Abstract
BACKGROUND/OBJECTIVE
The blue honeysuckle berry (Lonicera caerulea var. edulis L.) is a small deciduous shrub belonging to the Caprifoliaceae family that is native to Russia, China, Japan, and Korea. The berry of this shrub is edible, sweet and juicy and is commonly known as the blue honeyberry (BHB). This study examined the anti-diabetic potential of BHB on high-fat-diet-induced mild diabetic mice. The hypoglycemic, and nephroprotective effects of the 12-week oral administration of blue honeyberry extract were analyzed.
MATERIALS/METHODS
The hypoglycemic effects were based on the observed changes in insulin, blood glucose, and glycated hemoglobin (HbA1c). Furthermore, the changes in the weight of the pancreas, including its histopathology and immunohistochemical investigation were also performed. Moreover, the nephroprotective effects were analyzed by observing the changes in kidney weight, its histopathology, blood urea nitrogen (BUN), and serum creatinine levels.
RESULTS
The results showed that the high-fat diet (HFD)-induced control mice showed a noticeable increase in blood glucose, insulin, HbA1c, BUN, and creatinine levels. Furthermore, growth was observed in lipid droplet deposition related to the degenerative lesions in the vacuolated renal tubules with the evident enlargement and hyperplasia of the pancreatic islets. In addition, in the endocrine pancreas, there was an increase in the insulin-and glucagon-producing cells, as well as in the insulin/glucagon cell ratios. On the other hand, compared to the HFD-treated mice group, all these diabetic and related complications were ameliorated significantly in a dose-dependent manner after 84 days of the continuous oral administration of BHBe at 400, 200 and 100 mg/kg, and a dramatic resettlement in the hepatic glucose-regulating enzyme activities was observed.
CONCLUSIONS
By assessing the key parameters for T2DM, the present study showed that the BHBe could act as a potential herbal agent to cure diabetes (type II) and associated ailments in HFD-induced mice.
Keywords: Insulin, pancreas, kidney, glucagon, metformin, bun, creatinine
INTRODUCTION
Diabetes mellitus (DM) affected 451 million individuals worldwide in 2017, and the number is anticipated to increase to 693 million people by 2045 [1]. The important causes of this disease include age, poor dietary patterns, high caloric diet, and sedentary lifestyle [2,3]. DM can be categorized broadly into two etiopathogenetic classes: type I (insulin-dependent) and type II (insulin-independent). Type I diabetes mellitus (T1DM) is an autoimmune disorder that is attributed to insulin-scarcity owing to the dysfunction of β cells of the pancreas, and their incorrect destruction by the body's immune system [3]. Diabetes mellitus type II (T2DM), a global epidemic, is distinguished by hyperglycemia, as well as impaired carbohydrates, protein, lipids, and electrolyte metabolism [4]. In addition, an increased insulin resistance (IR) in the peripheral tissues and the relative reduction in the insulin secretion has also been reported [5]. If left untreated, T2DM can cause complications, such as myocardial infarction and stroke (macrovascular), and even retinopathy, neuropathy, and nephropathy (microvascular) problems [6]. Furthermore, the effects on the oral system, gastrointestinal system, genital system, skin, and soft tissues, and bone fracture risks have also been summarized [7]. Reports have shown that modification of the habits/dietary patterns and pharmacological intervention could avert or delay T2DM and related disorders [8]. Many different drugs for the treatment of T2DM are available in the market, but they are not without adverse effects, such as weight gain, gastrointestinal complications, hypoglycemia, and cardiovascular risks. [9]. Therefore, the quest for complementary and alternative medicine (CAM) for T2DM is expanding [10].
Metformin is the most prescribed anti-diabetic drug for T2DM treatment [11,12]. The drug is relatively safe and low cost, and has favorable effects on the insulin requirement, blood glucose, weight, and related cardiovascular ailments [13]. On the other hand, long-term use of metformin may lead to a vitamin B12 deficiency due to impaired absorption [14]. Furthermore, the unexpected effects of metformin use is lactic acidosis, which is an impending and rare condition accompanied by reduced kidney or liver function [15].
The sweet honeyberry species (Lonicera caerulea L. var. edulis), which is also known as haskap and blue honeyberry, is a shrub member of the Caprifoliaceae family. Its edible product, namely blueberries, are a rich source of primary and secondary metabolites [16,17], such as anthocyanins [18], phenolics, flavonoids [19], and saponins [20]. Traditionally, blue honeyberry (BHB) has been used as medicine in northern Russia, China, and Japan [17], and its extract has been reported to have the most potent antioxidant activity among 12 types of colored berries described [21]. Owing to its remarkable health benefits, BHB has emerged as a new functional food based on the research published over the last decade. The oral administration of blue honeyberry extract (BHBe) was reported to protect mice from ionizing radiation [22]. Other therapeutic applications of BHBe include hepatoprotective effects [23], anti-inflammatory effects [24,25], skin protective effects against ultraviolet-induced damages [24], impact on the hyperthyroidism [26], and amelioration of the abnormal lipid and glucose metabolism in rats [27]. Moreover, its effects on adjuvant-induced arthritis in a rat model have also been elucidated [28]. Furthermore, a few recent relevant studies include, the in vitro alleviation of silica particle (SP)-induced pulmonary inflammation by the administration of BHBe [29]. Similarly, the polyphenol extract of BHB resulted in the cholesterol-lowering and enhanced antioxidant capacity in vitro and in vivo [30]. Furthermore, research has shown that polyphenol supplementation increased the CYP7A1 activity, which augmented the metabolism of cholesterol to bile acids [31]. Another study revealed the dose-dependent amelioration effects of BHBe on high-fat diet (HFD)-induced obesity and hepatic fat deposition [32].
By realizing the importance of functional foods as herbal medicines, this study examined the anti-diabetic potential of BHBe on a HFD-induced mild diabetic mouse model by assessing the underlying hypoglycemic and nephroprotective effects.
MATERIAL AND METHODS
Animals and diets
Six-week-old female SPF ICR mice (n = 48) were purchased from OrientBio, Seongnam, Republic of Korea and allowed to acclimatize for one week in groups comprised four to five animals per polycarbonate cage in a light (12-h light per day), humidity (40–45%), and temperature (20–25℃) controlled room. The mice were fed a standardized rodent chow (Purina feed, Seongnam, Rep. of Korea) and given tap water ad libitum.
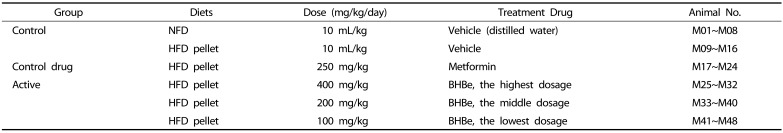
All adapted experimental animals were randomized into six groups (8 mice per group) based on the body weight: normal-pellet diet (NFD) group, high-fat diet (HFD) group, HFD+metformin group, HFD+BHBe (400 mg/kg) group, HFD+BHBe (200 mg/kg) group, and HFD+BHBe (100 mg/kg) group. Metformin hydrochloride purchased from Wako, Osaka, Japan was used as a standard. Table 1 provides details of the corresponding diets.
Table 1. Experimental test groups in this study.
NFD, Normal pellet diet; HFD, 45% kcal high-fat diet; BHB, Blue honeyberry; BHBe, lyophilized powder of BHB extract by enzyme.
