Abstract
Recently, we identified unique processing patterns of apolipoprotein A2 (ApoA2) in patients with pancreatic cancer. This study provides a first prospective evaluation of an ApoA2 isoform (“ApoA2-ATQ/AT”), alone and in combination with carbohydrate antigen 19-9 (CA19-9), as an early detection biomarker for pancreatic cancer.
We performed ELISA measurements of CA19-9 and ApoA2-ATQ/AT in 156 patients with pancreatic cancer and 217 matched controls within the European EPIC cohort, using plasma samples collected up to 60 months prior to diagnosis. The detection discrimination statistics were calculated for risk scores by strata of lag-time.
For CA19-9, in univariate marker analyses, C-statistics to distinguish future pancreatic cancer patients from cancer-free individuals were 0.80 for plasma taken ≤6 months before diagnosis, and 0.71 for >6-18 months; for ApoA2-ATQ/AT, C-statistics were 0.62, and 0.65, respectively. Joint models based on ApoA2-ATQ/AT plus CA19-9 significantly improved discrimination within >6-18 months (C = 0.74 vs. 0.71 for CA19-9 alone, p = 0.022) and ≤18 months (C = 0.75 vs. 0.74, p = 0.022). At 98% specificity, and for lag times of ≤6, >6-18 or ≤18 months, sensitivities were 57%, 36% and 43% for CA19-9 combined with ApoA2-ATQ/AT, respectively, vs. 50%, 29% and 36% for CA19-9 alone.
Compared to CA19-9 alone, the combination of CA19-9 and ApoA2-ATQ/AT may improve detection of pancreatic cancer up to 18 months prior to diagnosis under usual care, and may provide a useful first measure for pancreatic cancer detection prior to imaging.
Keywords: pancreatic cancer, early detection, CA19-9, Apolipoprotein A2, isoforms, prospective study
Introduction
Early detection of pancreatic ductal adenocarcinomas (PDAC) is difficult because the pancreas is located deep within the abdominal cavity, and because patients do not present unique symptoms 1. Given the low incidence of pancreatic cancer, general population screening is not cost-effective with current technology, which requires relatively expensive (magnetic resonance imaging) or invasive (e.g., endoscopic ultrasonography [EUS]) imaging modalities 2, 3. However, a feasible screening strategy could consist of a pre-screen based on noninvasive biomarkers, followed by imaging only among individuals who have a positive biomarker test.
Carbohydrate antigen 19-9 (CA19-9) is the conventional biomarker for the detection of PDAC, and is commonly used for monitoring therapy response in PDAC patients 4, 5. Limitations of CA19-9, however, are that it can be increased in several benign diseases and multiple types of advanced gastrointestinal adenocarcinoma 6, and that it may have only limited sensitivity for small tumors in still curable stage 7. Furthermore, CA19-9 is not expressed at all in individuals genetically expressing non-sialylated Lewis blood group antigens 8, 9.
Recently, we identified unique processing patterns of c-terminal amino acids of apolipoprotein A2 (ApoA2) in patients with pancreatic cancer 10–12. In the bloodstream ApoA2 can be found in 5 dimeric isoforms (ApoA2i) 10–13. In healthy subjects, 3 basic isoforms are found which we labeled ApoA2-ATQ/ATQ, ApoA2-ATQ/AT and ApoA2-AT/AT), by the lengths of each of the homomers. Patients with PDAC show additional isomers formed through two aberrant processing patterns of ApoA2i: a hyper-processing pattern of ApoA2i, which leads to predominantly light isoforms such as ApoA2-AT/AT, ApoA2-AT/A and ApoA2-A/A, and a hypo-processing pattern which leads to a predominance of heavy isoforms such as ApoA2-ATQ/ATQ 11, 12. The aberrant processing is likely a consequence of abnormal expression and release of carboxypeptidase A, a digestive enzyme that is primarily synthesized by the pancreas, and leads to a reduction in plasma levels of ApoA2-ATQ/AT, the major intermediate isoform of ApoA2i, in comparison with healthy subjects. Aberrant processing of ApoA2 is observed not only in relation to pancreatic malignancies (including early-stage cancers), but also in individuals with intraductal papillary mucinous neoplasia (IPMN) and other pancreatic conditions (e.g. chronic pancreatitis) predisposing to pancreatic cancer development 10–12.
In 2015, we developed an enzyme-linked immunosorbent assay (ELISA) method to determine blood concentrations of the intermediate ApoA2-ATQ/AT isoform. In validation studies jointly conducted in Japan and within the US National Cancer Center Early Detection Research Network (NCI EDRN), we then demonstrated the utility of this novel assay for pancreatic cancer detection, and showed that a combination of the ApoA2i assay with CA19-9 significantly improved diagnostic accuracy compared to CA19-9 alone 11. These studies, however, were based on case-control comparisons of patients already diagnosed with PDAC and cancer-free control subjects, and thus did not allow any evaluation of the lead time by which the markers may help anticipate cancer diagnosis.
Here, we present the results of a study using prospectively collected samples from the European Prospective Investigation into Cancer (EPIC) cohort. We measured ApoA2i and CA19-9 in 156 patients with PDAC diagnosed within 5 years after blood donation and 217 matched control subjects. The objectives of our study were to evaluate: (i) the early detection performance of the two markers in the short (within 0–6 months), middle (>6–18 months) and longer term (>18–60 months), and (ii) the improvement in detecting PDAC in patients using the combination assay with ApoA2i and CA19-9, as compared to CA19-9 alone.
Materials and Methods
Case control study nested within the European EPIC cohort
We conducted a case-control study nested within the European EPIC cohort (“European Prospective Investigation into Cancer”) – a population-based, multicenter prospective cohort study in 10 Western European countries 14, 15. A short description of data collection and prospective case ascertainment methods in the EPIC cohort is in the Supplemental Methods.
