavonoids [anthocyanidins, chalcones, dihydrochalcones, dihydroflavonols, flavanols (including flavan-3-ol monomers, proanthocyanidins, theaflavins), flavanones, flavones, flavonols, and isoflavones], phenolic acids (hydroxybenzoic acids, hydroxycinnamic acids, and hydroxyphenylacetic acids), lignans, stilbenes, and other minor polyphenols (alkylphenols, tyrosols, alkymethoxyphenols, furanocoumarins, hydroxybenzaldehydes, and hydroxycoumarins). The content of polyphenols was expressed in mg/100 g of food fresh weight.
Statistical analysis
Polyphenol intakes were analysed as categorical variables based on quintiles of the distribution among the entire EPIC cohort and by sex. Tests for linear trend were performed by assigning the medians of each quintile as scores. Polyphenol intakes were also analysed as continuous variables, after log2 transformation to improve normality of intake distributions. Each increase of one unit corresponded to a doubling in intake.
Multivariable Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (CIs) of the associations between total, classes and subclasses of polyphenol intakes and CRC risk. A chi-squared test based upon the scaled Schoenfeld residuals was used to ensure that the assumptions of proportional hazards were met. Age was the primary time variable in all models. Entry time was age at recruitment and exit time was age at diagnosis, death or censoring date (lost or end of follow-up), whichever came first. Model 1 was stratified by centre (to control for differences in questionnaires, follow-up procedures) and age at baseline (1-y interval). Model 2 was additionally adjusted for non-dietary variables: smoking status and intensity (never, former quit <11 years, former quit 11–20 years, former quit >20 years, current <16 cigarettes/d, current 16–25 cigarettes/d, current >25 cigarettes/d, current occasional, and not specified), physical activity (inactive, moderately inactive, moderately active, active, and not specified), education level (none, primary school, technical/professional school, secondary school, university or higher, and not specified), and body mass index (BMI, continuous kg/m2); and in women also for menopausal status (pre-, peri-, post-menopausal, surgical menopause), hormone replacement therapy use (yes, no, and unknown), and oral contraceptive use (yes, no, and unknown). Model 3 was further adjusted for dietary variables: total energy intake (kJ/d), alcohol (g/d), red and processed meat (g/d), fibre (g/d) and calcium (mg/d) intakes. The multivariable model for phenolic acids was additionally adjusted for coffee intake, because coffee is its main food source by far (17). Moreover, model 1 and 2 were also adjusted for total energy intake to assess the effect of absolute versus relative intakes of polyphenols in the diet. Results of Cox models with and without adjusting for total energy intake were almost identical. Furthermore, polyphenol intakes were also included in the statistical models as nutrient density (mg/8240kJ day) (24). This energy-adjustment method did not modify the results appreciably.
Interactions between polyphenol intakes (continuous as mg/day) and sex, age (<55 years, 55 to 65 years, or >65 years), BMI (BMI<25, 25 to <30, ≥30 kg/m2), tobacco smoking status (never, former, current smokers) and alcohol consumption (for women <15g/d and ≥15g/d; and for men <30g/d and ≥30g/d) were evaluated in separate analyses. The statistical significance of interactions on the multiplicative scale was assessed using the likelihood ratio test. Separate sex-specific models were fitted because a statistically significant interaction between sex and intake of total polyphenols was detected. In addition, we assessed separate models by smoking status category because a statistically significant interaction with smoking status (never, former, and current smokers) was observed. The Wald test statistic was used to evaluate heterogeneity by anatomical subsites of CRC (colon, proximal colon, distal colon, and rectum). Additional analyses by length of follow-up [censoring data at 3-, 6-, 9-, 12-, 15-, 18-years, and maximum of follow-up (22.8 years)] were performed. Sensitivity analyses were performed by repeating main analyses after the exclusion of 462 CRC cases diagnosed during the first 2 years of follow-up (279 colon and 183 rectum cancer cases). All P values presented are 2-tailed and were considered to be statistically significant when P <0.05. To account for multiple testing for the subclasses of polyphenols, Bonferroni correction was used and then results were considered statistically significant if P<0.05/26 (number of tests for the intakes of all polyphenol subclasses) <0.002. All statistical analyses were conducted using R 3.2.1 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
During 13.9 (4.0) years of mean (SD) follow-up, 5,991 (56.8% in women) incident primary CRC cases were diagnosed, of which 3,897 were identified as colon cancers (including 1,877 proximal, 1,743 distal, and 277 overlapping or unspecified colon cancers) and 2,094 as rectum cancers. The number of participants and distribution of CRC cases by country and sex are presented in Table 1. The highest estimated median of total polyphenol intakes among both sexes were in Denmark; whereas the lowest intakes amongst women and men were observed in Norway and Spain, respectively (Table 1). Phenolic acids were the main contributors to total polyphenols (51.0%), followed by flavonoids (44.2%), other minor polyphenol classes (4.4%), lignans (0.2%) and stilbenes (0.2%). Baseline characteristics of study participants by quintile of total polyphenol intake are shown in Supplementary Table 1. Men and women in the higher polyphenol intake groups were older, more physically active, had a lower BMI, higher educational level, and had a lower proportion of never smokers. Higher total polyphenol intake was also associated with higher average intakes of total energy, alcohol, calcium, fibre and red meat compared to participants with lower total polyphenol intakes. Furthermore, women with higher total polyphenol intakes were more likely to be post-menopausal and users of hormone replacement therapy and oral contraceptives than those with lower total polyphenol intakes.