The experimental animals were handled according to the national regulations of the use and welfare of laboratory animals and all the procedures were approved by the Institutional Animal Care and Use Committee (IACUC), Daegu Haany University, Gyeongsan, Gyeongbuk, Rep. of Korea [Approval No: DHU2017-022].
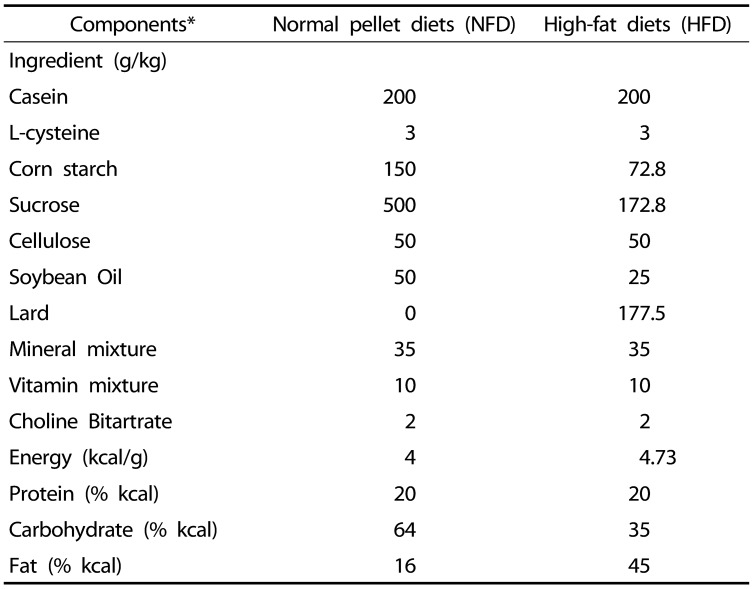
The animals were provided free access to a 45% kcal HFD (Research Diet, New Brunswick, NJ, USA) (Table 2) after 7 days of acclimatization. In the intact control group, the mice were given free access to a NFD (Purina feed, Seongnam, Rep. of Korea) instead of a HFD.
Table 2. Composition of normal and high-fat diets.
*45% kcal/fat pellet diets (Cat. No. D12451; Research Diet, New Brunswick, NJ, USA) was used as the high-fat diet (HFD), and the normal rodents pellet diet (Cat. No. 38057; Purina feed, Seongnam, Korea) were used as the normal fat pellet diets.
Preparations and administration of Blue honeyberry extract (BHBe)
BHBe was prepared and supplied by the sponsor (Aribio, Seongnam, Korea) as a deep purple colored powder, and stored at −20℃ to protect it from light and humidity until further use. Briefly, frozen BH fruits were heated at 45–55℃ for three minutes, pulverized, followed by enzymatic treatment with the mixture at a concentration of 0.05% (w/w) of Natuzyme olimax and Natuzyme DP ultra, incubated for 2–2.5 h at 50 rpm, centrifuged at 4,000 rpm and kept at 80℃ for 15–30 s. After the addition of chitosan (0.005%, w/w) and guar gum (0.005%, w/w), the mixture was filtered using (disc separation, diatomite filtration, and filter press), condensed (63 brix, 50℃, 1 min, 0.092 MPa), sterilized (90–95℃, 15–30 s), and then freeze-dried. The BHBe yield was 10.83%.
A white powder of metformin HCl (Wako, Osaka, Japan) was used as the reference drug. Metformin HCl was dissolved in deionized water at a concentration of 25 mg/mL and taken orally at 10 mL/kg (equivalent to 250 mg/kg) once in a day for 84 days from the 1st week after the initial HFD supply.
Similarly, the prepared berry extract was dissolved in distilled water at 10, 20, and 40 mg/mL, and a 10 mL/kg (equivalence to 400, 200 and 100 mg/kg) was administered orally once per day for 84 days using a stainless zoned (gastric gavage) from the 1st week after HFD supply. In this study, the selected doses and the volumes of BHBe were determined based on the general dosing instructions for the mice provided in the guidelines [KFDA Guidelines, Notification No: 2015-082]. The HFD and the intact vehicle control animals were administered orally an equal volume of deionized water, instead of the test substances to provide the same restrain stresses from gastric gavages (Table 1).
Body and organ weight measurement
The body weight of the experimental mice in each group was recorded on days 0, 8 (before the start of HFD supply), and 84 using an electronic measuring balance (Precisa Instrument, Zurich, Switzerland). After 12 weeks, the mice were fasted overnight, sacrificed on the next day and the left kidney and pancreas weights were recorded individually. To reduce the variances from the individual body weights, the relative body weights were also estimated using the absolute weight and body weight at sacrifice, as described in equation (1).
| (1) |
Measurement of serum biochemistry (glucose, BUN, creatinine)
For blood collection, all mice were anesthetized with 2% to 3% isoflurane in a mixture containing 28.5% O2 and 70% N2O using a rodent ventilator (Harvard Apparatus, Cambridge, UK) and the rodent inhalation anesthesia apparatus (Surgivet, Waukesha, WI, USA). At the end of 84 day treatment, blood was collected in NaF-containing vacuum tubes via the caudal vena cava (Becton Dickinson, Franklin Lakes, NJ, USA) for blood glucose analysis and plasma separation, and stored in an ultra-deep freezer at −150℃ (MDF-1156, Sanyo, Tokyo, Japan) until further analysis. An automated blood analyzer (Fuji Medical System Co., Ltd., Tokyo, Japan) was used to measure the blood glucose, BUN, and creatinine levels. For the BUN and creatinine measurements, clotting activated serum tubes were used to collect blood, and after collection, the tubes were centrifuged at 15,000 rpm for 10 min at room temperature (RT) to obtain serum.
Estimation of blood HbA1c and serum insulin levels
The serum insulin and blood hemoglobin A1c (HbA1c) levels were measured using automated HbA1c measuring equipment (Infopia, Anyang, Rep. of Korea) and the serum insulin levels were assayed using a mouse insulin ELISA kit (Alpco Diagnostics, Windham, NH, USA).
Histopathology
For organ histology, some parts of the splenic lobes of the pancreas and left lateral portions of the left kidney were fixed in 10% neutral buffered formalin. The fixed tissues were paraffin embedded using an automated tissue processor (Thermo Scientific, Waltham, MA, USA) and the embedding center (Thermo Scientific, Waltham, MA, USA), and 3–4 µm thick serial sections were prepared using a microtome (Leica Biosystems, Nussloch, Germany).
Hematoxylin and eosin stain (H&E) was used to stain representative sections, and the histological profile of the individual organs was measured by optical microscopy (Nikon, Tokyo, Japan), photographed, and analyzed. The mean numbers of the lipid droplets deposited in the vacuolated renal tubules were also calculated using automated image analysis software (IMT i-solution Inc., Vancouver, Quebec, Canada) and the diameters of pancreatic islets were also recorded using an automated image analysis process, as demonstrated previously [10,33,34]. At the time of analysis, the histopathologist was blinded to the group distribution.