The present study includes all incident cases of invasive, exocrine pancreatic cancer with ICD codes C25 (25.0–25.3, 25.7–25.9) who were clinically diagnosed within maximally 5 years after blood donation (N=156). Of these, 106 (68%) were microscopically confirmed, whereas the remaining diagnoses (33%) were based on a combination of clinical symptoms, physical examination and imaging. Exclusion criteria were the occurrence of other malignant tumors preceding the diagnosis of pancreatic cancer, except for non-melanoma skin cancer, and the non-availability of blood specimens. For each PDAC case, control participants were randomly selected among appropriate risk sets consisting of all cohort members with a blood sample, alive and free of cancer at the time of diagnosis of the index case. In view of cost-efficiency, one control was matched to cases with >2–5 years of follow-up, whereas two controls were matched to cases with 0–2 years of follow-up, where strongest discrimination was expected. An incidence density sampling protocol was used, such that in principle the controls could include study participants who became a case later in time and each control subject could be sampled more than once 16. The control participants actually drawn, however, did not include any of the future cases of pancreatic cancer detected so far in the EPIC cohort, and neither was any other form of cancer detected among the controls within their first three years of prospective follow-up. Case and control subjects were matched on study recruitment center, sex, length of follow-up, age at blood collection (±6 months), date of blood collection (±2 month), time of blood collection (±2 hours) and use of oral contraceptives or postmenopausal hormone replacement therapy (OC/HRT). The final sample size was 156 cases and 213 matched controls.
Laboratory assays
The plasma samples of pancreatic cancer cases and control subjects samples were split into batches such that matched case–control sets and samples from the same study center were kept together in the same batches, and with blinding of case-control status.
Measurements of CA19-9 were performed using an established ELISA kit (Lumipulse Presto CA19-9; Fujirebio, Inc., Tokyo, Japan). Laboratory values for CA19-9 of 37 samples (cases = 15, controls = 22) were below the detection limit value of 2 U/mL, and this lower threshold value was thereafter assigned to all 37 samples.
Measurements of ApoA2-ATQ/ATQ and ApoA2-AT/AT were performed by an ApoA2i measurement kit (Human ApoA2 C-terminal ApoA2 ELISA Kit; Toray Industries, Inc., Tokyo, Japan), which uses antibodies specific for each of the homodimers, according to the instruction manual. We then calculated the concentration of ApoA2-ATQ/AT hetero-dimers by the formula:
as described and in a previous report 11. Further details on ApoA2 isoform assays and calculation of ApoA2-ATQ/AT concentrations assay are in Supplemental Figure S1.
Informed consent and data protection
All participants had given their consent for future analyses of their blood samples and the present study was approved by the IARC Ethics Committee and the Institutional Review Board of the University of Heidelberg.
Statistical analyses
CA19-9 marker levels were log2-transformed, to achieve approximate normality of their distribution; statistical analyses of ApoA2 isoforms were all performed on the untransformed scale. To examine how the early detection and/or risk prediction capacities of the biomarkers changed with time between blood draw and clinical cancer diagnosis, all analyses were performed within strata of lag-time (≤6 months, >6-18 months, >18-36 months and >36-60 months). The difference of marker distributions among future pancreatic cancer cases and controls was tested with Wilcoxon’s signed rank test.
The discrimination between future cancer cases and control subjects was described using ROC (receiver operating characteristic) analyses, with the area under the curve (AUC), also known as the C-(concordance) statistic, as an overall measure for discrimination capacity. Additionally, we estimated the diagnostic (early detection) sensitivities of each marker at cut-off points corresponding to 95% and 98% specificity, determined on crude values of the biomarkers and after adjustment for matching factors in our full dataset for all control subjects (N = 213).
ROC curves were estimated either for crude marker measurements, without any adjustment, or for risk scores with CA19-9 or ApoA2-ATQ/AT markers as the major discrimination variables, using unconditional logistic regression models that included the matching factors as additional adjustments. Analyses directly based on marker measurements without further adjustments have the advantage that they allow use of pre-established marker cut-points, as used in other studies. As a complementary analysis, the adjusted model estimates account for the fact that the distribution of controls in our matched sample is not representative of the general population, and provide estimates of the general additional discriminative capacity of the markers over the risk factors included for matching17.
Multivariate models were also used to examine the discrimination capacity of CA19-9 and ApoA2 markers in combination. To test for improvement in discrimination for combined vs. single-marker models the statistical fit of nested models was compared with type-III F-tests within the logistic models. In addition, we calculated the continuous net reclassification improvement (NRI(>0)), which represents the net percent of case and control subjects correctly reclassified as a result of the added marker 18. Internal validation with 1000-fold bootstrapping was applied to adjust the results on discriminative capacity from multivariate models for over-estimation.
All analyses were conducted in SAS (version 9.4, SAS Institute).
Results
Of the 156 case patients examined in this study, 106 (68%) were microscopically confirmed, whereas the remaining 32% were of unknown morphology (Table 1). At clinical diagnosis, 14 patients (9%) had localized disease, 73 (47%) had metastatic disease and 69 (44%) were classified as having unknown disease spread. The median age at diagnosis was 60.9 years (range: 37.2–79.6). At the time of blood donation, case patients smoked significantly more often than controls. In addition, case patients had a marginally higher baseline BMI than controls. The prevalence of self-reported diabetes at time of recruitment was only marginally higher among future pancreatic cancer patients (9%) as compared to the controls (7%); for some of the cancer patients, these self-reports likely excluded undiagnosed diabetes that may have developed shortly before cancer diagnosis.