Table 1. Distribution of subjects and colorectal cancer cases according to anatomical subsite and medians (5th–95th percentiles) of total polyphenol intake in 10 participating countries in the EPIC Study.
| Country | N | Colorectal cancer cases, N |
Polyphenol intake (mg/d) | Flavonoid intake (mg/d) | Phenolic acid intake (mg/d) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Colon | Proximal | Distal | NOS | Rectum | |||||
| Women | ||||||||||
| Denmark | 28,720 | 533 | 363 | 170 | 161 | 32 | 170 | 1,552 (802-2,481) | 514 (133-1,459) | 890 (320-1,547) |
| France | 67,403 | 410 | 264 | 129 | 125 | 10 | 146 | 1,320 (552-2,603) | 514 (188-1,226) | 679 (165-1,848) |
| Germany | 27,379 | 177 | 121 | 66 | 53 | 2 | 56 | 1,033 (549-1,927) | 414 (153-1,051) | 504 (194-1,074) |
| Greece | 15,233 | 41 | 25 | 11 | 7 | 7 | 16 | 759 (345-1,556) | 247 (101-528) | 416 (105-1,105) |
| Italy | 30,513 | 342 | 264 | 119 | 116 | 29 | 78 | 853 (443-1,438) | 413 (175-791) | 377 (118-757) |
| Norway | 33,975 | 297 | 195 | 104 | 86 | 5 | 102 | 653 (263-1,090) | 184 (61-400) | 371 (66-844) |
| Spain | 24,850 | 218 | 154 | 57 | 79 | 18 | 64 | 671 (254-1,407) | 282 (80-684) | 311 (61-907) |
| Sweden | 26,368 | 442 | 305 | 182 | 108 | 15 | 137 | 838 (418-1,465) | 272 (89-678) | 488 (166-971) |
| The Netherlands | 26,912 | 387 | 268 | 154 | 109 | 5 | 119 | 1,158 (631-1,760) | 514 (185-1,008) | 574 (186-985) |
| United Kingdom | 52,566 | 555 | 381 | 216 | 132 | 33 | 174 | 1,443 (662-2,240) | 873 (317-1,495) | 469 (129-1,054) |
| TOTAL | 333,919 | 3,402 | 2,340 | 1,208 | 976 | 156 | 1,062 | 1,054 (415-2,148) | 420 (116-1,239) | 508 (123-1,318) |
| Men | ||||||||||
| Denmark | 26,294 | 709 | 395 | 161 | 202 | 32 | 314 | 1,594 (809-2,460) | 397 (107-1,271) | 993 (359-1,629) |
| France | - | - | - | - | - | - | - | - | - | - |
| Germany | 21,178 | 258 | 141 | 59 | 67 | 15 | 117 | 1,093 (554-2,079) | 402 (140-1,056) | 549 (199-1,226) |
| Greece | 10,815 | 51 | 31 | 10 | 10 | 11 | 20 | 967 (469-1,921) | 302 (126-614) | 538 (153-1,377) |
| Italy | 14,032 | 228 | 160 | 55 | 86 | 19 | 68 | 1,009 (522-1,695) | 493 (202-964) | 428 (156-805) |
| Norway | - | - | - | - | - | - | - | - | - | - |
| Spain | 15,139 | 339 | 220 | 81 | 126 | 13 | 119 | 834 (333-1,725) | 425 (118-1,085) | 315 (92-769) |
| Sweden | 22,306 | 473 | 284 | 142 | 136 | 6 | 189 | 888 (442-1,568) | 252 (75-664) | 544 (193-1,064) |
| The Netherlands | 9,627 | 119 | 58 | 29 | 26 | 3 | 61 | 1,155 (601-1,854) | 398 (137-910) | 674 (178-1,198) |
| United Kingdom | 22,850 | 412 | 268 | 132 | 114 | 22 | 144 | 1,509 (735-2,309) | 916 (334-1,519) | 517 (157-1,076) |
| TOTAL | 142,241 | 2,589 | 1,557 | 669 | 767 | 121 | 1,032 | 1,150 (505-2,159) | 419 (117-1,246) | 562 (162-1,396) |
In multivariable models, total polyphenol intake was not associated with CRC risk in either women (HRlog2 = 1.06, 95 % CI 0.99 - 1.14) or men (HRlog2 = 0.97, 95 % CI 0.90 - 1.05) (Psex-interaction < 0.001) (Table 2). Null associations were also observed with the risk of colon cancer and its anatomical subsites (proximal and distal) in women; although a borderline statistically significant inverse association was observed in men for colon cancer, especially for proximal cancer (HRlog2 = 0.85, 95 % CI 0.73 – 0.99). Higher intakes of total polyphenols were significantly associated with a higher rectal cancer in women (HRlog2 = 1.25, 95 % CI 1.10 - 1.41) but not in men (HRlog2 = 1.08, 95 % CI 0.95 - 1.23) (Psex-interaction = 0.026).
Table 2. HRs (95% CIs) for colorectal cancer (CRC) and subsites, according to quintile of intake of total polyphenols in women and men from the EPIC study.
| Overall CRC | Colon | Proximal | Distal | P-value1 | Rectum | P-value2 | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Women | ||||||||
| Model 1 | Quintile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Quintile 2 | 1.00 (0.89-1.13) | 0.96 (0.83-1.10) | 1.02 (0.84-1.24) | 0.86 (0.70-1.06) | 1.13 (0.91-1.40) | |||
| Quintile 3 | 1.11 (0.99-1.26) | 1.02 (0.89-1.18) | 1.06 (0.87-1.30) | 0.96 (0.77-1.19) | 1.37 (1.10-1.71) | |||
| Quintile 4 | 1.10 (0.97-1.25) | 0.99 (0.85-1.16) | 1.14 (0.92-1.41) | 0.81 (0.64-1.02) | 1.39 (1.10-1.76) | |||
| Quintile 5 | 1.12 (0.98-1.28) | 0.99 (0.85-1.17) | 1.12 (0.89-1.41) | 0.85 (0.66-1.09) | 1.45 (1.34-1.86) | |||
| P-trend | 0.09 | 0.93 | 0.26 | 0.22 | 0.004 | |||