Immunohistochemistry
The sectioned pancreatic tissues were immunostained by an avidin-biotin-complex (ABC) staining method [10,34] using guinea pig polyclonal insulin (1:100; Abcam, Cambridge, UK) or rabbit polyclonal glucagon (1:100; Abcam, Cambridge, UK) antiserum. Briefly, the endogenous peroxidase activity was limited by incubation in a 0.3% H2O2 and methanol solution for 30 min, whereas the tissues were incubated using a horse serum blocking solution (1:100; Vector Lab., Burlingame, CA, USA) for 1 h to block the non-specific binding of immunoglobulin. The mixture was treated overnight with the primary antiserum at 4℃, followed by the addition of the biotinylated universal secondary antibody (1:50; Vector Lab., Burlingame, CA, USA) and the required ABC reagents (1:50; Vectastain Elite ABC Kit, Vector Lab., Burlingame, CA, USA), incubated at RT for 1 h in a humid chamber. Finally, the peroxidase substrate from a kit (1:50; Vector Lab., Burlingame, CA, USA) was added to the mixture at RT for 3 min. After each step, tissue sections were rinsed three times with PBS (0.01 M). Compared to another naïve cell, an immunoreactive cell density > 20% of each antiserum (insulin and glucagon) was considered positive. As described previously, the insulin- and glucagon-positive immunoreactive cells were evaluated by measuring the mean area (mm2) of pancreatic parenchyma cells using an automated image analysis method [10,34,35]. In addition, the insulin/glucagon cell ratios were measured, as described in equation (2). The samples were coded to perform a blind test by the histopathologist.
| (2) |
Statistical analyses
Different doses of the test material were compared using multiple comparison tests. A Levene's test was used to examine the variance homogeneity [36]. If no significant deviation was observed by the Levene's test, the data were analyzed by one-way ANOVA using a least-significant differences multi-comparison (LSD) test to estimate the significantly different pairs among the groups compared. If a significant deviation from the variance was observed using the Levene's test, the groups were compared using a non-parametric comparison test (Kruskal-Wallis H test). If the Kruskal-Wallis H test showed a significant difference, a Mann-Whitney U test was performed to estimate the significantly different specific pairs of the group. SPSS ver. 14 (IBM-SPSS Inc., Chicago, IL, USA) was used for statistical analyses [37]. For the test materials effectiveness, the percentage changes were recorded and compared with the HFD control mice, whereas for disease induction, the percentage changes between the HFD and intact control mice were determined [10,38] using equations (3) and (4).
| (3) |
| (4) |
RESULTS
Effects on the pancreas and kidney weights
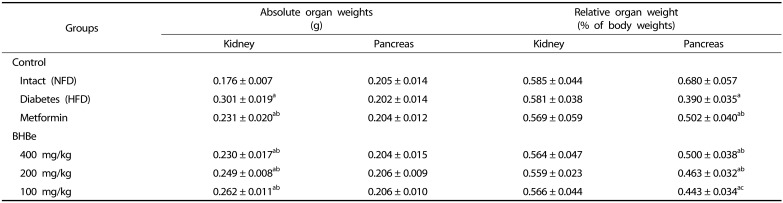
After treatment, the relative weights of the pancreas were significantly lower (P < 0.01) in the HFD-served mice group than the intact (NFD) control. In contrast, the relative weights of the pancreas in the groups supplemented with metformin and BHBe fractions, i.e., 400, 200 and 100 mg/kg, were 28.49% and 28.11, 18.71, 13.54% higher (P < 0.01), respectively, than that of the HFD-treated mice group. Compared to the intact control, a −42.62% change was observed in the relative weights of the pancreas in the HFD-treated mice group. On the other hand, compared to the NFD control, the absolute pancreatic weights did not show any significant change in the four mice treatment groups together with the HFD control (Table 3).
Table 3. Changes in absolute and relative organ weights in HFD-induced diabetes mice.
Values are expressed as mean ± SD (n = 8); NFD, Normal pellet diet; HFD, 45% kcal high-fat diet; BHB, Blue honeyberry; BHBe, lyophilized powder of BHB extract by enzyme.
aP < 0.01 as compared with the intact control by LSD test; bP < 0.01 and cP < 0.05 as compared with the HFD control by the LSD test.
Significantly (P < 0.01) higher absolute weights of the kidney were observed in the HFD treated mice group compared to that of the NFD control group. The absolute kidney weight in the HFD control was increased by 71.05% compared to the intact control. On the other hand, after treatment with all four test materials, it was normalized to that of the HFD-fed mice (P < 0.01). In particular, the absolute kidney weights with the supplementation of metformin and BHBe at 400, 200 and 100 mg/kg were reduced significantly by 23.12% and 23.53, 17.26, 12.77%, respectively. Compared to the NFD control group, the relative kidney weights were not changed significantly in different treatments together with the HFD control (Table 3).
Effects on the glycated hemoglobin and insulin concentrations
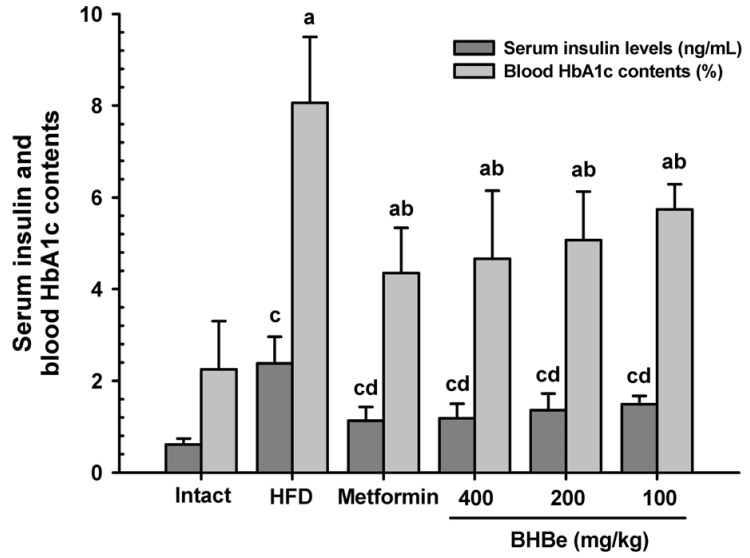
The serum insulin concentration was significantly higher (P < 0.01), by 291.58%, in the HFD-fed mice group than in the NFD control. On the other hand, these increases in the insulin concentrations were reduced by supplementation of all four agents in contrast to the HFD-fed group.
As shown in Fig. 1, mice treated with BHBe 400, 200 and 100 mg/kg agents displayed a dose-dependent decrease of 50.39, 43.10 and 37.44%, respectively, in the serum insulin levels. Also, a 52.75% decrease was observed in the metformin group.
Fig. 1. Effects of the blue honeyberry extract (BHBe) on the serum insulin and blood HbA1c contents in type 2 diabetic mice.
The values are expressed as mean ± SD (n = 8/group). NFD, Normal pellet diet; HFD, 45% kcal high-fat diet; HbA1c, Glycated hemoglobin, hemoglobin A1c; BHB, Blue honeyberry; BHBe, lyophilized powder of the BHB extract by enzyme. Metformin was administrated at a dose of 250 mg/kg; aP <0.01 compared to the intact control by a LSD test; bP <0.01 compared to the HFD control by a LSD test; cP < 0.01 compared to the intact control by a MW test; dP < 0.01 compared to the HFD control by a MW test.
Similarly, glycated hemoglobin (HbA1c) level was augmented significantly in the HFD-fed mice group in contrast to the intact control, and the percentage change was 258.93% compared to the NFD-fed control (P < 0.01). On the other hand, the HbA1c content of blood was decreased after four trial elements supplementation compared to that of the HFD-fed mice group (P < 0.01). A dose-dependent decrease in the HbA1c contents, i.e., 42.20, 37.13 and 28.87%, was observed with the BHBe treatments at 400, 200 and 100 mg/kg, respectively, compared to that of the HFD group (Fig. 1), whereas a 46.11% reduction was observed in the metformin-treated group.