Table 1. Baseline characteristics of cases and controls [median (min–max) or N (%)].
| Cases (N = 156) | Controls (N = 213) | Pa | |
|---|---|---|---|
| Men / Women | 85 (53%) / 74 (47%) | 115 (53%) / 102 (47%) | |
| Age at blood draw, years | 58.1 (34.9-75.7) | 58.0 (34.5-75.4) | |
| BMI, kg/m2 | 26.7 (19.0-38.9) | 26.0 (14.7-40.6) | |
| < 25 | 56 (36) | 84 (39) | 0.067 |
| ≥ 25 | 100 (64) | 129 (61) | |
| Smoking | |||
| Never | 61 (39) | 93 (44) | 0.024 |
| Former | 47 (30) | 77 (36) | |
| Current | 47 (30) | 40 (19) | |
| Unknown | 1 | 3 (1) | |
| Alcohol consumption | |||
| Yes | 133 (85) | 187 (88) | 0.446 |
| Non drinker | 22 (14) | 25 (12) | |
| Unknown | 1 | 1 | |
| Diabetesb | |||
| Yes | 14 (9) | 16 (7) | 0.095 |
| No | 125 (80) | 177 (83) | |
| Unknown | 17 (11) | 20 (10) | |
| Case characteristics | |||
| Age at diagnosis, median (range), years | 60.9 (37.2-79.6) | — | |
| Lag time, median (range), months | 35 (1-60) | — | |
| Morphology of the tumor Adenocarcinoma | 106 (68) | — | |
| Tumor site | |||
| Head | 82 (53) | — | |
| Body | 12 (8) | — | |
| Tail | 8 (5) | — | |
| Unspecific | 54 (35) | — | |
| Disease spread | |||
| Localized | 14 (9) | — | |
| Metastatic | 73 (47) | — | |
| Unknown | 69 (44) | — | |
| Basis of tumor diagnosis | |||
| Microscopically confirmed | 106 (68) | — | |
| Other (i.e. imaging, clinical symptoms) | 50 (32) | — | |
| Markerc | |||
| CA19-9 (U/mL) | 12.1 (10.1-14.6) | 6.8 (6.2-7.4) | 0.101 |
| ApoA2-AT/AT (μg/mL) | 43.2 (37.8-49.4) | 48.7 (45.6-52.0) | 0.480 |
| ApoA2-ATQ/ATQ (μg/mL) | 41.4 (38.7-44.2) | 42.9 (41.1-44.7) | 0.892 |
| ApoA2-ATQ/AT (μg/mL) | 42.2 (39.9-44.7) | 45.7 (44.5-46.8) | 0.193 |
P values determined using paired t-tests or generalized McNemar’s test.
Self-reported at baseline.
Presented as geometric mean (95% CI).
Note: BMI = body-mass-index
Between CA19-9 and ApoA2-ATQ/AT no meaningful correlations were observed, either among the controls (r = -0.04, Supplemental Table S1 and Supplemental Figure S2) or among the cases (r = -0.10), even when only cases were considered whose blood samples had been taken shortly before diagnosis. None of the markers showed significant associations with BMI or self-reported pre-existing diabetes; however, ApoA2-ATQ/AT was lower among current compared to never smokers (P = 0.02), and increased among controls within the higher alcohol intake categories (>12g/d; P = 0.005 for current consumption at baseline) (Supplemental Table S1).
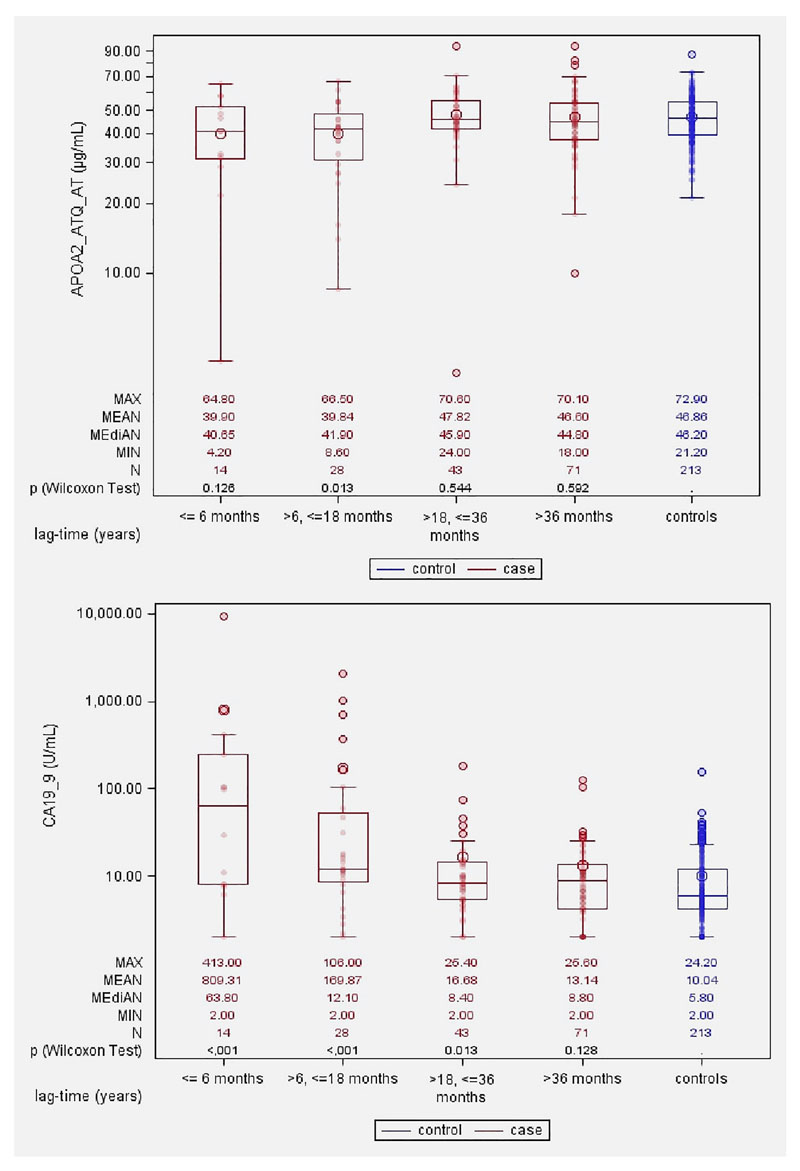
Box and whisker plots (Figure 1) show that, for CA19-9, the marker distribution among the future case patients started to diverge from that of the controls about 18 months prior to clinical diagnosis (>6-18 months, Wilcoxon’s p-value = <0.001) and this difference grew larger as the lag time diminished to 6 months or less (p=<0.001). For ApoA2-ATQ/AT, the marker distribution for future cancer patients also started diverging from that of the controls about 18 months prior to diagnosis ((>6-18 months, p=0.01) tending towards lower levels for future cases as compared to the controls.