| Continuous (log2) | 1.06 (0.99-1.13) | 0.99 (0.92-1.07) | 1.05 (0.94-1.16) | 0.92 (0.83-1.03) | 0.11 | 1.22 (1.09-1.37) | 0.002 | |
| Model 3 | Quintile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Quintile 2 | 1.00 (0.89-1.13) | 0.96 (0.83-1.10) | 1.02 (0.84-1.24) | 0.86 (0.70-1.07) | 1.13 (0.91-1.41) | |||
| Quintile 3 | 1.12 (0.99-1.26) | 1.02 (0.88-1.18) | 1.05 (0.86-1.30) | 0.97 (0.78-1.21) | 1.39 (1.11-1.74) | |||
| Quintile 4 | 1.10 (0.97-1.26) | 0.99 (0.85-1.17) | 1.13 (0.90-1.41) | 0.82 (0.64-1.06) | 1.41 (1.10-1.80) | |||
| Quintile 5 | 1.13 (0.97-1.30) | 1.00 (0.83-1.19) | 1.11 (0.86-1.42) | 0.87 (0.66-1.14) | 1.49 (1.14-1.94) | |||
| P-trend | 0.10 | 0.92 | 0.35 | 0.36 | 0.006 | |||
| Continuous (log2) | 1.06 (0.99-1.14) | 0.99 (0.91-1.07) | 1.04 (0.93-1.17) | 0.93 (0.82-1.06) | 0.22 | 1.25 (1.10-1.41) | 0.002 | |
| Men | ||||||||
| Model 1 | Quintile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Quintile 2 | 1.02 (0.90-1.16) | 1.08 (0.92-1.27) | 0.95 (0.74-1.22) | 1.23 (0.98-1.54) | 0.92 (0.75-1.13) | |||
| Quintile 3 | 0.96 (0.84-1.10) | 1.02 (0.86-1.21) | 0.94 (0.73-1.20) | 1.14 (0.90-1.44) | 0.88 (0.71-1.08) | |||
| Quintile 4 | 0.94 (0.82-1.08) | 0.93 (0.78-1.11) | 0.81 (0.62-1.06) | 1.07 (0.83-1.37) | 0.95 (0.77-1.18) | |||
| Quintile 5 | 0.89 (0.77-1.02) | 0.82 (0.68-0.99) | 0.78 (0.59-1.03) | 0.84 (0.64-1.11) | 0.97 (0.78-1.22) | |||
| P-trend | 0.05 | 0.010 | 0.05 | 0.07 | 0.94 | |||
| Continuous (log2) | 0.94 (0.88-1.01) | 0.88 (0.81-0.97) | 0.85 (0.74-0.97) | 0.91 (0.80-1.04) | 0.43 | 1.05 (0.93-1.17) | 0.022 | |
| Model 3 | Quintile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Quintile 2 | 1.03 (0.91-1.17) | 1.10 (0.93-1.29) | 0.95 (0.74-1.22) | 1.27 (1.01-1.59) | 0.93 (0.75-1.14) | |||
| Quintile 3 | 0.97 (0.85-1.11) | 1.04 (0.87-1.23) | 0.93 (0.72-1.21) | 1.18 (0.92-1.51) | 0.88 (0.71-1.10) | |||
| Quintile 4 | 0.96 (0.83-1.11) | 0.95 (0.79-1.15) | 0.80 (0.61-1.06) | 1.12 (0.86-1.46) | 0.96 (0.77-1.21) | |||
| Quintile 5 | 0.94 (0.80-1.10) | 0.89 (0.72-1.09) | 0.79 (0.58-1.08) | 0.95 (0.71-1.28) | 1.01 (0.79-1.29) | |||
| P-trend | 0.30 | 0.09 | 0.10 | 0.36 | 0.65 | |||
| Continuous (log2) | 0.97 (0.90-1.05) | 0.91 (0.82-1.01) | 0.85 (0.73-0.99) | 0.97 (0.84-1.12) | 0.21 | 1.08 (0.95-1.23) | 0.036 | |
P-value for heterogeneity for proximal vs distal colon cancer
P-value for heterogeneity for colon vs rectum cancer
Model 1: Cox model was stratified by age and centre.
Model 3: Cox model was additionally adjusted for smoking status and intensity, physical activity, education level, body mass index, total energy intake, alcohol, red and processed meat, fibre (g/d) and calcium (mg/d) intakes and in women also for menopausal status, hormone replacement therapy use, and oral contraceptive use.
For CRC, no statistically significant relationships were observed between any of the classes and subclasses of polyphenols neither in women nor in men (Table 3). For colon cancers, inverse associations with the intake of total phenolic acids (HRlog2 = 0.91, 95 % CI 0.85 - 0.97; P=0.005) (Psex-interaction < 0.001) and its main subclass hydroxycinnamic acids (HRlog2 = 0.92, 95 % CI 0.87 - 0.97; P=0.004), as well as for methoxyphenols (HRlog2 = 0.99, 95 % CI 0.98 – 1.00; P=0.007) were found only in men. For rectal cancers, positive associations were observed in women with the intake of phenolic acids (HRlog2 = 1.10, 95 % CI 1.02 - 1.19; P=0.013) (Psex-interaction = 0.22), and its subclasses hydroxybenzoic acids (HRlog2 = 1.05, 95 % CI 1.00 - 1.10; P=0.039), and hydroxycinnamic acids (HRlog2 = 1.07, 95 % CI 1.00 - 1.15; P=0.038), as well as for flavanones (HRlog2 = 1.03, 95 % CI 1.00 - 1.07; P=0.048), alkylmethoxyphenols (HRlog2 = 1.04, 95 % CI 1.00 - 1.08; P=0.031), and methoxyphenols (HRlog2 = 1.02, 95 % CI 1.00 - 1.03; P=0.036). In women, a significant positive association was also detected between the risk of rectal cancer and flavonoid intake using the continuous variable (HRlog2 = 1.09, 95 % CI 1.00 - 1.18; P=0.039), but not using the quintiles (HRQ5 vs Q1 = 1.23, 95 % CI 0.94 - 1.60; P-trend=0.41). In men, an inverse association was found between hydroxybenzaldehyde intake and rectal cancer (HRlog2 = 0.97, 95 % CI 0.95 – 1.00; P=0.035). However, none of these associations exceeded the Bonferroni significance threshold.
Table 3. Hazard ratios (95% CIs) for colorectal cancer and subsites, according to double the intake of polyphenol classes and subclasses by sex in the EPIC study.