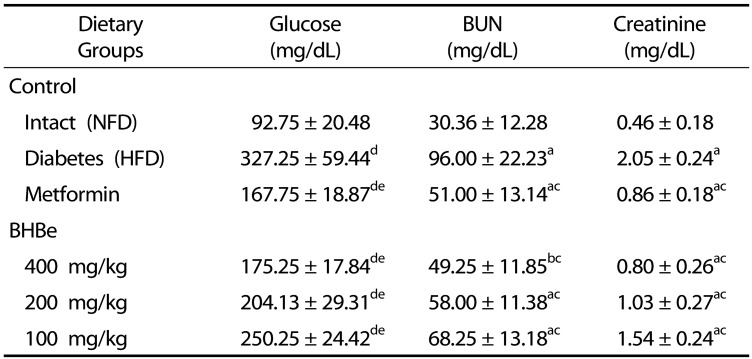
Effects on the glucose, creatinine and BUN proportions
Compared to the normal control, the blood glucose level was elevated significantly in the HFD-fed mice group, by a factor of 252.83% (P < 0.01). Nevertheless, significant recoveries of glucose were observed in the induced diabetic mice groups after the administration of four trial materials compared to the HFD-treated group (Table 4). In particular, a definite dose-dependent decrease in the blood glucose level was observed with three different test agents compared to the HFD group. As shown in table 4, with four different treatment agents, i.e., metformin 250 mg/kg, BHBe 400, 200 and 100 mg/kg, a 48.74, 46.45, 37.62, and 23.53% decrease in the blood glucose level, respectively, compared with the HFD control was observed.
Table 4. Changes in blood glucose, BUN and creatinine levels in HFD-induced diabetes mice.
Values are expressed as mean ± SD (n = 8); NFD, Normal pellet diet; HFD, 45% kcal high-fat diet; BHB, Blue honeyberry; BHBe, lyophilized powder of BHB extract by enzyme; BUN, Blood Urea Nitrogen.
aP < 0.01 and bP < 0.05 as compared with the intact control by LSD test; cP < 0.01 as compared with the HFD control by LSD test; dP < 0.01 as compared with the intact control by MW test; eP < 0.01 as compared with the HFD control by MW test.
The amounts of BUN in the serum and creatinine were significantly higher (P < 0.01) in the HFD-treated mice group than in the NFD group. A 213.47% increase in the serum BUN level was observed in the HFD-treated mice group compared to the NFD group. With the metformin 250 mg/kg, BHBe 400, 200 and 100 mg/kg treatments, the percentage BUN reduction was observed to be 46.88, 48.70, 39.58, and 28.91%, respectively.
Similarly, the serum creatinine level in the HFD control was 252.83% alleviated compared to the intact control (NFD-fed mice), and a 48.74, 46.45, 37.62, and 23.5% reduction was observed in the groups with metformin 250 mg/kg, BHBe supplementation at 400, 200 and 100 mg/kg, respectively, compared to the HFD-treated mice group (Table 4).
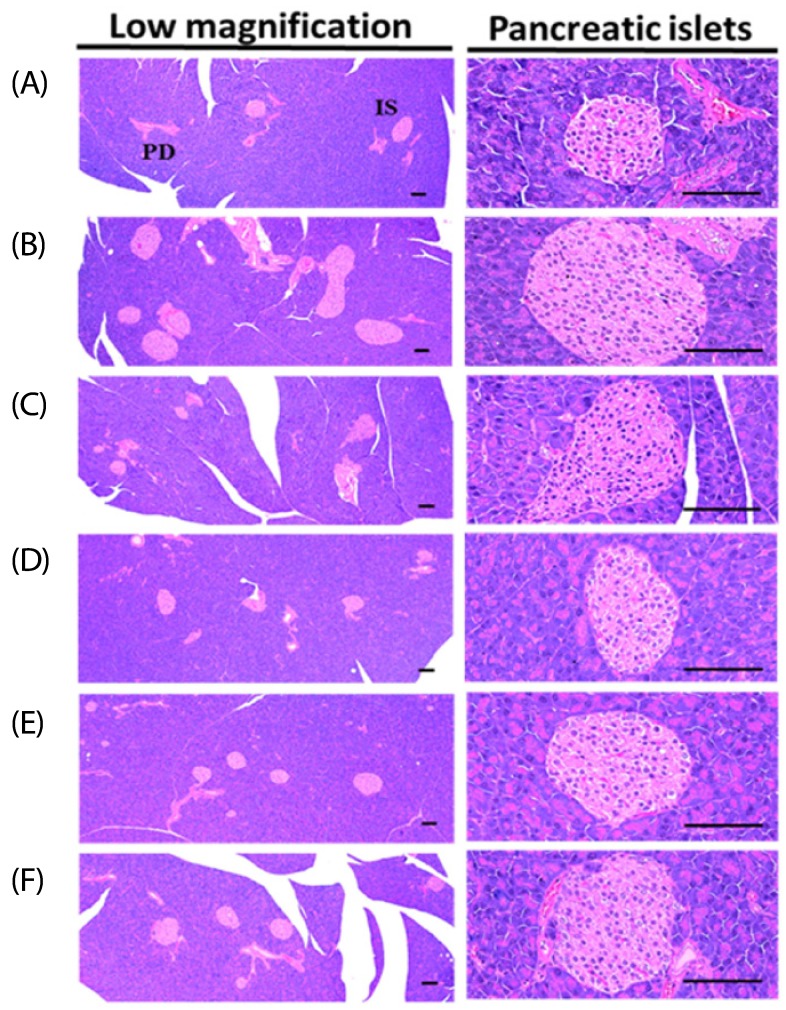
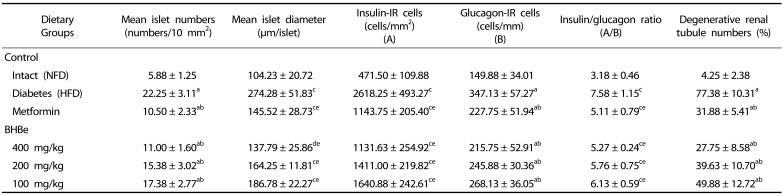
Effects on the pancreas histopathology
An increase in the mean diameters and numbers of islet cells of the pancreas was observed in the HFD-fed mice group compared to that of the normal control owing to the obvious hyperplasia of islet cells of the pancreas or constituent endocrine cells (P < 0.01).
Both the growth and hyperplasia of islet cells were decreased by the supplementation of various extracts compared to the HFD control. Mice treated with BHBe at 400, 200 and 100 mg/kg showed a dose-dependent decrease in the mean diameter and number of islet cells in the pancreas compared to the HFD group (Fig. 2; Table 5).
Fig. 2. Representative histological images of the pancreas from NFD or HFD supplied mice, stained with Hematoxylin & Eosin stain.