Figure 1. Box and whisker plots showing plasma levels of CA19-9 and ApoA2-ATQ/AT for pancreatic cancer cases and matched controls, by intervals of time from blood donation till diagnosis of (matched) case.
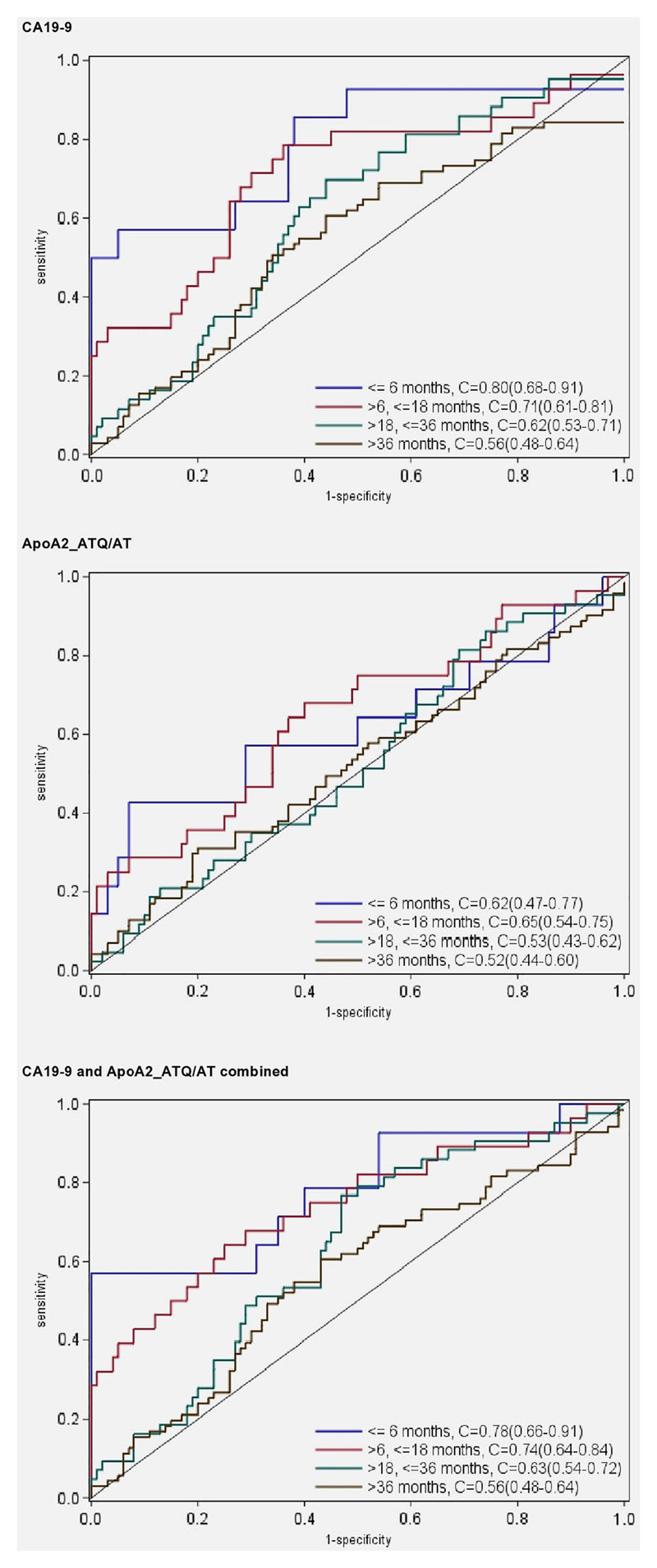
In basic univariate ROC analyses directly based on marker measurements, both biomarkers showed a diminishing capacity to discriminate between future case patients and cancer-free individuals with increasing time lags between blood donation and tumor diagnosis (Figure 2). For CA19-9, the C-statistic equaled 0.80 for plasma samples taken ≤6 months before diagnosis, 0.71 for lag times of >6-18 months, and less than 0.60 for lag times longer than 18 months. At the 98% specificity cut-point (38.0 U/mL) the sensitivity (SE98) estimate was 0.50 for lag times less than 6 months, and 0.29 for lag times of >6-18 months (Table 2). Using the predefined cut-point of 37 U/mL, frequently used in diagnostic settings 19, identical estimates for specificity (98%) and sensitivity (0.50) were obtained. For ApoA2-ATQ/AT, the C-statistics at lag times ≤6, >6-18 and >18 months were 0.62, 0.65 and 0.50, respectively, and using a 98% specificity cut-point (27.7 μg/mL) sensitivity (SE98) estimates were 0.14, 0.21 and 0.19, respectively. At more lenient 95% specificity cut-points the detection sensitivities were all slightly higher for both CA19-9 and ApoA2-ATQ/AT (Table 2). Focusing on biomarker measurements less than 18 months prior to cancer diagnosis, adding ApoA2-ATQ/AT to a logistic regression model with only CA19-9 significantly improved model fit (p=0.02), as well as the early detection discrimination (for the combined model, C=0.75 vs. C=0.74, with a continuous net reclassification improvement (NRI) of 25%. Further differentiating the analyses by lag-times of ≤6 months or >6-18 months showed that, especially for lag times of >6-18 months the joint marker discrimination was significantly better than for CA19-9 alone (C=0.75 vs. 0.71 P=0.022, NRI=35%). Combining CA19-9 and ApoA2-ATQ/AT using pre-defined cut-off values of 37 U/mL for CA19-9 19 or 27.7 μg/mL for ApoA2-ATQ/AT (based on the cut-point at 98% specificity within our data; Supplemental Figure S2), the sensitivity by which future cases were diagnosed within ≤18, >6-18 or >18-60 months was 45%, 39%, or 8%, respectively at an overall specificity of 96% (Table 3). With slightly modified cut points (38 U/mL for CA19-9, 25.0 μg/mL for ApoA2-ATQ/AT), fixing the joint specificity at 98%, the two markers combined yielded sensitivities of 43%, 36% and 7% respectively (Table 3), as compared to 36%, 29%, and 5% for CA19-9 alone (Table 2). Sensitivity analyses restricting to microscopically confirmed pancreas cancer (N=106) did not reveal any major discrepancies compared to analyses in the full dataset (all pancreas cancer outcomes; N=156) (supplementary Table S2).