| Women | Men | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intake (mg/d) median (P 5%-P 95%) | Colorectal HR (95% CI) | Colon HR (95% CI) | Rectum HR (95% CI) | P-value1 | Intake (mg/d) median (P 5%-P 95%) | Colorectal HR (95% CI) | Colon HR (95% CI) | Rectum HR (95% CI) | P-value1 | P-value2 | |
| Flavonoid subclasses | 419.7 (116.3-1238.9) | 1.03 (0.98-1.08) | 1.00 (0.95-1.06) | 1.09 (1.00-1.18)* | 0.10 | 418.8 (117.4-1245.8) | 1.00 (0.96-1.05) | 1.00 (0.94-1.06) | 1.01 (0.94-1.09) | 0.87 | 0.030 |
| Anthocyanins | 25.5 (3.7-116.1) | 1.01 (0.99-1.04) | 1.00 (0.97-1.03) | 1.02 (0.97-1.06) | 0.46 | 22.9 (2.8-120.5) | 0.99 (0.97-1.01) | 1.00 (0.97-1.02) | 0.98 (0.95-1.01) | 0.49 | 0.09 |
| Dihydrochalcones | 1.8 (0.1-6.3) | 1.00 (0.99-1.02) | 1.00 (0.99-1.02) | 1.01 (0.98-1.03) | 0.76 | 1.5 (0.1-6.9) | 1.00 (0.99-1.01) | 0.99 (0.98-1.01) | 1.00 (0.98-1.01) | 0.61 | 0.038 |
| Dihydroflavonols | 0.4 (0.0-9.6) | 1.00 (1.00-1.01) | 1.00 (0.99-1.00) | 1.00 (0.99-1.01) | 0.20 | 1.0 (0.0-18.4) | 0.99 (0.99-1.00) | 0.99 (0.99-1.00) | 0.99 (0.98-1.00) | 0.64 | 0.027 |
| Flavanols | 285.6 (62.4-1015.5) | 1.02 (0.98-1.06) | 1.00 (0.95-1.04) | 1.05 (0.98-1.13) | 0.19 | 283.5 (65.1-1028.8) | 1.01 (0.97-1.05) | 0.99 (0.94-1.04) | 1.01 (0.95-1.08) | 0.51 | 0.043 |
| Flavan-3-ol monomers | 39.8 (6.4-460.4) | 1.01 (0.99-1.03) | 0.99 (0.97-1.02) | 1.04 (1.00-1.09) | 0.08 | 42.8 (7.4-466.1) | 1.00 (0.97-1.02) | 0.99 (0.96-1.02) | 1.00 (0.96-1.04) | 0.63 | 0.08 |
| Proanthocyanidins | 202.9 (52.4-532.0) | 1.04 (0.99-1.08) | 1.01 (0.96-1.07) | 1.06 (0.98-1.15) | 0.29 | 203.7 (51.5-552.1) | 1.00 (0.96-1.05) | 0.99 (0.93-1.04) | 1.00 (0.93-1.07) | 0.56 | 0.020 |
| Theaflavins | 1.6 (0.0-106.4) | 1.00 (1.00-1.01) | 1.00 (1.00-1.00) | 1.01 (1.00-1.01) | 0.26 | 1.5 (0.0-112.0) | 1.00 (1.00-1.01) | 1.00 (1.00-1.01) | 1.00 (1.00-1.01) | 0.74 | 0.06 |
| Flavanones | 25.6 (1.8-118.3) | 1.00 (0.99-1.02) | 0.99 (0.97-1.02) | 1.03 (1.00-1.07)* | 0.05 | 24.2 (2.2-120.0) | 0.99 (0.97-1.01) | 0.99 (0.96-1.01) | 1.00 (0.96-1.03) | 0.69 | 0.10 |
| Flavones | 9.3 (2.7-26.6) | 1.02 (0.98-1.06) | 1.02 (0.97-1.07) | 1.02 (0.95-1.10) | 0.09 | 9.4 (2.3-30.4) | 0.98 (0.94-1.03) | 0.96 (0.91-1.02) | 0.97 (0.90-1.04) | 0.42 | 0.027 |
| Flavonols | 27.9 (6.9-112.0) | 1.01 (0.97-1.05) | 0.99 (0.94-1.04) | 1.07 (0.99-1.15) | 0.08 | 29.5 (7.9-113.3) | 1.01 (0.96-1.05) | 0.99 (0.94-1.05) | 1.00 (0.93-1.07) | 0.57 | 0.23 |
| Isoflavonoids | 0.0 (0.0-7.3) | 1.01 (1.00-1.02) | 1.01 (1.00-1.02) | 1.00 (0.99-1.02) | 0.48 | 0.0 (0.0-5.0) | 0.99 (0.98-1.01) | 0.99 (0.98-1.00) | 1.00 (0.98-1.02) | 0.28 | 0.001 |
| Phenolic acid subclasses | 508.2 (122.8-1317.8) | 1.03 (0.99-1.08) | 1.00 (0.95-1.05) | 1.10 (1.02-1.19)* | 0.038 | 561.9 (162.1-1395.7) | 0.96 (0.91-1.01) | 0.91 (0.85-0.97)** | 1.04 (0.95-1.14) | 0.015 | 0.001 |
| Hydroxybenzoics | 19.5 (1.3-155.0) | 1.00 (0.98-1.03) | 0.99 (0.96-1.02) | 1.05 (1.00-1.10)* | 0.03 | 23.0 (3.1-159.5) | 1.00 (0.97-1.04) | 1.00 (0.96-1.04) | 1.01 (0.96-1.06) | 0.93 | 0.10 |
| Hydroxycinnamic | 474.6 (95.5-1279.3) | 1.02 (0.98-1.06) | 1.01 (0.96-1.05) | 1.07 (1.00-1.15)* | 0.10 | 513.6 (118.2-1356.5) | 0.96 (0.92-1.01) | 0.92 (0.87-0.97)** | 1.03 (0.96-1.11) | 0.017 | 0.002 |
| Hydroxyphenylacetic | 0.1 (0.0-0.6) | 0.99 (0.98-1.01) | 0.99 (0.98-1.01) | 1.00 (0.98-1.03) | 0.54 | 0.2 (0.0-1.3) | 1.00 (0.98-1.01) | 0.99 (0.97-1.01) | 0.98 (0.96-1.01) | 0.12 | 0.40 |
| Stilbenes | 0.4 (0.0-6.6) | 1.00 (0.98-1.01) | 1.00 (0.98-1.02) | 1.00 (0.97-1.03) | 0.74 | 0.8 (0.0-11.8) | 0.98 (0.96-0.99) | 0.98 (0.96-1.00) | 0.97 (0.95-1.00) | 0.70 | 0.042 |