(A) Intact control: 10 mL/kg of distilled water orally administered mice with the NFD supply. (B) diabetes control: 10 mL/kg of distilled water orally administered mice with the HFD supply. (C) Metformin: 250 mg/kg of metformin orally administered mice with the HFD supply. (D) BHBe 400: 400 mg/kg of BHBe orally administered mice with the HFD supply. (E) BHBe 200: 200 mg/kg of BHBe orally administered mice with the HFD supply. (F) BHBe 100: 100 mg/kg of BHBe orally administered mice with the HFD supply. NFD, Normal pellet diet; HFD, 45% kcal high-fat diet; IS, Pancreatic islet; PD, Pancreatic secretory duct; BHB, Blue honeyberry; BHBe, lyophilized powder of the BHB extract by enzyme; Scale bars = 80 µm.
Table 5. Changes in the histopathology-histomorphometry of the pancreas and kidney in HFD-induced diabetes mice.
Values are expressed as mean ± SD (n = 8); NFD, Normal pellet diet; HFD, 45% kcal high-fat diet; BHB, Blue honeyberry; BHBe, lyophilized powder of BHB extract by enzyme.
aP < 0.01 as compared with the intact control by LSD test; bP < 0.01 as compared with the HFD control by LSD test; cP < 0.01; dP < 0.05 as compared with the intact control by MW test; eP < 0.01 as compared with the HFD control by MW test
The mean number value of islet cells was increased by 278.72% in the HFD-treated mice group compared to the NFD group, whereas 52.81 and 50.56, 30.90, 21.91% reductions were observed in the groups supplemented with metformin and BHBe at different concentrations compared to that of the HFD-fed group, respectively. On the other hand, compared to the normal control, HFD-fed group displayed a 163.15% increase in the islet engaged regions, and a 46.95, 49.76, 40.12, and 31.90% reduction was observed in mice after treatment with metformin at 250 mg/kg, BHBe at 400, 200 and 100 mg/kg, respectively, compared to the HFD group.
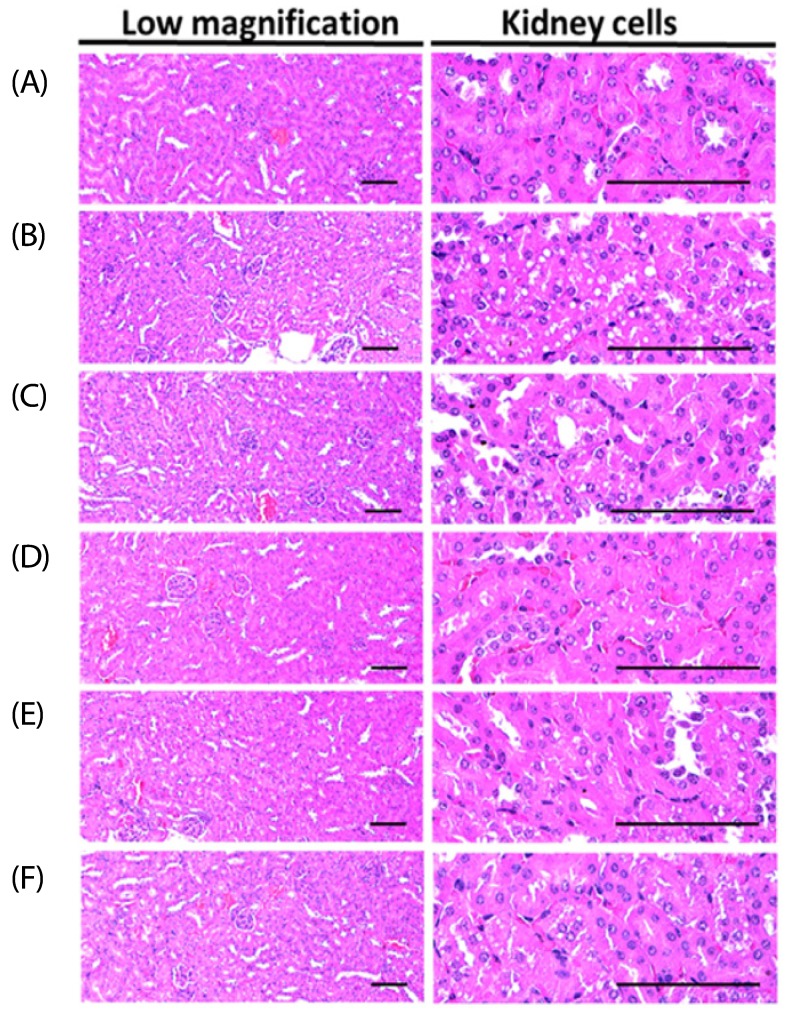
Effects on the histopathology of the kidney
In the HFD-fed mice group, the number of vacuolated renal tubules (degenerative lesion of the kidney) was increased significantly (P < 0.01) by a factor of 1720.59% compared to that of the normal control, resulting from lipid droplets deposited in nephropathies due to diabetes. However, after treatment with all four supplementations, including metformin, nephropathies due to diabetes were resettled compared to the induced diabetic mice group (P < 0.01).
For the metformin (250 mg/kg)-treated group, a 58.80% decrease in the number of vacuolated renal tubules was observed. Moreover, a clear dose-dependent decrease, 64.14, 48.79 and 35.56%, in the number of vacuolated renal tubules in BHBe administered mice groups at concentrations of 400, 200 and 100 mg/kg, respectively, compared to the HFD group was noted (Fig. 3; Table 5).
Fig. 3. Representative histological images of the kidney from the NFD or HFD supplied mice, stained with Hematoxylin & Eosin stain.
(A) Intact control: 10 mL/kg of distilled water orally administered mice with the NFD supply. (B) Diabetes control: 10 mL/kg of distilled water orally administered mice with the HFD supply. (C) Metformin: 250 mg/kg of metformin orally administered mice with the HFD supply. (D) BHBe 400: 400 mg/kg of BHBe orally administered mice with the HFD supply. (E) BHBe 200: 200 mg/kg of BHBe orally administered mice with the HFD supply. (F) BHBe 100: 100 mg/kg of BHBe orally administered mice with the HFD supply. NFD, Normal pellet diet; HFD, 45% kcal high-fat diet; BHB, Blue honeyberry; BHBe, lyophilized powder of the BHB extract by enzyme; Scale bars = 80 µm.
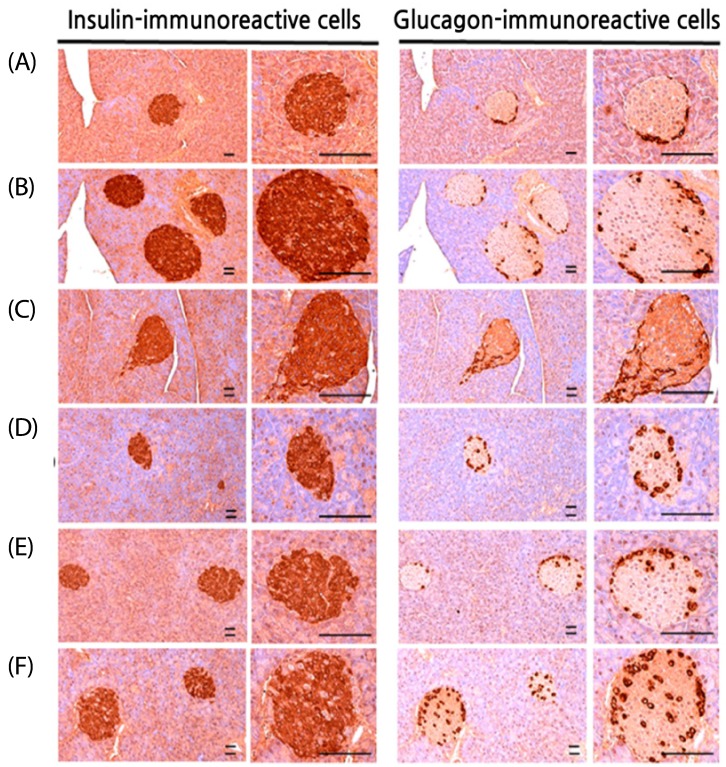
Effects on the insulin- and glucagon-producing islet cells of the pancreas
In the HFD-fed mice group, significant augmentation (P < 0.01) in the number and ratio was observed in the insulin-immunoreactive and glucagon-immunoreactive cells compared to that of the normal control. This unusual growth of cells in the pancreas was regularized by the supplementation of four trial materials with a significant P-value (< 0.01) compared to the induced diabetic mice group.