Figure 2. ROC curves and C-statistics for blood samples taken ≤6 months, >6-18 months, >18-36 months and >36-60 months before cancer diagnosis.
Table 2. Sensitivity at 95% and 98% specificity of pancreatic cancer detection by time between blood draw and diagnosis, for crude marker measurements and with adjustments for case-control matching factorsa.
| Lag-time (months) | threshold 95 crude | SE95 (95% CI) crude | SE95 (95% CI) adjusted | threshold 98 crude | SE98 (95% CI) crude | SE98 (95% CI) adjusteda |
|---|---|---|---|---|---|---|
| CA19-9 (U/mL) | ||||||
| ≤6 | 29.2 | 0.57 (0.30-0.81) | 0.54 (0.36-0.64) | 38.0 | 0.50 (0.23-0.77) | 0.50 (0.29-0.57) |
| >6-18 | 29.2 | 0.32 (0.16-0.54) | 0.34 (0.25-0.46) | 38.0 | 0.29 (0.12-0.53) | 0.27 (0.18-0.36) |
| ≤18 | 29.2 | 0.40 (0.24-0.59) | 0.39 (0.36–0.43) | 38.0 | 0.36 (0.19-0.58) | 0.35 (0.31-0.38) |
| >18-36 | 29.2 | 0.12 (0.04-0.28) | 0.14 (0.07-0.21) | 38.0 | 0.07 (0.02-0.24) | 0.10 (0.05-0.16) |
| >36-60 | 29.2 | 0.07 (0.02-0.18) | 0.07 (0.03-0.14) | 38.0 | 0.03 (0.01-0.13) | 0.04 (0.01-0.10) |
| ApoA2-ATQ/AT (μg/mL) | ||||||
| ≤6 | 30.3 | 0.21 (0.07-0.52) | 0.27 (0.07-0.43) | 27.7 | 0.14 (0.03-0.47) | 0.19 (0.07-0.35) |
| >6-18 | 30.3 | 0.25 (0.11-0.47) | 0.22 (0.11-0.32) | 27.7 | 0.21 (0.08-0.46) | 0.15 (0.04-0.21) |
| ≤18 | 30.3 | 0.24 (0.12-0.42) | 0.23 (0.12-0.31) | 27.7 | 0.19 (0.08-0.40) | 0.16 (0.07-0.21) |
| >18-36 | 30.3 | 0.05 (0.01-0.19) | 0.09 (0.02-0.16) | 27.7 | 0.05 (0.01-0.20) | 0.05 (0.00-0.09) |
| >36-60 | 30.3 | 0.07 (0.02-0.18) | 0.18 (0.03-0.14) | 27.7 | 0.04 (0.01-0.15) | 0.05 (0.00-0.10) |
adjustment factors were: study recruitment country, sex, age at blood collection, and exogenous hormone use (contraceptive OC/HRT) at time of blood donation
Note: SE95 = sensitivity at 95% specificity; SE98 = sensitivity at 98% specificity; CI = confidence interval
Table 3. Joint sensitivity and specificity of pancreatic cancer detection by time between blood draw and diagnosis, for pre-defined cut-points.
| Lag-time (months) |
Threshold crude | Threshold crude | Sensitivity (95% CI) |
Specificity (95% CI) |
|
|---|---|---|---|---|---|
|
CA19-9 (U/mL) |
ApoA2-ATQ/AT (μg/mL) |
||||
| ≤6 | 37 | 27.7 | 0.57 (0.29-0.82) | 0.96 (0.92-0.98) | |
| >6-18 | 37 | 27.7 | 0.39 (0.22-0.59) | 0.96 (0.92-0.98) | |
| ≤18 | 37 | 27.7 | 0.45 (0.30-0.61) | 0.96 (0.92-0.98) | |
| >18-36 | 37 | 27.7 | 0.09 (0.03-0.22) | 0.96 (0.92-0.98) | |
| >36-60 | 37 | 27.7 | 0.07 (0.02-0.16) | 0.96 (0.92-0.98) | |
|
CA19-9 (U/mL) |
ApoA2-ATQ/AT (μg/mL) |
||||
| ≤6 | 38 | 25 | 0.57 (0.29-0.82) | 0.98 (0.95-0.99) | |
| >6-18 | 38 | 25 | 0.36 (0.19-0.56) | 0.98 (0.95-0.99) | |
| ≤18 | 38 | 25 | 0.43 (0.28-0.59) | 0.98 (0.95-0.99) | |
| >18-36 | 38 | 25 | 0.07 (0.01-0.19) | 0.98 (0.95-0.99) | |
| >36-60 | 38 | 25 | 0.07 (0.02-0.16) | 0.98 (0.95-0.99) |
In multivariable models adjusting for the matching factors as co-variates, and using bootstrapping to correct for possible overfitting, ROC curves (C-statistics) and estimates of SE98 or SE95 were generally comparable to univariate analyses based directly on the marker measurements (see Table 2 and Supplemental Figure S3). Similar to the unadjusted analyses, estimated C-statistics from the adjusted models show on increase in detection discrimination within time windows ≤18 months when the two biomarkers are combined, as compared to either biomarker alone (Supplementary Figure S3). Further model adjustments for smoking status (current, past, never), alcohol consumption, BMI or prevalent diabetes, or excluding individuals with a history of heavy alcohol drinking [>60g/d, 5% prevalence, N=26] or with self-reported baseline history of prevalent diabetes [N=30], did not substantially change any of the above discrimination estimates [results not reported in tables].