| Lignans | 1.4 (0.7-4.9) | 1.01 (0.94-1.08) | 0.98 (0.90-1.06) | 1.08 (0.95-1.21) | 0.20 | 1.6 (0.8-5.3) | 1.06 (0.98-1.15) | 1.11 (1.01-1.22) | 0.99 (0.86-1.13) | 0.17 | 0.83 |
| Other polyphenol classes | |||||||||||
| Alkylphenols | 24.4 (2.0-80.1) | 1.00 (0.97-1.03) | 1.00 (0.97-1.04) | 1.00 (0.95-1.06) | 0.95 | 39.7 (2.3-113.5) | 0.99 (0.96-1.02) | 0.99 (0.95-1.02) | 1.01 (0.95-1.06) | 0.57 | < 0.001 |
| Tyrosol | 3.5 (0.3-30.2) | 0.99 (0.97-1.00) | 0.99 (0.97-1.01) | 0.97 (0.94-1.00) | 0.26 | 4.5 (0.4-49.8) | 0.99 (0.97-1.01) | 1.00 (0.97-1.02) | 0.97 (0.94-1.00) | 0.22 | 0.20 |
| Alkymethoxyphenols | 2.2 (0.1-6.2) | 1.01 (0.99-1.02) | 1.00 (0.98-1.02) | 1.04 (1.00-1.08)* | 0.036 | 2.7 (0.3-7.3) | 0.99 (0.98-1.01) | 0.99 (0.97-1.01) | 1.00 (0.97-1.03) | 0.49 | 0.005 |
| Furanocoumarins | 0.0 (0.0-0.4) | 1.00 (0.99-1.00) | 1.00 (0.99-1.01) | 0.99 (0.98-1.00) | 0.39 | 0.0 (0.0-0.3) | 1.00 (0.99-1.01) | 1.00 (0.99-1.01) | 1.01 (0.99-1.02) | 0.31 | 0.87 |
| Hydroxybenzaldehydes | 0.1 (0.0-1.5) | 0.99 (0.98-1.01) | 0.99 (0.98-1.01) | 1.01 (0.98-1.03) | 0.39 | 0.3 (0.0-2.5) | 0.99 (0.98-1.01) | 0.98 (0.96-1.00) | 0.97 (0.95-1.00)* | 0.10 | 0.008 |
| Hydroxycoumarins | 0.0 (0.0-0.4) | 0.99 (0.99-1.00) | 1.00 (0.99-1.01) | 0.99 (0.98-1.01) | 0.42 | 0.2 (0.0-1.3) | 1.00 (0.99-1.01) | 0.99 (0.98-1.01) | 0.99 (0.98-1.00) | 0.13 | 0.003 |
| Hydroxyphenylpropenes | 0.0 (0.0-4.0) | 1.00 (1.00-1.01) | 1.00 (1.00-1.01) | 1.00 (0.99-1.01) | 0.59 | 0.2 (0.0-5.8) | 1.00 (1.00-1.01) | 1.01 (1.00-1.01) | 1.00 (0.99-1.01) | 0.22 | 0.18 |
| Methoxyphenols | 0.3 (0.0-0.8) | 1.01 (1.00-1.02) | 1.00 (0.99-1.01) | 1.02 (1.00-1.03)* | 0.17 | 0.3 (0.0-0.8) | 0.99 (0.98-1.00) | 0.99 (0.98-1.00)** | 0.99 (0.98-1.00) | 0.73 | < 0.001 |
P-value<0.05
P-value<0.01; any association exceeds the Bonferroni threshold (P<0.05/26) < 0.002
P-value for heterogeneity for colon vs rectum cancer
P-value for interaction by sex in colorectal cancer
Cox model was stratified by age and centre, and additionally adjusted for smoking status and intensity, physical activity, education level, body mass index, total energy intake, alcohol, red and processed meat, fibre (g/d) and calcium (mg/d) intakes and in women also for menopausal status, hormone replacement therapy use, and oral contraceptive use
There were no evidence that age, BMI, and baseline alcohol intake modified the association between total polyphenol intake and CRC risk in the multivariable models. Since a statistically significant interaction between smoking status (never, former, and current smoker) and total polyphenol (Pinteraction = 0.033) and flavonoid (Pinteraction = 0.037) intake in relation to CRC risk was observed in women, we stratified the statistical models by smoking status (Supplementary table 2). In most of cases, stronger associations were detected in either never or current smokers, although the results obtained were similar to those of the entire cohort.
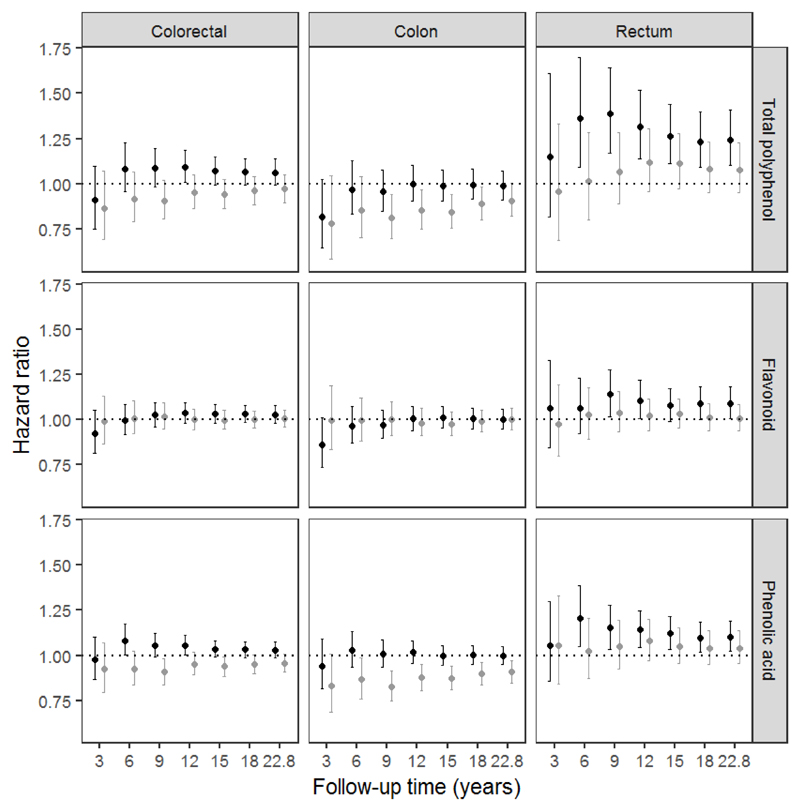
In additional analysis, the relationships between the intake of total polyphenols and their main classes (flavonoids and phenolic acids) and the risk of overall CRC and by anatomical subsite (colon and rectal cancers) (Figure 1) were performed by length of follow-up [at 3 years, 6 years, 9 years, 12 years, 15 years, 18 years, and maximum of follow-up (22.8 years)]. When censoring data at 3 years of follow-up, no associations were observed. At 6 years, all associations were similar to those found after the longest follow-up, although not all of them were statistically significant. The strongest results were found censoring data at 9 years of follow-up, while in longer follow-ups (>9 years) the associations were progressively attenuated.
Figure 1.