A definite dose-dependent decrease was observed in the insulin-and glucagon-immunoreactive cell number as well as in the insulin/glucagon cell ratios, mainly using three doses of BHBe (400, 200 and 100 mg/kg) compared to HFD control (Table 5; Fig. 4).
Fig. 4. Histological images of the insulin- and glucagon-immunoreactive cells in the pancreas and all cells were immunostained by the avidin-biotin-peroxidase complex.
(A) Intact control: 10 mL/kg of distilled water orally administered mice with the NFD supply. (B) Diabetes control: 10 mL/kg of distilled water orally administered mice with the HFD supply. (C) Metformin: 250 mg/kg of metformin orally administered mice with the HFD supply. (D) BHBe 400: 400 mg/kg of BHe orally administered mice with the HFD supply. (E) BHBe 200: 200 mg/kg of BHBe orally administered mice with the HFD supply. (F) BHBe 100: 100 mg/kg of BHBe orally administered mice with HFD supply. NFD, Normal pellet diet; HFD, 45% kcal high-fat diet; IS, Pancreatic islet; BHB, Blue honeyberry; BHBe, lyophilized powder of the BHB extract by enzyme; Scale bars = 80 µm.
Moreover, the mean number of insulin-immunoreactive cells and glucagon-immunoreactive cells in the HFD controls was increased to 455.30% and 131.61%, respectively, compared to the intact controls. On the other hand, the mice fed with the BHBe 400, 200 and 100 mg/kg agents showed 56.78, 46.11, 37.33%, and 37.75, 29.17, 22.76% reductions in the number of insulin-immunoreactive cells and glucagon-immunoreactive cells, respectively, than the HFD control. Furthermore, the metformin 250 mg/kg group showed a 56.32% reduction for the insulin-immunoreactive cells and a 34.39% decrease for the glucagon-immunoreactive cells.
Similarly, the insulin/glucagon cells ratio was increased in the HFD controls to 138.01% compared with the intact control mice fed the NFD. In contrast, treatment of the mice with metformin 250 mg/kg, BHBe 400, 200 and 100 mg/kg agents showed a decline in the ratio, i.e., 32.61, 30.50, 23.97, and 19.11%, respectively, compared to the HFD control (Table 5).
DISCUSSION
Globally, DM has emerged as a health pandemic issue owing to its high incidence, mortality and related complications [2]. Reports showed that the treatment of T2DM with metformin (Biguanides) is associated with side effects, such as gastric complications and vitamin B12 deficiency [14,15]. Therefore, worldwide, the need for natural products/functional food has increased to develop a physiologically active anti-diabetic herbal drug with fewer side effects [10].
Recently, BHB has attracted attention as a valuable source of bioactive constituents. Reports have shown that the major components of BHB consisted of polyphenols, saccharides, lipids, proteins, and organic acids demonstrating various bioactivities, such as anti-inflammation, anti-diabetic, antioxidant, and anticancer [17,39]. Among polyphenols, phenolic acids (major chlorogenic acid) and the anthocyanins (major cyanidin-3-O-glucoside; C3G) are the main constituents [40]. C3G along with other anthocyanins are absorbed mainly as glycosides in the stomach and small intestine, as shown by various in vivo and clinical studies. On the other hand, the percentage bioavailability is considered to be low and dependent of the presence of anthocyanidin and sugar molecules [41].
Previous review study has shown that the phytochemicals of berries have potential health activities against T2DM [17]. The prevention of α-amylase and α-glucosidase activities in the pancreas and intestine, respectively, were reported to be useful for the management of T2DM, and the HB concentration corresponding to its IC50 value (39.91 mg/mL) was reported to have the strongest α-glucosidase inhibitory activity [42]. This study examined the pharmacological effect of the BHBe on a mouse model with induced diabetes. There are reports that the animal prototypes fed with a HFD display high blood sugar and mild obesity; therefore, animal fed with a HFD can be used as suitable animal model to study the protective effects of bioactive compounds against induced type II diabetes [43,44].
The HFD-consumption leads to excessive blood/hepatic lipids that alleviate insulin resistance in the liver, eventually leading to DM (Type II) and related cardiovascular disorders [45,46]. The essential suggestive for the cure of diabetes includes the prevention of oxidative stress and the supervision of “after eating” hyperglycemia [10,47].
In the present study, after 91 days of HFD-induction, the mice group with diabetes displayed a noticeable increase in the insulin, blood glucose, HbA1c, BUN, and creatinine levels. In addition, the elevated degenerative lesions, i.e., vacuolization of the renal tubules with lipid droplet deposition, was observed with a marked increase and hyperplasia of the islets of Langerhans. Similarly, during the histopathological observation, an increase in the number and ratio of the pancreatic islet cells producing insulin/glucagon were observed in the HFD control mice. On the other hand, these diabetic and related nephritic complications were inhibited significantly by the BHBe treatments. Among them, the BHBe 400 mg/kg treatment exhibited favorable inhibitory action on type II diabetes and related complications compared to the HFD-induced diabetic mice. These findings provide direct evidence of the favorable anti-diabetic effect of BHBe on a type II diabetic mice model compared to the HFD-supplemented group.
In chronic diabetes, an elevation in the serum BUN and creatinine levels leads to weight gain of the kidneys owing to puffiness, necrotic processes, and chronic inflammation, which are termed diabetic nephropathy. Therefore, any agent that leads to an improvement in these complications provides direct evidence of the treatment of diabetic nephropathies [10]. Similarly, in the present study, the kidney weight and the related complications were reduced in a dose-dependent manner after treatment with different concentrations of BHBe compared to the HFD-treated group. Therefore, these results may be considered as direct evidence of the nephroprotective effects of BHBe on the HFD-induced mild diabetic mice. Metformin is the most prescribed medication for DM (type II) patients [11]. In diabetes, due to the low response of tissues to insulin, the need for increased insulin secretion from the pancreas leads to the activation of more insulin-producing cells as well as an increase in the region of pancreatic islets to maintain glucose homeostasis [38]. On the other hand, this increased pancreatic islets region does not affect the weight of the pancreas. Rather, the relative weight of the pancreas due to body weight gain will be reduced. This phenomenon appears to be a common symptom observed in the HFD-induced diabetic model [48]. In this study, the weight of the pancreas was lower in the HFD-induced diabetic mice group. Nevertheless, treatments with the test agents led to resettlement of the pancreas weight, including metformin compared to the HFD control.