Discussion
In this prospective study the combination of CA19-9 with ApoA2-ATQ/AT showed a moderate but significant improvement in early detection discrimination for pancreatic cancer, compared to CA19-9 alone. In plasma samples predating cancer diagnosis up to 18 months, the two markers combined provided a detection sensitivity of 43% at 98% specificity vs. 36% for CA19-9 alone. This discrimination improvement was driven mostly by cases diagnosed within a >6-18 months lag time after blood donation (C-statistic of 0.74 for the markers combined [adjusted model: 0.76] vs. 0.71 [adjusted: 0.73] for CA19-9 and 0.72 [adjusted: 0.71] for ApoA2-ATQ/AT respectively). For both markers, the discrimination capacity waned to insignificant levels at lag times between blood sampling and diagnosis greater than 18 months.
For CA19-9, two further prospective studies have recently investigated early detection capacity in pre-diagnostic blood samples 20, 21. In the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), using the standard cut-point of 37 U/ml O’Brien et al. observed sensitivities of 53%, 59% and 18%, respectively, in blood samples drawn ≤6, >6-12 and >12-24 months prior to diagnosis, at specificities of 96-100% – findings very similar to ours – and the authors concluded that CA19-9 has encouraging sensitivity for detecting preclinical pancreatic cancer. By contrast, an investigation in the US PLCO cohort revealed lower sensitivity (38%) and specificity (93%) compared to O’Brien`s and our studies, and a C-statistic of only 0.695, for cases diagnosed within 1-12 months after blood draw.
For ApoA2-ATQ/AT, our previous studies in Japan showed a strong capacity to distinguish patients with stage-I, -II, -III, or -IV of PDAC from healthy controls, with estimated C-statistics greater than 0.92 11. In this previous study, diagnostic discrimination by ApoA2-ATQ/AT measurements was as good as, or even stronger than, that by CA19-9 for both early and late-stage PDAC (C-statistics all cases 0.94 vs. 0.90, stage-I 0.94 vs. 0.83, stage-II 0.96 vs. 0.95, stage-III 0.93 vs. 0.90, stage-IV 0.95 vs. 0.88, respectively). These initial findings were largely confirmed in a further, blinded validation study of diagnostic accuracy for distinguishing PDAC of stage-I and -II from healthy controls, in collaboration with the NCI EDRN, which also showed higher C-statistics for ApoA2-ATQ/AT than for CA19-9 (0.81 vs. 0.78) 11. Finally, our previous study in the NCI EDRN showed that the combined assays for ApoA2-ATQ/AT and CA19-9 improved diagnostic discrimination as compared to either marker alone (0.88, 0.81, and 0.78 for the combined assay, ApoA2-ATQ/AT and CA19-9, respectively).
Pancreatic cancer screening efforts currently focus on high-risk groups with familial pancreatic cancer clustering due to heritable cancer syndromes. However, 90% of pancreatic cancers develop as sporadic tumors with much lower population incidence rates, prohibiting the direct use of expensive (MRI) or potentially invasive (e.g, EUS) imaging modalities as tools for generalized pancreatic cancer screening. Thus, current research focuses on strategies for multimodal screening, using blood-based markers to enrich the screening population with individuals at increased risk of having PDAC and to target diagnostic imaging towards a much smaller part of the population while still capturing a majority of pancreatic cancer cases.
Data from screening studies among high-risk individuals indicate a sensitivity of about 56% at about 97% specificity for MRI-based detection of resectable, early-stage (N0-M0) PDAC 22, and in other prospective screening studies the general population prevalence of detectable pancreatic cancer has been estimated to be around 0.03 - 0.07% 23. Based on these data, it can be calculated 24 that complementary biomarkers should have a minimal sensitivity at least 15 times their false-positive detection rate (e.g., a sensitivity of 30% at a specificity of 98%) to yield an overall positive predictive value (PPV) for multi-modal biomarker-plus-MRI screening greater than 0.10 – a PPV threshold at which screening will prompt no more than 9 invasive diagnostic procedures (e.g., EUS, biopsies) for one true positive case of pancreatic cancer diagnosed. For blood samples taken >6-18 months before usual diagnosis – a time window that may include a high proportion of patients with tumors in still resectable stage 25 – our data indicate 36% detection sensitivity at 98% specificity [0.43 for the period 0-18 months] for CA19-9 combined with ApoA2-ATQ/AT.
Evaluation of early detection markers in clinical context, comparing between clinically diagnosed cases and controls, often has the limitation that either cases have already advanced disease or, if disease is still early-stage (which for pancreas cancer is very rare), these cases may not represent average early-stage patients in the general population. For example, it is possible that early-stage tumors spontaneously diagnosed after symptoms include a higher than average proportion of more slowly growing, comparatively less aggressive tumors. Often, markers that were initially found to distinguish clinical cancer cases (even in early stage) from cancer-free controls failed upon cross-validation in prospective cohort studies.
The prospective design of our study ensures rigorous internal validity for the evaluation of marker differences between case and control participants, and allowed analyses by lagtime since blood donation, while adjusting for potential confounders. The combination of ApoA2-ATQ/AT and CA19-9 showed 43% sensitivity at 98% specificity for cases diagnosed >6-18 months after blood donation. This finding suggests diagnostic sensitivity of this marker combination for earlier stage disease, as detecting cancer sufficiently in advance of usual symptomatic diagnosis is generally believed to improve chances for successful surgical intervention and long-term survival. However, a limitation of our and other population-based cohort studies is that no information is available about the patients’ tumor stages at the time they provided their blood samples. Thus, although our data suggest that a meaningful proportion of cases could have been detected at least 6 months earlier, it remains speculative whether indeed those patients whose tumor might have been detectable earlier would have had a survival benefit if detected at that time point. Independent, prospective screening trials will be required to answer the question, whether screening by CA19-9 and ApoA2-ATQ/AT will lead to a significant shift in tumor stage at diagnosis and improved survival. A further limitation of our study may be that we had no information on prevalent chronic pancreatitis or other non-malignant conditions that could have affected CA19-9 or ApoA2-ATQ/AT measurements, although general population prevalence of such conditions is known to be low. During follow-up, all control subjects have so far remained free of pancreas cancer up to 15 years after blood donation, and none of the control subjects developed any other cancer within less than three years. Finally, in spite of the very large size of the European EPIC cohort, due to the relatively low incidence rate of pancreas cancer the numbers of cases detected within short lag times after blood donation remain modest, and more precise estimation of the diagnostic performances of CA19-9 and other detection markers eventually may require the combined resources of larger cohort consortia. External cross-validation of the combined CA19-9 plus ApoA2-ATQ/AT marker set will also be needed in view of possible over-estimation of their joint detection prediction, which may result when the prediction measure is computed in the same population where the value of the marker was assessed and its threshold decided, as in our single study.