Hazard ratios and (95% CI) for colorectal cancer and subsites by sex and length of follow-up, according to double the intake (log2) of total polyphenol, flavonoid, and phenolic acid in women (black circles) and men (grey circles) from the EPIC study.
In a separate sensitivity analysis in which the 462 CRC cases diagnosed within the first 2 years of follow-up were excluded, the associations between the intake of total polyphenols and polyphenol classes and overall CRC risk and by anatomical subsite were practically identical to results based on the whole cohort (data not shown).
Discussion
In the present European prospective multi-country study, no statistically significant association between total polyphenol intake and overall CRC risk was observed. This is in line with findings of the Fukuoka colorectal case-control study (15). However, we observed a suggestive inverse association between total polyphenols intake and colon cancer risk in men and a positive one with rectal cancer risk in women. These findings for total polyphenol intake were almost identical to those found for phenolic acid intake.
Phenolic acids are the main contributors to total polyphenol intake (49.0% and 54.7% in Mediterranean and non-Mediterranean EPIC countries, respectively) and coffee is, by far, their principal food source (70.6-74.6%) (17). In the current study, we did not see an association between phenolic acid intake and CRC risk in either men or women. Similar results were also observed after adjustment for coffee intake, implying that other food sources of phenolic acids were not related to CRC risk. In a nested case-control study within EPIC, no associations were found between concentrations of phenolic acids in plasma (including caffeic and ferulic acids which are major phenolic acids associated with coffee intake) (25) and colon cancer risk, except that homovanillic acid was associated with an increased risk (26). Plasma homovanillic acid is most probably associated with the metabolism of catecholamines and cannot be directly linked to phenolic acid intake. In the Fukuoka colorectal case-control study a borderline statistically significant inverse association between coffee polyphenol intake (which accounts for most phenolic acids) and colon cancer risk was reported in both sexes, but not for rectal cancer risk (15). In the EPIC study, null results were previously shown between coffee intake and overall CRC risk (27) and CRC mortality (28), although inverse associations with colon cancer risk in men and positive associations with rectal cancer risk in women (27) and CRC mortality in women (28) were noted. In two recent meta-analyses, coffee intake was not associated with the risk of both overall CRC and rectum cancers in cohort studies (29;30); although higher doses of coffee (>5cups/day) has been reported to decrease the risk of colon cancer (30). However, the evidence is inconsistent; in an Australian-based case-control study, iced coffee consumption was associated with a higher risk of rectal cancer (31). Interestingly, in a recent meta-analysis of coffee intake, including 8 Japanese cohorts, a significant decreased risk of colon cancer was observed in women, but not in men (32). Moreover, no association was observed with rectal cancer risk in both sexes; although a significant increase was detected after excluding cases diagnosed within 3 years of the baseline only in women. Despite the suggestive epidemiological evidence regarding sex and anatomical location, there is heterogeneity in the association between phenolic acid and coffee in relation to CRC, thus further research is needed to confirm these results and to elucidate the underlying mechanisms of action. Part of these discrepancies might be because different types of coffee have different polyphenol compositions and contents, which are difficult to take into account in large epidemiological studies, such as in EPIC (33). In an Israeli-based case-control study, a significant inverse association was found between CRC risk and the intake of boiled and expresso coffees but not instant and filter coffees, with stronger associations for colon cancer (34). Phenolic acid intake is highly correlated with coffee intake (35) and therefore, other coffee constituents such as caffeine, cafestol and kahweol may also contribute to any association with CRC risk (36). No associations between total, caffeinated or decaffeinated coffee and CRC risk were found in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (37). Indeed, CYP1A2 and NAT2 genotypes, enzymes involved in caffeine metabolism, did not affect associations between coffee consumption and CRC risk (27). Therefore, caffeine does not seem to play a role in CRC pathogenesis. Another potential explanation for these differences in the relationships between cancer sites and sexes is due to endogenous factors, such as metabolic heterogeneity and gut microbiota, which may influences coffee bioavailability and therefore the bioactivity and bioefficacy of its constituents. Gut microbiota composition slightly varies between sexes (38), and especially, depend on the interaction between sex and diet (39).
We did not observe clear associations between flavonoid intake, the second major contributor to total polyphenols (44.3%), and CRC risk, and anatomical subsites in both men and women. These results were in concordance with our previous study with shorter follow-up (13), and three meta-analyses of prospective studies (40–42), although some protective associations have been systematically reported in case-control studies (41;42). In these prospective studies and in agreement with the present findings, no association was observed either with any of the flavonoid subclasses. However, some inverse associations have been reported between CRC risk and specific flavonoid compounds such as tea polyphenols and isoflavones. Urinary biomarkers of green tea polyphenols were also associated with a reduced risk of developing colon cancer in Chinese men (43); however, in Europe black tea is the type usually consumed. Plasma equol concentration, but not other isoflavones, was inversely related to colon cancer risk in a previous nested case-control study within EPIC (26). In contrast, no association was found with plasma and urinary isoflavone levels in the EPIC-Norfolk study (44) or with dietary isoflavone intakes in a meta-analysis of cohort studies (11).
No association between lignan intake and CRC risk was observed in our study, as previously reported in a meta-analysis of cohort studies. No association was found with urinary and plasma lignan concentrations in EPIC (26;44) and in a Dutch cohort (45). However an inverse association between intakes of dietary enterolignan and enterodiol and CRC risk were found in women but not in men from EPIC-Norfolk (44).
No significant association between any minor subclasses of polyphenols and CRC risk was observed in our study. Methoxyphenols (guaiacol is the only polyphenol in this class) showed a similar pattern of associations to phenolic acids, because the main food source is coffee (17). In agreement with present observations, plasma concentrations of stilbenes and tyrosols were not related to colon cancer (26), although an inverse association between plasma alkylresorcinols, biomarkers of whole-grain wheat and rye intake, and distal colon cancer risk (46) was observed in a previous nested case-control study within EPIC.
We also investigated the relationships between polyphenol intake and CRC risk over the years of follow-up. The strongest associations were found from 6 to 9 years of follow-up, which may be the presumable period of progression from asymptomatic precancerous polyps to CRC (47;48). Results from longer follow-ups tended to be attenuated, which could be due to misclassification bias. The longer the follow-up the higher the chance of change of dietary and lifestyle habits by the participants. This can be evaluated with periodic reassessments of the main exposure and the cofounders. Despite this attenuation, our findings after a mean of 14 years of follow-up maintained their significance because accrual of more cases meant there was greater statistical power to detect associations.