HbA1c, a form of hemoglobin, is used as the gold standard to observe the glycemic control and its higher content is an indication of poor blood glucose control, which leads to multiple diabetic complications [49]. For diabetes, a high blood sugar level is a key concern and should be monitored to treat the ailment [10]. Furthermore, for diabetic patients, an adjustment of drugs or dosages is based on a regular evaluation of the HbA1c levels [50]. Rodents fed a HFD have been a frequently used animal model for research [51] and revealed a high content of HbA1c and blood insulin following the continuous administration of a HFD [10,52].
Furthermore, in this study, an apparent increase in the insulin, blood glucose, and HbA1c levels were observed in the HFD-fed mice group compared to the normal control. In addition, after the histopathological observations, the increase in the number and ratio of islet cells of the pancreas producing insulin and glucagon hormones was observed, suggested an insulin-resistant type II diabetic ailment.
Previous studies showed that the islet of Langerhans hyperplasia is associated with an augmented insulin level due to the development of insulin-resistance in a HFD-treated mice model [53]. Therefore, to maintain glucose homeostasis, an increase in the insulin-producing pancreatic islet cells (both number and area) after chronic consumption of HFD was reported [54] with noticeable hypertrophy or hyperplasia of pancreatic endocrine cells [53,54]. Similarly, an increased percentage was also reported for glucagon-producing cells [53].
On the other hand, the continuous oral administration of BHBe at 400, 200 and 100 mg/kg for 84 days resulted in the dose-dependent inhibition of these abnormal histopathological changes, including an increase in the blood glucose, insulin, and the HbA1c contents, compared to the HFD group. These findings are considered evidence that the test agents BHBe at 400, 200 and 100 mg/kg concentrations have favorable hypoglycemic effects on HFD mice, which may be due to the inhibition of pancreatic endocrine changes.
In conclusion, the present analysis indicates that BHBe test agents showed a favorable response against T2DM and the related complications in the HFD-induced type II diabetic mice compared to the HFD group. Therefore, it is expected that BHBe can act as a potential herbal drug or medicinal food to cure type II diabetes and its related complications in the near future.
Footnotes
This study was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, and partially supported by MAFRA (Ministry of Agriculture, Food and Rural Affairs) with grant number-116019-3 and partly supported by the Gachon University research fund of 2018 (GCU-2018-0369).
CONFLICT OF INTEREST: The authors declare no potential conflicts of interest.
References
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Sami W, Ansari T, Butt NS, Hamid MRA. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci (Qassim) 2017;11:65–71. [PMC free article] [PubMed] [Google Scholar]
- 3.Enk J, Mandelboim O. The role of natural cytotoxicity receptors in various pathologies: emphasis on type I diabetes. Front Immunol. 2014;5:4. doi: 10.3389/fimmu.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright JJ, Tylee TS. Pharmacologic therapy of type 2 diabetes. Med Clin North Am. 2016;100:647–663. doi: 10.1016/j.mcna.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev. 2017;13:3–10. doi: 10.2174/1573399812666151016101622. [DOI] [PubMed] [Google Scholar]
- 8.Lindström J, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, Uusitupa M, Tuomilehto J Finnish Diabetes Prevention Study (DPS) Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS) Diabetologia. 2013;56:284–293. doi: 10.1007/s00125-012-2752-5. [DOI] [PubMed] [Google Scholar]
- 9.Shah P, Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin Drug Saf. 2010;9:347–354. doi: 10.1517/14740331003623218. [DOI] [PubMed] [Google Scholar]
- 10.Kang SJ, Lee JE, Lee EK, Jung DH, Song CH, Park SJ, Choi SH, Han CH, Ku SK, Lee YJ. Fermentation with Aquilariae lignum enhances the anti-diabetic activity of green tea in type II diabetic db/db mouse. Nutrients. 2014;6:3536–3571. doi: 10.3390/nu6093536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mottillo EP, Desjardins EM, Fritzen AM, Zou VZ, Crane JD, Yabut JM, Kiens B, Erion DM, Lanba A, Granneman JG, Talukdar S, Steinberg GR. FGF21 does not require adipocyte AMP-activated protein kinase (AMPK) or the phosphorylation of acetyl-CoA carboxylase (ACC) to mediate improvements in whole-body glucose homeostasis. Mol Metab. 2017;6:471–481. doi: 10.1016/j.molmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma A, Wang J, Yang L, An Y, Zhu H. AMPK activation enhances the anti-atherogenic effects of high density lipoproteins in apoE−/− mice. J Lipid Res. 2017;58:1536–1547. doi: 10.1194/jlr.M073270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: Post hoc analysis of a randomized controlled 4.3year trial. J Diabetes Complications. 2018;32:171–178. doi: 10.1016/j.jdiacomp.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Zdilla MJ. Metformin with either histamine H2-receptor antagonists or proton pump inhibitors: A polypharmacy recipe for neuropathy via vitamin B12 depletion. Clin Diabetes. 2015;33:90–95. doi: 10.2337/diaclin.33.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khurana R, Malik IS. Metformin: safety in cardiac patients. Heart. 2010;96:99–102. doi: 10.1136/hrt.2009.173773. [DOI] [PubMed] [Google Scholar]
- 16.Chaovanalikit A, Thompson MM, Wrolstad RE. Characterization and quantification of anthocyanins and polyphenolics in blue honeysuckle (Lonicera caerulea L.) J Agric Food Chem. 2004;52:848–852. doi: 10.1021/jf030509o. [DOI] [PubMed] [Google Scholar]
- 17.Svarcova I, Heinrich J, Valentova K. Berry fruits as a source of biologically active compounds: the case of Lonicera caerulea. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:163–174. doi: 10.5507/bp.2007.031. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Xu Q, Li X, Wang Y, Zhu J, Ning C, Chang X, Meng X. Effects of high hydrostatic pressure on physicochemical properties, enzymes activity, and antioxidant capacities of anthocyanins extracts of wild Lonicera caerulea berry. Innov Food Sci Emerg Technol. 2016;36:48–58. [Google Scholar]
- 19.Oszmiański J, Wojdyło A, Lachowicz S. Effect of dried powder preparation process on polyphenolic content and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L. var. kamtschatica) LWT-Food Sci Technol. 2016;67:214–222. [Google Scholar]
- 20.Becker R, Pączkowski C, Szakiel A. Triterpenoid profile of fruit and leaf cuticular waxes of edible honeysuckle Lonicera caerulea var. kamtschatica. Acta Soc Bot Pol. 2017;86:3539 [Google Scholar]
- 21.Chen L, Xin X, Yuan Q, Su D, Liu W. Phytochemical properties and antioxidant capacities of various colored berries. J Sci Food Agric. 2014;94:180–188. doi: 10.1002/jsfa.6216. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Wang Z, Ma F, Yang X, Cheng C, Yao L. Protective effect of anthocyanin from Lonicera caerulea var. edulis on radiation-induced damage in mice. Int J Mol Sci. 2012;13:11773–11782. doi: 10.3390/ijms130911773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palíková I, Valentová K, Oborná I, Ulrichová J. Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage. J Agric Food Chem. 2009;57:6584–6589. doi: 10.1021/jf9003994. [DOI] [PubMed] [Google Scholar]