In conclusion, we found that compared to CA19-9 alone the combination of CA19-9 and ApoA2-ATQ/AT can significantly improve discrimination for early detection of pancreatic cancer, as judged by the increase in sensitivity, at elevated specificity, for plasma measurements up to 18 months before diagnosis under usual care. This improvement in sensitivity may allow a significant enrichment of a general-population screening sample before further examination by non-invasive (e.g. MRI) imaging. The absolute sensitivity at high (e.g. 98%) specificity remained modest, however, even for the combination of CA19-9 and ApoA2-ATQ/AT. The discovery and validation of other complementary markers 26,27,28 may further improve the sensitivity for identification of individuals with preclinical pancreatic cancer in multi-modal screening strategies.
Supplementary Material
Novelty and Impact.
Using pre-diagnostic blood samples of pancreas cancer cases and controls from the EPIC cohort, we examined the prospective detection capacity for pancreas cancer by apolipoprotein A2 isoforms in combination with CA19-9. Compared to CA19-9 alone, the combined markers showed significantly improved detection discrimination up to 18 months before usual diagnosis. The combined markers could be used in multi-modal screening strategies, to enrich a general-population screening sample with pancreas cancer cases before further examination by imaging.
Acknowledgements
Since 1992 the coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF) (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); ERC-2009-AdG 232997 and Nordforsk, Nordic Centre of Excellence programme on Food, Nutrition and Health (Norway); Health Research Fund (FIS), PI13/00061 to Granada, PI13/01162 to EPIC-Murcia, Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom).
Funding
This work was funded and supported by the Practical Research for Innovative Cancer Control (18ck0106280h0002), and the Project for Cancer Research And Therapeutic Evolution (P-CREATE), and CREST from the Japan Agency for Medical Research and development, AMED (Japan).
Abbreviations
- PDAC
Pancreatic ductal adenocarcinomas
- EUS
Endoscopic ultrasonography
- IPMN
Intraductal papillary mucinous neoplasia
- NCI EDRN
US National Cancer Center Early Detection Research Network
- EPIC
European Prospective Investigation into Cancer
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- UKCTOCS
UK Collaborative Trial of Ovarian Cancer Screening
Footnotes
Conflicts of interest: The authors declare no conflict of interest and no specific disclosures to be made. Kazufumi Honda is co-inventor of a patent, held by Tokyo National Cancer Center Research Institute and Tory company, for the use of “apolipoprotein-A2 isoforms” for early detection of pancreatic disease.
Authors’ Contributions
Conception and design: K. Honda, V. Katzke, A. Hüsing, F. Canzian, R. Kaaks
Development of methodology: K. Honda, S. Okaya, H. Shoji, K. Onidani
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Anne Tjønneland, Kim Overvad, Elisabete Weiderpass, Domenico Palli, Valeria Pala, Rosario Tumino, Alessio Naccarati, Salvatore Panico, Heiner Boeing, H Bas Bueno-de-Mesquita, Petra H Peeters, Antonia Trichopoulou, Kay-Tee Khaw, Nick J Wareham, Ruth C. Travis, Susana Merino, Eric J. Duell, Miguel Rodríguez-Barranco, María Dolores Chirlaque, Eva Ardanaz, Marie-Christine Boutron-Ruault, Jonas Manjer, Malin Sund, Rudolf Kaaks
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A. Hüsing, V. Katzke, R. Kaaks
Writing, review, and/or revision of the manuscript: Kazufumi Honda, Verena A Katzke, Anika Hüsing, Shinobu Okaya, Hirokazu Shoji, Kaoru Onidani, Anja Olsen, Anne Tjønneland, Kim Overvad, Elisabete Weiderpass, Paolo Vineis, David Muller, Kostas Tsilidis, Domenico Palli, Valeria Pala, Rosario Tumino, Alessio Naccarati, Salvatore Panico, Krasimira Aleksandrova, Heiner Boeing, H Bas Bueno-de-Mesquita, Petra H Peeters, Antonia Trichopoulou, Pagona Lagiou, Kay-Tee Khaw, Nick J Wareham, Ruth C. Travis, Susana Merino, Eric J. Duell, Miguel Rodríguez-Barranco, María Dolores Chirlaque, Eva Ardanaz, Vinciane Rebours, Francesca Romana, Marie-Christine Boutron-Ruault, Paul Brennan, Ghislaine Scelo, Jonas Manjer, Malin Sund, Daniel Öhlund, Federico Canzian, Rudolf Kaaks
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): V. Katzke, A. Hüsing, R. Kaaks
Study supervision: K Honda, R Kaaks
Other (Development and performance of laboratory assays): K. Honda, S. Okaya, H. Shoji, K. Onidani
Core writing team: R Kaaks, VA Katzke, A Hüsing, K Honda.