The major strengths of the present study are its prospective design, its long follow-up, its large size and number of cases, and the coverage of several European countries with large dietary heterogeneity. This study also has several potential limitations. First, diet and other lifestyle variables were only available at baseline, and therefore, changes in these variables could not be taken into account in these analyses. The second limitation may be the measurement error in collecting dietary intake, but centre/country-specific validated questionnaires for polyphenol-rich foods were used (19). Moreover, the Phenol-Explorer is the most comprehensive food composition database on polyphenols available nowadays (16). The third limitation is the potential modification of diet during the early prediagnostic period of the disease; however, sensitivity analyses excluding incident cases diagnosed in the first 2 years of follow-up did not alter the associations. The fourth limitation is the potential impact of residual confounding, since several lifestyle and other dietary factors related to CRC were different according to polyphenol intake. Although we have included them in the statistical models, measurement error and changes during follow-up may affect our results. Finally, we realize that our study is prone to the well-known drawback of multiple comparisons. We have therefore applied the Bonferroni correction and none of the tested associations remained statistically significant. Despite this rather conservative method, we were still able to observe borderline statistically significant associations.
In summary, we found that higher intakes of phenolic acids, reflecting high coffee consumption, were associated with a lower risk of colon cancer in men and a higher risk of rectal cancer in women, although the findings were no longer significant after Bonferroni correction. Further studies are warranted to evaluate the potential role of the intakes of phenolic acids and coffee in CRC development.
Supplementary Material
Acknowlegdements
We thank Mr Bertrand Hémon for his valuable help with the EPIC database. We also acknowledge the Northern Sweden Diet Database.
Funding
This study was supported by the Institut National du Cancer, Paris (INCa grants 2011-105). The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF), Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); European Research Council (ERC-2009-AdG 232997); Health Research Fund (FIS): PI13/00061 to Granada; PI13/01162 to EPIC-Murcia, Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, AGAUR - Generalitat de Catalunya (exp. 2014 SGR 726), The Health Research Funds RD12/0036/0018, cofunded by European Regional Development Fund (ERDF) “A way to build Europe (Spain); the Swedish Research Council for Health, Working Life and Welfare (FORTE), Swedish Cancer Society, Swedish Research Council (VR) and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom). RZ-R was supported by the “Miguel Servet” program (CP15/00100) from the Institute of Health Carlos III and European Social Fund (ESF).
List of Abbreviations
- BMI
body mass index
- CRC
colorectal cancer
- CI
confidence interval
- EPIC
European Prospective Investigation into Cancer and Nutrition
- HR
hazard ratio
- ICD
International Classification of Diseases
- NOS
not otherwise specified
- SD
standard deviation
Footnotes
ORCID ID’s:
Raul Zamora-Ros: 0000-0002-6236-6804
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund and American Institute for Cancer Research. Continuous Update Project Report: Diet, Nutrition, Physical Activity and Colorectal Cancer. [Accessed 06 December 2017];2017 http://www.wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/colorectal-bowel-cancer.
- 3.Zamora-Ros R, Touillaud M, Rothwell JA, Romieu I, Scalbert A. Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits. Am J Clin Nutr. 2014;100(1):11–26. doi: 10.3945/ajcn.113.077743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Kok TM, van Breda SG, Manson MM. Mechanisms of combined action of different chemopreventive dietary compounds: a review. Eur J Nutr. 2008;47(Suppl 2):51–9. doi: 10.1007/s00394-008-2006-y. [DOI] [PubMed] [Google Scholar]
- 5.Kampa M, Nifli AP, Notas G, Castanas E. Polyphenols and cancer cell growth. Rev Physiol Biochem Pharmacol. 2007;159:79–113. doi: 10.1007/112_2006_0702. [DOI] [PubMed] [Google Scholar]
- 6.Thomasset SC, Berry DP, Garcea G, et al. Dietary polyphenolic phytochemicals-promising cancer chemopreventive agents in humans? Int J Cancer. 2007;120(3):451–8. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 7.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–9. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S–42S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 9.Selma MV, Espin JC, Tomas-Barberan FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57(15):6485–501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 10.Nunez-Sanchez MA, Gonzalez-Sarrias A, Romo-Vaquero M, et al. Dietary phenolics against colorectal cancer--From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol Nutr Food Res. 2015;59(7):1274–91. doi: 10.1002/mnfr.201400866. [DOI] [PubMed] [Google Scholar]
- 11.Jiang R, Botma A, Rudolph A, Husing A, Chang-Claude J. Phyto-oestrogens and colorectal cancer risk: a systematic review and dose-response meta-analysis of observational studies. Br J Nutr. 2016;116(15):2115–28. doi: 10.1017/S0007114516004360. [DOI] [PubMed] [Google Scholar]
- 12.Nimptsch K, Zhang X, Cassidy A, et al. Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. Am J Clin Nutr. 2016;103(1):184–91. doi: 10.3945/ajcn.115.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamora-Ros R, Barupal DK, Rothwell JA, et al. Dietary flavonoid intake and colorectal cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int J Cancer. 2017;140(8):1836–44. doi: 10.1002/ijc.30582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arts IC, Jacobs DR, Jr, Gross M, Harnack LJ, Folsom AR. Dietary catechins and cancer incidence among postmenopausal women: the Iowa Women's Health Study (United States) Cancer Causes Control. 2002;13(4):373–82. doi: 10.1023/a:1015290131096. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZJ, Ohnaka K, Morita M, et al. Dietary polyphenols and colorectal cancer risk: the Fukuoka colorectal cancer study. World J Gastroenterol. 2013;19(17):2683–90. doi: 10.3748/wjg.v19.i17.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neveu V, Perez-Jimenez J, Vos F, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:bap024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamora-Ros R, Knaze V, Rothwell JA, et al. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr. 2015;55:1359–75. doi: 10.1007/s00394-015-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 19.Margetts BM, Pietinen P. European Prospective Investigation into Cancer and Nutrition: validity studies on dietary assessment methods. Int J Epidemiol. 1997;26(Suppl 1):S1–S5. doi: 10.1093/ije/26.suppl_1.s1. [DOI] [PubMed] [Google Scholar]