- 24.Vostálová J, Galandáková A, Palíková I, Ulrichová J, Doležal D, Lichnovská R, Vrbková J, Rajnochová SA. Lonicera caerulea fruits reduce UVA-induced damage in hairless mice. J Photochem Photobiol B. 2013;128:1–11. doi: 10.1016/j.jphotobiol.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Wu D, Zheng N, Qi K, Cheng H, Sun Z, Gao B, Zhang Y, Pang W, Huangfu C, Ji S, Xue M, Ji A, Li Y. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med Gas Res. 2015;5:1. doi: 10.1186/s13618-014-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SI, Lee YJ, Choi SH, Park SJ, Song CH, Ku SK. Therapeutic effects of blue honeysuckle on lesions of hyperthyroidism in rats. Am J Chin Med. 2016;44:1441–1456. doi: 10.1142/S0192415X16500804. [DOI] [PubMed] [Google Scholar]
- 27.Jurgoński A, Juśkiewicz J, Zduńczyk Z. An anthocyanin-rich extract from Kamchatka honeysuckle increases enzymatic activity within the gut and ameliorates abnormal lipid and glucose metabolism in rats. Nutrition. 2013;29:898–902. doi: 10.1016/j.nut.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Wu S, He X, Wu X, Qin S, He J, Zhang S, Hou DX. Inhibitory effects of blue honeysuckle (Lonicera caerulea L) on adjuvant-induced arthritis in rats: crosstalk of anti-inflammatory and antioxidant effects. J Funct Foods. 2015;17:514–523. [Google Scholar]
- 29.Zhao J, Lin Y, Zhao Y, Wang Y, Ning C, Ma Y, Meng X. Polyphenol-rich blue honeysuckle extract alleviates silica particle-induced inflammatory responses and macrophage apoptosis via NRF2/HO-1 and MAPK signaling. J Funct Foods. 2018;46:463–474. [Google Scholar]
- 30.Liu S, You L, Zhao Y, Chang X. Wild Lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Res Int. 2018;107:73–83. doi: 10.1016/j.foodres.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Wu Z, Guo S, Meng X, Chang X. Polyphenol-rich extract from wild Lonicera caerulea berry reduces cholesterol accumulation by mediating the expression of hepatic miR-33 and miR-122, HMGCR, and CYP7A1 in rats. J Funct Foods. 2018;40:648–658. [Google Scholar]
- 32.Liu M, Tan J, He Z, He X, Hou DX, He J, Wu S. Inhibitory effect of blue honeysuckle extract on high-fat-diet-induced fatty liver in mice. Anim Nutr. 2018;4:288–293. doi: 10.1016/j.aninu.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HS, Chang JH, Ku SK. An immunohistochemical study of the pancreatic endocrine cells of the ddN mouse. Folia Histochem Cytobiol. 2010;48:387–393. doi: 10.2478/v10042-010-0026-y. [DOI] [PubMed] [Google Scholar]
- 34.Lee JE, Kang SJ, Choi SH, Song CH, Lee YJ, Ku SK. Fermentation of green tea with 2% Aquilariae lignum increases the anti-diabetic activity of green tea aqueous extracts in the high fat-fed mouse. Nutrients. 2015;7:9046–9078. doi: 10.3390/nu7115447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HS, Chang JH, Ku SK. An immunohistochemical study of the pancreatic endocrine cells of the ddN mouse. Folia Histochem Cytobiol. 2010;48:387–393. doi: 10.2478/v10042-010-0026-y. [DOI] [PubMed] [Google Scholar]
- 36.Levene A. Pathological factors influencing excision of tumours in the head and neck. Part I. Clin Otolaryngol Allied Sci. 1981;6:145–151. doi: 10.1111/j.1365-2273.1981.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 37.Ludbrook J. Update: microcomputer statistics packages. A personal view. Clin Exp Pharmacol Physiol. 1997;24:294–296. doi: 10.1111/j.1440-1681.1997.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 38.Choi JS, Kim JW, Park JB, Pyo SE, Hong YK, Ku SK, Kim MR. Blood glycemia-modulating effects of melanian snail protein hydrolysates in mice with type II diabetes. Int J Mol Med. 2017;39:1437–1451. doi: 10.3892/ijmm.2017.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jurikova T, Rop O, Mlcek J, Sochor J, Balla S, Szekeres L, Hegedusova A, Hubalek J, Adam V, Kizek R. Phenolic profile of edible honeysuckle berries (Genus Lonicera) and their biological effects. Molecules. 2011;17:61–79. doi: 10.3390/molecules17010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rupasinghe HPV, Arumuggam N, Amararathna M, De Silva ABKH. The potential health benefits of haskap (Lonicera caerulea L.): role of cyanidin-3-O-glucoside. J Funct Foods. 2018;44:24–39. [Google Scholar]
- 41.Sancho RAS, Pastore GM. Evaluation of the effects of anthocyanins in type 2 diabetes. Food Res Int. 2012;46:378–386. [Google Scholar]
- 42.Podsędek A, Majewska I, Redzynia M, Sosnowska D, Koziołkiewicz M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J Agric Food Chem. 2014;62:4610–4617. doi: 10.1021/jf5008264. [DOI] [PubMed] [Google Scholar]
- 43.Tan Y, Kim J, Cheng J, Ong M, Lao WG, Jin XL, Lin YG, Xiao L, Zhu XQ, Qu XQ. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J Gastroenterol. 2017;23:3805–3814. doi: 10.3748/wjg.v23.i21.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim UH, Yoon JH, Li H, Kang JH, Ji HS, Park KH, Shin DH, Park HY, Jeong TS. Pterocarpan-enriched soy leaf extract ameliorates insulin sensitivity and pancreatic β-cell proliferation in type 2 diabetic mice. Molecules. 2014;19:18493–18510. doi: 10.3390/molecules191118493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 46.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Qu Z, Fu L, Dong P, Zhang X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J Food Sci. 2009;74:C469–C474. doi: 10.1111/j.1750-3841.2009.01231.x. [DOI] [PubMed] [Google Scholar]
- 48.Ku SK, Sung SH, Choung JJ, Choi JS, Shin YK, Kim JW. Anti-obesity and anti-diabetic effects of a standardized potato extract in ob/ob mice. Exp Ther Med. 2016;12:354–364. doi: 10.3892/etm.2016.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo HY, Hsiang CY, Li TC, Li CC, Huang HC, Chen JC, Ho TY. A novel glycated hemoglobin A1c-lowering traditional Chinese medicinal formula, identified by translational medicine study. PLoS One. 2014;9:e104650. doi: 10.1371/journal.pone.0104650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, Imperatore G, Williams DE, Albright AL. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33:1665–1673. doi: 10.2337/dc09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim CM, Yi SJ, Cho IJ, Ku SK. Red-koji fermented red ginseng ameliorates high fat diet-induced metabolic disorders in mice. Nutrients. 2013;5:4316–4332. doi: 10.3390/nu5114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung SI, Rico CW, Kang MY. Comparative study on the hypoglycemic and antioxidative effects of fermented paste (doenjang) prepared from soybean and brown rice mixed with rice bran or red ginseng marc in mice fed with high fat diet. Nutrients. 2014;6:4610–4624. doi: 10.3390/nu6104610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007;117:246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noriega-López L, Tovar AR, Gonzalez-Granillo M, Hernández-Pando R, Escalante B, Santillán-Doherty P, Torres N. Pancreatic insulin secretion in rats fed a soy protein high fat diet depends on the interaction between the amino acid pattern and isoflavones. J Biol Chem. 2007;282:20657–20666. doi: 10.1074/jbc.M701045200. [DOI] [PubMed] [Google Scholar]