References
- 1.Modolell I, Guarner L, Malagelada JR. Vagaries of clinical presentation of pancreatic and biliary tract cancer. Ann Oncol. 1999;10(Suppl 4):82–4. [PubMed] [Google Scholar]
- 2.Chari ST, Kelly K, Hollingsworth MA, Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto M, Cleeter DF, Firpo MA, et al. Early Detection of Sporadic Pancreatic Cancer: Summative Review. Pancreas. 2015;44:693–712. doi: 10.1097/MPA.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182–98. doi: 10.3748/wjg.v20.i32.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, Firpo MA, Mulvihill SJ. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med. 2013;13:340–51. doi: 10.2174/1566524011313030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballehaninna UK, Chamberlain RS. Serum CA 19-9 as a Biomarker for Pancreatic Cancer-A Comprehensive Review. Indian journal of surgical oncology. 2011;2:88–100. doi: 10.1007/s13193-011-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, Nicolini A, Topolcan O, Heinemann V. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441–7. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 7.Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–902. doi: 10.1200/JCO.2005.05.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takasaki H, Uchida E, Tempero MA, Burnett DA, Metzgar RS, Pour PM. Correlative study on expression of CA 19-9 and DU-PAN-2 in tumor tissue and in serum of pancreatic cancer patients. Cancer Res. 1988;48:1435–8. [PubMed] [Google Scholar]
- 9.Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501–3. [PubMed] [Google Scholar]
- 10.Honda K, Hayashida Y, Umaki T, Okusaka T, Kosuge T, Kikuchi S, Endo M, Tsuchida A, Aoki T, Itoi T, Moriyasu F, et al. Possible detection of pancreatic cancer by plasma protein profiling. Cancer Res. 2005;65:10613–22. doi: 10.1158/0008-5472.CAN-05-1851. [DOI] [PubMed] [Google Scholar]
- 11.Honda K, Kobayashi M, Okusaka T, Rinaudo JA, Huang Y, Marsh T, Sanada M, Sasajima Y, Nakamori S, Shimahara M, Ueno T, et al. Plasma biomarker for detection of early stage pancreatic cancer and risk factors for pancreatic malignancy using antibodies for apolipoprotein-AII isoforms. Sci Rep. 2015;5 doi: 10.1038/srep15921. 15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda K, Okusaka T, Felix K, Nakamori S, Sata N, Nagai H, Ioka T, Tsuchida A, Shimahara T, Shimahara M, Yasunami Y, et al. Altered plasma apolipoprotein modifications in patients with pancreatic cancer: protein characterization and multiinstitutional validation. PLoS One. 2012;7:e46908. doi: 10.1371/journal.pone.0046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honda K, Srivastava S. Potential usefulness of apolipoprotein A2 isoforms for screening and risk stratification of pancreatic cancer. Biomark Med. 2016;10:1197–207. doi: 10.2217/bmm-2016-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bingham S, Riboli E. Diet and cancer--the European Prospective Investigation into Cancer and Nutrition. Nat Rev Cancer. 2004;4:206–15. doi: 10.1038/nrc1298. [DOI] [PubMed] [Google Scholar]
- 15.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public health nutrition. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 16.Lubin JH. Extensions of analytic methods for nested and population-based incident casecontrol studies. Journal of chronic diseases. 1986;39:379–88. doi: 10.1016/0021-9681(86)90124-4. [DOI] [PubMed] [Google Scholar]
- 17.Pepe MS, Fan J, Seymour CW. Estimating the receiver operating characteristic curve in studies that match controls to cases on covariates. Acad Radiol. 2013;20:863–73. doi: 10.1016/j.acra.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. The American journal of gastroenterology. 1990;85:350–5. [PubMed] [Google Scholar]
- 20.Nolen BM, Brand RE, Prosser D, Velikokhatnaya L, Allen PJ, Zeh HJ, Grizzle WE, Huang Y, Lomakin A, Lokshin AE. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One. 2014;9:e94928. doi: 10.1371/journal.pone.0094928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien DP, Sandanayake NS, Jenkinson C, Gentry-Maharaj A, Apostolidou S, Fourkala EO, Camuzeaux S, Blyuss O, Gunu R, Dawnay A, Zaikin A, et al. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: implications for early disease detection. Clin Cancer Res. 2015;21:622–31. doi: 10.1158/1078-0432.CCR-14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandharipande PV, Heberle C, Dowling EC, Kong CY, Tramontano A, Perzan KE, Brugge W, Hur C. Targeted screening of individuals at high risk for pancreatic cancer: results of a simulation model. Radiology. 2015;275:177–87. doi: 10.1148/radiol.14141282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenner BJ, Chari ST, Maitra A, Srivastava S, Cleeter DF, Go VL, Rothschild LJ, Goldberg AE. Early Detection of Pancreatic Cancer-a Defined Future Using Lessons From Other Cancers: A White Paper. Pancreas. 2016;45:1073–9. doi: 10.1097/MPA.0000000000000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepe MS, Janes H, Li CI, Bossuyt PM, Feng Z, Hilden J. Early-Phase Studies of Biomarkers: What Target Sensitivity and Specificity Values Might Confer Clinical Utility? Clin Chem. 2016;62:737–42. doi: 10.1373/clinchem.2015.252163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas. 2008;36:e15–20. doi: 10.1097/mpa.0b013e31814de421. [DOI] [PubMed] [Google Scholar]
- 26.Balasenthil S, Huang Y, Liu S, Marsh T, Chen J, Stass SA, KuKuruga D, Brand R, Chen N, Frazier ML, Jack Lee J, et al. A Plasma Biomarker Panel to Identify Surgically Resectable Early-Stage Pancreatic Cancer. Journal of the National Cancer Institute. 2017;109 doi: 10.1093/jnci/djw341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capello M, Bantis LE, Scelo G, Zhao Y, Li P, Dhillon DS, Patel NJ, Kundnani DL, Wang H, Abbruzzese JL, Maitra A, et al. Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. Journal of the National Cancer Institute. 2017;109 doi: 10.1093/jnci/djw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkinson C, Elliott VL, Evans A, Oldfield L, Jenkins RE, O'Brien DP, Apostolidou S, Gentry-Maharaj A, Fourkala EO, Jacobs IJ, Menon U, et al. Decreased Serum Thrombospondin-1 Levels in Pancreatic Cancer Patients Up to 24 Months Prior to Clinical Diagnosis: Association with Diabetes Mellitus. Clin Cancer Res. 2016;22:1734–43. doi: 10.1158/1078-0432.CCR-15-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.