- 20.Slimani N, Deharveng G, Unwin I, et al. The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr. 2007;61(9):1037–56. doi: 10.1038/sj.ejcn.1602679. [DOI] [PubMed] [Google Scholar]
- 21.Wareham NJ, Jakes RW, Rennie KL, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6(4):407–13. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 22.Rothwell JA, Perez-Jimenez J, Neveu V, et al. The Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford) 2013:bat070. doi: 10.1093/database/bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knaze V, Rothwell JA, Zamora-Ros R, et al. A new food composition database for 437 polyphenols in 19,899 raw and prepared foods used to estimate polyphenol intakes in adults from 10 European countries. Am J Clin Nutr. 2018 doi: 10.1093/ajcn/nqy098. Submitted. [DOI] [PubMed] [Google Scholar]
- 24.Brown CC, Kipnis V, Freedman LS, et al. Energy adjustment methods for nutritional epidemiology: the effect of categorization. Am J Epidemiol. 1994;139(3):323–38. doi: 10.1093/oxfordjournals.aje.a117000. [DOI] [PubMed] [Google Scholar]
- 25.Edmands WM, Ferrari P, Rothwell JA, et al. Polyphenol metabolome in human urine and its association with intake of polyphenol-rich foods across European countries. Am J Clin Nutr. 2015;102(4):905–13. doi: 10.3945/ajcn.114.101881. [DOI] [PubMed] [Google Scholar]
- 26.Murphy N, Achaintre D, Zamora-Ros R, et al. A prospective evaluation of plasma polyphenol levels and colon cancer risk. Int J Cancer. 2018 doi: 10.1002/ijc.31563. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dik VK, Bueno-de-Mesquita HB, Van Oijen MG, et al. Coffee and tea consumption, genotype-based CYP1A2 and NAT2 activity and colorectal cancer risk-results from the EPIC cohort study. Int J Cancer. 2014;135(2):401–12. doi: 10.1002/ijc.28655. [DOI] [PubMed] [Google Scholar]
- 28.Gunter MJ, Murphy N, Cross AJ, et al. Coffee Drinking and Mortality in 10 European Countries: A Multinational Cohort Study. Ann Intern Med. 2017;167(4):236–47. doi: 10.7326/M16-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieira AR, Abar L, Chan D, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017;28(8):1788–802. doi: 10.1093/annonc/mdx171. [DOI] [PubMed] [Google Scholar]
- 30.Gan Y, Wu J, Zhang S, et al. Association of coffee consumption with risk of colorectal cancer: a meta-analysis of prospective cohort studies. Oncotarget. 2017;8(12):18699–711. doi: 10.18632/oncotarget.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green CJ, de DP, Boyle T, Tabatabaei SM, Fritschi L, Heyworth JS. Tea, coffee, and milk consumption and colorectal cancer risk. J Epidemiol. 2014;24(2):146–53. doi: 10.2188/jea.JE20130063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashino I, Akter S, Mizoue T, et al. Coffee drinking and colorectal cancer and its subsites: A pooled analysis of 8 cohort studies in Japan. Int J Cancer. 2018 doi: 10.1002/ijc.31320. In press. [DOI] [PubMed] [Google Scholar]
- 33.Zamora-Ros R, Rothwell JA, Scalbert A, et al. Dietary intakes and food sources of phenolic acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2013;110(8):1500–11. doi: 10.1017/S0007114513000688. [DOI] [PubMed] [Google Scholar]
- 34.Schmit SL, Rennert HS, Rennert G, Gruber SB. Coffee Consumption and the Risk of Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(4):634–9. doi: 10.1158/1055-9965.EPI-15-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamora-Ros R, Achaintre D, Rothwell JA, et al. Urinary excretions of 34 dietary polyphenols and their associations with lifestyle factors in the EPIC cohort study. Sci Rep. 2016;6:26905. doi: 10.1038/srep26905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guertin KA, Loftfield E, Boca SM, et al. Serum biomarkers of habitual coffee consumption may provide insight into the mechanism underlying the association between coffee consumption and colorectal cancer. Am J Clin Nutr. 2015;101(5):1000–11. doi: 10.3945/ajcn.114.096099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dominianni C, Huang WY, Berndt S, Hayes RB, Ahn J. Prospective study of the relationship between coffee and tea with colorectal cancer risk: the PLCO Cancer Screening Trial. Br J Cancer. 2013;109(5):1352–9. doi: 10.1038/bjc.2013.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Org E, Mehrabian M, Parks BW, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016 Jul 3;7(4):313–22. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolnick DI, Snowberg LK, Hirsch PE, et al. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun. 2014;5:4500. doi: 10.1038/ncomms5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bo Y, Sun J, Wang M, Ding J, Lu Q, Yuan L. Dietary flavonoid intake and the risk of digestive tract cancers: a systematic review and meta-analysis. Sci Rep. 2016;6:24836. doi: 10.1038/srep24836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He X, Sun LM. Dietary intake of flavonoid subclasses and risk of colorectal cancer: evidence from population studies. Oncotarget. 2016;7(18):26617–27. doi: 10.18632/oncotarget.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo HD, Kim J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J Gastroenterol. 2013;19(7):1011–9. doi: 10.3748/wjg.v19.i7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. Int J Cancer. 2007;120(6):1344–50. doi: 10.1002/ijc.22460. [DOI] [PubMed] [Google Scholar]
- 44.Ward HA, Kuhnle GG, Mulligan AA, et al. Breast, colorectal, and prostate cancer risk in the European Prospective Investigation into Cancer and Nutrition-Norfolk in relation to phytoestrogen intake derived from an improved database. Am J Clin Nutr. 2010;91(2):440–8. doi: 10.3945/ajcn.2009.28282. [DOI] [PubMed] [Google Scholar]
- 45.Kuijsten A, Hollman PC, Boshuizen HC, et al. Plasma enterolignan concentrations and colorectal cancer risk in a nested case-control study. Am J Epidemiol. 2008;167(6):734–42. doi: 10.1093/aje/kwm349. [DOI] [PubMed] [Google Scholar]
- 46.Kyro C, Olsen A, Landberg R, et al. Plasma alkylresorcinols, biomarkers of whole-grain wheat and rye intake, and incidence of colorectal cancer. J Natl Cancer Inst. 2014;106(1):djt352. doi: 10.1093/jnci/djt352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stracci F, Zorzi M, Grazzini G. Colorectal cancer screening: tests, strategies, and perspectives. Front Public Health. 2014;2:210. doi: 10.3389/fpubh.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neugut AI, Jacobson JS, De V I. Epidemiology of colorectal adenomatous polyps. Cancer Epidemiol Biomarkers Prev. 1993;2(2):159–76. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.